Abstract
Cerebellar degeneration (abiotrophy) (CD) is a spontaneous and accelerated degeneration of one or several mature cerebellar neuronal cell populations and has been described in many domestic animals, especially in dogs, with numerous breed-related cases. In cats, CD is mentioned as a rare sporadic entity. Late onset CDs are exceptionally uncommon and only two cases are reported in young adults, both aged 18 months. This report describes clinical and pathological findings of a late onset feline CD in a 9-year-old male Persian cat. The cat was presented with a history of progressive ataxia lasting 2 years. Neurological examination revealed severe neurological deficits such as generalised and severe ataxia, hypermetria in all four limbs, and bilateral absence of menace response. The lesion was diffusely localised in cerebellum. On gross pathology, the cerebellum appeared of normal size and shape and kidneys were characterised by mild hyperaemia. Histologically, lesions were limited to the cerebellum and kidneys. In the cerebellum, all cerebellar folia of both hemispheres and the vermis were affected. Changes were characterised by severe and diffuse loss of Purkinje cells, loss of cellularity in the granular layer, mild astrogliosis associated with moderate hypertrophy of Bergmann's glia. Immunohistochemistry for feline parvovirus antigen revealed a negative result. Renal lesions consisted of chronic fibrosis associated with chronic interstitial nephritis. CD is a rare disease and occurs commonly in puppies or young animals, who are clinically normal at birth and usually develop neurological signs within a few weeks or months after birth. This report represents the first case of CD in a middle-aged cat.
Cerebellar cortical degeneration (abiotrophy) (CD) is commonly considered a neurological condition characterised by premature degeneration and death of one or several cerebellar neuronal cell populations (Jubb and Huxtable 1993, Summers et al 1995). The term CD, used in its original definition, refers to a neurological condition that occurs in cerebellar neurons that have reached complete maturity and are functionally normal. CD has to be differentiated from cerebellar hypoplasia, a term indicating a failure of complete neuronal development (Jubb and Huxtable 1993, Summers et al 1995).
In human neuropathology, the term cerebellar ‘abiotrophy’ is rarely used; some degenerative conditions, such as Parkinson's and Alzheimer's disease (Summers et al 1995), are considered examples of abiotrophic diseases. A particular form of cerebellar degeneration, called subacute cerebellar degeneration (SCD) seems to be associated with two different conditions: paraneoplastic cerebellar degeneration, which sometimes precedes the diagnosis of neoplasia, and alcoholic or nutritional cerebellar degeneration, caused, eg, by vitamin B1 deficiency (Gray et al 2004).
CD has been described in many domestic animals, including dogs, cattle, sheep, horses, and pigs. In several cases of CD, a hereditary mechanism has been assumed and numerous canine breed-related abiotrophies are described (De Lahunta 1990, Jubb and Huxtable 1993). CD commonly occurs in puppies or young animals, who are clinically normal at birth and usually develop neurological signs within the first few weeks or months after birth; cases of late onset or geriatric CD have been described in some canine breeds comprising Brittany Spaniels and American Staffordshire Terriers (LeCouter et al 1988, Jubb and Huxtable 1993, Higgins et al 1998, Olby et al 2004).
In cats, CD is considered a rare neurological condition. In Japan, several cases of early onset cerebellar cortical degeneration has been observed in different kitten populations; a feline parvovirus infection was ruled out in these kittens. For some of them, a hereditary condition has been suggested (Inada et al 1996, Aye et al 1998, Barone et al 2002). Only two cases, involving young adults have been reported to date (Shamir et al 1999, Barone et al 2002). Other cases of feline CD have been referred as anecdotal (De Lahunta 1980; Summers et al 1995) without complete breed, age, clinical and pathological descriptions. In the present report, the clinical and pathological findings in a feline late onset cerebellar abiotrophy are described, and to the authors' knowledge, this is the first CD report in a middle-aged cat.
