Abstract
The clinical findings, treatment and outcome of suspected clindamycin-associated oesophageal injury in five cats are reported. All cats were treated with one 75 mg clindamycin capsule twice daily (dose range 12–19 mg/kg). Capsules were administered without food or a water bolus. Dysphagia, regurgitation, choking or gagging were seen 3–9 days after starting clindamycin. On oesophagoscopy, three cats had oesophagitis, one of which progressed to stricture formation. Two cats had an oesophageal stricture at first presentation. This is the first report of suspected clindamycin-associated oesophageal injury in cats. It serves to further alert practitioners to the potential for drug-induced oesophageal disorders (DIOD) in cats treated with oral medications and to urge prevention by promoting a change in dosing practices.
Drug-induced oesophageal disorders (DIOD) are a common clinical diagnosis in humans with normal oesophageal function (Boyce 1998). Over 70 medications have been implicated and more than half of all cases are caused by antibiotics (Levine 1999). Improper ingestion promoting prolonged oesophageal retention is crucial in the pathogenesis (Kirkendall 1999). Severe oesophageal damage resulting in stricture formation has been associated with the administration of doxycycline hydrochloride by dry swallow to cats (Melendez et al 2000, Leib et al 2001, McGrotty and Knottenbelt 2002, German et al 2005). We report the clinical findings, treatment and outcome of five cases of suspected clindamycin-associated oesophageal injury in cats.
Case reports
Case 1
An 8-year-old female neutered Birman cat was presented with a 2-week history of regurgitation and weight loss. Seventeen days prior to presentation, the cat had been treated for chronic, intermittent bilateral nasal discharge with clindamycin hydrochloride capsules (Antirobe; Pfizer) (17 mg/kg per os every 12 h). After 3 days of treatment, the cat was noted by the owner, a veterinary student, to regurgitate during eating. Treatment was stopped but the cat continued to regurgitate and was presented to the Valentine Charlton Cat Centre (VCCC). No major abnormalities were detected on physical examination, routine haematology, serum biochemistry or thoracic radiographs. On oesophagoscopy, an area of circumferential, oesophageal ulceration was observed 3–5 cm distal to the proximal oesophageal sphincter (Fig 1). The cat was treated with parenteral prednisolone sodium succinate (Solu Delta Cortef; Pharmacia) (1 mg/kg subcutaneously every 24 h) amoxycillin–clavulanate (Clavulox; Pfizer) (14 mg/kg SC every 12 h), omeprazole (Losec; Astra Zeneca) 1 mg/kg intravenously over 1 h every 24 h) for 2 days and sucralfate suspension (Carafate; Aspen Pharmacare) (250 mg PO every 12 h×7 days). Solids were withheld for 24 h and intravenous fluids were instituted. The next morning the cat regurgitated solid food but tolerated liquidised food. The cat was discharged on amoxycillin–clavulanate oral drops (Clavulox; Pfizer) (12.5 mg/kg every 12 h×7 days), omeprazole (Losec; Astra Zeneca) (1 mg/kg PO every 24 h×4 days), prednisolone (Pred-X5; Apex Labs) (1 mg/kg PO every 24 h×14 days), with instructions to give a 5 ml water bolus with solid oral medications. Intermittent regurgitation was noted during the week following discharge. At the time of writing, 5 months later, the owner reports that there are no oesophageal signs and the cat can tolerate tinned and dry food.
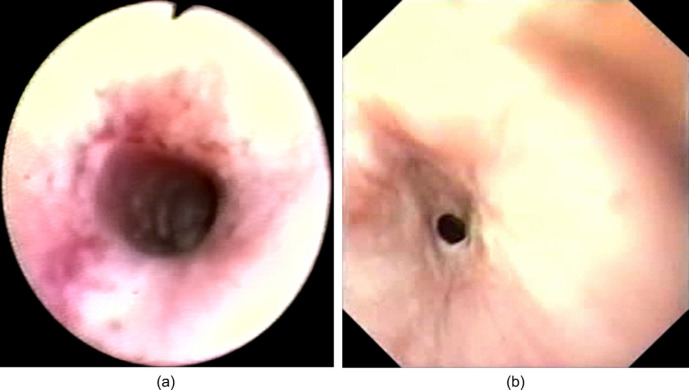
Fig 1.
Oesophagoscopy (Case 1). Circumferential, oesophageal ulceration was observed 3 to 5 cm distal to the upper oesophageal sphincter. Focal areas of deeper ulceration were associated with moderate haemorrhage.
