Abstract
Fourteen mixed-breed domestic cats underwent laparoscopic ovariectomy (lapOVE) using paediatric equipment, an Nd:YAG laser and a bipolar electrocoagulation forceps. Cats were placed in 10° Trendelenburg position (head down) and insufflation pressure was kept at 4 mmHg, while surgery was performed through three midline portals. Randomly-assigned unilateral laser resection of one ovary (laserOVE) and bipolar electrocoagulation (BECOVE) of the contralateral ovary were performed. Duration of predetermined surgery intervals was recorded, as well as occurrence of intra- and postoperative complications. Both methods were successful and without complications. Duration of laserOVE was significantly increased compared to BECOVE. The right ovary was more difficult to access; however, both ovaries were easy to manipulate because of the relatively long suspensory ligament. The ovarian ligaments contained minimal amounts of fat and obesity did not influence surgery duration. Convalescence period was short (0.9±0.4 days) and owner satisfaction high.
Ovariectomy (OVE) is the standard procedure for neutering the domestic cat and dog in The Netherlands (van Goethem et al 2006). The canine laparoscopic OVE (lapOVE) is a well-documented procedure (Van Goethem et al 2003, Van Nimwegen et al 2005, Wenkel et al 2005) that is routinely performed at specialised clinics. Research in dogs, pigs and humans indicates that laparoscopic procedures are superior to open surgery due to reduced morbidity caused by less incisional trauma, wound complications, adhesion formation (Schippers et al 1998, Gamal et al 2001), peri- and postoperative pain (Devitt et al 2005, Hancock et al 2005) and physical stress response (Marcovich et al 2001, Matsumoto et al 2005). Furthermore, laparoscopy is associated with better aesthetic results and increased visibility of abdominal structures (Wildt et al 1977). The shorter convalescence period (Barkun et al 1992, Bohm et al 1995) has resulted in improved cost-effectiveness in human medicine (Vilos and Alshimmiri 1995).
Often a mini-laparotomy (key hole) technique is used to perform OVE in the cat. In this procedure, the ovaries may be exteriorised (by some surgeons) in a blind fashion, using a spay hook and ligated extra-abdominally. The amount of intra-abdominal trauma caused by the blind technique does not lead to major complications and is suggested to be minor, but has not been investigated. The lapOVE technique should allow a more controlled excision of the ovaries under direct visualisation causing minimal trauma when specialised laparoscopic equipment is used. Laparoscopic-assisted cryptorchidectomy has been described in cats (Vannozzi et al 2002, Miller et al 2004) and, in addition to our own experiences with feline laparoscopic cryptorchidectomy (unpublished data), has shown that laparoscopy is feasible in cats. To the authors' knowledge, neither laparoscopic-assisted OVE nor lapOVE has been described in the domestic cat.
Multiple techniques of canine lapOVE have been published. Laser dissection (Van Nimwegen et al 2005) and bipolar electrocoagulation (BEC) (Van Goethem et al 2003) are most commonly used at the authors' institution. Laser can be applied in laparoscopic surgery, because the beam passes along a flexible optical fibre. This fibre-guided laser beam can be used to coagulate (non-contact mode) and to cut tissue (contact mode). Cutting, and especially coagulating ability, depends on the type of laser used; being wavelength-dependant (Peavy 2002). The Nd:YAG laser wavelength of 1064 nm shows relatively deep tissue penetration, being absorbed by tissue constituents but not by water and can be used in several procedures requiring deep tissue coagulation. In contact mode, the fibre tip is used in contact with tissue, which is comparable to the use of an electrical (monopolar) knife. The high energy density of the diverging laser beam at the tip leads to rapid vaporisation of the tissue while at the same time the heat produced coagulates small blood vessels in the cut surface (Janda et al 2003).
Currently, the use of bipolar electrocoagulation forceps is considered superior in canine lapOVE because of excellent coagulation of mesovarial vessels and short duration of surgery. In dogs, the Nd:YAG laser is associated with more intra-operative haemorrhage. It was unable to coagulate the larger vessels in the ovarian pedicle and could, therefore, not be used as a stand-alone device for ovary excision. The laser technique required additional use of bipolar electrocoagulation, which resulted in increased surgery duration (Van Nimwegen et al 2005). Neither of these techniques has been described in cats.
The aim of this study was to prove that lapOVE is possible in cats and is a relatively simple method, despite the small size of the feline abdomen. Additionally, the use of surgical laser dissection was compared to bipolar electrocoagulation in a prospective clinical trial.