A 9-year-old male Persian indoor cat was presented with a history of ataxia lasting for 2 years. The cat was adopted by the owner at 5 years of age and was found to be clinically normal at that time. At 7 years of age, the cat showed a depressed mental status, associated with an episode of vomiting and diarrhoea. Two weeks later a mild ataxia in the hind limbs was observed, but no diagnostic investigations were performed at this stage to investigate these neurological signs. Vomiting and diarrhoea were interpreted as signs of small intestinal bacterial overgrowth and a symptomatic treatment was instituted. The cat's overall performance temporally improved but neurological signs persisted. The ataxia progressively involved the fore limbs and the neurological signs slowly progressed over 2 years, when the cat was presented to his referring veterinarian for persisting episodes of vomiting. Cell blood count and biochemical parameters (aspartate aminotransferase, alanine aminotransferase, creatine kinase (CK), creatinine, urea, glucose, total bilirubin, triglycerides, total cholesterol, γ-glutamyltransferase, total proteins, albumin, albumin/globulin, calcium, phosphorus, sodium, potassium, chloride) were within normal limits except for an increase of urea (79.9 mg/dl, reference value: 20–65 mg/dl), creatinine (2.58 mg/dl, reference value: 0.8–1.8 mg/dl), total cholesterol (258 mg/dl, reference value: 95–130 mg/dl) and CK (518 UI/l, reference value: 18–230 UI/l), suggesting a possible renal disease. Serologically, titres for feline infectious peritonitis virus, feline leukaemia virus and feline immunodeficiency virus as well as Toxoplasma gondii were not detected. No history of past trauma or toxic ingestion was reported. No treatments were instituted and then, the cat was referred to our Institution for neurological evaluation.
Physical examination revealed a poorly groomed coat and moderate dehydration. At neurological examination, generalised and severe ataxia, intention tremor of the head, severe hypermetria in all four limbs, wide based stance, and loss of balance with frequent events of falling and tumbling were recorded. In addition, the cat exhibited a bilateral absence of the menace response with normal pupillary light reflex. Clinically, the lesion was diffusely localised in the cerebellum. Differential diagnoses included degenerative diseases, including late onset lysosomal storage diseases, such as gangliosidosis, and feline cerebellar abiotrophy. Neuroaxonal dystrophy was included in degenerative differential diagnoses even if, with the late onset of neurological signs, it was considered unlikely. Moreover, a metabolic condition or a chronic toxicity was not included, despite the possible underlying renal dysfunction previously detected by blood tests, as cerebellar signs as unique neurological manifestations of a systemic disorder are not well defined in veterinary medicine. Based on the slow progression and protracted clinical signs, an inflammatory and neoplastic process was considered unlikely. Due to the poor prognosis, the owner requested euthanasia and a complete necropsy was performed.
Gross lesions were observed only in the kidneys, which presented diffuse hyperaemia associated to mild and diffuse atrophy. On gross examination, the cerebellum appeared to be of expected size and shape. Tissues of various organs, such as brain, spinal cord, digestive tract, trachea, lungs, liver, spleen, bladder, kidneys, adrenals, pancreas, popliteal lymph nodes, mesenteric lymph nodes, bronchial lymph nodes, myocardium, sciatic nerves, samples of femoral quadriceps, gastrocnemius and brachial triceps were collected and placed in neutral buffered 10% formalin for histopathology. Tissue samples were processed routinely, embedded in paraffin and stained with haematoxylin and eosin and Luxol fast blue. Immunohistochemical staining for detection of glial fibrillary acidic protein (GFAP, DAKO, mouse monoclonal anti-human, clone 6F2) and feline parvovirus antigen (monoclonal antibody, clone CPV1-2A1, from Chris K. Grant, Harbor Blvd, W. Sacramento, CA) was performed on paraffin-embedded sections using EnVision system (DAKO EnVision, peroxidase; Mouse).
