Abstract
The pathogenesis of chronic rhinitis in cats is poorly understood and consistently effective therapies are not currently available. Therefore, randomized clinical trials were conducted to evaluate a novel immunotherapy for treatment of chronic rhinitis in adult (n=12) and young cats (n=28). In addition, cytokine profiles in cats with chronic rhinitis were compared to those of healthy cats. Cats were treated with a potent stimulator of innate immunity (liposome–IL-2 DNA complexes) and the effects of treatment on clinical signs and immune function were assessed. In adult cats with chronic rhinitis, immunotherapy led to significant improvement in frequency of sneezing but not in other clinical signs when compared to the placebo group, whereas immunotherapy failed to improve clinical signs in younger cats. Analysis of cytokine expression in cats with rhinitis did not reveal evidence of a Th2 cytokine bias in cats with rhinitis. We conclude that chronic rhinitis in cats is not a Th2-biased disease and that immunotherapy may lead to clinical improvement in adult cats with the disease.
Chronic rhinitis in cats is a syndrome associated with inflammation of the nasal cavity; it is frequently associated with secondary infection of the frontal sinuses, and it typically lasts more than 1 month (Cape 1992). Clinical signs in cats include nasal discharge, sneezing, ocular discharge, anorexia, and stertorous breathing. Possible causes for chronic rhinitis include neoplasia, foreign bodies, viral infections (feline herpesvirus-1 [FHV-1], calicivirus), mechanical obstruction (dental disease, polyps, or nasopharyngeal stenosis), bacterial infections (Bordetella bronchiseptica, Chlamydophila felis and others), allergic diseases, or fungal diseases (Cryptococcus neoformans or less commonly, Aspergillus or Penicillium species). It is relatively easy to remove foreign body, neoplasia, fungal infection, dental disease, polyps, and nasopharyngeal stenosis from the differential list with a diagnostic work-up. Once these diseases are excluded, many chronic rhinitis cases have been attributed to the effects of chronic viral infections or bacterial infections (Helps et al 2005, Johnson et al 2005b); however, it is virtually impossible to prove a cause and effect relationship, as many potentially pathogenic organisms are also detected in clinically normal cats (Van Pelt and Lappin 1994). In addition, multiple causes of chronic rhinitis may coexist in some cats.
Once foreign bodies, dental disease, neoplasia, polyps, fungal disease, and nasopharyngeal stenosis are ruled out or considered unlikely, multiple treatments for chronic rhinitis can be attempted. These include use of recombinant human interferon (IFN)-α, anti-viral drugs, systemic or inhaled corticosteroids, topical decongestants, antibiotics, rhinotomy, or nasal sinus trephination (Van Pelt and Lappin 1994). However, none of these treatments have been shown to be consistently effective and recurrences are common.
New treatments are needed for cats with chronic rhinitis. Because many cases are thought to have viral or bacterial involvement, enhancement of innate immune responses may be of benefit. Modulation of the innate immune responses through the systemic administration of liposomal DNA complexes, containing either non-coding plasmid DNA or plasmid DNA encoding the gene for interleukin-2 (IL-2), has been demonstrated to have substantial activity in rodent tumor models (Dow et al 1999a). In addition, complexes of cationic liposomes and plasmid DNA (CLDC) are extremely potent inducers of Th1 cytokine responses, triggering the release of several cytokines with potent anti-viral activity, including IFN-γ, IFN-α, and IL-12. Moreover, systemic administration of these complexes has also been shown to partially reverse the Th2 mediated immunological abnormalities present in a mouse model of asthma (Dow et al 1999b). More recently, we have also found that CLDC can be used as vaccine adjuvants in dogs with refractory atopy (Mueller et al 2005). Studies in mice have used treatment with recombinant interferon-α to successfully reduce the severity of clinical signs in experimentally induced herpesvirus infection (Lenzo et al 2003). Moreover, scid-hu mice infected with γ-herpesvirus have been treated effectively with recombinant human IL-2, illustrating that several different Th1 cytokines may elicit potent anti-viral activity (Baiocchi et al 2001).
The results of these prior studies suggested that liposome–DNA complexes might be useful as an immunotherapeutic in feline chronic rhinitis, particularly in cases for which inflammation is related to viral or bacterial infections. We hypothesized that chronic rhinitis in cats could be mediated at least in part by an ineffective or subverted immune response triggered initially by chronic viral or bacterial infection and that stimulation of Th1 immune responses may aid in the treatment of affected cats. The purpose of this study was to administer CLDC to different groups of cats to determine the immunological and clinical effects.
Materials and methods
Animals and study inclusion criteria
All protocols and procedures were approved by the Animal Care and Use Committee of Colorado State University. In total, four groups of healthy or clinically ill cats were studied in a series of four experiments.
Group 1 cats
The six cats in this group were laboratory-reared and apparently healthy when evaluated at 6–8 months of age. There was no history of upper respiratory disease, a complete blood cell count, serum biochemistry profile, and urinalysis were normal, and the cats were negative for feline leukemia virus (FeLV) p27 antigen and Feline Immunodeficiency Virus (FIV) antibodies in serum (IDEXX, Westbrook, ME).
Group 2 cats
The eight domestic shorthair, laboratory-reared cats (five males, neutered; three females, spayed) in this group were evaluated at 1–2 years of age (median age 17 months, range 15–25 months) and were apparently healthy on physical examination. There was no history of upper respiratory disease, a complete blood cell count, serum biochemistry profile, and urinalysis were normal, and the cats were negative for FeLV p27 antigen and FIV antibodies in serum (IDEXX, Westbrook, ME).
Group 3 cats
The 12 older cats in this group (median age 102 months, range 12–168 months; five males, neutered; seven females, spayed) were client-owned and had clinical signs of chronic rhinitis, the cause of which was most consistent with chronic viral disease. Cats were owned by clients of either a university teaching hospital (n=5) or a private secondary referral center (n=7). To be entered into the study, the cats had to have: (1) a non-progressive nasal discharge of more than 1 year duration that was incompletely cleared after administration of oral antibiotics for 4–6 weeks; (2) minimal to no dental disease on oral examination; (3) lack of other systemic signs besides anorexia and lethargy; (4) lack of evidence of facial deformity; and (5) discontinuation of antibiotic therapy or immunomodulating therapy for at least 3 weeks. All 12 cats were determined to be free of concurrent systemic disease by means of a routine complete blood count, serum biochemistry profile, urinalysis, and results of FeLV p27 antigen and FIV antibody testing (IDEXX, Westbrook, ME). For some cats, other diagnostic tests were performed to further exclude other common causes of chronic rhinitis. These included Cryptococcus neoformans antigen test on serum for cats with travel to endemic areas (n=2; both negative), thoracic radiographs (n=9), nasal radiographs (n=8), computed tomography of the nasal cavity and sinuses (n=3), or rhinoscopy with biopsy, histopathology, and bacterial culture (n=8).