Methods
Ethics and owner consent
All procedures were approved by the Ethics Committee of the University of Utrecht and conducted in a manner consistent with Dutch law concerning care and use of animals. Approval of owners was obtained before surgery in all cats.
Animals
Both techniques were performed and refined in a pilot study in four healthy, client-owned, intact, female cats. After the pilot study, 14 healthy client-owned female cats, presented for elective OVE entered the lapOVE clinical trial comparing the two methods. A standard questionnaire was completed by the owner concerning the patient's age, breed, general health status, diet, previous oestrus cycles and pregnancies. All cats underwent a full physical examination. Body weight was measured and obesity was scored, ranging from 1 (thin) to 5 (obese), to subjectively differentiate obese from non-obese cats. The cats were randomly assigned to two groups. Group 1 cats had a laser dissection (laserOVE) performed of the left ovary and bipolar electrocoagulation (BECOVE) of the right ovary. Group 2 cats had a BECOVE performed of the left ovary and laserOVE of the right ovary. The cats served as their own controls in comparing the two techniques. Only cats with normal anatomy of the uterus and ovaries during laparoscopy were allowed to participate in the study. As this was a routine surgical procedure on normal cats, no selection bias was present.
Anaesthesia
All cats received general anaesthesia according to a standard protocol. The cats were sedated using medetomidine (Domitor; Pfizer), 40 μg/kg IM. After placement of a catheter in the cephalic vein, the cats were induced using propofol (Propovet; Abbott Laboratories), 1–2 mg/kg IV, administered to effect preceding tracheal intubation. Anaesthesia was maintained using isoflurane (IsoFlo; Abbot Laboratories) in a mixture of oxygen/air by means of mechanical IPPV ventilation. Additional analgesia was accomplished by administering carprofen (Rimadyl; Pfizer), 4 mg/kg IV, single dose. Monitoring consisted of continuous ECG, gas analyser (measuring CO2 and isoflurane concentrations), pulse-oximetry and body temperature measurements using an oesophageal probe. Sedation was reversed using atipamezole (Antisedan; Pfizer), 100 μg/kg IM.
Surgery
All surgery was performed by the same surgeon (JK). Before surgery, the urinary bladder was emptied manually, to decrease the chance of puncture during trocar insertion and increase the visibility during laparoscopy. The surgical field was prepared aseptically after clipping the hair between the nipples from sternum to os pubis. Patients were positioned in dorsal recumbency in a 10° Trendelenburg position (head down) for video-assisted laparoscopic surgery through three midline portals as described for the dog (Van Nimwegen et al 2005). A variation of the standard closed trocar technique was used for the first trocar, without the use of a Veress needle. The first trocar was placed just caudal to the umbilicus. A 4 mm skin incision was made and the subcutis was carefully prepared using scissors, revealing the linea alba. After suture placement in the linea, the abdominal wall was pulled upward. Two stay-sutures were placed at the cranial and caudal border of the skin incision, penetrating skin, subcutis, abdominal wall and peritoneum. The first suture in the linea was removed and the two stay-sutures were used to pull up the ventral abdominal wall while inserting the primary camera trocar (4.5 mm diameter sheath, 6 cm length; Karl Storz-Endoscopy, Vianen, The Netherlands) avoiding trauma to visceral organs. Insufflation with CO2 was started at 1 l/min to check for proper trocar placement. Insufflation was then set to 6 l/min and intra-abdominal pressure was kept at 4 mmHg. A 4 mm diameter 0° rigid telescope (18 cm length, Hopkins II; Karl Storz-Endoscopy) was inserted and the abdominal space was inspected. The second and third trocar (3.5 mm diameter sheath, 5 cm length; Karl Storz-Endoscopy) were inserted under laparoscopic guidance through a 3 mm skin incision midway between os pubis and umbilicus and midway between umbilicus and xyphoid. Care was taken not to traumatise the ventral ligament of the urinary bladder. Surgery always started with the left ovary with the surgeon and assistant on the right side of the cat. The ovary was located using either the uterus or teres ligament that originated from the inguinal canal. The uterus was grasped using a 3 mm diameter paediatric grasping forceps (20 cm length, Click-Line; Karl Storz-Endoscopy) through the cranial portal and a 3 mm diameter paediatric self-retaining grasping forceps (20 cm length, Click-Line; Karl Storz-Endoscopy) through the caudal portal. The uterus was followed cranially until the ovary was visualised. The ovary was grasped and lifted upward using the self-retaining grasping forceps through the cranial portal (Fig 1).