Microscopically, lesions were limited to the cerebellum and kidney. In the cerebellum, all cerebellar folia of both cerebellar hemispheres and the vermis were affected. Histological changes were characterised by severe and diffuse loss of Purkinje cells and small neurons in the granular layer (Fig 1). Few degenerated Purkinje cells, with dark eosinophilic cytoplasm, were present in the granular layer. A mild to moderate gliosis, presumably astrogliosis, in the molecular and granular layers was present. In addition, a moderate to severe hypertrophy and hyperplasia of Bergmann's glial cells and their processes in the Purkinje cell layer were noticed (Fig 2). Those cells displayed an irregular round shape, with distinct cell borders and a moderate homogenous eosinophilic cytoplasm and a centrally placed, round–oval, vesicular nucleus; a single and central nucleolus was commonly present. Luxol fast blue stain revealed a loss of positivity of Purkinje cells processes (Fig 3) and a generalised reduction of staining positivity also in the granular layer (Fig 4). These features detected the loss of myelin that is likely secondary to the severe loss of neurons in Purkinje and granular layers. In addition, a moderate Wallerian-type degeneration was present in the white matter of the folia, as confirmed by the Luxol fast blue stain. In the brain stem, no lesions were observed in any nuclei that projected to cerebellum. Immunohistochemistry for GFAP confirmed the diffuse astrogliosis and proliferation Bergmann's glial cells. Feline parvovirus antigen could not be detected in various brain regions including the cerebellum. The morphological diagnosis was a cerebellar degeneration.
Fig 1.
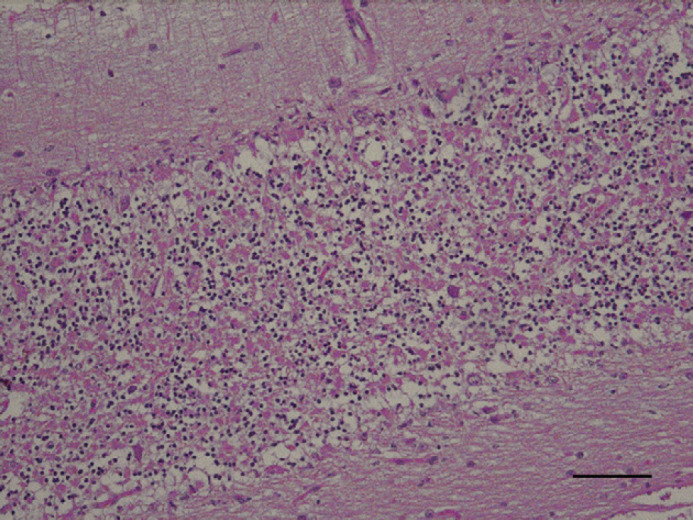
Cerebellar vermis; feline. Severe and diffuse loss of cellularity in cerebellar cortex, mainly in Purkinje granular cell layers. H&E. Bar=40 μm.
Fig 2.
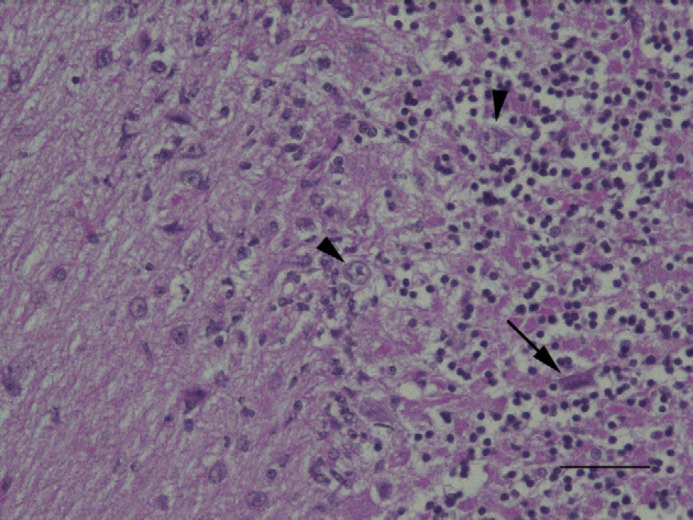
Left cerebellar hemisphere; feline. Note a pycnotic, shrunken Purkinje cell (arrow) in granular layer and the overall loss of cellularity. Hypertrophic Bergmann's glial cells (arrowheads) are also present. H&E. Bar=20 μm.
Fig 3.