Group 4 cats
The 28 cats in this group (estimated median age 17 months, range 6–96 months; 13 males, neutered; 15 females, spayed) came from a local animal shelter and had clinical signs of rhinitis, the cause of which was most consistent with viral disease. To be entered into the study, group 4 cats had to have: (1) persistence of clinical signs precluding adoption despite treatment with two different antibiotics (amoxycillin–clavulanic acid and azithromycin) over 21 days; (2) minimal to no dental disease on oral examination; (3) lack of systemic signs besides anorexia and lethargy; (4) lack of evidence of facial deformity; and (5) discontinuation of antibiotic therapy or immunomodulating therapy for at least 4 days. All 28 cats were determined to be free of concurrent systemic disease by means of a routine complete blood count, serum biochemistry profile, urinalysis, and results of FeLV p27 antigen and FIV antibody testing.
Preparation of cationic liposome–DNA complexes
The feline IL-2 cDNA was cloned by real-time polymerase chain reaction (RT-PCR) from normal cat peripheral blood mononuclear cells (PBMC), based on published sequence data. The cDNA was cloned into a eukaryotic expression vector (pMB75.6) that utilized the cytomegalovirus (CMV) immediate–early promoter-enhancer region, a synthetic intron (pGL3) immediately upstream of the start site, an SV40 early poly(A) site, and the kanamycin resistance gene, as described previously (Fairman et al 1999). Expression was confirmed by assaying the ability of supernatants from transiently transfected feline Crandall Rees Feline Kidney (CRFK) cells to support proliferation of normal feline PBMC in vitro (data not shown). Plasmid DNA encoding feline IL-2 was prepared by alkaline lysis followed by chromatographic purification, as described previously (Dow et al 1999a). The endotoxin content of the DNA was less than 0.1 EU/μg DNA, as determined by the LAL kinetic assay (Biowhittaker, Walkersville, MD). Cationic liposomes comprising DOTIM (octadecenoyloxy [ethyl-2-heptadecenyl-3-hydroxyethyl] imidazolinium chloride) (Sigma–Aldrich Chemical Co, St Louis, MO) and cholesterol (Avanti Polar Lipids, Alabaster, AL) were prepared as described previously by Templeton et al (2003). Briefly, equimolar amounts of DOTIM and cholesterol were evaporated to dryness, then rehydrated in 5% dextrose in water (D5W) at 50°C, then extruded sequentially through filters of decreasing diameter (1000 nm, 450 nm, and 200 nm) to form extruded liposomes. Immediately before treatment of cats, CLDC were prepared by addition of 100 μl of liposomes to 1.5 ml D5W. Then, 150 μg of feline IL-2 plasmid DNA was added to the liposomes and mixed by gentle pipetting of the solution. Additional D5W was then added to bring the solution to a final volume of 3 ml prior to injection. The CLDC solution was administered to non-anesthetized cats by intraperitoneal injection.
Treatment protocols
Group 1 cats
These six cats were randomly assigned by coin flip to two treatment groups in an unblinded randomized trial. Group 1 cats were administered either CLDC (treatment group, n=3) or 0.9% NaCl solution (placebo group, n=3) by intraperitoneal injection (IP) once weekly for 6 weeks. This route of administration was selected because pilot studies in mice indicated that this route gave the highest degree of systemic immune activation with minimal toxicity (data not shown).
Group 2 cats
These eight cats were randomly assigned by coin flip and assessed in a double-blinded trial. Group 2 cats were administered either CLDC (treatment group, n=4) or cationic liposomes (placebo group, n=4) by IP injection, once weekly for 4 weeks. To determine humoral responses to a novel antigen, the cats were administered 100 μg of keyhole limpet hemocyanin (KLH) (Sigma–Aldrich Chemical Co, St Louis, MO) dissolved in sterile phosphate buffered saline solution (PBS) intradermally (ID) at week 0 and week 2.
Group 3 cats
These 12 cats were randomly assigned by coin flip and assessed in a double-blinded trial. Group 3 cats were administered either CLDC (treatment group, n=7) or cationic liposomes (placebo group, n=5) by IP injection once weekly for 4 weeks. To determine humoral responses to a novel antigen, the cats were administered 100 μg of KLH dissolved in PBS ID at week 0 and week 2.
Group 4 cats
These 28 cats were randomly assigned by coin flip and assessed in a double-blinded trial. Group 4 cats were administered either CLDC (treatment group, n=13) or cationic liposomes (placebo group, n=15) by IP injection once weekly for 4 weeks. To determine humoral responses to a novel antigen, the cats were administered 100 μg of KLH dissolved in PBS, ID at week 0 and week 2.
Clinical monitoring and sample collections
Group 1 cats
Body temperature was assessed before treatment and at 2–4 h intervals after injection for a 12-h period and then again at 24 h after injection. A complete blood cell count was performed on blood collected on days −14, 0, 1, 7, and 21. Percentage of the total lymphocyte population that was CD4+ and CD8+ was determined by flow cytometry on samples collected on days 0, 21, and 42.
Group 2 cats
Body temperature was assessed before treatment and at 4-h intervals after injection for a 24-h period. A complete blood cell count was performed on blood collected on days 0, 1, 7, and 28. Antibodies to KLH were determined on serum collected on days 0 and 28. Cytokine profiles were determined on samples collected prior to treatment as well as 8 h after the first treatment and on day 28.
Group 3 cats
Each of these 12 cats was monitored closely for clinical abnormalities in the hospital for 12–24 h following the first injection of CLDC or liposomes. The cats were then monitored by their owners at home for the duration of the study. Owners were blinded to the treatment groups and were asked to assess any changes in clinical signs relative to the cat's clinical condition at the start of the study. Clinical responses were measured in 25% increments of improvement or decline from baseline over the preceding week in specific clinical signs, based on standardized scoring criteria as follows: serous discharge alone occurring on at least 1 day but less than 4 days: one point; serous discharge occurring anymore than 3 days of the preceding 7 days: two points; mucopurulent discharge present for either 1 or 2 days: two points were assigned for the period; 3–5 days of mucopurulent discharge: four points, and the same discharge for 5–7 days: five points. Any day on which sneezing was observed was also recorded and contributed one point per day observed. Other clinical signs anticipated but not analyzed due to infrequency of occurrence were severity of conjunctivitis, amount of coughing, and increase or decrease in appetite. Complete blood counts and serum biochemical profile values were also determined on blood samples collected on days 0 and 28. Percentage of the total lymphocyte population that was CD4+ and CD8+ was determined by flow cytometry on samples collected on days 0, 1, 7, and 28. Antibodies to KLH were determined on serum collected on days 0 and 28. Cytokine profiles were determined on samples collected prior to treatment as well as 8 h after the first treatment and on day 28. Samples for concanavalin A stimulated whole blood proliferation were collected on days 0, 14, and 28.
