Abstract
A young adult male domestic shorthair cat was presented for physical examination, routine vaccinations, and a fecal examination. Physical examination revealed no significant abnormalities. Eggs of the raccoon pancreatic fluke Eurytrema procyonis were detected by fecal flotation. Results of a complete blood count and serum biochemistry panel were normal. Abdominal sonography revealed an enlarged hypoechoic pancreas with a hyperechoic rim, and a distended and thickened pancreatic duct. Serum pancreatic lipase immunoreactivity (PLI) was increased. These findings supported the possibility of fluke-associated pancreatitis. Treatment with praziquantel/pyrantel/febantel was associated with resolution of sonographic abnormalities and normalization of PLI.
A young adult, castrated male, domestic shorthair cat was presented to the Community Practice Service of the Cornell University Hospital for Animals for routine vaccinations and a fecal examination. He had been adopted from a shelter by his owner 2 months previously, and had since remained indoors sharing the household with one other cat. A month prior to presentation, the owner noticed ‘worms’ in the feces, and a veterinarian prescribed two doses of a treatment for roundworms, given 2 weeks apart. The client did not remember the product's name and did not notice any worms in the feces after treatment.
On initial presentation, there were no significant findings upon physical examination. The owner reported no abnormalities in the feces and no vomiting. The cat tested negative for feline leukemia and feline immunodeficiency viruses. He received routine annual vaccinations. A fecal specimen examined using centrifugal flotations (solutions: zinc sulfate and sugar, specific gravities of 1.18 and 1.33, respectively) contained eggs of Aonchotheca (Capillaria) putorii (an epithelial parasite of the stomach and small intestine of cats, mustelids, raccoons, and bears (Bowman et al 2002)) and eggs of the trematode Eurytrema procyonis (Fig 1). E procyonis can be common in some cat populations (10.7% of 290 stray cats in St Louis, MO, Fox et al 1981), and the authors were interested in any potential clinical implications. Client consent was obtained to perform additional diagnostic testing at no charge.
Fig 1.
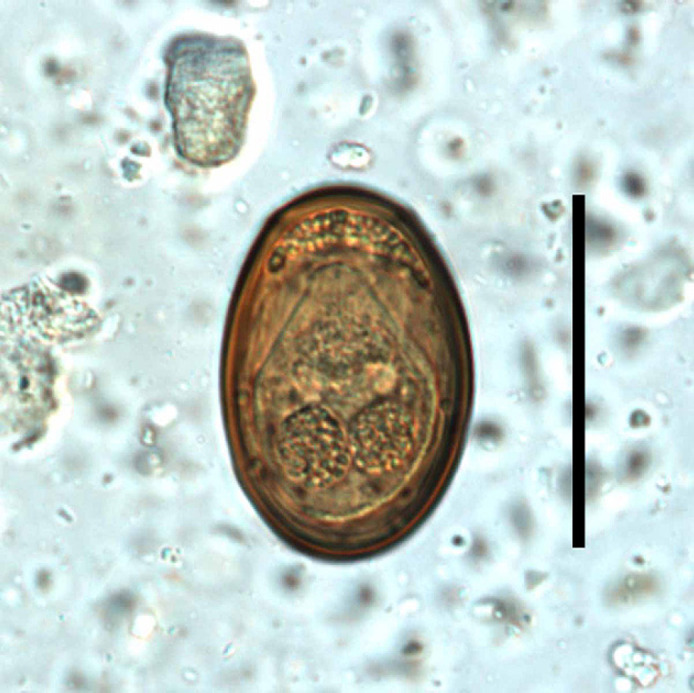
A Eurytrema procyonis egg passed in the feces of the cat in this case (bar represents 50 μm).
The cat returned the following week, at which time mild intestinal thickening was palpated. No significant abnormalities were noted on the complete blood count and serum biochemistry panel. Urine sediment was normal; urine specific gravity was 1.030. A mild glucosuria (100 mg/dl) was observed, but was not present on repeat urinalysis at a follow-up visit. The glucosuria was, therefore, presumed to have been residual evidence of a transient stress-induced hyperglycemia (Jackson 2007).