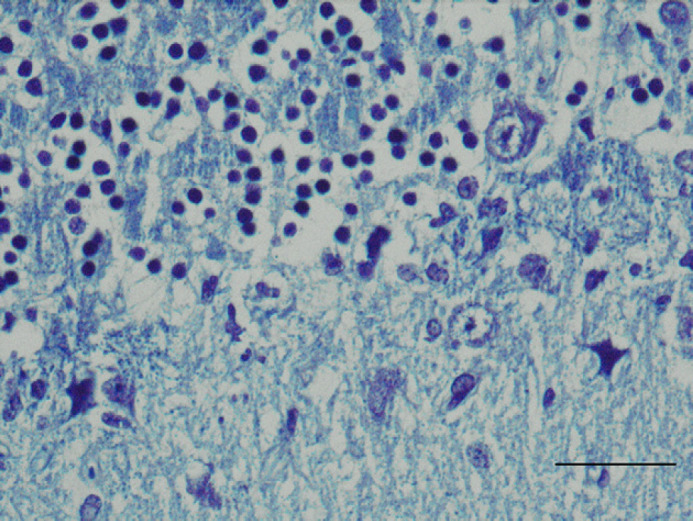
Cerebellum; feline. Loss of myelin of the Purkinje cells processes. Luxol fast blue. Bar=15 μm.
Fig 4.
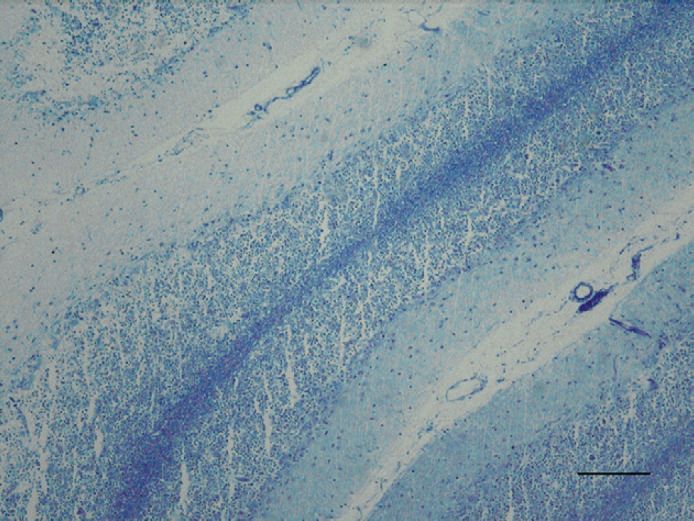
Cerebellum; feline. Generalised reduction of myelin in the granular layer due to severe loss of Purkinje and granular cells. Luxol fast blue. Bar=100 μm.
In the kidney, multifocal and moderate interstitial fibrosis was observed in association with a multifocal mild to moderate infiltration of lymphocytes and plasma cells.
Neurological signs such as generalised ataxia, hypermetria and bilateral absence of the menace reaction resulted from severe and diffuse involvement of the cerebellum. For the slow progression of the signs, the main differential diagnosis was degenerative disease, such as cerebellar degeneration (CD) or lysosomal storage disease. These features are similar to both feline cerebellar abiotrophy (CA) cases reported in literature (Shamir et al 1999, Barone et al 2002).
Comparing the two late onset CA reports (Shamir et al 1999, Barone et al 2002) to the present case, several similarities can be recognised. Within cerebellum, no gross lesions were observed in the first report (Shamir et al 1999), while, in 2002, Barone detected a moderate loss of cerebellar volume, which was valuated as two-thirds of normal size. In the present case, no macroscopical alterations on size or structure of the cerebellum were recorded. In general, abnormal cerebellar size has been reported to be frequently present in the late stage of the disease and, in particular, with the progression, cerebellar shrinkage can be observed (Summers et al 1995). From a histopathological point of view, in the present case, both cerebellar hemispheres were affected by lesions comparable for type, distribution and amount to those present in vermis. These features are typical of late stage of the disease (Summers et al 1995). Purkinje cells are usually firstly affected and reduced in number, followed by a reduction of the granule cells and shrinkage of the molecular layer can also be observed (Shamir et al 1999). Loss of cellularity in molecular and granular layers is usually directly related to the amount of Purkinje cells loss, as the integrity of granular cells depends on its synaptic relationships with Purkinje dendrites (De Lahunta 1990). Histological type of lesions observed in the present case were extremely similar to those reported by Barone in 2002 and by Shamir in 1999. Lack of Luxol fast blue staining in the Purkinje cell processes confirmed severe loss of these synaptic relationships.