Group 4 cats
Each of these 28 cats was hospitalized for the duration of the study. On day 1 of the study and then weekly, a nasal swab was collected from each cat, placed in sterile PBS (0.01 M; pH=7.2), incubated at room temperature for 2 h, and then stored at −70°C until assayed for FHV-1 by quantitative PCR assay. Clinical findings were scored as for group 3 cats and the person determining the score was blinded to the treatment groups. Complete blood counts and serum biochemical profile values were also determined on blood samples collected on days 0 and 28. Percentage of the total lymphocyte population that was CD4+ and CD8+ was determined by flow cytometry on samples collected on days 0, 1, 7, and 28. Antibodies to KLH were determined on serum collected on days 0 and 28. Cytokine profiles were determined on samples collected before treatment as well as 8 h after the first treatment and on day 28. Samples for concanavalin A stimulated whole blood proliferation were collected on days 0, 14, and 28.
Quantitation of FHV-1 copy number by quantitative PCR
Quantitation of FHV-1 copy number in nasal swabs taken weekly from group 4 cats was performed by quantitative PCR, using a previously published assay (Vogtlin et al 2002) adapted for nasal swabs. DNA was extracted from samples using a commercially available kit (Qiagen, Valencia, CA) according to the manufacturer's protocols with 50 μg salmon sperm DNA (Invitrogen Corporation, Carlsbad, CA) added per ml of manufacturer's lysis buffer. The 25 μl PCR reaction contained 12.5 μl TaqMan Mastermix (Applied Biosystems), 0.5 ml (400 nM) of each primer, 0.2 μl (80 nM) of probe 6-FAM (6-carboxyfluorescein)/TAMRA (carboxy-tetramethylrhodamine), 1.3 μl of sterile water, and 10 μl of extracted template DNA. All reactions were carried out on an ABI 7000 (Applied Biosystems, Foster City, CA) 0.5 μl using the following conditions: 2 min at 50°C, 10 min at 95°C, and then 40 cycles consisting of a 15 s 95°C denaturation step followed by a 1 min 60°C annealing step. Parallel reactions for feline glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using a previously published protocol (Leutenegger et al 1999) were performed on the same plate to account for variations in DNA quantity in each sample secondary to different yields of cells during swab sampling. GAPDH reaction conditions were the same as for FHV-1 with the exception that only 5 μl of template DNA was added with a corresponding increase in the volume of sterile water (6.3 μl). Data were analyzed with the instrument software (ABI Prism 7000 SDS Software Version 1.0 (build 81 rev 3): Applied Biosystems, Foster City, CA). Signals were considered positive if the fluorescence intensity exceeded 10 times the standard deviation of the baseline fluorescence (threshold cycle, Ct). All reactions were run in duplicate. A control sample consisting of DNA pooled from swabs from 10 normal cats spiked with plasmid derived FHV-1 DNA was run on each plate to ensure repeatable thermocycler conditions.
A standard curve for GAPDH-cell equivalent was generated using DNA isolated from a feline lung epithelial cell line (Dow, unpublished data) that was digested in the same manner as the test samples. The standard curve for FHV-1 was generated using a 10-fold dilution series using plasmid generated DNA. The FHV-1 pDNA was produced using a commercially available vector (TA Cloning Vector: Invitrogen Corporation, Carlsbad, CA) after purification (Qiagen, Valencia, CA) of product obtained from a conventional PCR reaction using the primers used in the real-time assay. Viral load was then defined as the calculated FHV copy number divided by the calculated GAPDH-cell equivalent copy number of each sample.
KLH antibody ELISA
Sera for use in this assay was stored at −70°C until assayed. An indirect ELISA was developed to measure antibodies to KLH. Sera collected from 11 cats prior to inoculation and on day 28 (after receiving two inoculations of KLH) were used for ELISA titrations. Multiple antigen concentrations, micro-ELISA plates, buffers, blocks, and secondary antibody concentrations were assessed while optimizing the ELISAs. In the optimized ELISA, 100 μl of a 1:1600 dilution of 1 mg/ml KLH solution in 0.01 M phosphate buffered saline solution (PBS; pH=7.2) was added to a micro-ELISA plate (Immulon-1 96-well microtitre plates, ThermoElectron Corporation, Milford, MA) and incubated at 4°C for a minimum of 16 h. Plates were washed three times with 200 μl PBS solution containing 0.05% Tween 20 (Sigma–Aldrich Chemical Co, St Louis, MO) (PBS–TW solution). The sera from the study cats were serially diluted 1:50 to 1:51200 in PBS–TW solution in a final volume per well of 100 μl. The plate was then incubated at 37°C for 30 min. Plates were then washed three times with 200 μl of PBS–TW solution. Horseradish peroxidase conjugated goat anti-cat IgG (heavy chain specific) (Kirkegaard and Perry Laboratories Inc, Gaithersburg, MD) was diluted 1:2000 in PBS–TW solution and 100 μl was pipetted into the appropriate wells. After a 30 min incubation at 37°C, the plates were washed three times (as before) and 100 μl of substrate (TMB microwell peroxidase system, Kirkegaard and Perry Laboratories Inc, Gaithersburg, MD) was added to all wells. The enzyme reaction was stopped after an incubation period of 10 min at room temperature (approximately 20°C) by pipetting 100 μl of 0.18 M H2SO4 into each well. The optical density of each well (compared to a substrate control blank row) was read at 450 nm with an automated micro-ELISA reader (Multiskan Ascent plate reader, ThermoElectron Corporation, Milford, MA). Mean values for this calculation were used in statistical comparisons. Positive (sera from previously inoculated cats) and negative (sera from naïve cats) controls were included on each plate.
Flow cytometric evaluation of peripheral blood mononuclear cells
All samples for PBMC collection were frozen and stored in liquid nitrogen until analysis. The PBMC were separated from heparinized whole blood by density gradient centrifugation using lymphocyte separation medium (ICN Biomedical, Aurora, OH). Prior to immunostaining and flow cytometric analysis, PBMC samples were rapidly thawed and washed in complete medium. Cells were placed in round-bottom 96-well plates for immunostaining and non-specific binding was first blocked by addition of 10% normal cat serum. Immunostaining was done at 4°C in flow cytometry buffer containing PBS, 0.1% sodium azide and 2% fetal bovine serum. Cat lymphocytes were immunostained with fluorescein isothiocyanate (FITC) conjugated antibodies to feline CD4 (vpg34; CD4-FITC: Serotec, Raleigh, NC) and phycoerythrin-conjugated antibodies to feline CD8 (vpg9; CD8-rpe; Serotec, Raleigh, NC) and with a cross-reactive PE/cy5 labeled antibody to CD44 (IM7; CD44-rpe/cy5 Pharmingen, Franklin Lakes, NJ). For analysis of monocytes, PBMC were immunostained with a cross-reactive antibody to CD11b (5C6, CD11b-rpe; Serotec, Raleigh, NC) and a cross-reactive antibody to MHC class II (CAG5-3D1; MHC Class II: Serotec, Raleigh, NC). Analysis gates were set on the live lymphocyte population based on forward and side-scatter characteristics. Data were collected using either a FACSCalibur cytometer (BD Biosciences, San Jose, CA) or a Cyan MLE cytometer (DAKO-Cytomation, Fort Collins, CO) and data were analyzed using either CellQuest (BD Biosciences, San Jose, CA) or Summit software (DAKO-Cytomation, Fort Collins, CO).