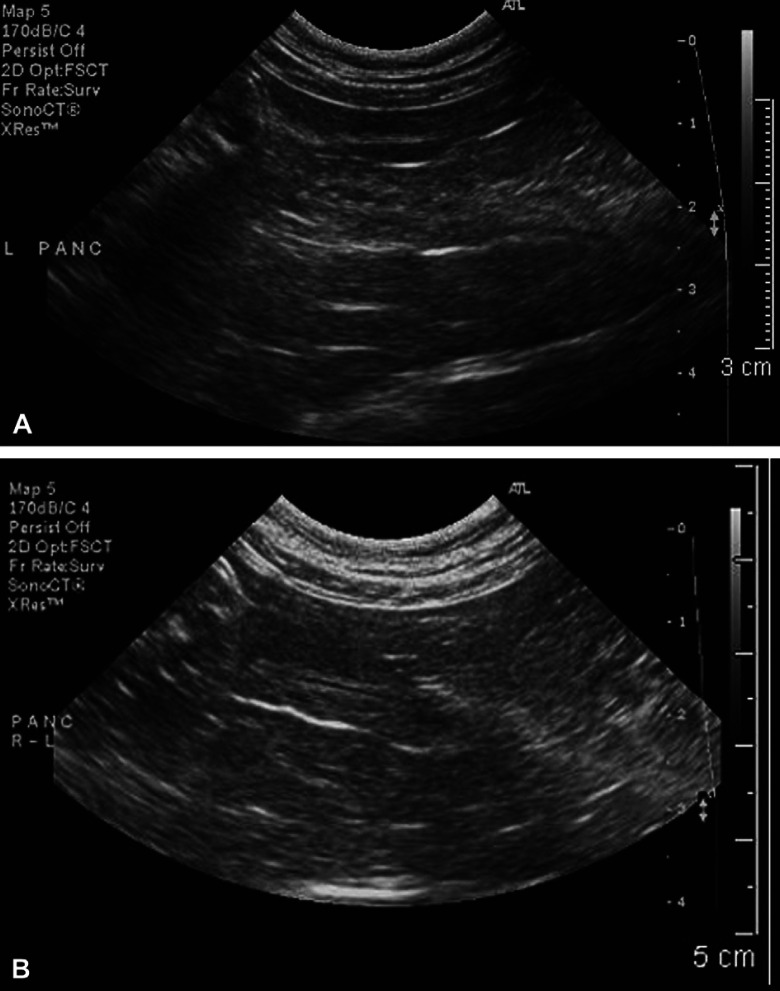
Abdominal sonography revealed several abnormalities. Portions of the pancreas were mildly enlarged (body 1 cm thick), and the contour of the left limb was irregular and surrounded by a thin hyperechoic rim, suggestive of saponified fat. Findings were consistent with active pancreatic inflammation (Simpson et al 1994, Saunders et al 2002). The pancreatic duct was diffusely abnormal: the lumen was distended to a diameter of 1–2 mm with hypoechoic contents and an irregular ‘beaded’ shape, and the duct wall was 1–2 mm thick and abnormally hyperechoic (Fig 2A). The ductal changes were considered consistent with inflammation, fibrosis, or edema (Larson et al 2005), and it was suspected that the pancreatic abnormalities were caused by the E procyonis infection. Other changes included: mild hepatomegaly, with portal veins notably smaller (1.8 mm) than the hepatic veins (4.3 mm), but with normal color flow; mild diffuse thickening of the small intestine (3.4–4.2 mm), consistent with physical examination findings; mild thickening of the cecal wall (3.3 mm); moderately enlarged gastric and jejunal lymph nodes (5–7.5 mm diameter); and abnormal linear material approximately 1 mm thick in the gastric lumen, suggestive of ascarids.
Fig 2.
Long-axis sonographic view of the left limb of the pancreas in the E procyonis infected cat. (A) At time of presentation. Note the mildly enlarged pancreas (1.0 cm thick) with its irregular contour. The pancreatic duct has thickened walls, an irregular, ‘beaded’, mildly distended lumen, and hypoechoic lumen contents. (B) Eighteen weeks after treatment with praziquantel, pyrantel, and febantel. The pancreas has a smooth contour and is reduced in size (0.8 cm thick). The pancreatic duct is now of normal size and architecture.
To further define the pancreatic abnormalities, serum was submitted to the Gastrointestinal Laboratory at Texas A&M University, College of Veterinary Medicine, for the measurement of feline pancreatic lipase immunoreactivity (PLI). Serum PLI was markedly increased (84.9 μg/dl; reference range 2–6.8 μg/dl), consistent with pancreatic inflammation (Forman et al 2004).
