History and physical findings
A 6-year-old spayed domestic shorthaired cat (3.7 kg) was presented for ‘pain’ and stiffness of the hindquarters. The lameness first became evident 3–4 weeks earlier and progressed subsequently. Stiffness became more prominent 2 weeks prior to presentation. Lameness was asymmetrical and worse on the right. There was no history of trauma. The patient was somewhat inappetent. The cat had been taken to another veterinarian 3 weeks previously; no investigations were undertaken and amoxycillin clavulanate and carprofen (doses unrecorded) had been administered empirically, but without benefit.
The patient was initially investigated by the orthopaedic service. Abnormal physical findings included discomfort/hyperaesthesia, which was hard to localise, a 3 mm nodule on the upper eyelid and hind limb muscle atrophy. The cat was plainly uncomfortable in the hindquarters, but initial physical examination failed to localise the pain to the lumbosacral region, pelvis or the hind limbs. On re-evaluation, both hocks felt prominent and sore on manipulation. The cat was normothermic (38.2°C after hospitalisation).
Initial assessment
The cat was considered to have musculoskeletal or neurological lesion(s) causing pain. It seemed most likely that the problem was skeletal, muscular, vertebral/spinal or neuropathic in origin, and the hock joints were considered to be the most logical anatomic region to concentrate on. The underlying disease process was thought to be traumatic, inflammatory (infectious or auto-immune), degenerative, developmental or neoplastic. The chronic progressive clinical course suggested the disease process was not self-limiting.
Investigation
Haematology and routine serum biochemistries demonstrated hyperproteinaemia (81 g/l; reference interval 54–73 g/l), mature neutrophilia (22.4×109/l; RI 3.8 to 10.8×109/l), monocytosis (0.84×109/l; RI 0.08 to 0.56×109/l) and eosinophilia (1.12×109/l; RI 0 to 0.14×109/l). Unfortunately, urine was not collected for analysis.
Palpation under inhalational anaesthesia confirmed the impression that both hocks were slightly swollen. Radiographs were taken of the hind limbs, lumbosacral spine and pelvis, and a small volume of clear colourless fluid was obtained from both tibiotarsal joints. An aspirate was also obtained from a subtle swelling in the subcutaneous tissues medial to the right hock.
The radiographic report, supplied by a board certified radiologist, noted bilateral soft tissue swelling of both hocks and the distal hind limbs, more pronounced on the right. Periosteal new bone formation was evident on the caudal and lateral surfaces of both tarsometatarsal joints, and also the tibiotarsal joint (Fig 1). The radiologist offered a likely diagnosis of immune-mediated polyarthropathy.
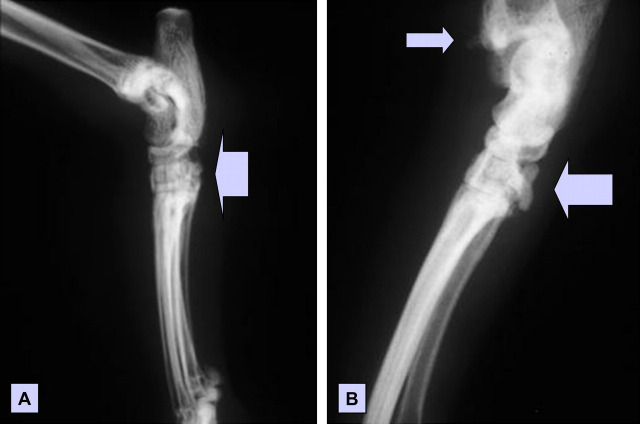
Fig 1.
Lateral radiographs of the left (A) and right (B) tibiotarsal region of a cat with pain and swelling of the hocks. Note the periosteal new bone formation (arrows), which is especially prominent on the caudal aspect of the tarsometarsal joint of the right hind limb. There was also diffuse soft tissue swelling in the vicinity of the hocks, which is difficult to appreciate from these photographic images.