In addition, Barone et al (2002) reported renal lesions characterised by chronic fibrosis. Similar histological findings, in association with moderate and diffuse interstitial nephritis, were recorded in the present case. In both cases, these lesions were not considered to be related to the neuropathological findings, and in the present case, they could be age-related. Moreover, in 2002, Barone recorded retinal photoreceptor degeneration, and also this condition is not related to the cerebellar degenerative disease. In the present case, retinal status was not investigated due to the lack of clinical evidence of visual deficits.
The aetiology of cerebellar cortical abiotrophy is mostly unknown. In some species, such as canine, a hereditary condition has been suggested and an autosomal recessive model of inheritance has been confirmed or strongly suspected (De Lahunta 1990, Jubb and Huxtable 1993). Similarly, in Japan, some recent cases of feline hereditary cerebellar degeneration have been reported and an autosomal recessive transmission has been assumed (Taniyama et al 1994, Inada et al 1996). Those cats developed cerebellar signs around 6–7 weeks of age, characterised by intention tremor, wide based stance and ataxia. The clinical course was progressive in few months. Macroscopically, a severe reduction in size was observed, and histological findings included loss of Purkinje cells associated with an increase in Bergmann's glia. These lesions were more pronounced in the cerebellar hemispheres than in vermis. Moreover, moderate neuronal depletion in the olivary nuclei was detected. This condition has been recently considered a hereditary form of cerebellar abiotrophy (Barone et al 2002). In humans, hereditary cerebellar degenerative conditions, such as spinocerebellar ataxia (SCA) have been investigated. Six forms of spinocerebellar ataxia have been reported and lesions in the brain invariably affect the cerebellar cortex, the dentate nuclei, and the inferior olivary nuclei (Koeppen 2005).
Moreover, in animals, cerebellar signs, together with other neurological manifestations, can be present in extrinsic metabolic alterations, such as toxics (Jubb and Huxtable 1993, Summers et al 1995), even if chronic toxicity causing specifically cerebellar degeneration has not been well defined. Global environmental pollutants may have a role in humans and animals brain degeneration. There is evidence, in human medicine, for example, that heavy metal intoxications, such as methyl mercury, used as pesticide, can cause degeneration of different brain sites, such as cerebellum (Castoldi et al 2003). In the present study, chronic toxicity was not considered within the differential diagnoses due to history and clinical manifestations, restricted to cerebellar involvement only.
Intrinsic metabolic conditions as causes of cerebellar degeneration have not been described in veterinary medicine and rarely reported in human medicine. Moreover, to the authors' knowledge, in veterinary medicine, there are no reports regarding cerebellar degeneration as unique neurological abnormality in a metabolic scenario or relating the cerebellar degeneration with a chronic renal failure.
In animals, to the authors' knowledge, no paraneoplastic conditions are reported as causes of CD as in human medicine.
CD has to be differentiated by cerebellar hypoplasia (CH), in which several intrinsic or extrinsic factors can modify the normal development of cerebellar neuronal populations (Summers et al 1995). The most frequent causes of CHs are anti-mitotic agents and intrauterine viral infections, such as parvoviruses and pestiviruses (Summers et al 1995). In cats, feline panleucopenia virus infection during pregnancy is one of the most common cause of CH, with a selective destruction of proliferating cells of the external granular layer and occasionally the Purkinje cells layer too (Summers et al 1995). In the present case, CH due to parvovirus infection was ruled out by the late onset of the neurological signs, the slow progression of the cerebellar deficits, the histological features, and immunohistochemistry.
Neuroaxonal dystrophy (NA), another cerebellar degenerative disease has to be differentiated from cerebellar abiotrophy (Carmichael et al 1993, Woodard et al 1994, Summers et al 1995, Rodriguez et al 1996). NA is usually seen in cats less than 1 year of age and is supposed to be linked to coat colour dilution. This disease involves many CNS structures, comprising cerebellum, and it is characterised by axonal spheroids. However, it has been described in kittens only.
The present case is similar to the recently reported cases of feline cerebellar abiotrophy (Shamir et al 1999, Barone et al 2002). However, on the basis of the late onset and progression of neurological signs, it represents the first case of late onset CD in a middle-aged cat.