Examination of feces from the day of the second visit revealed large numbers of E procyonis eggs and a few A putorii eggs. The cat was treated with oral fenbendazole (Panacur Horse and Cattle Dewormer Suspension 10%; Intervet), 30 mg/kg once daily for 6 days. The plan had been to repeat hematology, biochemistry, and sonography in 1 month; however, this was delayed because weekly fecal samples continued to be positive for E procyonis eggs over the next 6 weeks.
Five weeks later, the cat had gained 1.0 kg in body weight, and had a 7/9 body condition score. Physical examination was normal. Abdominal sonography showed persistence of the pancreatic and intestinal abnormalities. Hematology and serum biochemistry were normal apart from mild hyperglycemia (145 mg/dl; reference range 63–140 mg/dl) consistent with stress. PLI remained increased (24.0 μg/dl). Serum cobalamin was >3000 pg/ml (reference range 950–2000 pg/ml) and did not support the presence of intestinal malabsorption (Simpson et al 2001).
Two weeks later (8 weeks after starting fenbendazole), fecal examination revealed the eggs of E procyonis, A putorii, Toxocara cati and Ancylostoma tubaeforme. The cat was treated with praziquantel and started on praziquantel/pyrantel/febantel therapy (Drontal Plus; Bayer: one-and-a-half tablets, 34 mg praziquantel, 34 mg pyrantel, and 170 mg febantel, PO sid for 5 days).
The cat was re-examined at 8 and 18 weeks after treatment with praziquantel/pyrantel/febantel, and samples from these visits were negative for all parasites. PLI concentrations at 8 (4.8 μg/dl) and 18 (4.3 μg/dl) weeks were within reference range. Abdominal sonography at 18 weeks showed the majority of the pancreas being of normal thickness and echogenicity, although the left limb remained slightly irregular and focally enlarged. The hyperechoic rim suggestive of saponified fat had resolved and the pancreatic duct was smooth and of normal diameter; the peri-ductular hyperechogenicty had partially resolved (Fig 2B).
Typically, there is little frank disease observed in response to infection by E procyonis. This trematode of the raccoon, Procyon lotor, is sometimes found in foxes and cats (Roudebush and Schmidt 1982, Wade et al 1989). The life cycle is incompletely known, but the final host is probably infected via ingestion of an arthropod (Denton 1944). As adults, the flukes commonly live within the pancreatic ducts, but they have also been reported in the gall bladder and bile ducts. In a number of feline reports, diagnosis has been made post mortem (Burrows and Lillis 1960, Sheldon 1966, Anderson et al 1987). Pancreatic dysfunction via cannulation was examined in 31 naturally infected cats prior to euthanasia. Impaired pancreatic function was demonstrated by measuring total secretory volume and bicarbonate ion and protein concentrations of the pancreatic fluid after the administration of secretin and/or cholecystokinin (Fox et al 1981). One cat with a subclinical infection, diagnosed by detection of E procyonis eggs in its feces, had normal serum amylase, lipase, and fecal trypsin activities (Roudebush and Schmidt 1982). Another cat that was euthanased and necropsied after 2 years of persistent weight loss, vomiting, and ultimately incoordination, had a fibrotic pancreas containing numerous E procyonis in its ducts that were lined with hyperplastic epithelium infiltrated with eosinophils and mononuclear cells (Anderson et al 1987).
The cat in this case report was infected subclinically with E procyonis. Pancreatic disease was only evident after a thorough investigation. Clinical findings in pancreatitis are often non-specific and do not necessarily include vomiting and cranial abdominal pain, signs that are commonly associated with canine pancreatitis. Routine clinicopathological tests are also non-specific (Forman et al 2004).
The initial treatment with fenbendazole did not stop egg shedding, although it may have damaged or killed some of the trematodes. The remaining trematodes may have been of decreased bulk allowing duct activity to return towards normal. Because there is not a linear relationship between the quantitative PLI value and the actual severity of inflammation (Forman et al 2004), the decrease in PLI from 84.9 to 24.0 μg/dl after fenbendazole treatment can be considered proof neither for nor against partial resolution of infection. Following treatment with praziquantel/pyrantel/febantel, the cat was cleared of its infection as evidenced by a cessation of egg shedding, and there was sonographic improvement and normalization of PLI.
