Abstract
This study investigated the effect of inhaled fluticasone on lower airway inflammation and bronchial responsiveness (BR) to inhaled carbachol in cats with very mild, chronic bronchitis (n=5) that were compared with healthy cats serving as controls (n=6). Chest radiographs, BR tests performed non-invasively by barometric whole body plethysmography (BWBP) and bronchoalveolar lavage (BAL) were performed before and after treatment. BR was quantified by calculating the concentration of carbachol inducing bronchoconstriction (C-Penh300%), defined as a 300% increase of baseline Penh, an index of bronchoconstriction obtained by BWBP. BAL fluid was analyzed cytologically and the oxidant marker 8-iso-PGF2α was determined. At test 1, healthy cats and cats with bronchitis were untreated, whereas for test 2 inhalant fluticasone (250 μg once daily) was administrated for 2 consecutive weeks to cats with bronchitis. Control cats remained untreated. Inhaled fluticasone induced a significant increase in C-Penh300% and a significant decrease of BAL fluid total cells, macrophages, neutrophils and 8-iso-PGF2α in cats with bronchitis, whilst untreated control cats did not show significant changes over time. This study shows that a 2-week fluticasone treatment significantly reduced lower airway inflammation in very mild bronchitis. BR could be successfully monitored in cats using BWPB and decreased significantly in response to inhaled fluticasone. 8-Iso-PGF2α in BAL fluid was responsive to treatment and appeared as a sensitive biomarker of lower airway inflammation in cats.
Feline chronic bronchitis and feline asthma are to the most common respiratory diseases in cats (Moise et al 1989, Corcoran et al 1995, Dye et al 1996, Bay and Johnson 2004). Although clinical signs such as coughing, wheezing and exercise intolerance are similar, it remains controversial whether chronic bronchitis and asthma in cats are two different pathological conditions or result from a similar pathology with different inflammatory profiles, especially with regard to bronchoalveolar lavage (BAL) fluid cytology (Moise et al 1989, Corcoran et al 1995, Dye et al 1996). Whilst chronic bronchitis is only described in clinical studies and characterized by a neutrophilic airway inflammation (Moise et al 1989, Corcoran et al 1995, Dye et al 1996), clinical cases and experimental models of feline asthma have shown that asthma is an essentially eosinophilic inflammatory condition (Padrid et al 1995, Norris et al 2003, Kirschvink et al 2004a,b), even if a significant increase in neutrophils is found (Padrid et al 1995, Kirschvink et al 2004a,b). The experimental models of asthma further provide evidence of an increased bronchial responsiveness (BR) after allergen exposure (Padrid et al 1995, Norris et al 2003, Kirschvink et al 2004a,b), which can be modulated using anti-inflammatory therapeutic agents such as cyclosporine (Padrid et al 1996).
The most frequently used anti-inflammatory drugs in human patients suffering from asthma or chronic bronchitis are corticosteroids administered preferentially as inhalant therapy in order to reduce systemic side effects (Busse 1998). Despite a consistent lack of experimental or clinical studies investigating efficiency and dose regimens of anti-inflammatory therapeutics in feline respiratory diseases, corticosteroids are successfully used (Padrid 2000, Boothe 2004). They are often administrated orally or by injection, but recent development of adapted inhalation devices offers the possibility of efficient aerosol administration for cats (Padrid 2000, Schulman et al 2004).
The evaluation of therapy efficiency in feline patients suffering from chronic bronchitis or asthma is strongly dependent on the owner's opinion and is mainly based on improvement of clinical and radiographic signs. Bronchoscopy and analysis of BAL fluid cells or inflammatory markers provide important information about the evolution of a bronchial inflammatory process (Hawkins 2004), but these investigations are rarely repeated in practice once the patient's status is improved. Among the recent biological markers of lower airway inflammation, the lipid peroxidation product 8-iso-PGF2α determined in BAL fluid or exhaled breath condensate has gained attention as sensitive index of oxidative processes occurring in respiratory disease, such as asthma, chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis in humans (Morrow and Roberts 2002, Wood et al 2003), and heaves in equines (Kirschvink et al 2002a).
Since bronchial inflammation is likely to be reflected by increased BR, lung function tests allowing assessment of BR might be an alternative or complement to bronchoscopy and BAL (O'Byrne 1998). However, conventional lung function tests measuring pulmonary resistance (R L) and dynamic compliance (C Dyn) require anaesthesia in cats and are therefore unsuitable for clinical use (Padrid et al 1995, Norris et al 2003, Rozanski and Hoffman 2004). Barometric whole body plethysmography (BWBP) for unsedated and unrestrained animals offers the possibility of a non-invasive, well tolerated and repeatable assessment of BR in cats (Hoffman et al 1999, Hirt et al 2003). Although BWBP cannot be considered as a measure of the mechanical properties of the airways, the index of airflow limitation calculated by this technique, the enhanced pause or Penh, increases in different species in response to cholinergic stimulation of the respiratory system (Hamelmann et al 1997, Hoffman et al 1999, Halloy et al 2004, Kirschvink et al 2005a) and is correlated with R L in mice, pigs and rats (Hamelmann et al 1997, Halloy et al 2004, Kirschvink et al 2005a). Assessment of BR using BWBP might therefore be useful in monitoring quantitatively the response to an anti-inflammatory treatment in feline patients suffering from lower airway inflammation.