References
- Aye M.M., Izumo S., Inada S., et al. Histopathological and ultrastructural features of feline hereditary cerebellar cortical atrophy: a novel animal model of human spinocerebellar degeneration, Acta Neuropathologica 96, 1998, 379–387. [DOI] [PubMed] [Google Scholar]
- Barone G., Foureman P., de Lahunta A. Adult-onset cerebellar cortical abiotrophy and retinal degeneration in a domestic shorthair cat, Journal of the American Animal Hospital Association 38, 2002, 51–54. [DOI] [PubMed] [Google Scholar]
- Carmichael K.P., Howerth E.W., Oliver J.E., Jr., Klappenbach K. Neuroaxonal dystrophy in a group of related cats, Journal of Veterinary Diagnostic Investigation 5, 1993, 585–590. [DOI] [PubMed] [Google Scholar]
- Castoldi A.F., Coccini T., Manzo L. Neurotoxic and molecular effects of methylmercury in humans, Reviews on Environmental Health 18, 2003, 19–31. [DOI] [PubMed] [Google Scholar]
- De Lahunta A. Comparative cerebellar disease in domestic animals, Compendium of Continuing Education in Practice Veterinary 2, 1980, 8–19. [Google Scholar]
- De Lahunta A. Abiotrophy in domestic animals, a review, Canadian Journal of Veterinary Research 54, 1990, 65–76. [PMC free article] [PubMed] [Google Scholar]
- Gray F., De Girolami U., Poirier J. Manual of Basic Neuropathology, 4th edn, 2004, Butterworth-Heinemann, Elsevier; pp. 205–215 [Google Scholar]
- Higgins R.J., LeCouteur R.A., Kornegay J.N., Coates J.R. Late-onset progressive spinocerebellar degeneration in Brittany Spaniel dogs, Acta Neuropathologica 96, 1998, 97–101. [DOI] [PubMed] [Google Scholar]
- Inada S., Mochizuki M., Izumo S., Kuriyama M., Sakamoto H., Kawasaki Y., Osame M. Study of hereditary cerebellar degeneration in cats, American Journal of Veterinary Research 57, 1996, 296–301. [PubMed] [Google Scholar]
- Jubb K.V.F., Huxtable C.R. The nervous system. Jubb K.V.F., Kennedy P.C., Palmer N. 4th edn, Pathology of Domestic Animals Vol. 1, 1993, Academic Press, Inc.: San Diego, California, 283–284. [Google Scholar]
- Koeppen A.H. The pathogenesis of spinocerebellar ataxia, Cerebellum 4 (1), 2005, 62–73. [DOI] [PubMed] [Google Scholar]
- LeCouter R., Kornegay J., Higgins R. Late onset progressive cerebellar degeneration of Brittany spaniel dogs, Proceedings of the 6th Annual Medical Forum of the American College Veterinary Internal Medicine, 1988, 331–334.
- Olby N., Blot S., Thibaud J.L., Phillips J., O'Brien D.P., Burr J., Berg J., Brown T., Breen M. Cerebellar cortical degeneration in adult American Staffordshire Terriers, Journal of Veterinary Internal Medicine 18 (2), 2004, 201–208. [DOI] [PubMed] [Google Scholar]
- Rodriguez F., de los Monteros A. Espinosa, Morales M., et al. Neuroaxonal dystrophy in two Siamese kitten littermates, Veterinary Research 138, 1996, 548–549. [DOI] [PubMed] [Google Scholar]
- Shamir M., Perl S., Sharon L. Late onset of cerebellar abiotrophy in a Siamese cat, Journal of Small Animal Practice 40, 1999, 343–345. [DOI] [PubMed] [Google Scholar]
- Summers B.A., Cummings J.F., de Lahunta A. Degenerative diseases of the central nervous system, Veterinary Neuropathology, 1995, Mosby-Year Book Inc.: St Louis, Missouri, pp. 300–307 [Google Scholar]
- Taniyama H., Takayanagi S., Izumisawa Y., Kotani T., Kaji Y., Okada H., Matsukawa K. Cerebellar cortical atrophy in a kitten, Veterinary Pathology 31, 1994, 710–713. [DOI] [PubMed] [Google Scholar]
- Woodard J.C., Collins G.H., Hessler J.R. Feline hereditary neuroaxonal dystrophy, American Journal of Pathology 74, 1994, 551–566. [PMC free article] [PubMed] [Google Scholar]