A combination of sonographic abnormalities and increased serum PLI is currently considered the best evidence to support a non-invasive diagnosis of pancreatic inflammation (Forman et al 2004). The parenchymal irregularity and hyperechoic rim observed during initial sonography of this cat, coupled with the markedly increased PLI value, strongly supported a diagnosis of pancreatitis, despite the lack of clinical signs. Distension and thickening of the pancreatic duct is not usually associated with pancreatitis, and in this case it was probably due to the presence of pancreatic flukes (Simpson et al 1994, 2001, Saunders et al 2002). Previous terminal studies have indicated that pancreatic fibrosis and atrophy associated with chronic E procyonis infection can be sufficient to cause exocrine pancreatic insufficiency, and in rare cases even diabetes (Fox et al 1981, Anderson et al 1987). Sonography of asymptomatic, infected cats may assist in determining the extent of pancreatic disease and resolution after treatment.
Acknowledgements
The students on CPS rotation: Drs Vanessa Philson, Collin Wolff, Christina Kehas, Tim Bartsch, Laura Cromal, Jee-Yon Pyun, and Courtney Grandner; Alice C. Y. Lee, DVM, Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA.
References
- Anderson W.I., Georgi M.E., Car B.D. Pancreatic atrophy and fibrosis associated with Eurytrema procyonis in a domestic cat, Veterinary Record 120, 1987, 235–236. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Hendriz C.M., Lindsay D.S., Barr S.C. Aonchotheca putorii (Rudolphi, 1819) Lopez-Neyra, 1947, Feline Clinical Parasitology, 2002, Iowa State University Press: Ames, IA, 340–342. [Google Scholar]
- Burrows R.B., Lillis W.G. Eurytrema procyonis Denton, 1942 (Trematoda: Dicrocoelidae), from the domestic cat, Journal of Parasitology 45, 1960, 810–812. [PubMed] [Google Scholar]
- Denton J.F. Studies on the life history of Eurytrema procyonis Denton, 1942, Journal of Parasitology 30, 1944, 277–286. [Google Scholar]
- Forman M.A., Marks S.L., De Cock H.E.V., Hergesell E.J., Wisner E.R., Baker T.W., Kass P.H., Steiner J.M., Williams D.A. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis, Journal of Veterinary Internal Medicine 18, 2004, 807–815. [DOI] [PubMed] [Google Scholar]
- Fox J.N., Mosley J.G., Vogler G.A., Austin J.A., Reber H.A. Pancreatic function in domestic cats with pancreatic fluke infection, Journal of the American Veterinary Medical Association 178, 1981, 58–60. [PubMed] [Google Scholar]
- Jackson M.L. Renal system, Veterinary Clinical Pathology, an Introduction, 2007, Blackwell Publishing: Oxford, 194–222. [Google Scholar]
- Larson M.M., Panciera D.L., Ward D.L., Stenier J.M., Williams D.A. Age-related changes in the ultrasound appearance of the normal feline pancreas, Veterinary Radiology and Ultrasound 46 (3), 2005, 238–242. [DOI] [PubMed] [Google Scholar]
- Roudebush P., Schmidt D.A. Fenbendazole for treatment of pancreatic fluke infection in a cat, Journal of the American Veterinary Medical Association 180, 1982, 545–546. [PubMed] [Google Scholar]
- Saunders H.M., VanWinkle T.J., Drobatz K., Kimmel S.E., Washabau R.J. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994–2001), Journal of the American Veterinary Medical Association 221, 2002, 1724–1730. [DOI] [PubMed] [Google Scholar]
- Sheldon W.G. Pancreatic flukes (Eurytrema procyonis) in domestic cats, Journal of the American Veterinary Medical Association 148, 1966, 251–253. [PubMed] [Google Scholar]
- Simpson K.W., Fyfe J., Cornetta A., Sachs A., Strauss-Ayali D., Lamb S.V., Reimers T.J. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease, Journal of Veterinary Internal Medicine 15, 2001, 26–32. [DOI] [PubMed] [Google Scholar]
- Simpson K.W., Shiroma J.T., Biller D.S., Wicks J., Johnson S.E., Dimski D., Chew D. Ante mortem diagnosis of pancreatitis in four cats, Journal of Small Animal Practice 35, 1994, 93–99. [Google Scholar]
- Wade S.E., Anderson W.I., Kidder J.D. Eurytrema procyonis in a raccoon (Procyon lotor) from New York State – a first report, Journal of Wildlife Diseases 25 (2), 1989, 270–272. [DOI] [PubMed] [Google Scholar]