Real-time PCR analysis for cytokines
The level of mRNA transcript expression for the cytokines IL-4, IL-6, IL-10 and IL-12 and IFN-γ and IFN-α and tumor necrosis factor (TNF)-α was assessed with quantitative real-time PCR using previously published assays (Leutenegger et al 1999, Kipar et al 2001). Samples of PBMC were resuspended in complete tissue culture medium (CTM: modified Eagle's medium supplemented with essential and non-essential amino acids+10% FBS) at 37°C for 10 min immediately after a rapid thaw and then washed two times with PBS. Total RNA was extracted using a commercially available kit (Qiagen, Valencia, CA) according to the manufacturer's directions. Residual genomic DNA was removed using the optional on-column DNase 1 treatment. The final product was eluted into 30 μl of DEPC treated water. The cDNA was synthesized the same day using 10 μl of the above RNA product, random primers (Invitrogen Corporation, Carlsbad, CA), dNTP mix (2.5 mM each dNTP) (Invitrogen Corporation, Carlsbad, CA), MMLV reverse transcriptase (Invitrogen Corporation), and an RNase inhibitor (Invitrogen Corporation, Carlsbad, CA). Final product was brought up to volume (60 μl) in DEPC treated water.
TaqMan primer probe pairs were prepared based on previously published sequences (Leutenegger et al 1999, Kipar et al 2001). The probes were labeled at the 5′ and 3′ ends with a reporter fluorescent dye, FAM, and a quencher, TAMRA, respectively. Amplifications were carried out in duplicate in 25 μl reaction mixtures containing (final concentration) 12.5 μl Mastermix (Applied Biosystems, Foster City, CA), 0.5 μl (400 nM) of each primer, 0.2 μl (80 nM) of probe, 6.3 μl PCR grade water, and 5 μl template cDNA. Thermocycler conditions and background were set using the same parameters as for FHV-1. Relative quantification was carried out using the comparative Ct method (Applied Biosystems 2001) with feline GAPDH as the endogenous reference gene and each cat's pre-treatment PBMC sample as the control sample for across time comparisons. Analysis was undertaken using either unstimulated PBMC (controls) or following stimulation for 6 h with phorbol myristate acetate (PMA: 10 ng/ml; Sigma–Aldrich Chemical Co, St Louis, MO) plus ionomycin (500 ng/ml; Sigma–Aldrich Chemical Co, St Louis, MO) (week 0 samples only). Samples from group 3 and group 4 cats obtained at times 0, 8 h, 2 weeks, and 4 weeks were analyzed for cytokine mRNA expression.
Whole blood proliferation assays
Assays were performed in 96-well plates using 10 μl whole heparinized blood diluted in 100 μl CTM per well. Cells were incubated with media only (control) or 10 μg/ml concanavalin A and media (ConA). After 96 h of incubation at 37°C with 5% CO2, wells were pulsed with 1 μCi tritiated thymidine for 24 h before being harvested onto filter mats (Wallac-Microbeta; Perkin–Elmer, Boston, MA) and counted. All samples were run in triplicate and stimulation indexes (SI) were calculated by dividing the average of the stimulated cell count by the control count for each cat and time point.
Statistical analysis
Statistical differences in clinical scores were assessed using the sign rank test. KLH antibody titers were log transformed in order to normalize data and then compared using a paired t-test. One-way ANOVA with Tukey's multiple comparison test was used to identify significant changes between more than two groups of variables. The level of significance was set at P<0.05 for all comparisons.
Results
CLDC treatment effects on innate and adaptive immune responses in healthy cats
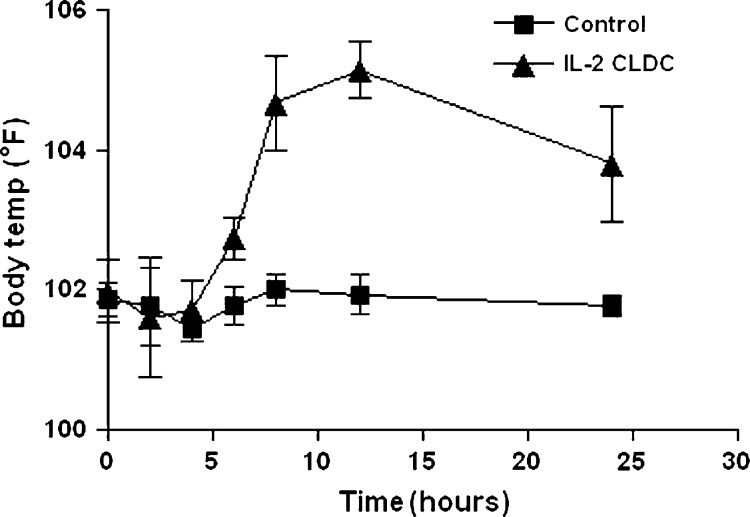
Intraperitoneal administration of CLDC rapidly induced a febrile response in group 1 cats that was significantly different than group 1 cats inoculated with saline. Fever was detectable by 6 h post-injection, peaked by 10 h, and declined thereafter (Fig 1). Fever was detected in some CLDC-inoculated, group 2 cats (three episodes) and some liposome-inoculated group 2 cats (two episodes) but statistically significant differences between the groups were not noted.
Fig 1.
Effects of injection of IL-2 CLDC on body temperature in healthy cats. Six healthy, 6–8-month-old purpose-bred cats were randomized into two treatment groups of three cats each (group 1). One subgroup of three cats was injected intraperitoneally once weekly with liposome–DNA complexes (CLDC) containing 150 μg plasmid DNA encoding the feline IL-2 cDNA (IL-2 CLDC group). A second subgroup of three cats was injected intraperitoneally with saline only (control). Body temperature was assessed before treatment and at 2–4-h intervals after injection for a 24-h period. The mean body temperature (±SD) for each group was plotted. Body temperature increased significantly (P<0.05) in the IL-2 CLDC group beginning 6 h after injection and remained significantly elevated for a 24-h period.