The aim of this study was to test whether fluticasone, an inhalant corticosteroid, decreases airway inflammation and BR in cats with very mild, but persistent bronchitis. Bronchial inflammation was assessed by analysing BAL fluid for cytology and the oxidant marker 8-iso-PGF2α, whereas BR was determined by measuring Penh after inhalation of a cholinergic challenge.
Material and methods
Animals
Eleven experimental cats (Harlan, Horst, Netherlands) were enrolled for 4 months in the study (median age: 2 years [1.5–2.4]; median weight: 4.0 kg [3.4–5.7]). The animals were housed and cared for according to the national guidelines and the principles advised by the European Council for the care of laboratory animals. They lived in appropriate rooms where they were allowed to move freely and were fed twice daily with commercially available food. Among those animals, five cats (median age: 2.1 years [1.8–2.4]; median weight: 5.0 kg [3.8–5.7]) presented radiographic, bronchoscopic and pulmonary cytological signs of a very mild non-infectious bronchitis. These abnormal findings were present for a period lasting at least 6 months, during which the cats underwent no treatment but were followed by radiography and bronchoscopy and BAL. No clinical signs of respiratory disease were detectable when the cats were included in the study. The six other cats with normal radiographic, bronchoscopic and cytological findings were used as healthy controls (median age: 1.5 years [1.2–2.2]; median weight: 3.7 kg [2.8–4.6]). The study was approved by the Animal Ethical Committee of the University of Liège.
Study design
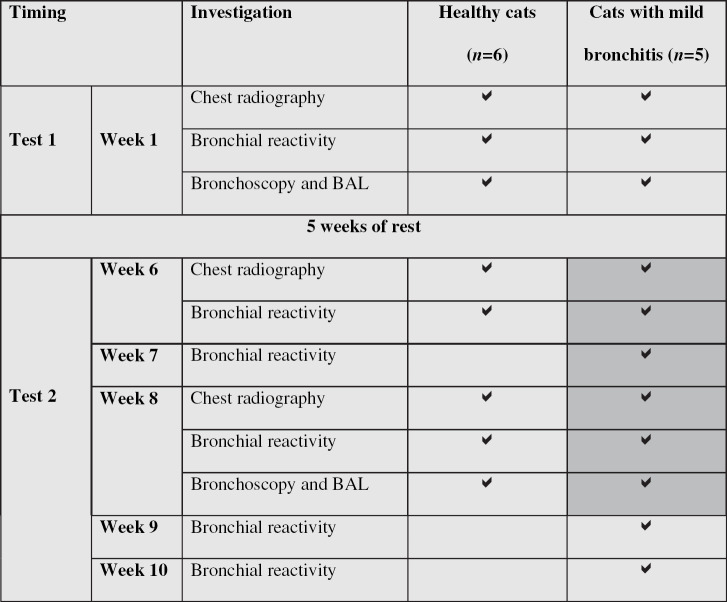
All cats underwent chest radiography, BR testing and bronchoscopy including BAL at regular intervals. At week 1, both cat groups underwent each test once. After a pause period of 5 weeks allowing the cats to recover from bronchoscopy and BAL, both groups underwent BR testing and chest radiography during week 6. The cats with bronchitis were then treated daily during 14 consecutive days by 250 μg inhaled fluticasone. Both groups underwent chest radiography, BR testing and bronchoscopy at week 8. In cats with bronchitis, BR was further determined after 7 days of treatment (week 7), as well as 7 (week 9) and 14 days (week 10) after end of treatment. Based on earlier investigations, the variables investigated in this study were expected to remain unchanged in healthy control cats. Therefore, BR tests at week 7, week 9 and week 10 were not performed in control cats. Study design is shown in Table 1.
Table 1.
Inhalant fluticasone (Fluticasone propionate ‘Flixotide’, 250 μg/dose, GSK, Belgium) was administrated each morning to bronchitic cats by use of a pressurized metered dose inhaler (pMDI) and a spacing chamber connected through an inspiratory valve to a facemask (Aerokat, Trudell Medical Int., London, Canada). The facemask was carefully placed on the cat's nose and mouth, the pMDI was activated once and the facemask was removed when the cat had taken minimum five deep breaths.
Barometric whole body plethysmography and determination of bronchial responsiveness
Barometric whole body plethysmography (BWBP)
The system of BWBP for cats as previously described was used (Hoffman et al 1999, Hirt et al 2003, Kirschvink et al 2005b, in press). The cats were placed in the transparent plexiglas chamber of a barometric whole body plethysmograph (Buxco Electronics, Sharon, CO, USA; height: 25 cm, length: 51 cm, width: 30 cm) that was ventilated by continuous bias flow (Air Control Industries Ltd, Somerset, UK) of 4 L/min, which was adjustable by a flowmeter (Influx, Caché Instrumentation Ltd, Wakefield, UK). The air inlet was via a screen pneumotachograph (35 mm diameter) positioned on one wall of the main chamber, whereas the air outlet was at the opposite end through a 30 mm outlet in the chamber's cover. One pole of a differential pressure transducer (Emka Technologies, Paris, France) was open to the main chamber, whereas the other pole was open to a reference chamber equilibrated with atmospheric pressure by a small channel (1.5 mm). Transduced signals were amplified (Emka Technologies, Paris, France), digitized and sampled at 100 Hz by use of the IOX software version 1.569 (Emka Technologies, Paris, France), which provided a breath-by-breath analysis of waveforms. The following respiratory variables were recorded: respiratory rate (RR), calculated from inspiratory time (Ti [ms]) and expiratory time (Te [ms]), peak inspiratory and expiratory pressure (expressed as pseudoflows: PIF and PEF [mL/s]), estimated tidal volume (V T [mL]) and enhanced pause or Penh=([Te−RT]/RT×PEF/PIF), RT being the relaxation time (ms), which is the time point where 65% of V T is expired. Penh is a unitless variable or index that increases during bronchoconstriction. Waveforms disturbed by movements and sniffing were automatically eliminated when V T was smaller than 10 mL, Ti less than 150 ms or greater than 10000 ms or when the difference between inspiratory and expiratory volume exceeded 20%. The chamber pressure signal was calibrated each day of measurement by dynamic injection of 50 mL of room air via a syringe into the main chamber of the plethysmograph.