Joint fluid collected from both hocks was similar cytologically. There was only enough material aspirated to prepare smears and perform aerobic culture; insufficient fluid remained to perform quantitative cell counts. Microscopically, against a purple to pink, clumped granular background there was a mild to moderate increase in the number of nucleated cells—70% neutrophils, 23% macrophages and 7% lymphocytes (Fig 2). No microorganisms were apparent in Diff-Quik and Gram-stained smears. The viscosity of fluid from the right hock was considered to be reduced slightly. The material aspirated from the swelling medial to the right hock was reported as ‘just blood and maybe some joint fluid’ (Fig 3). The cytological diagnosis was polyarticular inflammatory joint disease. A sample of joint fluid was incubated aerobically on blood agar and in broth. No organisms were isolated after 3 days incubation. The material aspirated from the subcutaneous tissues was not cultured.
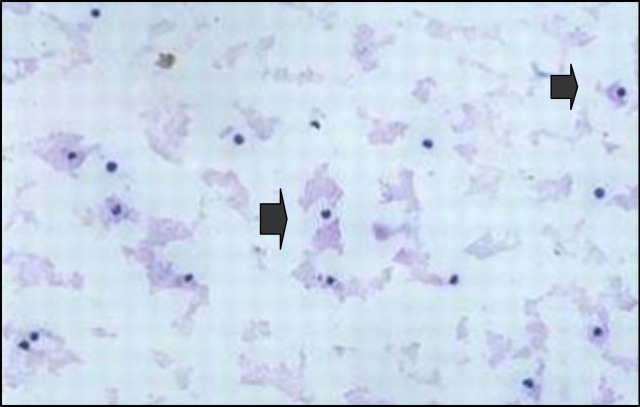
Fig 2.
Diff-Quik stained smear of joint fluid obtained from the right tibiotarsal joint. Note the clumped background granular material and the presence of nucleated cells, the majority of which (70%) were neutrophils (arrows). Grossly, the joint fluid was clear, colourless and considered possibly to be of reduced viscosity. Only a very small volume of fluid could be aspirated.
Fig 3.
Diff-Quik stained smear from an aspirate of the subcutaneous swelling medial to the right hock. Some joint fluid can be seen on the left, while coagulated blood dominates the rest of the low power microscopic field.
The cat was discharged on doxycycline monohydrate (25 mg orally before meals twice daily) pending results of culture and subsequently referred to the feline medicine service. There was no improvement in the cat's status after 10 days of doxycycline; the right hock remained sore on manipulation, body weight was stable (3.7 kg) but temperature was now elevated (39.4°C). On the presumption that the cat had feline progressive polyarthritis (an aseptic, immune-mediated disease; Pedersen et al 1980), immunosuppressive therapy was commenced using prednisolone (15 mg orally once daily). There was, however, no improvement, and 12 days after starting prednisolone the cat was probably worse. According to the owners, the cat's hindquarters appeared weak, the left leg was held out at an angle to avoid weight bearing and the patient would vocalise as if distressed when patted on its lower back. On physical examination, the cat was febrile (39.7°C); previous abnormalities persisted, while the popliteal lymph nodes were considered prominent. The immunosuppressive drug chlorambucil (2 mg three times a week) was added to the therapeutic regimen.
Although there was some initial improvement following addition of chlorambucil, 20 days later the cat remained in considerable discomfort, with extreme spinal pain, pain on manipulation of both hocks and a swelling (8 mm diameter) on the medial aspect of the right hock, plus persistent fever (39.5°C). Cyclosporine (25 mg once daily) was added to the drug regimen, but the cat did not improve and after 18 days the cat remained febrile (40.1°C), with palpable distention of both hock joints. Rifampicin (75 mg orally once daily) and doxycycline (25 mg orally twice daily) were added in case the immune-mediated joint disease was secondary to a systemic Bartonella henselae infection. Haematology was repeated, but despite the prednisolone and other agents, haematological values were largely as before, including a comparable eosinophilia (0.73×109/l).