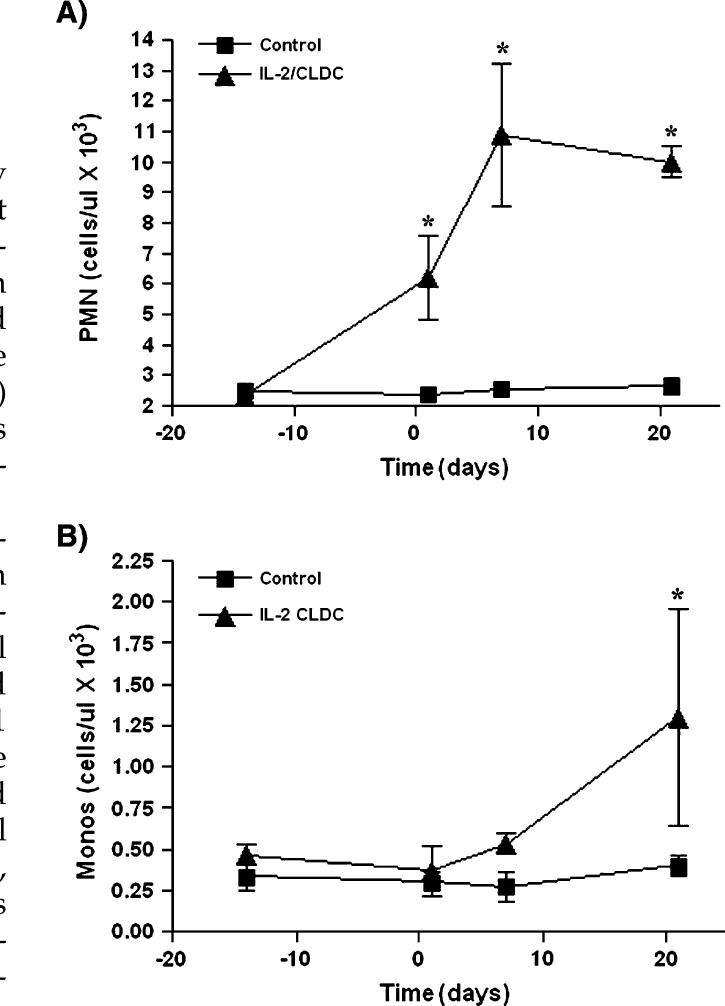
CLDC-inoculated, group 1 cats had a significant increase in neutrophil numbers within 24 h of injection of CLDC when compared to saline-inoculated group 1 cats (Fig 2A). The neutrophil numbers continued to increase until day 7 and plateaued by day 21. CLDC-inoculated, group 1 cats also had a significant increase in monocyte numbers when compared to saline-inoculated group 1 cats, but differences were not noted until day 7 (Fig 2B). Compared to baseline values, a significant increase in neutrophil counts was detected in healthy adult group 2 cats administered CLDC and group 2 cats administered liposomes alone by day 14 (data not shown). However, differences between CLDC-inoculated and liposome-inoculated group 2 cats were not detected. Changes in monocyte counts were not detected in group 2 cats regardless of treatment assignment.
Fig 2.
Effects of IL-2 CLDC treatment on neutrophil and monocyte counts. Six healthy, 6–8-month-old purpose-bred cats were randomized into two treatment groups of three cats each (group 1). One subgroup of three cats was injected intraperitoneally once weekly with 150 μg IL-2 CLDC (IL-2 CLDC). A second subgroup of three cats was injected intraperitoneally with saline only (control). Neutrophil (reference range 2–12.0×103/μl) and monocyte (reference range 0.0–0.8×103/μl) counts were determined for each group of animals and the mean (±SD) was plotted. (A) Neutrophil (‘PMN’) counts were significantly increased (P<0.05) in cats treated with IL-2 CLDC, beginning within 24 h of the first injection. (B) Monocyte (‘Monos‘) counts were significantly increased (P<0.05) by the first week after treatment.
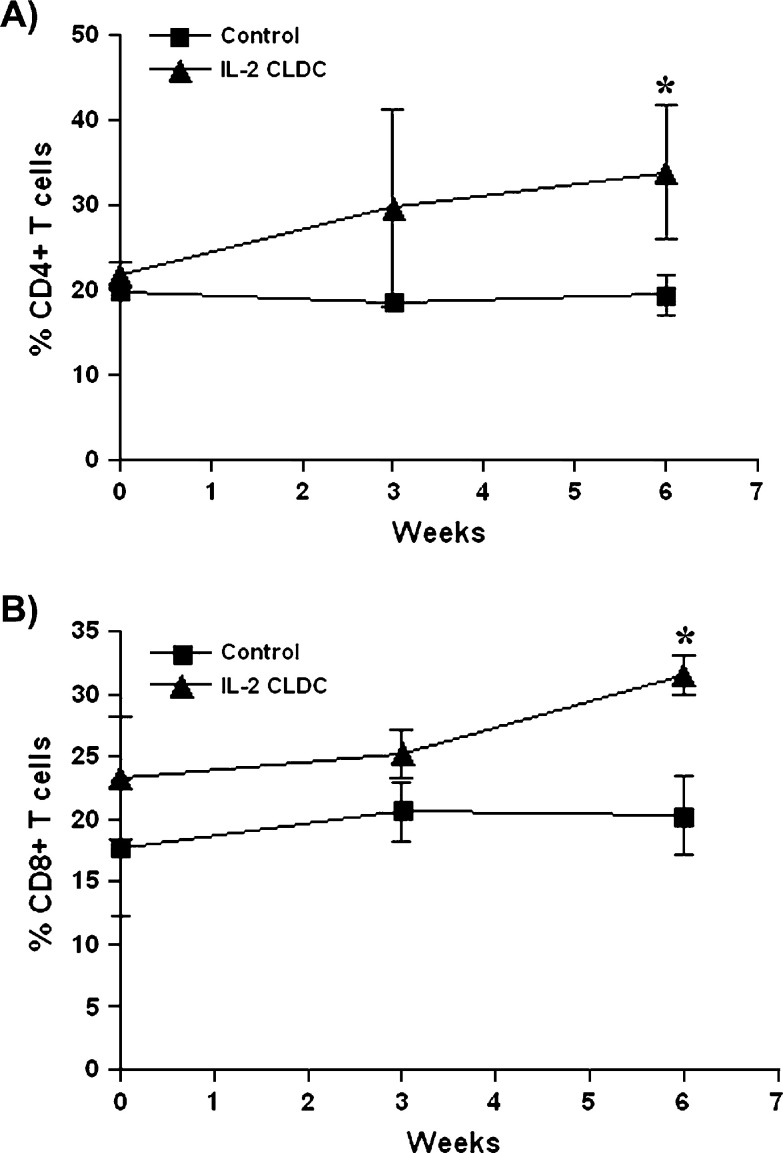
Group 1 cats administered CLDC weekly had a significantly higher percentage of CD4+ lymphocytes (Fig 3A) and a significantly higher percentage of CD8+ lymphocytes (Fig 3B) than group 1 cats administered saline by 6 weeks of treatment. KLH antibody responses were similar in group 2 cats regardless of treatment.
Fig 3.
Effects of IL-2 CLDC treatment on CD4+ and CD8+ lymphocytes in cats. Six healthy, 6–8-month-old purpose-bred cats were randomized into two treatment groups of three cats each (group 1). One subgroup of three cats was injected intraperitoneally once weekly with 150 μg IL-2 CLDC (IL-2 CLDC). A second subgroup of three cats was injected intraperitoneally with saline only (control). Peripheral blood mononuclear cells were collected before treatment and again at 3 weeks and 6 weeks of treatment. (A) The percentage of CD4+ lymphocytes in each cat was assessed by flow cytometry, as described in Materials and methods, and the mean (±SD) percentage of CD4+ lymphocytes for each group was plotted. (B) The mean (±SD) percentage of CD8+ lymphocytes was determined and plotted as for CD4+ lymphocytes. ∗ Denotes values that are significantly (P<0.05) different between control and IL-2 CLDC-treated cats.