Case 2
A 1-year-old male neutered Burmese cat presented to the referring veterinarian with a 3-day history of lethargy and anorexia. Pyrexia (40.1°C) was the only abnormality on physical examination. Neutropenia (1.8×109/l; reference range 3.8–10.1×109/l) was detected on haematology. Serum biochemistry was unremarkable. A Toxoplasma gondii IgG titre was 1280. The cat was hospitalised and supported with intravenous fluids. Oral clindamycin (19 mg/kg every 12 h×20 days) was prescribed (day 1). On days 4 and 5 of hospitalisation, small amounts of undigested food were found in the cat's cage within 15 min of eating. The cat's appetite and demeanour gradually improved and it was discharged from hospital on day 6 and then boarded for 2 weeks. The cat was represented to the referring veterinarian 25 days after initial presentation because it had been unable to tolerate solid food during boarding. It had lost 12% of its body weight. The cat was ravenously hungry and was seen to regurgitate solid food immediately after eating. An oesophageal stricture was suspected. Ranitidine syrup was prescribed (Zantac; GlaxoSmithKline) (2.5 mg PO every 12 h) and the cat was referred to the Veterinary Specialist Centre. On oesophagoscopy, a single benign stricture with a lumen of 5 mm was visualised 3 cm from the proximal oesophageal sphincter. An 8-mm balloon dilation catheter was used to dilate the stricture. There was moderate post-dilation bleeding and the stricture was 1 cm long. Solid food was withheld for 24 h and analgesia was provided with buprenorphine. Ranitidine syrup was continued and the cat was discharged on amoxycillin–clavulanate oral drops (17 mg/kg PO every 12 h×7 days) and sucralfate suspension (250 mg PO every 8 h×5 days). On oesophagoscopy, 1 week later the stricture had partially reformed with a lumen of 7 mm. The dilation procedure was repeated using a 12.5-mm balloon. A longitudinal mucosal tear was identified after the dilation. Oesophagitis was managed as previously except that dexamethasone (Dexadreson; Intervet) (0.1 mg/kg SC) was given at the time of dilation and prednisolone was give crushed in food (0.8 mg/kg PO every 24 h×14 days). Two weeks later the owner reported that the cat was able to tolerate tinned food but not dry food. Further endoscopy was declined. At 3-month follow-up the cat was able to tolerate some dry food.
Case 3
A 14-year-old female neutered Burmese cat was presented to the referring veterinarian with acute, unilateral, peripheral vestibular signs (day 1). Haematology and serum biochemistry were unremarkable. A T gondii IgG titre was positive (10,240). The cat was treated with clindamycin (15 mg/kg PO every 12 h×10 days). Presenting signs resolved within 12 h. On day 4, the owners reported that the cat was bringing up solid food during or immediately after eating but was able to tolerate liquids. On day 10, the cat developed mild sneezing and epiphora. Clindamycin treatment was stopped and doxycycline monohydrate (Vibravet paste; Pfizer) was prescribed (5 mg/kg every 24 h×7 days). One week later, upper respiratory tract (URT) signs had resolved and the frequency of bringing up solid food had decreased to once every 2–3 days. Clindamycin treatment was reinstituted at the previous dose for a week. The cat was referred 4 days after treatment stopped (25 days from initial presentation). Physical examination at the VCCC was unremarkable. On test feeding, the cat readily attempted to ingest tinned food but had episodes of dysphagia lasting up to 30 s followed by gagging, regurgitation and coughing. Results of haematology were unremarkable. Thoracic radiographs showed consolidation of the right middle lung lobe. On oesophagoscopy, circumferential, patchy erythema, erosion and ulceration of the oesophageal mucosa was observed 3 cm from the proximal oesophageal sphincter (Fig 2a). Cytology of bronchoalveolar lavage fluid showed active, suppurative inflammation (95% neutrophils, <5% macrophages). Gram-negative bacterial rods were seen extracellularly and within neutrophils. A facultative anaerobe sensitive to penicillin was isolated. The cat was supported with intravenous fluids and treated for aspiration pneumonia (amoxycillin–clavulanate, 17 mg/kg SC every 12 h for 2 days then oral drops for 21 days) and for oesophagitis (omeprazole, 1 mg/kg over 1 h IV every 24 h for 2 days, sucralfate suspension 250 mg every 12 h for 21 days). The cat continued to gag and regurgitate immediately after gaining access to solid food but could tolerate liquid or semisolid food. Repeat oesophagoscopy was declined until 4 weeks from referral when the cat was seen to choke on solid food. A benign oesophageal stricture with a lumen of 3–4 mm was detected 3 cm distal to the proximal oesophageal sphincter (Fig 2b). The stricture was dilated using a 12-mm balloon dilation catheter (Leib et al 2001, German et al 2005). There was mild mucosal bleeding post-dilation and the stricture was estimated to be 2 cm in length. The cat was treated with parenteral prednisolone sodium succinate (1 mg/kg SC every 24 h), amoxycillin–clavulanate and omeprazole (as previously) for 2 days. Solid food was withheld for 24 h and intravenous fluids were instituted. The cat was discharged on amoxycillin–clavulanate oral drops, prednisolone suspension (1 mg/kg PO every 24 h), omeprazole (1 mg/kg PO every 24 h) and sucralfate suspension (as previously). The owners declined a fixed dilation schedule. Repeat balloon dilations were performed when the cat was seen to regurgitate on days 9, 23, 35, and 70 from the first dilation procedure. After the fifth dilation, medications were stopped apart from prednisolone (1 mg/kg every 12 h for 14 days, tapered to every 24 h for 7 days, then stopped). At the time of writing, 7 months later, the cat tolerated tinned but not dry food.