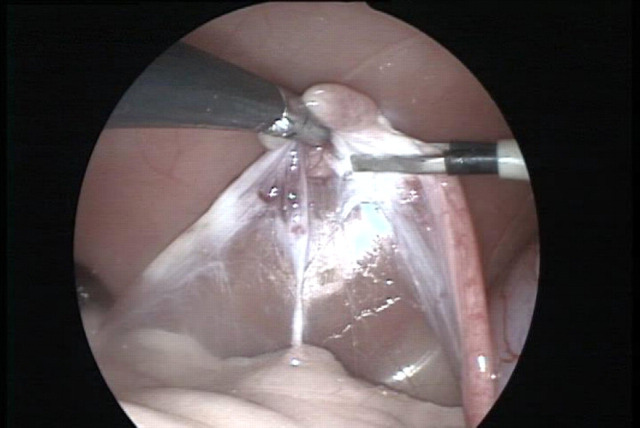
Fig 1.
The left ovary is grasped and held upward by the self-retaining grasping forceps (left). The proper ligament is being coagulated using the bipolar electrocoagulation forceps (right). Tissues from left to right: suspensory ligament, pedicle vessels, proper ligament and uterus.
During BECOVE, the proper ligament, the suspensory ligament and the ovarian pedicle were coagulated using a 3 mm diameter bipolar electrocoagulation forceps (Take-Apart; Karl Storz-Endoscopy) through the caudal portal (Fig 1). These structures were then cut using 3 mm diameter endoscopic scissors (20 cm length, Click-Line; Karl Storz-Endoscopy) (Fig 2). During the laserOVE, major blood vessels of the ovarian pedicle were coagulated using the BEC-forceps. The laser fibre (600 μm diameter optical fibre, Ultraline (side-firing tip removed); Heraeus LaserSonics, Milpitas, CA) connected to an Nd:YAG surgical laser (Medilas 40 N; MBB Medizintechnik GmbH, München, Germany) was inserted through the caudal portal. The ovary was resected by cutting the proper ligament, pedicle and suspensory ligament, respectively, using the laser fibre in contact mode with 10 W continuous wave laser power (Fig 3).
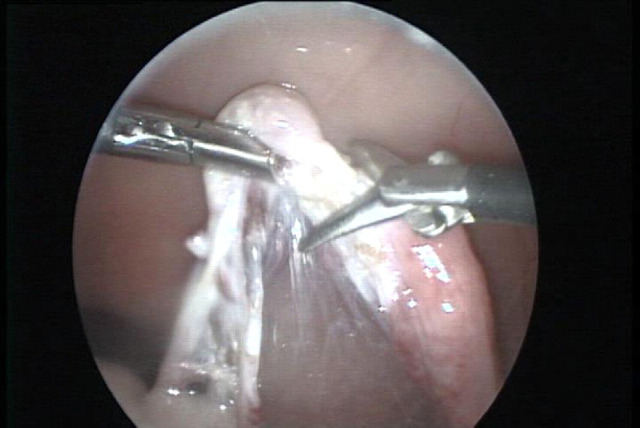
Fig 2.
The bursa and ovary are excised using endoscopic scissors (right). The grasping forceps holds the ovary (left). At this stage the proper ligament is cut.
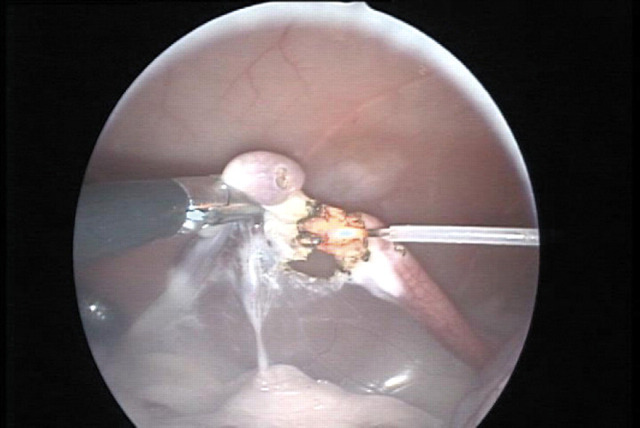
Fig 3.