Clinical effects of CLDC administration to cats with rhinitis
In group 3 cats, fever was detected in 4/7 cats (57%) administered CLDC and 2/5 cats (40%) administered liposomes. Vomiting was detected in 0/7 group 3 cats administered CLDC and 1/5 cats (20%) administered liposomes. In group 4 cats, fever was detected in 1/13 cats (8%) administered CLDC and 1/15 cats (7%) administered liposomes. Vomiting was detected in 3/13 (23%) group 4 cats administered CLDC and 2/15 cats (13%) administered liposomes. None of these differences were statistically significant. Differences between complete blood cell counts, serum biochemical panel, and urinalysis between the baseline and day 28 samples or between treatment groups at any time points in group 3 and group 4 cats were not detected regardless of whether CLDC or liposomes were administered.
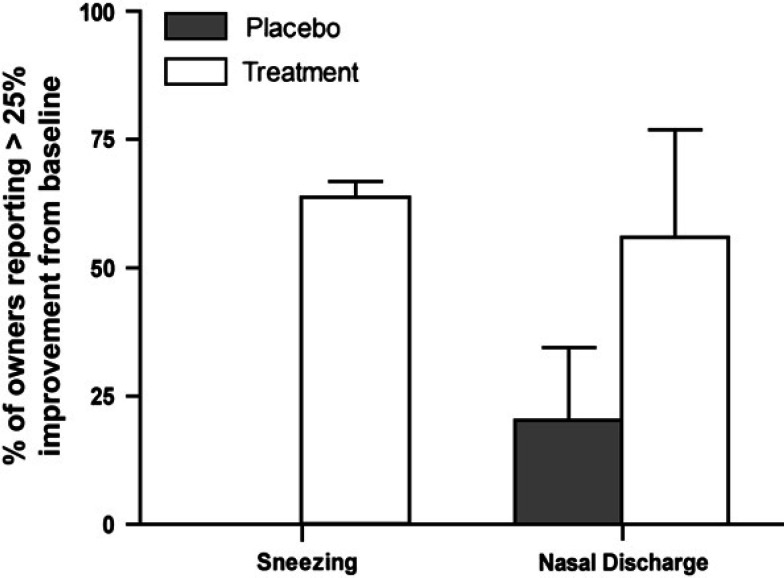
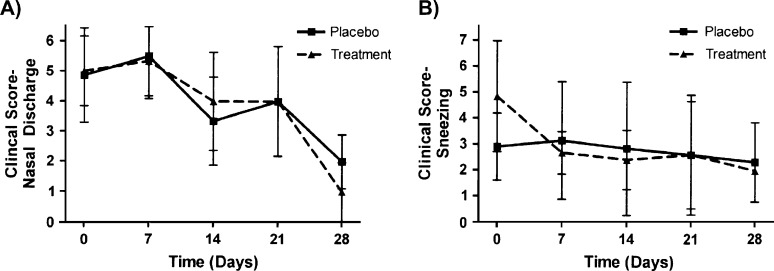
For group 3, 5/7 (71%) of owners of cats administered CLDC reported at least a 25% improvement in the rate of sneezing, whereas none of the five cats administered liposomes alone were reported by the owners to have improved (Fig 4); this difference was significantly different (P<0.05). However, there were no differences between CLDC-inoculated and liposome-inoculated group 3 cats with regard to nasal discharge (Fig 4) or weekly clinical score (data not shown). There were also no differences in nasal discharge (Fig 5A), sneezing (Fig 5B), or weekly clinical score (data not shown) between CLDC-inoculated and liposome-inoculated group 4 cats. Differences in FHV-1 viral load (data not shown) between CLDC-inoculated and liposome-inoculated group 4 cats were not detected. Similar numbers of group 4 cats were positive for FHV-1 DNA at the beginning of the study (CLDC cats=2/13; liposome cats=1/15) and the end of the study (CLDC cats=0/13; liposome cats=0/15), regardless of whether the cats were administered CLDC or liposomes.
Fig 4.
Effect of IL-2 CLDC treatment on clinical signs in adult client-owned cats with chronic rhinitis. Client-owned adult cats (group 3) were randomized to 4 weeks of treatment with intraperitoneal injection once weekly with 150 μg IL-2 CLDC (Treatment, n=5), or were randomized to treatment with liposomes only (Placebo, n=7). The mean (+SD) of the owners' evaluations of changes in clinical scores at each week, compared to baseline values, were tabulated and plotted. Compared to pre-treatment signs, cats randomized to the IL-2 CLDC treatment group had a significant improvement in sneezing (P=0.016) but not in the frequency of nasal discharge (P=0.063).
Fig 5.
Effect of IL-2 CLDC treatment on clinical scores in shelter-owned cats with chronic rhinitis. Shelter cats were randomized to 4 weeks of treatment with intraperitoneal injection once weekly with 150 μg IL-2 CLDC or with liposomes only, as described in Materials and methods (group 4). The clinical scores were assessed by assistants blinded to the treatment groups. The mean (±SD) scores for nasal discharge (A) and sneezing (B) were plotted. Differences in placebo (n=13) and treatment (n=15) groups were not statistically different at any time point (P>0.05).
Th1 and Th2 cytokine profiles in cats with and without rhinitis
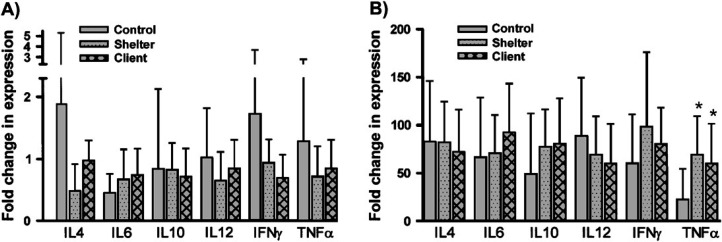
Results for IL-4, IL-6, IL-10, IL-12, IFN-γ, and TNF-α cytokine expression from PMBC samples from cats in groups 2–4 collected prior to treatment were compared before and after activation with PMA plus ionomycin at time zero. Results from unstimulated PBMC did not vary between the three groups of cats for any of the six cytokines evaluated (Fig 6A). However, after stimulation, both group 3 and group 4 cats had significantly higher levels of mRNA transcript expression of TNF-α than group 2 cats (Fig 6B).
Fig 6.
Cytokine mRNA expression in PBMC from normal cats and cats with rhinitis. PBMC were isolated by density centrifugation from blood of group 2 cats (control, n=8), group 3 cats (client, n=9), and group 4 cats (shelter, n=28), prior to treatment based on sample availability. Total RNA was isolated from cells immediately after isolation (A) or following stimulation for 6 h with phorbol myristate acetate (PMA: 10 ng/ml) plus ionomycin (500 ng/ml) (B) and cytokine mRNA expression was quantitated as described in Materials and methods. Mean cytokine expression levels (±SD) were expressed relative to a sample of pooled cDNA and plotted. ∗ Denotes values that are statistically different (P<0.05) from the control group.