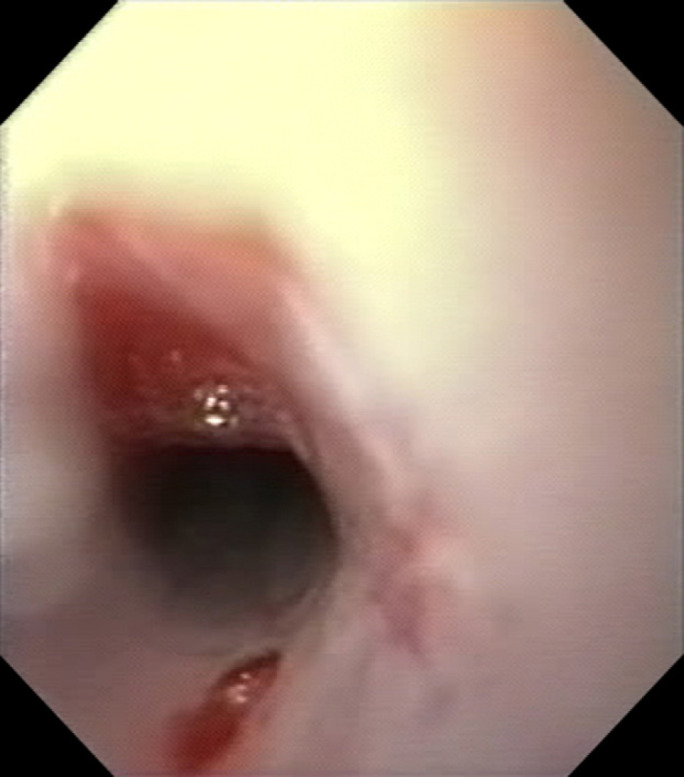
Fig 2.
(a) Oesophagoscopy prior to development of an oesophageal stricture (Case 3). Circumferential, patchy, erythema, erosion and ulceration of the oesophageal mucosa was observed 3 cm distal to the proximal oesophageal sphincter. (b) Oesophagoscopy 4 weeks later (Case 3). A benign oesophageal stricture with a lumen of 3 to 4 mm was detected 3 cm distal to the proximal oesophageal sphincter.
Case 4
A 6-year-old female neutered Burmese cat was treated for an anal sac abscess with clindamycin (17 mg/kg PO every 12 h for 14 days). Nine days after clindamycin treatment began dysphagia was noted. The cat had a normal appetite but had difficulty swallowing and brought up undigested food during or immediately after eating. Liquidised foods were tolerated. No abnormalities were seen on plain thoracic radiographs taken 1 month from the beginning of treatment with clindamycin. A barium series showed normal gastric emptying. The cervical oesophagus was not included in this study. A small amount of barium was aspirated and amoxycillin–clavulanate tablets (14 mg/kg PO every 12 h for 3 days) were prescribed. The cat was unable to keep the tablets down so medication was changed to doxycycline monohydrate paste (5 mg/kg PO every 24 h for 10 days) which was tolerated. The cat was referred to the Animal Referral Hospital, 2 months from the onset of signs. On oesophagoscopy, a benign circumferential stricture with a lumen of 3 mm was identified 4 cm distal to the proximal oesophageal sphincter. The stricture was dilated using a 16-mm balloon dilation catheter and a gastrostomy tube was placed. Dexamethasone was given (0.1 mg/kg IV). Other medications given parenterally after the first dilation were cephazolin, ranitidine and metoclopramide. Dilations were carried out three times weekly initially and the frequency was gradually reduced. Eleven dilations were performed in total over a 14-week period. At the last two dilation procedures, depot methylprednisolone acetate (10 mg) (Depo-Medrol; Pharmacia) was injected radially into the submucosa at the stricture site. On final endoscopy, the oesophageal lumen was 8–10 mm at the stricture site. The residual stricture was seen as a focal band traversing 180° of the oesophageal lumen. The gastrostomy tube was removed. The cat continues to do well but is unable to tolerate dry food at 4-month follow-up.