The bursa and ovary are excised using Nd:YAG laser irradiation through a 600 μm diameter optical fibre (right). At this stage the proper ligament is cut using the laser fibre in contact mode.
In both techniques, iatrogenic haemorrhage was controlled using the BEC-forceps. Care was taken to remove the complete ovary, excising at least 10 mm of non-ovarial tissue around the ovary. After complete resection, the ovary was removed from the abdomen through the caudal portal, after removal of the trocar sheath. After reinsertion of the caudal trocar, the surgeon and assistant turned to the other side of the operating table and the same procedure was performed for the right ovary using the laser method instead of BEC (group 2) or vice-versa (group 1). The excised tissue was checked to ensure that both ovaries had been removed in entirety. A routine, exploratory laparoscopy was performed of the abdomen, excess CO2 was removed and each portal was closed in a routine fashion with one 4-0 inverted, absorbable, monofilament suture (Monocryl; Ethicon, Amersfoort, The Netherlands).
Postoperative care and follow-up
Patients were released from the hospital immediately after recovery. Ketoprofen (Ketofen; Fort Dodge) was prescribed for pain management (1 mg/kg orally once a day for 3 days). The owners were contacted 7–10 days after surgery using a standard follow-up questionnaire concerning time taken for complete recovery, appetite (decreased, same, increased), activity (decreased, same, increased), number of days on pain medication, evaluation of the surgery wounds, satisfaction (unsatisfied, neutral, satisfied, very satisfied), whether they would recommend the laparoscopic treatment to other owners and whether they would choose lapOVE again in the future instead of a regular laparotomy. Cases of minor wound swelling, redness and/or discharge were considered wound complications.
Experimental data
During surgery the following time points were recorded:
| First skin incision: | t 0 |
| Insertion first trocar: | t 1 |
| Start searching for the left ovary: | t 2 |
| Start excision of the left ovary: | t 3 |
| End excision of the left ovary: | t 4 |
| End removal of the left ovary: | t 5 |
| Start searching for the right ovary: | t 6 |
| Start excision of the right ovary: | t 7 |
| End excision of the right ovary: | t 8 |
| End removal of the right ovary: | t 9 |
Other recorded intra-operative parameters were accessibility of the ovaries, occurrence of haemorrhage during surgery, abnormalities of ovaries or uterus, the amount of fat in the ovarian ligament (fat score: 0 (no fat), 1 (moderate amount of fat), 2 (large amount of fat)) and loss of vision because of smoke production or insufficient insufflation.
Data analysis
Paired data (laser versus BEC and left versus right) were tested for equality using Student's t-test for paired samples. Influences of obesity, haemorrhage and access difficulty on surgery duration were evaluated using one-way ANOVA tests. Differences were assumed significant if P<0.05, two-tailed. Results are reported as means±SD.
Results
Patients were all healthy, mixed-breed, domestic shorthair cats. It was not necessary to exclude any of the cats from the study because of ovarial or uterine abnormalities. Mean age was 1.5±1.9 years (range, 0.3–7.6 years). Mean body weight was 2.8±0.5 kg (range, 2.3–3.8 kg). The distension of the feline abdomen was relatively extensive at lower pressures. An intra-abdominal pressure of 4 mmHg was sufficient to create enough abdominal distension for an adequate surgical working space and good laparoscopic visibility. The distension did not cause any problems with maintaining the anaesthetic plane. Production of smoke impairing visibility during surgery was minimal. None of the ovarian ligaments contained much fat, even in obese cats. This made the excision of the ovaries relatively easy. The relatively long suspensory and mesovarial ligaments allowed easy manipulation of the ovary. Rather large depositions of fat were found at the mid-ventral abdominal wall, in the falciform ligament, especially in obese cats.
Surgery time intervals
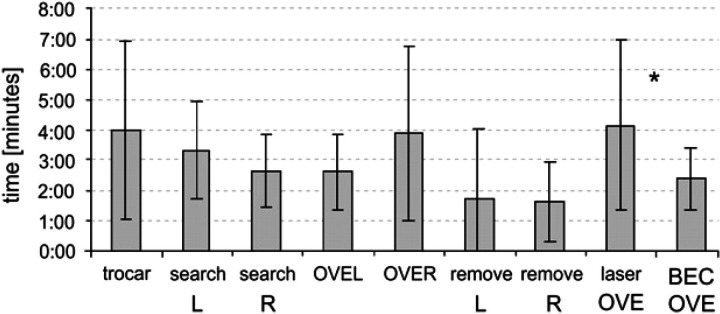
The time points recorded during surgery were used to reconstruct the duration of selected time intervals (for instance, searching left ovary= t 3− t 2). The time intervals were compared for the left side versus the right side and excision (OVE) time was also compared for laserOVE versus BECOVE (Fig 4).