Determination of bronchial responsiveness
After a 5-min baseline recording of respiratory variables, an ultrasonic nebulizer (Ultraneb Devilbiss 2000, Devilbiss Healthycare Inc., Somerset, USA) was connected to the nebulizer port of the plethysmograph and sterile saline (0.9% NaCl) was nebulized during 1 min. A post-saline record of 5 min was performed and saline solutions containing increasing concentrations of carbachol (Sigma, St Louis, MO, USA) (0.005, 0.01, 0.02, 0.04, 0.06, 0.08%) were successively nebulized during 1 min and were followed each by 5 min recordings until bronchoconstriction occurred. Bronchoconstriction was defined as an increase of 300% or more of post-saline Penh during 20 s or longer (Hoffman et al 1999). The carbachol concentration inducing a 300% increase of post-saline Penh (C-Penh300%) was calculated by interpolation of the dose-response curve between the two final concentrations used (Hoffman et al 1999, Hirt et al 2003).
Chest radiographs
When cats underwent chest radiography, they were not sedated and a ventral–dorsal and a right lateral view were performed. A scoring system ranging for the total score from 0 to 6 aimed to evaluate separately bronchial pattern (0: absence of signs, 1: mild [first generation of bronchi visible], 2: moderate [second generation visible], 3: severe [third generation visible]) and interstitial pattern (0: absence of signs, 1: mild [mild interstitial framework visible], 2: moderate [interstitial framework distinguishable from a bronchial pattern], 3: severe [clearly apparent interstitial pattern]) (Foster et al 2004). This scoring system was applied to each couple of views by a radiologist (FS) blinded to the animals' identity and treatment.
Bronchoscopy and bronchoalveolar lavage
The cats were sedated by intramuscular injection of medetomidine (100 μg/kg, ‘Domitor’, Pharmacia Animal Health, Puurs, Belgium). Venous blood was sampled for haematological analysis by jugular puncture. The cats were equipped with an antebrachial intravenous catheter and anaesthesia was induced and maintained by intravenous administration of propofol (1–2 mg/kg, ‘Diprivan’, Zeneca, Destelbergen, Belgium). Each cat breathed for 1 min oxygen-enriched air. After topical anaesthesia of the larynx (lidocaine ‘Linisol 1%’, Braun Medical, Degem, Belgium), a paediatric 4.8 mm diameter video-endoscope (Fujinon EB-4105, ONYS SA, Brussels, Belgium) was introduced through the oral cavity into the trachea. Trachea, right and left main bronchi were visualised and allowed a macroscopic evaluation of the large airways. A bronchoscopy score taking into account the mucosal aspect (0: normal, 1: moderate mucosal reddening and oedematous appearance, 2: severe reddening and oedematous appearance), the presence of mucus (0: absent, 1: moderate, 2: abundant) and the airway reactivity (ie, coughing, tracheal and bronchial mobility or collapse: 0: absent, 1: moderate, 2: severe) was established by the same person (NK) after reviewing of video recordings of each bronchoscopy. The bronchoscope was first wedged in the right lung and 10 mL of preheated (37°C) sterile saline (NaCl 0.9%) was instilled into the right main bronchus and recovered by gentle suction. The same procedure was repeated in the left main bronchus. Recovery rate of BAL fluid ranged between 50 and 65%. Recovered BAL fluid was kept on ice until being processed within 1 h of collection. After extraction of the bronchoscope, the cats were oxygenated by breathing spontaneously oxygen-enriched air. Anaesthesia was reversed by intravenous administration of atipamezole (250 μg/kg, “Antisedan”, Pharmacia Animal Health, Puurs, Belgium).
Sample processing and analysis
Venous blood
Blood samples were analyzed within 6 h after collection for haematology (differential blood cell count, packed cell volume), which ranged within normal values for all cats throughout the study.
BAL fluid cytology
Cytology and total nucleated cell count were performed by light microscopy within 4 h after collection. The BAL fluid was Cytospin (Cytospin 2, Shandon Inc, Pittsburgh, PA, USA) centrifuged and Giemsa-stained. At least 100 cells were counted. For total cell count Türck coloration and a Thomas cell were used and repeated four times.
BAL fluid bacteriology
A semi-quantitative bacteriological analysis of BAL fluid (aerobes and anaerobes) was systematically performed. Results were considered as negative in all cases (<1.7×103 colonies/mL) (Peeters et al 2000).