Case 5
A 3-year-old female neutered domestic shorthair cat was presented to referring veterinarian with a 10-day history of intermittent lethargy and inappetence. Pyrexia (40.5°C) was detected on physical examination. No major abnormalities were detected on haematology, serum biochemistry or plain thoracic and abdominal radiographs. Serology for feline leukaemia virus, feline immunodeficiency virus and cryptococcal antigen were negative. Supportive treatment with intravenous fluids and amoxycillin–clavulanate tablets (14 mg/kg PO every 12 h for 7 days) was initiated. The cat was discharged from the hospital 4 days after admission, on amoxycillin–clavulanate and orbifloxacin (2.5 mg/kg PO every 24 h for 5 days). Titres for T gondii IgG and IgM were >2560 and 32, respectively. Three days later, clindamycin (14 mg/kg PO every 12 h) was prescribed, and amoxycillin–clavulanate was stopped. Seven days after starting clindamycin the cat was seen to regurgitate food and a small amount of fresh blood and was referred. On physical examination at the VCCC, mild pyrexia (39.4°C) and generalised joint pain were identified. On oesophagoscopy two focal areas of mucosal erythema and erosion were identified in the caudal oropharynx adjacent to and involving the proximal oesophageal sphincter. Oral medications were withdrawn. The cat was supported with intravenous fluids and omeprazole and oral sucralfate suspension. A repeat T gondii IgM titre was 128. Clindamycin was given intravenously (12.5 mg/kg every 12 h) for suspected toxoplasmosis. No further oesophageal signs were noted.
Discussion
DIOD are commonly recognised in humans and are likely to be underreported (Jaspersen 2000, Petersen and Jaspersen 2003, Kadayifci et al 2004). More than 70 medications have been implicated and over half of all cases involve antibiotic administration (Levine 1999). Doxycycline, clindamycin and trimethoprim–sulphonamide are most commonly implicated (Sutton and Gosnold 1977, Froese 1979, Boyce 1998, Rivera Vaquerizo et al 2004). Antibiotic-associated oesophagitis occurs in patients with no pre-existing structural or functional oesophageal abnormalities (Bokey and Hugh 1975). A history of taking medications with an inadequate volume of liquid immediately prior to becoming recumbent is typical. Recumbency increases oesophageal contact time and increases the chance of gastrooesophageal reflux, while sleep reduces salivation and swallowing (Petersen and Jaspersen 2003). Signs of DIOD are typically acute and severe. The most common signs are marked odynophagia and retrosternal pain, which occur within hours to days after ingestion (Boyce 1998). The diagnosis is usually made from a consistent history and clinical signs and, in an otherwise healthy patient, endoscopy is generally considered unnecessary (Levine 1999, Jaspersen 2000). Oesophagitis is usually mild and self-limiting and resolves on withdrawal of medication, although liquid or parenteral analgesics and medical management may be prescribed (Boyce 1998).
In the veterinary literature, antibiotic-associated oesophageal injury has been documented in cats treated with doxycycline hyclate (hydrochloride). All 10 cases had oesophageal strictures at the time of diagnosis (Melendez et al 2000, Leib et al 2001, McGrotty and Knottenbelt 2002, German et al 2005). DIOD have not been reported in the dog. In a study of balloon dilation of benign oesophageal strictures in dogs and cats, clindamycin is reported as a possible inciting cause but no clinical details are provided (Leib et al 2001). The cases presented here represent the first published clinical reports of suspected clindamycin-associated oesophageal injury in cats.