Fig 4.
Surgery time intervals±SD for placement of the first trocar, searching, resection (OVE) and removal of the ovary from the abdomen, laser resection (laserOVE) and bipolar resection (BECOVE) of the ovary (R=right, L=left; *indicates significant difference).
There were no significant differences in surgery time intervals between the left and the right sides. The increased duration of OVE of the right ovary (3:54±2:52 min) compared to the left ovary (2:37±1:16 min) was not significant (P=0.135). Laser resection of the ovary (laserOVE, 4:09±2:50 min) took significantly longer compared to the bipolar technique (BECOVE, 2:23±1:01 min, P=0.032). Mean overall surgery duration was 30:01±5:58 min (range, 19:41–40:02 min).
Obesity, accessibility, haemorrhage
Five of 14 cats were considered obese. Obesity was not correlated with a higher fat score of the ovary and ligaments. Neither obesity nor fat score had a significant effect on duration of any surgery interval. The left ovary was generally more easily accessible than the right ovary, although both ovaries were easily manipulated when grasped by forceps. Notable difficulty accessing the ovary occurred in three cats, all concerning the right ovary. Difficult access significantly lengthened duration in searching for the right ovary in these three cats compared to cats that had an ovary that was easily accessible (3:59±1:05 versus 2:17±1:00 min, P=0.025). There was no overall difference in mean duration of searching, however, for the left ovary compared to the right ovary (Fig 4). Difficult access had no significant effect on other surgery intervals. Occurrence of haemorrhage was not a problem in BECOVE or laserOVE. Only one notable bleeding occurred with BECOVE, which was managed properly using the BEC-forceps and had no obvious effect on surgery duration.
Postoperative variables
Mean period for complete recovery was 0.9±0.4 days (range, 0.5–2 days) and all cat owners were very satisfied with the results. Ketoprofen was given 2.9±0.3 days (range, 2–3 days). Postoperative appetite was unchanged compared to eating behaviour of the cats before surgery. Postoperative activity did vary from cats becoming sweeter and calmer (three cats), no change (nine cats) to increased activity (two cats). A wound complication occurred in one of 42 wounds in which the suture did not properly hold the edges together. Surgical intervention was not required, and the wound healed without further complications. All cat owners reported that they would choose the laparoscopic approach over laparotomy again in future cases of OVE, and that they would recommend it to other cat owners.
Discussion
Fourteen cats received lapOVE through three midline portals, comparing the use of laser and bipolar electrocoagulation. An intra-abdominal CO2 pressure of 4 mmHg was sufficient to create a comprehensive surgical space compared to 10–12 mmHg noted in other publications (Vannozzi et al 2002, Miller et al 2004). This is an encouraging finding, considering that pneumoperitoneum is responsible for many negative haemodynamic effects (Bailey and Pablo 1999), and could be the main factor causing surgical stress in laparoscopic procedures (Marcovich et al 2001). An intra-abdominal pressure of 8–12 mmHg is considered safe in dogs (Ishizaki et al 1993), with no major haemodynamic derangements. Such safety limits have not been determined for cats, but we expect them to be lower for cats compared to dogs. The 4 mmHg CO2 pressure allows full visibility of all essential abdominal organs, mainly caused by the flexibility of the feline abdominal wall. In order to reduce the risk of injuring the urinary bladder, the position of the first trocar was more cranial compared to the technique used in dogs and it was necessary to empty the urinary bladder manually before surgery to reduce the risk of bladder injury and to create an appropriate surgical space. The stay suture technique for trocar placement was also modified, by placing the sutures through and through to enable sufficient tension during trocar insertion. Because of a less tight fit of the trocar in the abdominal wall, more effort had to be made to keep the trocars in place during surgery compared to laparoscopy in dogs. There was also more difficulty directing the laser fibre towards the ovary, because an additional laser-guide could not be used with the paediatric trocars. Fibre movements had to be guided by the trocar, leading to a relatively large degree of flexibility.