BAL fluid 8-iso-PGF2α determination
Untreated BAL fluid was rapidly frozen and stored at −80°C until analysis. As described elsewhere in detail (Kirschvink et al 2002b), 8-iso-PGF2α was purified and concentrated before being analyzed by an enzyme immuno-assay kit (Cayman, Ann Arbor, MI, USA). Briefly, defrosted samples were adjusted for pH 2–2.5 before centrifugation for 10 min at 200 g and 4°C. One millilitre of supernatant was run through a C18 1 mL·100 mg−1 column (Bond Elut, Varian, Harbor City, CA, USA). The column was rinsed with water and hexane; elution was performed with ethyl-acetate/methanol (95:5 V/V). Eluate was vacuum dried and restituted with 200 μL of buffer provided by the kit manufacturer. The kit was consequently used according to manufacturer's instructions. Each sample was analyzed in triplicate and recovery rate of the purification procedure was checked by spiking one sample per assay.
Statistical analysis
BWBP and BAL fluid variables were normally distributed and are shown as mean values±SD, whereas radiography and bronchoscopy scores are shown as medians with ranges. Parametric data were analyzed by analysis for variance (ANOVA) for repeated measures and t-tests, whereas non-parametric data were analyzed by Friedman, Wilcoxon and Mann–Whitney tests (Statview, SAS Institute Inc., Cary, NC, USA). Correlation analyses were performed using Spearman correlation (Statview, SAS Institute Inc., Cary, NC, USA). A P-value lower than 0.05 was considered to be significant.
Results
Bronchial responsiveness
Baseline measurements of respiratory variables recorded by BWBP, such as RR, V T, PIF, PEF and Penh were within normal ranges (Kirschvink et al, in press) and did not change throughout the protocols (data not shown). In healthy cats, C-Penh300% remained unchanged over the investigation period but was not significantly higher than that of cats with bronchitis before they were treated (Fig 1). In cats with bronchitis, C-Penh300% was similar at week 1 and week 6. After 7 days of fluticasone treatment, C-Penh300% was increased in a non-significant manner (P=0.07), whereas after 14 days of treatment as well as 7 days after end of treatment, C-Penh300% was significantly higher than at week 1 and week 6. Fourteen days after the end of treatment, difference from week 6 was no more significant (P=0.06).
Fig 1.
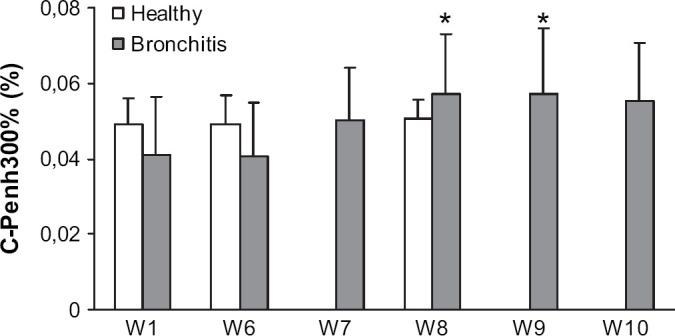
Carbachol concentration inducing a 300% increase of Penh (C-Penh300%) determined at different occasions in six healthy cats and five cats with mild bronchitis. Healthy cats were untreated at W1, W6 and W8. Cats with bronchitis were untreated at W1 and W6 and underwent daily inhalant fluticasone treatment for W7 and W8. W9 and W10 corresponded to 1 and 2 weeks post-treatment, respectively. *Significantly different from C-Penh300% of cats with bronchitis at W1 and W6, P<0.05.
Chest radiographs
At week 1, healthy cats had significantly lower bronchial and interstitial scores than cats with bronchitis (Table 2). Radiographic scores obtained at week 6 (before starting fluticasone treatment in cats with bronchitis) did not differ from those obtained at week 1 (data not shown). At week 8, scores of healthy cats were similar to those of week 1, whereas in cats with bronchitis the total score was significantly lower than that obtained at week 1. Bronchial pattern was similar to that of healthy control cats, whereas interstitial pattern remained significantly higher.
Table 2.
Cytological analysis of bronchoalveolar lavage fluid, bronchoscopy and radiography scores in healthy control cats (n=6) and cats with mild bronchitis (n=5) at week 1 and week 8
| Variable/Time | Healthy controls (n=6) | Cats with mild bronchitis (n=5) | ||
|---|---|---|---|---|
| Week 1 | Week 8 | Week 1 | Week 8 | |
| Bronchoalveolar lavage fluid | ||||
| Macrophages (%) | 87.0±9.1 | 89.8±5.5 | 74.6±8.3 † | 85.2±2.5 * |
| Lymphocytes (%) | 0.8±1.0 | 0.7±1.0 | 0.6±0.5 | 0.6±0.5 |
| Neutrophils (%) | 7.5±4.3 | 7.8±3.3 | 24.2±7.8 † | 13.6±1.5 * † |
| Eosinophils (%) | 4.7±2.4 | 1.7±3.2 | 0.6±0.9 | 0.6±1.3 |
| Total cell count (103/ml) | 83±81 | 48±54 | 150±52 | 79±13 * |
| Macrophages (103/ml) | 71±69 | 44±54 | 109±33 | 68±12 * |
| Lymphocytes (103/ml) | 0.9±1.7 | 0.7±1.0 | 0.9±0.9 | 0.5±0.4 |
| Neutrophils (103/ml) | 6.7±7.7 | 3.0±2.7 | 38±26 † | 11±1.6 * † |
| Eosinophils (103/ml) | 4.2±2.6 | 0.2±0.3 | 0.8±1.1 | 0.4±0.8 |
| Bronchoscopy score (0–6) | 1 (0–1) | 1 (0–2) | 3 (1–3)† | 1 (0–3) * |
| Radiography scores | ||||
| Bronchial pattern (0–3) | 1 (0–1) | 1 (0–1) | 1 (1–2) † | 1 (0–1) |
| Interstitial pattern (0–3) | 1 (0–1) | 1 (0–1) | 2 (1–2) † | 2 (1–2) † |
| Total score (0–6) | 1 (1–2) | 1 (1–2) | 4 (2–4) † | 3 (1–3) * † |
Prior week 8, cats with mild bronchitis had been treated with inhalant fluticasone (250 μg/day) during 14 consecutive days. Data are shown as means±SD or medians with ranges.