Effect of repeated CLDC administration on cytokine mRNA expression in cats with and without rhinitis
Results for IL-4, IL-6, IL-10, IL-12, IFN-γ, and TNF-α cytokine expression from unstimulated PMBC samples from cats in groups 2–4 collected before treatment were compared to those in samples collected 8 h after the first treatment and on day 28 of the treatment period. Significant differences in the expression of IL-4, IL-6, IL-10, IL-12, or IFN-γ transcripts were not noted in CLDC-treated cats compared to liposome-only treated cats in either group 3 or group 4, when compared to pre-treatment levels (data not shown). Expression of TNF-α mRNA decreased between week 0 and week 4 in CLDC-inoculated group 3 cats (P=0.025) but not in CLDC-inoculated group 4 cats (P=0.42). In cats treated with liposomes only, there were no significant differences in TNF-α mRNA levels in either group 3 (P=0.39) or group 4 cats (P=0.38) when compared to pre-treatment levels.
Effects of CLDC treatment on adaptive immune responses in cats with chronic rhinitis
Whole blood proliferative responses to concanavalin A were significantly increased (P=0.05) from baseline in CLDC-inoculated group 4 cats but not liposome-inoculated group 4 cats or CLDC-inoculated or liposome-inoculated group 3 cats (data not shown).
KLH antibody levels from day 28 were significantly greater than day 0 in group 4 cats inoculated with liposomes only (P=0.0004) or CLDC (P<0.0001). In addition, there were no detectable differences between treatment groups within group 2 or group 3.
Discussion
There are several significant findings from the experiments performed on the healthy cats in group 1 and group 2. Detection of fever, increased neutrophil count, monocytosis, increased percentage of CD4+ lymphocytes, and increased percentage of CD8+ lymphocytes in group 1 healthy cats after the administration of CLDC document that CLDC are activators of innate immunity in cats, similar to what was previously shown in mice (Dow et al 1999a). These effects may vary by the age of the cat as evidenced by detection of persistent monocytosis in the CLDC-inoculated young group 1 cats but not the CLDC-inoculated older group 2 cats. Previous studies in mice had shown that injections of cationic liposomes alone were inert in vivo (Dow et al 1999a). Data from this study suggest the liposome component of CLDC is not inert when administered to cats. For example, both liposome-inoculated and CLDC-inoculated group 2 cats developed fever and increase in neutrophil count, as did CLDC-inoculated group 1 cats. However, cats in the group 1 placebo group received saline in contrast to liposomes and did not develop fever or change in neutrophil count. Though the CLDC used in these studies encoded the feline IL-2 gene, the immune stimulatory effects observed were most likely the result of the CLDC themselves, rather than due to DNA specifically coding for IL-2. In support of this, we have previously observed equivalent degrees of immune activation in mice administered IL-2 encoding CLDC or CLDC prepared with non-coding DNA (data not shown) by IP injection.
In general, the IP inoculations were well tolerated by the cats. Fever, lethargy, and vomiting following administration of liposomes or CLDC to cats were generally transient and mild; we attributed the findings to the rapid induction of innate immunity. Serum biochemical profiles remained normal over time and so there appeared to be no adverse effects on liver or kidney function. The results should be viewed cautiously as the duration of this study was relatively short; it is possible that longer treatment might have elicited changes. However, we have treated several cats repeatedly with CLDC over a 2–4-year period without induction of notable side effects (unpublished data).
The biggest limitations to the experiments performed in the group 3 and group 4 cats with rhinitis are sample size and inability to perform a complete diagnostic work-up in all cats which did not allow us to further stratify the cases by cause (ie, bacterial vs viral). However, based on the entry criteria used, we believe the majority of otherwise treatable diseases such as nasopharyngeal polyp, dental disease, neoplasia, and fungal disease were unlikely. Because all cats had previously been treated with antibiotics considered appropriate for bacterial rhinitis, we believe that chronic viral disease or another undetermined cause of chronic inflammatory disease was the cause of illness in these cats. In future prospective studies of this type, it would be optimal to apply a standardized work-up and pre-study therapeutic trials to all cats.
Administration of CLDC but not liposomes to group 3 cats with chronic rhinitis resulted in decreased sneezing which suggests that the CLDC protocol used here may be a useful adjunctive therapy for these patients. As virological data were not available for this group of cats, it cannot be determined whether induction of anti-viral activity could have accounted for the lessening of sneezing. Based on results of the experiments in healthy group 1 and group 2 cats, we believe activation of innate immunity is most likely responsible for the clinical improvement observed, though the exact mechanism of this effect could not be ascertained in this group of study animals. However, CLDC administration may also improve T cell mediated immunity as evidenced by increased percentages of CD4+ and CD8+ lymphocytes in healthy group 1 kittens and by increased concanavalin A stimulated whole blood proliferative responses in CLDC-treated group 4 cats.
In group 4 cats, improvement in clinical parameters was noted over time regardless of administration of CLDC or liposomes alone. It is possible that the clinical abnormalities were naturally resolving as these were young cats with a relatively short duration of clinical illness compared to group 3 cats. However, as we showed evidence of enhanced innate immunity in healthy group 2 cats after administration of liposomes alone, the liposomes may have had a treatment effect in group 4 cats. Group 4 cats developed detectable KLH antibody responses regardless of treatment with CLDC or liposomes whereas KLH antibody responses were not detected in group 2 or group 3 cats. The failure of healthy group 2 cats to develop detectable KLH responses suggests that CLDC or liposome associated immune stimulation alone was not potent enough to enhance responses to this non-adjuvanted antigen in normal cats. The detection of KLH antibodies in group 4 cats with rhinitis, regardless of treatment with CLDC or liposomes suggests the cats had non-specific immune enhancement induced by their disease process. The failure to detect the same KLH responses in group 3 cats with chronic rhinitis may relate to their age and immune function. Alternately, because group 3 cats had very chronic illnesses, any non-specific immune enhancement associated with acute rhinitis may have waned.
Because complete virological assessments were not performed, we cannot make definitive statements about the role viruses played in the disease process. In group 4 cats, FHV-1 DNA was detected in some cats but because healthy cats can also be positive, this finding does not prove disease causation. Similar viral loads and percentage positive results were detected before and after CLDC or liposome administration to group 4 cats, suggesting that these protocols did not influence the infection rate or shedding rates of FHV-1. The FHV-1 infection rate of our group 4 cats with persistent disease was much lower than a group of cats with acute, treatment responsive disease (52 of 61 cats) evaluated at the same shelter during the same time period (Veir et al 2004). This finding may indicate that FHV-1 was not associated with the syndrome in our population of shelter cats with resistant disease or that the superficial swabs lead to falsely negative results. However, it is also possible that FHV-1 had caused the initial insult but was not necessary for the persistence of clinical signs or that FHV-1 copy numbers within the sensitivity limits of the PCR assay used are only present during acute disease. We believe the latter hypothesis is most likely because similar methods were used in our study of cats with acute, treatment responsive disease.