In all the cats, including the obese ones, there was a relatively small amount of fat deposited in the ligaments supporting the ovary and uterus. Only one cat had a pedicle fat score of 1, the rest had a fat score of 0. In contrast, obese dogs always had pedicle fat scores of 2 or 3 in previous studies (Van Goethem et al 2003, Van Nimwegen et al 2005). This probably explains why duration of lapOVE was not affected by obesity in cats, whereas in dogs obesity significantly increased surgery duration by 35% (Van Nimwegen et al 2005). The minimal amount of fat in the ligaments improved surgical access. More fat was located against the abdominal wall in obese cats, mainly in the mid-ventral region including the falciform ligament. In the cat, initial access to the right ovary was more demanding compared to the left ovary. A distinct access difficulty occurred three times on the right side, causing a significant increase in duration of searching for the right ovary compared to normal access of the right ovary. No significant difference, however, was found in searching time or resection time overall between the right and the left ovaries. Furthermore, after grasping the ovary and if necessary, creating more working space by gently uncovering the ovary and pedicle from viscera, both ovaries could easily be retracted and manipulated for surgery. Laser resection was combined with BEC-forceps for proper haemostasis, instead of using the surgical laser as a stand-alone device for OVE. Using the laser in non-contact mode to coagulate blood vessels running in the pedicle had been achieved in a pilot case. However, the authors were not confident that surrounding tissues would not be damaged by the diverging (non-contact) laser beam and the BEC-forceps were chosen for their safer, more rapid and localised method of coagulation. Apart from the extra instrument exchange (laser versus BEC-forceps), most of the increased duration of laserOVE compared to BECOVE is probably caused by a distinct delay that occurred during each first attempt to use the laser fibre in contact mode. When a clean laser fibre is used for contact mode surgery, some char-formation has to occur at the fibre tip, in order to catalyse further tissue carbonisation and vaporisation during surgery. The speed at which this initial tissue char is formed is largely dependant on laser absorption characteristics of the tissue (Janda et al 2003). Formation of this initial layer of charred tissue may take a relatively long time, compared to the time needed for ovary excision. Apparently, absorption of Nd:YAG irradiation is relatively weak in the pale-coloured tissue of the feline pedicle and initial charring is somewhat delayed. The amount of smoke produced during either laser or bipolar OVE did not remarkably decrease visibility during feline lapOVE as compared to lapOVE in dogs (Van Nimwegen et al 2005), probably because of the much smaller dimensions of the feline ovary and the reduced amount of lipoid tissue.
Duration of lapOVE is probably not reduced compared to OVE or ovariohysterectomy by means of laparotomy, although this was not the scope of the research reported here. Equipment setup and initial laparoscopic abdominal access require more effort compared to a plain laparotomy. Additionally, equipment requirements are more demanding for laparoscopic procedures including the need for laparoscopy unit, insufflator, bipolar electrocoagulation unit, extra help during surgery, and the need for mechanical ventilation. On the other hand, besides the decrease in trauma to the patient, excision of the ovaries is probably faster because of improved accessibility and visibility and the absence for the need of ligatures. Additionally, the improved visibility greatly enhances detection and control of pedicle haemorrhage and allows a quick scan of the abdomen for subclinical abnormalities. LapOVE is most likely not going to replace normal OVE in the cat but the minimal invasive technique is appreciated by cat owners and can be offered as a successful alternative to the standard OVE.
This study shows the feasibility of feline lapOVE. The authors did not encounter major difficulties or complications during or after surgery. In fact, low-pressure pneumoperitoneum, easy manipulation of the ovaries and a minimal amount of fat in the ovarian ligament facilitated excellent surgical access and outcome. Furthermore, convalescence time was short and owner satisfaction was high. The use of laser, although effective, did not significantly enhance the procedure or outcomes.