Significantly different from respective week 1-value.
Significantly different from respective control-value, P<0.05. Radiography scores established at week 6 (before starting fluticasone treatment in cats with bronchitis) did not differ from those established on week 1 and are not shown.
Bronchoscopy and bronchoalveolar lavage fluid analysis
The bronchoscopy score established in healthy control cats did not differ between week 1 and week 8 but was significantly lower than that of cats with bronchitis at week 1 (Table 2). After fluticasone treatment at week 8, bronchoscopy score of cats with bronchitis was significantly decreased and did not differ from healthy control cats.
Cytology of BAL fluid is shown in Table 2. Neutrophil percentage in cats with bronchitis was significantly higher than that in controls at week 1, but was significantly reduced after fluticasone treatment at week 8. Neutrophil count was also significantly decreased after treatment, as well as total nucleated cell count and macrophage count.
By concentrating BAL fluid five times, the oxidant marker 8-iso-PGF2α could be detected in all cats (Fig 2). Cats with bronchitis showed significantly higher concentrations of 8-iso-PGF2α than control cats before and after treatment, but a significant decrease between week 1 and week 8 was observed.
Fig 2.
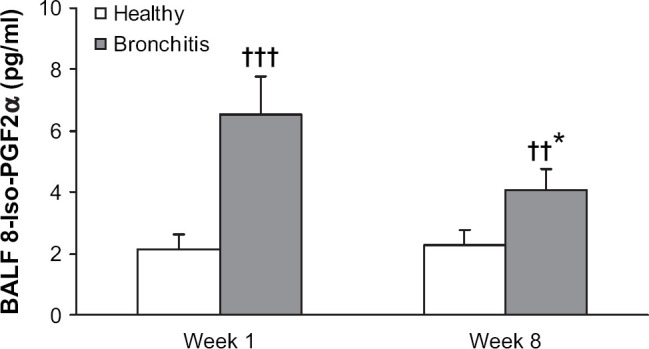
8-Iso-PGF2α concentration in bronchoalveolar lavage (BAL) fluid sampled in six healthy control cats and five cats with mild bronchitis at week 1 (all cats untreated) and week 8, where cats with bronchitis had been treated for 14 consecutive days with inhalant fluticasone. Data are shown as means±SD. Significantly different from values obtained in healthy cats at the same time point, ††P<0.001, †††P<0.0001; significantly different from values obtained in cats with bronchitis at week 1, *P<0.01.
Correlation analyses
Whilst 8-iso-PGF2α concentration in BAL fluid was positively and significantly correlated with radiography score (φ=0.70, P<0.005), bronchoscopy score (φ=0.57, P<0.01), BAL fluid total cells (φ=0.64, P<0.005) and neutrophils (φ=0.84, P<0.005), none of the correlations for C-Penh300% reached significance. 8-Iso-PGF2α and C-Penh300% were significantly and negatively correlated (φ=−0.65, P<0.05).
Discussion
The present study aimed to test whether inhaled fluticasone might modulate BR and lower airway inflammation in cats presenting a very mild, but persistent neutrophilic bronchitis.
A major limitation of this study is the fact that our experimental cats considered having mild and chronic bronchitis did not fulfil the criteria defining this disease in practice (Bay and Johnson 2004). These cats were in fact free from clinical respiratory signs and only showed marginal modifications of radiographic, bronchoscopic and cytological BAL fluid findings. Nevertheless, these cats were abnormal in comparison to healthy control animals of our cat colony. As these abnormal findings had been repeatedly detected over several months preceding this study (data not shown), they were suggestive of a stabilized and mild inflammatory condition of unknown origin. Although an infection with Bordatella bronchiseptica or Mycoplasma species cannot be excluded, it seems unlikely that only a part of the cats, which were all housed in a same room, would have developed neutrophilic airway inflammation in response to one of these infectious organisms. The cats with bronchitis were not much older than the control cats used in this study and initially belonged to the healthy experimental animals. Even if similar cytological findings have been reported to be normal in healthy, client-owned cats (Padrid et al 1991, Dye et al 1996), these cats were significantly different in comparison to healthy control cats from our colony and were therefore considered as presenting a very mild, chronic bronchitis.
This study aimed at evaluating whether inhaled fluticasone given during 2 consecutive weeks had a significant anti-inflammatory effect. Although the comparison with pre-treatment values and values obtained from healthy control cats provided evidence for this effect, inclusion of a placebo-treatment of the cat group with bronchitis and fluticasone treatment of the control group would have been ideal to further strengthen the results of this study. It is, however, unlikely that 5–10 deep inspirations of nebulized saline solution during 14 consecutive days would have changed the results of our cats with bronchitis. Data available in placebo-treated healthy cats exposed for 5 min to nebulized saline solution indicate that radiography, bronchoscopy, BAL cytology and BR results remain unchanged (unpublished data). Inhalation of fluticasone might also have unspecific effects, which may not be directly related to its anti-inflammatory properties and which could be evidenced in healthy animals. Such an experimental design would be ideal but should in our opinion be tested in cats with clinically apparent bronchitis and not in our model with a small number of individuals and relatively small differences between groups.