The oesophageal injuries reported in this case series most likely result from the administration of clindamycin capsules by dry swallow. The timing of the onset of signs related to treatment and the absence of evidence of other causes of oesophageal injury are consistent with this. In no case was there a history of oesophageal signs prior to the administration of clindamycin. No underlying disorder was identified that might predispose to increased oesophageal transit time. Furthermore, clindamycin is a known cause of DIOD in humans (Sutton and Gosnold 1977, Froese 1979, Rivera Vaquerizo et al 2004) and the propensity of cats to develop antibiotic-associated oesophageal injury has been documented. The resolution of oesophageal signs in two of five cases after withdrawal of medication and medical management for oesophagitis is also compelling. In cases 1–4, clindamycin was the only oral medication given prior to the onset of signs. Two cases (cases 3 and 4) received oral medications in addition to clindamycin. In these cases, doxycycline and amoxycillin–clavulanate were given after the onset of signs and thus are unlikely to have initiated the lesions but a role in maintenance or progression of the lesion cannot be ruled out. However, it should be noted that the veterinary preparation of doxycycline available in Australia, doxycycline monohydrate (Vibravet; Pfizer) has minimal propensity to cause DIOD compared with the hydrochloride (or hyclate) commonly used in the United Kingdom and North America, with one unconfirmed case on file since 1993 (Trumble 2005). In case 5, oral amoxycillin–clavulanate and orbifloxacin had also been administered in the 2 weeks preceding documentation of mucosal ulceration. An initiating role for these medications cannot be completely ruled out.
Comparative data on the mechanisms involved in the pathogenesis of DIOD in humans are relevant to the cat as the gross anatomy of feline and human oesophagus are similar (Geisinger et al 1990). Furthermore, the use of the feline oesophagus as an experimental model for human DIOD provides specific information regarding this species. Prolonged oesophageal contact in combination with a direct injurious effect on the oesophageal mucosa is important; parenteral medications do not cause oesophageal injury. Some medications are highly irritant. For example, potassium chloride can cause oesophageal perforation in human patients even in its liquid form (Pemberton 1970). In contrast, oesophageal damage caused by antibiotics in humans is usually restricted to mild erosion and ulceration. Some antibiotics implicated in DIOD have a local irritant effect. Doxycycline hydrochloride produces an acidic solution in the normally neutral pH of oesophagus (Boyce 1998) and its ulcerogenic potential in the feline oesophagus has been demonstrated (Carlborg et al 1983). Clindamycin, however, does not alter oesophageal pH (Jaspersen 2000) and clindamycin hydrochloride solution is minimally irritant in a rabbit cornea model (Pfizer, data on file). Compounds which cause minimal local irritation can cause problems if they are in prolonged mucosal contact. The latter may be promoted by dry swallow or capsule formulation. Westfall et al (2001) reported that capsules given to cats by dry swallow were retained in the cervical oesophagus (88%) or oropharynx (8%). In another study, more than 50% of capsules given to normal cats became entrapped in the mid-cervical oesophagus (Graham et al 2000). This is consistent with our finding that the lesions were located in the proximal oesophagus in the cases presented here. Capsules are more likely to be retained than tablets and are harder to dislodge once adhered (Perkins et al 1999, Jaspersen 2000). In experimental studies of the feline oesophagus, local damage from doxycycline hydrochloride was more severe with capsules than the same salt in tablet formulation (Carlborg et al 1983).
All cats were treated with clindamycin at greater than the manufacturers' recommended dose rate. In three cats this was because, given the difficulties of diagnosing toxoplasmosis antemortem, they were undergoing therapeutic trials for toxoplasmosis. A dose range of 12.5–25 mg/kg every 12 h has been recommended for treating toxoplasmosis with clindamycin (Lappin et al 1989). In the other cases, it may have been that the dose was ‘rounded up’ to allow dosing with a single 75 mg capsule rather than two 25 mg capsules. Whether the potential for causing oesophageal injury is influenced by increasing dose is not known; in humans antibiotic-associated oesophagitis occurs at therapeutic doses.
Oesophageal stricture following antibiotic-associated DIOD in humans has not been reported. In contrast, the 10 published cases of doxycycline-associated oesophageal disease in cats and two of five cases in this series had oesophageal strictures at the time of presentation. In addition, case 3 presented with oesophagitis but went on to develop a stricture despite medical management. Stricture formation implies severe oesophageal damage which penetrates beyond the mucosa and heals by fibrosis. The propensity for feline patients to develop strictures following DIOD may be a reflection of prolonged damage due to delayed diagnosis. Alternatively, there may be interspecies differences that render the feline oesophagus more susceptible to direct injury. Certainly, experimental studies of the feline oesophagus have shown that antibiotics, including doxycycline hyclate, can cause severe oesophageal damage with stricture formation within 4 days (Carlborg and Densert 1980).