References
- Bailey J.E., Pablo L.S. Anesthetic and physiologic considerations for veterinary endosurgery. Freeman L.J. Veterinary Endosurgery, 1999, Mosby: St. Louis, MO, pp. 24–43. [Google Scholar]
- Barkun J.S., Barkun A.N., Sampalis J.S., Fried G., Taylor B., Wexler M.J., Goresky C.A., Meakins J.L. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group, Lancet 340, 1992, 1116–1125. [DOI] [PubMed] [Google Scholar]
- Bohm B., Milsom J.W., Fazio V.W. Postoperative intestinal motility following conventional and laparoscopic intestinal surgery, Archives of Surgery 130, 1995, 415–424. [DOI] [PubMed] [Google Scholar]
- Devitt C.M., Cox R.E., Hailey J.J. Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs, Journal of the American Veterinary Medical Association 227, 2005, 921–928. [DOI] [PubMed] [Google Scholar]
- Gamal E.M., Metzger P., Szabo G., Brath E., Peto K., Olah A., Kiss J., Furka I., Miko I. The influence of intraoperative complications on adhesion formation during laparoscopic and conventional cholecystectomy in an animal model, Surgical Endoscopy 15, 2001, 873–880. [DOI] [PubMed] [Google Scholar]
- van Goethem B., Schaefers-Okkens A., Kirpensteijn J. Making a rational choice between ovariectomy and ovariohysterectomy in the dog: a discussion of the benefits of either technique, Veterinary Surgery 35, 2006, 136–143. [DOI] [PubMed] [Google Scholar]
- Hancock R.B., Lanz O.I., Waldron D.R., Duncan R.B., Broadstone R.V., Hendrix P.K. Comparison of postoperative pain after ovariohysterectomy by harmonic scalpel-assisted laparoscopy compared with median celiotomy and ligation in dogs, Veterinary Surgery 34, 2005, 273–282. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y., Bandai Y., Shimomura K., Abe H., Ohtomo Y., Idezuki Y. Safe intra-abdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery, Surgery 114, 1993, 549–554. [PubMed] [Google Scholar]
- Janda P., Sroka R., Mundweil B., Betz C.S., Baumgartner R., Leunig A. Comparison of thermal tissue effects induced by contact application of fiber guided laser systems, Lasers in Surgery and Medicine 33, 2003, 93–101. [DOI] [PubMed] [Google Scholar]
- Marcovich R., Williams A.L., Seifman B.D., Wolf J.S., Jr. A canine model to assess the biochemical stress response to laparoscopic and open surgery, Journal of Endourology 15, 2001, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Matsumoto E.D., Margulis V., Tunc L., Taylor G.D., Duchene D., Johnson D.B., Pearle M.S., Cadeddu J.A. Cytokine response to surgical stress: comparison of pure laparoscopic, hand-assisted laparoscopic, and open nephrectomy, Journal of Endourology 19, 2005, 1140–1145. [DOI] [PubMed] [Google Scholar]
- Miller N.A., Van Lue S.J., Rawlings C.A. Use of laparoscopic-assisted cryptorchidectomy in dogs and cats, Journal of the American Veterinary Medical Association 224, 2004, 875–883. [DOI] [PubMed] [Google Scholar]
- Peavy G.M. Lasers and laser-tissue interaction, Veterinary Clinics of North America. Small Animal Practice 32, 2002, 517–534. [DOI] [PubMed] [Google Scholar]
- Schippers E., Tittel A., Ottinger A., Schumpelick V. Laparoscopy versus laparotomy: comparison of adhesion-formation after bowel resection in a canine model, Digestive Surgery 15, 1998, 145–152. [DOI] [PubMed] [Google Scholar]
- Van Goethem B.E., Rosenveldt K.W., Kirpensteijn J. Monopolar versus bipolar electrocoagulation in canine laparoscopic ovariectomy: a non-randomized, prospective, clinical trial, Veterinary Surgery 32, 2003, 464–470. [DOI] [PubMed] [Google Scholar]
- Van Nimwegen S.A., Van Swol C.F., Kirpensteijn J. Neodymium:yttrium aluminum garnet surgical laser versus bipolar electrocoagulation for laparoscopic ovariectomy in dogs, Veterinary Surgery 34, 2005, 353–360. [DOI] [PubMed] [Google Scholar]
- Vannozzi I., Benetti C., Rota A. Laparoscopic cryptorchidectomy in a cat, Journal of Feline Medicine and Surgery 4, 2002, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilos G.A., Alshimmiri M.M. Cost–benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease, Journal of the American Association of Gynecologic Laparoscopists 2, 1995, 299–303. [DOI] [PubMed] [Google Scholar]
- Wenkel R., Ziemann U., Thielebein J., Prange H. Laparoskopische Kastration der Hündin Darstellung neuer Verfahren zur minimal invasiven Ovariohysterektomie, Tierarztliche Praxis 33, 2005, 177–188. [Google Scholar]
- Wildt D.E., Kinney G.M., Seager S.W. Laparoscopy for direct observation of internal organs of the domestic cat and dog, American Journal of Veterinary Research 38, 1977, 1429–1432. [PubMed] [Google Scholar]