The feasibility of BR testing by BWBP has already been described in cats (Hoffman et al 1999, Hirt et al 2003), but this is to our knowledge the first study using non-invasive BR testing to assess response to treatment. The tests were well tolerated by all cats; they were lying quietly in the plethysmograph until onset of the response to the carbachol-challenge inducing airflow limitation. Bronchoconstriction occurred within 1–5 min after the inhalation challenge and was sometimes preceded by nervousness, movements and mild cholinergic signs, such as swallowing saliva. The cats remained then seated and bronchoconstriction was clinically visible by an expiratory dyspnoea. Coughing was seen in some but not all cats. Peak response, measured as the maximal value of Penh, was always recorded within 10 min after the last challenge and completely resolved within 15 min. Use of bronchodilators or oxygen supply was never necessary and repetition of the tests at 1 week intervals was well accepted by all cats.
In comparison with other studies using BWBP (Hoffman et al 1999, Hirt et al 2003), we obtained in our healthy cats lower baseline C-Penh300% values. This might be explained firstly by a longer carbachol nebulization period, which was 60 s instead of 30 s in order to achieve a homogenous aerosol dispersion in the plethysmograph, secondly by another type of nebulizer (ultrasonic versus jet nebulizer) used and thirdly by the young age of our cats; young cats being more responsive than older animals (Hirt et al 2003). C-Penh300% values of the healthy cats were very repeatable throughout the study. Cats with bronchitis also showed very similar values before treatment. The fluticasone treatment induced a significant increase of C-Penh300%, indicating that BR was successfully decreased. According to veterinary literature, the peak effect of inhaled corticosteroids is only achieved after 10–14 days of consecutive treatment (Padrid 2000, Boothe 2004). Our data support this observation because C-Penh300% further increased during the second week of fluticasone treatment and remained stable for 1 week after end of the treatment.
Cytological analysis of BAL fluid of healthy cats did not change over time, whereas total cells, macrophages and neutrophils significantly decreased after fluticasone treatment. Nevertheless, BAL fluid neutrophil count remained significantly higher than that of control cats, indicating that the inflammatory process did not completely resolve within 2 weeks of treatment.
8-Iso-PGF2α results were in agreement with BAL cytology and significant correlations with the BAL fluid neutrophil count and C-Penh300% were found. Interestingly, this marker of lipid peroxidation appeared to be sensitive because it decreased in response to fluticasone therapy but remained significantly different from control cats. In comparison to findings made in human patients suffering from chronic lower airway disease (Morrow and Roberts 2002, Wood et al 2003), these results suggest that 8-iso-PGF2α might be also of use in cats. However, even if some preliminary studies suggest that 8-iso-PGF2α concentration in BAL fluid of healthy cats is similar to that of our control cats and increases in response to lower airway inflammation (Hirt et al 2004, Kirschvink et al 2004b), this marker would need to be validated in felines.
Given that BR is described as a predictor of inflammatory processes within the airways (O'Byrne 1998), a correlation between C-Penh300% and inflammatory variables such as 8-iso-PGF2α was expected. The lack of correlation with BAL fluid cytology was surprising and disagrees with observations made in experimentally sensitised asthmatic cats (Kirschvink et al 2004a,b), but the mildness of inflammatory changes and the small number of cats investigated might account for this.
Radiographic scores revealed no time-related changes in healthy cats, but suggested that inhaled fluticasone significantly reduced radiographically detectable signs of airway inflammation. In comparison with clinical studies investigating feline bronchopulmonary disease (Corcoran et al 1995, Dye et al 1996, Foster et al 2004), the interstitial pattern of our cats with bronchitis was more important than the bronchial pattern. Improvement of the radiographic score after fluticasone treatment was mainly due to a decrease of the interstitial pattern. Given that our cats were in a very early stage of developing chronic bronchitis, only mild radiographic lesions could be expected. It might be possible that a bronchial pattern, such as described in clinical cases, develops throughout longer time periods and implies the presence of respiratory clinical signs (Corcoran et al 1995, Dye et al 1996, Foster et al 2004). Indeed, in our studies investigating feline asthma using Ascaris suum-sensitised cats, the first radiographic lesions that appear concomitantly with clinical signs in response to allergen exposure are also interstitial rather than a bronchial (unpublished data).
It is important to point out that radiographic scores were attributed by an investigator who was blinded to the cat's identity and treatment, whereas bronchoscopic scoring might have been less objective because perfect blinding of the evaluator was technically impossible. Consequently, the improvement of the bronchoscopy scores should not be overestimated. Nevertheless, the radiographic and bronchoscopic scores show, on one hand, that no changes should be expected in healthy cats within several weeks, whereas reduced airway inflammation might be related to decreased radiographic and bronchoscopic scores.
Fluticasone belongs to the group of ‘soft’ corticosteroids with a very efficient first-pass hepatic inactivation and has little systemic effects in humans (Derendorf et al 2002). We observed no clinically detectable side effects after treatment in our cats and bacteriological analysis of BAL fluid indicated absence of infection after 2 weeks of daily treatment. Although fluticasone is essentially used for control of eosinophil-driven inflammatory processes as asthma (Busse 1998), we found a significant anti-inflammatory effect in mild neutrophilic bronchitis. In veterinary medicine, fluticasone has been successfully tested in heaves-affected horses by decreasing BAL fluid neutrophils and cytokines and by improving lung function (Giguere et al 2002). In retrospective studies (Corcoran et al 1995, Foster et al 2004), most of the asthmatic and bronchitic cats positively responded to oral prednisolone, suggesting that corticosteroids are efficient anti-inflammatory drugs for both eosinophilic and neutrophilic processes in felines.