Given the apparent propensity of cats to develop oesophageal strictures, aggressive medical management of oesophagitis in cats is advised. Optimal medical management for oesophagitis has not been determined in human or veterinary medicine. Controlled clinical trials in humans are limited and often conflicting (Vermeijden et al 1992, Vigneri et al 1995). In this series, medical management was instituted on a case by case basis at the discretion of the clinician. The goals of treatment are to reduce any further damage from gastric reflux and to promote mucosal healing without stricture formation. A gastrostomy tube may be indicated to provide nutrition and a convenient method of drug delivery. Proton-pump inhibitors or H2-receptor antagonists can be used to reduce gastric acid secretion and prevent ongoing damage from gastrooesophageal reflux. Sucralfate forms a mucosal protectant layer in an acidic environment and a cytoprotective effect in experimental feline oesophagitis has been demonstrated (Clark et al 1987). Timing of treatments should take into account that sucralfate requires acid to become activated (administer 30 min prior to antacids) and may reduce absorption of drugs given concurrently. Prolonged use can cause constipation. Antibiotics, in parenteral, liquid or paste form, are indicated if there is evidence of aspiration or deep ulceration (Baskin et al 2004). The utility of glucocorticoids to inhibit the inflammatory response and fibroblast proliferation is controversial. Controlled studies in children show no beneficial effect of systemic glucocorticoids in preventing stricture formation following accidental caustic ingestion (Anderson et al 1990, Ulman and Mutaf 1998). Glucocorticoids would be relatively contraindicated where there is concurrent aspiration. Use of systemic corticosteroids did not prevent stricture formation in case 3 or prevent stricture recurrence in case 4. Experimental investigations into the prevention of stricture formation may identify novel agents for future use (Ozcelik et al 2004, Turkyilmaz et al 2005).
The technique of endoscopically guided balloon dilation to treat oesophageal strictures in dogs and cats has been well described (Burk et al 1987, Melendez et al 1998, Weyrauch and Willard 1998, Leib et al 2001, Adamama-Moraitou et al 2002, Sellon and Willard 2003). Multiple dilations are often required. Potential complications include oesophageal tearing, particularly in cats (Willard et al 1994). As tears may not be apparent on endoscopic inspection after the procedure, patient monitoring for pneumomediastinum, pneumothorax and pyothorax is prudent (Willard et al 1994, Harai et al 1995). Mild to moderate bleeding and mucosal tearing were seen on post-dilation inspection of the strictures sites in this series. For refractory strictures, electrocautery or intralesional corticosteroids may augment the effects of dilation (Melendez et al 1998, Kochhar and Makharia 2002, Sellon and Willard 2003). The use of intralesional depot corticosteroids may have contributed to the eventual good outcome in case 4. All cats in this series had a good clinical outcome although those with strictures remained unable to tolerate dry food.
Thus far, the potential for DIOD in cats has been demonstrated for doxycycline and clindamycin. These drugs are extremely useful in feline medicine and should not be avoided. Prevention of DIOD in cats can be achieved by a change in dosing practices. Careful instructions to give a small amount of food or a 5–6 ml water bolus with solid oral medications should be given (Graham et al 2000, Westfall et al 2001). The contents of clindamycin capsules can be combined with a small amount of food without affecting absorption of the drug or the palatability of the food (Pfizer, personal communication, 2005). These instructions should be extended to all solid oral medications in cats given the wide range of medications that is associated with DIOD in humans.
Acknowledgements
The authors are grateful to Drs Stephanie Armstrong, Antony Kery, Romy Feldman and Emma Holmes-Walker for their role in the clinical management of these cases.