Cytokine profiles showed no bias towards a Th2 type response in cats with chronic rhinitis in this study. Since the completion of this study, this has been supported by other publications in which a bias towards a Th1 type response was demonstrated in nasal biopsies from cats with chronic rhinitis (Johnson et al 2005a). The decrease in TNF-α mRNA expression over time in older group 3 cats with chronic rhinitis following CLDC treatment may actually be a beneficial effect, as excessive concentrations of TNF-α can lead to chronic weight loss and other signs associated with chronic rhinitis. Overproduction of TNF-α can also lead to an inappropriate inflammatory response to pathogens, which could be a contributing factor to disease in cats with chronic rhinitis. The failure to observe changes in cytokine mRNA expression by PBMC in response to treatment with CLDC may be attributable to the effects of sample collection timing as activation of innate immunity by CLDC in mice is typically transient (Dow et al 1999a). In addition, CLDC are more likely to traffic to lymph nodes than to the bloodstream following IP injection (Dow, unpublished data), so that assessment of PBMC for cytokine changes may fail to sample the relevant cell populations.
In summary, we have shown that relatively low doses of CLDC, which are a potent activator of innate immunity, are capable of eliciting activation of both innate and adaptive immune responses in cats. Adult, client-owned cats with chronic rhinitis of greater than 1 year appear to be more likely to experience clinical benefit from treatment with CLDC compared to younger shelter cats. However, ages in the humane society group were estimated and so this age comparison must be interpreted cautiously. The potency of CLDC and their favorable safety profile in both healthy and sick cats suggest that further evaluation as an immunotherapeutic in chronic infectious or inflammatory diseases of cats is warranted.
Acknowledgements
This research was supported by a grant from the Morris Animal Foundation.
The authors wish to acknowledge Jennifer Hawley and Melissa Brewer for technical assistance and Drs. Steve Wheeler and Kelly Diehl (Veterinary Referral Center of Colorado, Englewood, CO) for assistance with enrolling and treating client-owned cats for these studies.
References
- Applied Biosystems User Bulletin #2: ABI PRISM 7700 Sequence Detection System, 2001, Applied Biosystems [Google Scholar]
- Baiocchi R.A., Ward J.S., Carrodeguas L., Eisenbeis C.F., Peng R., Roychowdhury S., Vourganti S., Sekula T., O'Brien M., Moeschberger M., Caligiuri M.A. GM-CSF and IL-2 induce specific cellular immunity and provide protection against Epstein–Barr virus lymphoproliferative disorder, Journal of Clinical Investigations 108, 2001, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape L. Feline idiopathic chronic rhinosinusitis: a retrospective study of 30 cases, Journal of American Animal Hospital Association 28, 1992, 149–155. [Google Scholar]
- Dow S.W., Fradkin L.G., Liggitt D.H., Willson A.P., Heath T.D., Potter T.A. Lipid–DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously, Journal of Immunology 163, 1999a, 1552–1561. [PubMed] [Google Scholar]
- Dow S.W., Schwarze J., Heath T.D., Potter T.A., Gelfand E.W. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice, Human Gene Therapy 10, 1999b, 1905–1914. [DOI] [PubMed] [Google Scholar]
- Fairman J., Roche L., Pieslak I., Lay M., Corson S., Fox E., Luong C., Koe G., Lemos B., Grove R., Fradkin L., Vernachio J. Quantitative RT-PCR to evaluate in vivo expression of multiple transgenes using a common intron, Biotechniques 27, 1999, 566–570, 572–574. [DOI] [PubMed] [Google Scholar]
- Helps C.R., Lait P., Damhuis A., Bjornehammar U., Bolta D., Brovida C., Chabanne L., Egberink H., Ferrand G., Fontbonne A., Pennisi M.G., Gruffydd-Jones T., Gunn-Moore D., Hartmann K., Lutz H., Malandain E., Mostl K., Stengel C., Harbour D.A., Graat E.A. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries, Veterinary Record 156, 2005, 669–673. [DOI] [PubMed] [Google Scholar]
- Johnson L.R., De Cock H.E., Sykes J.E., Kass P.H., Maggs D.J., Leutenegger C.M. Cytokine gene transcription in feline nasal tissue with histologic evidence of inflammation, American Journal of Veterinary Research 66, 2005a, 996–1001. [DOI] [PubMed] [Google Scholar]
- Johnson L.R., Foley J.E., De Cock H.E., Clarke H.E., Maggs D.J. Assessment of infectious organisms associated with chronic rhinosinusitis in cats, Journal of the American Veterinary Medical Association 227, 2005b, 579–585. [DOI] [PubMed] [Google Scholar]
- Kipar A., Leutenegger C.M., Hetzel U., Akens M.K., Mislin C.N., Reinacher M., Lutz H. Cytokine mRNA levels in isolated feline monocytes, Veterinary Immunology and Immunopathology 78, 2001, 305–315. [DOI] [PubMed] [Google Scholar]
- Lenzo J.C., Mansfield J.P., Sivamoorthy S., Cull V.S., James C.M. Cytokine expression in murine cytomegalovirus-induced myocarditis: modulation with interferon-alpha therapy, Cell Immunology 223, 2003, 77–86. [DOI] [PubMed] [Google Scholar]
- Leutenegger C.M., Mislin C.N., Sigrist B., Ehrengruber M.U., Hofmann-Lehmann R., Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA, Veterinary Immunology and Immunopathology 71, 1999, 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R.S., Veir J., Fieseler K.V., Dow S.W. Use of immunostimulatory liposome–nucleic acid complexes in allergen-specific immunotherapy of dogs with refractory atopic dermatitis – a pilot study, Veterinary Dermatology 16, 2005, 61–68. [DOI] [PubMed] [Google Scholar]
- Templeton K.E., Scheltinga S.A., Graffelman A.W., Van Schie J.M., Crielaard J.W., Sillekens P., Van Den Broek P.J., Goossens H., Beersma M.F., Claas E.C. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae, Journal of Clinical Microbiology 41, 2003, 4366–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt D.R., Lappin M.R. Pathogenesis and treatment of feline rhinitis, Veterinary Clinics of North America – Small Animal Practice 24, 1994, 807–823. [DOI] [PubMed] [Google Scholar]
- Veir J.K., Ruch-Gallie R., Spindel M.E., Lappin M.R. Prevalence of FHV-1, Mycoplasma spp., and aerobic bacteria in shelter cats with acute upper respiratory tract disease, Journal of Veterinary Internal Medicine 18 (3), 2004, 197. [Google Scholar]
- Vogtlin A., Fraefel C., Albini S., Leutenegger C.M., Schraner E., Spiess B., Lutz H., Ackermann M. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR, Journal of Clinical Microbiology 40, 2002, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]