The decision to administrate once daily 250 μg of fluticasone per cat was based on general recommendations and the dose used in asthmatic children (Masoli et al 2004). As the cats were adult and had a similar body weight, the same dose was used for all cats. A recent study investigated pulmonary deposition of a nebulized radioactive agent in awake cats (Schulman et al 2004). The authors used a spacer chamber and a facemask similar to ours and demonstrated a homogenous aerosol deposition in the lungs of healthy cats. Even if we cannot presume that inhaled fluticasone will undergo a similar pulmonary deposition, these study results encourage use of inhalants in cats.
In conclusion, this study has shown that inhaled fluticasone exerts a significant anti-inflammatory effect in experimental cats presenting very mild signs of neutrophilic bronchitis. The oxidant marker 8-iso-PGF2α determined in BAL fluid was decreased in response to treatment. Bronchial responsiveness was non-invasively assessed by BWBP and decreased in a treatment duration dependent manner in response to fluticasone. Although these results should be considered as preliminary and need to be confirmed in clinical settings, they provide at least a basis for a treatment regimen using inhaled fluticasone in felines.
Acknowledgements
This study was supported by a grant ‘Région wallonne DGTRE’, Belgium. Jérôme Leemans is supported by a grant from FRIA (Belgium).
References
- Bay J.D., Johnson L.R. Feline bronchial disease/asthma. King L.G. Respiratory Disease in Dogs and Cats, 2004, Saunders: St. Louis, Missouri, 338–396. [Google Scholar]
- Boothe D.M. Drugs affecting the respiratory system. King L.G. Respiratory Disease in Dogs and Cats, 2004, Saunders: St. Louis, Missouri, 229–252. [Google Scholar]
- Busse W.W. How inhaled corticosteroids changed asthma therapy. Holgate S.T., Busse W.W. Lung Biology in Health and Disease: Inflammatory Mechanisms in Asthma Vol. 117, 1998, Marcel Dekker: New York, 37–64. [Google Scholar]
- Corcoran B.M., Foster D.J., Fuentes V.L. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats, Journal of Small Animal Practice 36, 1995, 481–488. [DOI] [PubMed] [Google Scholar]
- Derendorf H., Hochhaus G., Krishnaswami S., Möllmann H. Systemic disposition and effects of inhaled corticosteroids. Schleimer R.P., O'Byrne P.M., Szefler S.J., Brattsand R. Lung Biology in Health and Disease: Inhaled Steroids in Asthma Vol. 163, 2002, Marcel Dekker: New York, 247–272. [Google Scholar]
- Dye J.A., McKiernan B.C., Rozanski E.A., Hoffman W.E., Losonsky J.M., Homco L.D., Weisiger R.M., Kakoma I. Bronchopulmonary disease in the cat: historical, physical, radiographic, clinicopathologic, and pulmonary functional evaluation of 24 affected and 15 healthy cats, Journal of Veterinary Internal Medicine 10, 1996, 385–400. [DOI] [PubMed] [Google Scholar]
- Foster S.F., Allan G.S., Martin P., Robertson I.D., Malik R. Twenty-five cases of feline bronchial disease (1995–2000), Journal of Feline Medicine and Surgery 6, 2004, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere S., Viel L., Lee E., MacKay R.J., Hernandez J., Franchini M. Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate, Veterinary Immunology and Immunopathology 85, 2002, 147–158. [DOI] [PubMed] [Google Scholar]
- Halloy D., Kirschvink N., Vincke G., Hamoir J.N., Delvaux F., Gustin P. Whole body barometric plethysmography: a screening method to investigate airway reactivity and acute lung injuries in freely moving pigs, Veterinary Journal 168, 2004, 276–284. [DOI] [PubMed] [Google Scholar]
- Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. Non-invasive measurement of airway responsiveness in allergic mice using barometric plethysmography, American Journal of Respiratory Critical Care Medicine 156, 1997, 766–775. [DOI] [PubMed] [Google Scholar]
- Hawkins E.C. Bronchoalveolar lavage. King L.G. Respiratory Disease in Dogs and Cats, 2004, Saunders: St. Louis, Missouri, 118–128. [Google Scholar]
- Hirt R.A., Dederichs D., Boehler A., Hoffman A.M. Relationship of age, sex, body weight, and hematologic and respiratory variables with airway reactivity in adult cats, American Journal of Veterinary Research 64, 2003, 26–31. [DOI] [PubMed] [Google Scholar]
- Hirt RA, Gütl A, Delvaux F, Gustin P, Kirschvink N. (2004) 8-Isoprostaglandin-F2alpha in BAL is a direct marker of free radical induced lipid peroxidation in feline asthma. In Proceedings: 22nd Annual ACVIM Forum, Minneapolis, USA, June 9–12, 2004, p. 841, A157 (abstract).