References
- Adamama-Moraitou K.K., Rallis T.S., Prassinos N.N., Galatos A.D. Benign esophageal stricture in the dog and cat: a retrospective study of 20 cases, Canadian Journal of Veterinary Research 66, 2002, 55–59. [PMC free article] [PubMed] [Google Scholar]
- Anderson K.D., Rouse T.M., Randolph J.G. A controlled trial of corticosteroids in children with corrosive injury of the esophagus, New England Journal of Medicine 323, 1990, 637–640. [DOI] [PubMed] [Google Scholar]
- Baskin D., Urganci N., Abbasoglu L., Alkim C., Yalcin M., Karadag C., Sever N. A standardised protocol for the acute management of corrosive ingestion in children, Pediatric Surgery International 20, 2004, 824–828. [DOI] [PubMed] [Google Scholar]
- Bokey L., Hugh T.B. Oesophageal ulceration associated with doxycycline therapy, Medical Journal of Australia 1, 1975, 236–237. [DOI] [PubMed] [Google Scholar]
- Boyce H.W. Drug-induced esophageal damage: diseases of medical progress, Gastrointestinal Endoscopy 47, 1998, 547–550. [DOI] [PubMed] [Google Scholar]
- Burk R.L., Zawie D.A., Garvey M.S. Balloon catheter dilation of intramural esophageal strictures in the dog and cat: a description of the procedure and a report of six cases, Seminars in Veterinary Medicine and Surgery (Small Animal) 2, 1987, 241–247. [PubMed] [Google Scholar]
- Carlborg B., Densert O. Esophageal lesions caused by orally administered drugs. An experimental study in the cat, European Surgical Research 12, 1980, 270–282. [DOI] [PubMed] [Google Scholar]
- Carlborg B., Densert O., Lindqvist C. Tetracycline induced esophageal ulcers. A clinical and experimental study, Laryngoscope 93, 1983, 184–187. [DOI] [PubMed] [Google Scholar]
- Clark S., Katz P.O., Wu W.C., Geisinger K.R., Castell D.O. Comparison of potential cytoprotective action of sucralfate and cimetidine. Studies with experimental feline esophagitis, American Journal of Medicine 83, 1987, 56–60. [DOI] [PubMed] [Google Scholar]
- Froese E.H. Oesophagitis with clindamycin, South African Medical Journal 56, 1979, 826. [PubMed] [Google Scholar]
- Geisinger K.R., Cassidy K.T., Nardi R., Castell D.O. The histologic development of acid-induced esophagitis in the cat, Modern Pathology 3, 1990, 619–624. [PubMed] [Google Scholar]
- German A.J., Cannon M.J., Dye C., Booth M.J., Pearson G.R., Reay C.A., Gruffydd-Jones T.J. Oesophageal strictures in cats associated with doxycycline therapy, Journal of Feline Medicine and Surgery 7, 2005, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.P., Lipman A.H., Newell S.M., Roberts G.D. Esophageal transit of capsules in clinically normal cats, American Journal of Veterinary Research 61, 2000, 655–657. [DOI] [PubMed] [Google Scholar]
- Harai B.H., Johnson S.E., Sherding R.G. Endoscopically guided balloon dilatation of benign esophageal strictures in 6 cats and 7 dogs, Journal of Veterinary Internal Medicine 9, 1995, 332–335. [DOI] [PubMed] [Google Scholar]
- Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management, Drug safety: An International Journal of Medical Toxicology and Drug Experience 22, 2000, 237–249. [DOI] [PubMed] [Google Scholar]
- Kadayifci A., Gulsen M.T., Koruk M., Savas M.C. Doxycycline-induced pill esophagitis, Diseases of the Esophagus 17, 2004, 168–171. [DOI] [PubMed] [Google Scholar]
- Kirkendall J.W. Pill esophagitis, Journal of Clinical Gastroenterology 28, 1999, 298–305. [DOI] [PubMed] [Google Scholar]
- Kochhar R., Makharia G.K. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures, Gastrointestinal Endoscopy 56, 2002, 829–834. [DOI] [PubMed] [Google Scholar]
- Lappin M.R., Greene C.E., Winston S., Toll S.L., Epstein M.R. Clinical feline toxoplasmosis. Serologic diagnosis and therapeutic management of 15 cases, Journal of Veterinary Internal Medicine 3, 1989, 139–143. [DOI] [PubMed] [Google Scholar]
- Leib M.S., Dinnel H., Ward D.L., Reimer M.E., Towell T.L., Monroe W.E. Endoscopic balloon dilation of benign esophageal strictures in dogs and cats, Journal of Veterinary Internal Medicine 15, 2001, 547–552. [DOI] [PubMed] [Google Scholar]
- Levine M.S. Drug-induced disorders of the esophagus, Abdominal Imaging 24, 1999, 3–8. [DOI] [PubMed] [Google Scholar]
- McGrotty Y.L., Knottenbelt C.M. Oesophageal stricture in a cat due to oral administration of tetracyclines, Journal of Small Animal Practice 43, 2002, 221–223. [DOI] [PubMed] [Google Scholar]
- Melendez L.D., Twedt D.C., Weyrauch E.A., Millard M.D. Conservative therapy using balloon dilation for intramural, inflammatory esophageal strictures in dogs and cast: a retrospective study of 23 cases (1987–1997), European Journal of Comparative Gastroenterology 3, 1998, 31–36. [Google Scholar]
- Melendez L., Twedt D., Wright M. Suspected doxycycline-induced esophagitis with esophageal stricture formation in three cats, Feline Practice 28, 2000, 10–12. [Google Scholar]
- Ozcelik M.F., Pekmezci S., Saribeyoglu K., Unal E., Gumustas K., Dogusoy G. The effect of halofuginone, a specific inhibitor of collagen type 1 synthesis, in the prevention of esophageal strictures related to caustic injury, American Journal of Surgery 187, 2004, 257–260. [DOI] [PubMed] [Google Scholar]
- Pemberton J. Oesophageal obstruction and ulceration caused by oral potassium therapy, British Heart Journal 32, 1970, 267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A.C., Wilson C.G., Frier M., Vincent R.M., Blackshaw P.E., Dansereau R.J., Juhlin K.D., Bekker P.J., Spiller R.C. Esophageal transit of risedronate cellulose-coated tablet and gelatin capsule formulations, International Journal of Pharmaceutics 186, 1999, 169–175. [DOI] [PubMed] [Google Scholar]
- Petersen K.U., Jaspersen D. Medication-induced oesophageal disorders, Expert Opinion on Drug Safety 2, 2003, 495–507. [DOI] [PubMed] [Google Scholar]
- Vaquerizo P.A. Rivera, Lopez Y. Santisteban, Colmenarejo M. Blasco, Gutierrez M. Vicente, Garcia V. Garcia, Perez-Flores R. Clindamycin-induced esophageal ulcer, Revista Espanola de Enfermedades Digestivas 96, 2004, 143–145. [DOI] [PubMed] [Google Scholar]
- Sellon R.K., Willard M.D. Esophagitis and esophageal strictures, Veterinary Clinics of North America, Small Animal Practice 33, 2003, 945–967. [DOI] [PubMed] [Google Scholar]
- Sutton D.R., Gosnold J.K. Oesophageal ulceration due to clindamycin, British Medical Journal 1 (6076), 1977, 1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble C. Oesophageal stricture in cats associated with use of the hyclate (hydrochloride) salt of doxycycline, Journal of Feline Medicine and Surgery 7, 2005, 241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkyilmaz Z., Sonmez K., Demirtola A., Karabulut R., Poyraz A., Gulen S., Dincer S., Basaklar A.C., Kale N. Mitomycin C prevents strictures in caustic esophageal burns in rats, Journal of Surgical Research 123, 2005, 182–187. [DOI] [PubMed] [Google Scholar]
- Ulman I., Mutaf O. A critique of systemic steroids in the management of caustic esophageal burns in children, European Journal of Pediatric Surgery 8, 1998, 71–74. [DOI] [PubMed] [Google Scholar]
- Vermeijden J.R., Tytgat G.N., Schotborgh R.H., Dekker W., vd Boomgaard D.M., van Olffen G.H., Schrijver M., Vosmaer G.D., Dekkers C.P. Combination therapy of sucralfate and ranitidine, compared with sucralfate monotherapy, in patients with peptic reflux esophagitis, Scandinavian Journal of Gastroenterology 27, 1992, 81–84. [DOI] [PubMed] [Google Scholar]
- Vigneri S., Termini R., Leandro G., Badalamenti S., Pantalena M., Savarino V., Di Mario F., Battaglia G., Mela G.S., Pilotto A., Plebani M., Giovanni D. A comparison of five maintenance therapies for reflux esophagitis, New England Journal of Medicine 333, 1995, 1106–1110. [DOI] [PubMed] [Google Scholar]
- Westfall D.S., Twedt D.C., Steyn P.F., Oberhauser E.B., VanCleave J.W. Evaluation of esophageal transit of tablets and capsules in 30 cats, Journal of Veterinary Internal Medicine 15, 2001, 467–470. [DOI] [PubMed] [Google Scholar]
- Weyrauch E.A., Willard M.D. Esophagitis and benign esophageal strictures, Compendium of Continuing Education for the Practicing Veterinarian 20, 1998, 203–212. [Google Scholar]
- Willard M.D., Delles E.K., Fossum T.W. Iatrogenic tears associated with ballooning of esophageal strictures, Journal of the American Animal Hospital Association 30, 1994, 431–435. [Google Scholar]