- Hoffman A.M., Dhupa N., Cimetti L. Airway reactivity measured by barometric whole-body plethysmography in healthy cats, American Journal of Veterinary Research 60, 1999, 1487–1492. [PubMed] [Google Scholar]
- Kirschvink N., Smith N., Fiévez L., Bougnet V., Art T., Degand G., Marlin D., Roberts C., Génicot B., Lindsey P., Lekeux P. Effect of chronic airway inflammation and exercise on pulmonary and systemic antioxidant status of healthy and heaves-affected horses, Equine Veterinary Journal 34, 2002a, 563–571. [DOI] [PubMed] [Google Scholar]
- Kirschvink N., Fievez L., Bureau F., Degand G., Maghuin-Rogister G., Smith N., Art T., Lekeux P. Adaptation to multiday ozone exposure is associated with a sustained increase of bronchoalveolar uric acid, Free Radical Research 36, 2002b, 23–32. [DOI] [PubMed] [Google Scholar]
- Kirschvink N., Leemans J., Delvaux F., Snaps F., Clercx C., Gustin P. Bronchial reactivity assessed by whole body barometric plethysmography is correlated with lower airway inflammation in cats, Journal of Veterinary Internal Medicine 18, 2004a, 784, (abstract) [Google Scholar]
- Kirschvink N., Bureau F., Fievez F., Leemans J., Delvaux F., Clercx C., Marlin D., Gustin P. Indices of oxidative stress in a feline model of asthma: 8-iso-PGF2alpha in bronchoalveolar lavage fluid and H2O2 in exhaled breath condensate, European Respiratory Journal 24 (S48), 2004b, 680s, (abstract) [Google Scholar]
- Kirschvink N., Vincke G., Onclinx C., Peck M., Gustin P. Comparison between pulmonary resistance and Penh in anaesthetised rats with tracheal diameter reduction and after carbachol inhalation, Journal of Pharmacological and Toxicological Methods 51, 2005a, 123–128. [DOI] [PubMed] [Google Scholar]
- Kirschvink N., Marlin D., Delvaux F., Leemans J., Clercx C., Sparkes A., Gustin P. Collection of exhaled breath condensate and analysis of hydrogen peroxide as a potential marker of lower airway inflammation in cats, The Veterinary Journal 169, 2005b, 385–396. [DOI] [PubMed] [Google Scholar]
- Kirschvink N, Leemans J, Delvaux F, Snaps F, Marlin D, Sparkes A, Clercx C, Gustin P. Non-invasive assessment of growth, gender and diurnal changes of respiratory pattern in healthy cats by use of barometric whole body plethysmography. The Veterinary Journal, in press. [DOI] [PubMed]
- Masoli M., Weatherall M., Holt S., Beasley R. Systematic review of the dose-response relation of inhaled fluticasone propionate, Archives of Disease in Childhood 89, 2004, 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise N.S., Wiedenkeller D., Yeager A.E., Blue J.T., Scarlett J. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980–1986), Journal of the American Veterinary Medical Association 194, 1989, 1467–1473. [PubMed] [Google Scholar]
- Morrow J.D., Roberts L.J. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease, American Journal of Respiratory Critical Care Medicine 166, 2002, S25–S30. [DOI] [PubMed] [Google Scholar]
- Norris C.R., Decile K.C., Berghaus L.J., Berghaus R.D., Walby W.F., Schelegle E.S., Hyde D.M., Gershwin L.J. Concentrations of cysteinyl leukotrienes in urine and bronchoalveolar lavage fluid of cats with experimentally induced asthma, American Journal of Veterinary Research 64, 2003, 1449–1453. [DOI] [PubMed] [Google Scholar]
- O'Byrne P.M. Mechanisms of airway hyperresponsiveness. Holgate S.T., Busse W.W. Lung Biology in Health and Disease: Inflammatory Mechanisms in Asthma Vol. 117, 1998, Marcel Dekker: New York, 891–906. [Google Scholar]
- Padrid P.A., Feldman B.F., Funk K., Samitz E.M., Reil D., Cross C.E. Cytologic, microbiologic, and biochemical analysis of bronchoalveolar lavage fluid obtained from 24 healthy cats, American Journal of Veterinary Research 52, 1991, 1300–1307. [PubMed] [Google Scholar]
- Padrid P.A., Snook S., Finucane T., Shiue P., Cozzi P., Solway J., Leff A.R. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats, American Journal of Respiratory Critical Care Medicine 151, 1995, 184–193. [DOI] [PubMed] [Google Scholar]
- Padrid P.A., Cozzi P., Leff A.R. Cyclosporine A inhibits airway reactivity and remodeling after chronic antigen challenge in cats, American Journal of Respiratory Critical Care Medicine 154, 1996, 1812–1818. [DOI] [PubMed] [Google Scholar]
- Padrid P.A. Feline asthma: diagnosis and treatment, The Veterinary Clinics of North America: Small Animal Practice 30, 2000, 1279–1294. [DOI] [PubMed] [Google Scholar]
- Peeters D.E., McKiernan B.C., Weisiger R.M., Schaeffer D.J., Clercx C. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens in dogs, Journal of Veterinary Internal Medicine 14, 2000, 534–541. [DOI] [PubMed] [Google Scholar]
- Rozanski E.A., Hoffman A.M. Lung Mechanics using plethysmography and spirometry. King L.G. Respiratory Disease in Dogs and Cats, 2004, Saunders: St. Louis, Missouri, 175–193. [Google Scholar]
- Schulman R.L., Crochik S.S., Kneller S.K., McKiernan B.C., Schaeffer D.J., Marks S.L. Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats, American Journal of Veterinary Research 65, 2004, 806–809. [DOI] [PubMed] [Google Scholar]
- Wood L.G., Gibson P.G., Garg M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma, European Respiratory Journal 21, 2003, 177–186. [DOI] [PubMed] [Google Scholar]