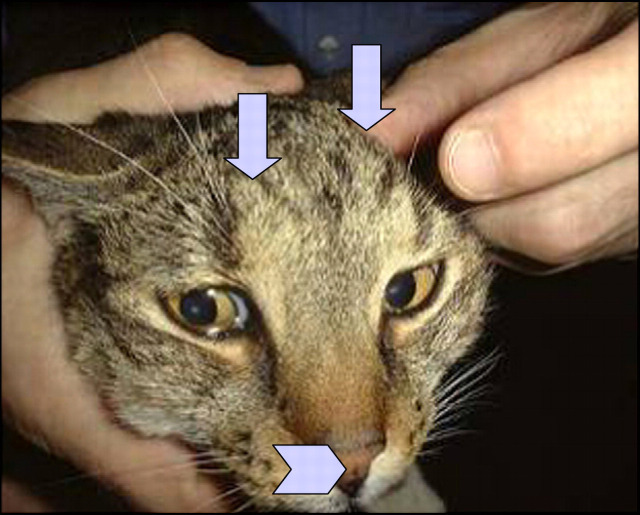
Importantly, the owner noted a small subcutaneous lump on the side of the cat's face, roughly over the zygomatic arch (Fig 4). A fine needle aspirate from this mass demonstrated abundant, capsulate yeasts with narrow-necked budding, furnishing a diagnosis of cryptococcosis (Fig 5). There was a heavy pure growth of Cryptococcus neoformans var grubii from material aspirated from the subcutaneous facial lesion. A repeat physical examination revealed a small nodule on the nasal philtrum, and two small subcutaneous lesions on the cat's forehead above the frontal sinus (Fig 6). The lesion that had been noted initially on the eyelid could not be appreciated.
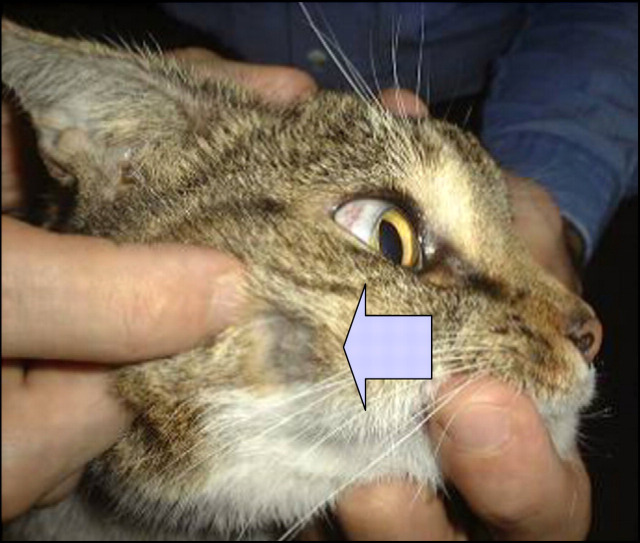
Fig 4.
Note the small subcutaneous lump on the side of the cat's face—some fur has been clipped from over the lesion to facilitate fine needle aspiration biopsy. The slight protrusion of subcutaneous tissue over the frontal sinus is evident also (see Fig 6).
Fig 5.
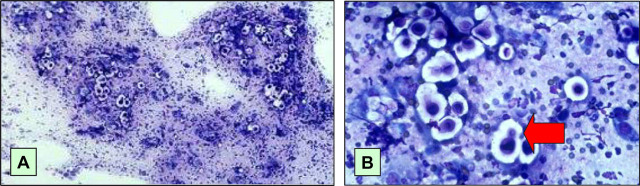
Diff-Quik stained smears at low (A) and high (B) magnification of the fine needle aspirate from the subcutaneous nodule on the side of the patient's face. This nodule developed during the course of immunosuppressive therapy, and was detected by the owner while she was visiting the cat in hospital. In (A), the round to oval cryptococcal organisms stand out in relief because they are surrounded by a non-staining capsule, admixed with some macrophages. In (B), budding forms (arrow) can be appreciated, as well as the non-staining capsule.
Fig 6.
In addition to the lump on the side of the cat's face, there were two subtle parallel subcutaneous ridges on the forehead (arrows) and a small lesion on the nasal philtrum (arrow head); in retrospect, the forehead lumps had been evident for some time and were considered to be a normal part of the cranium.
Discussion
Was disseminated cryptococcosis the principal problem from the outset (?), or did the cat develop an opportunistic infection as a consequence of treatment of immune-mediated polyarthritis with combination immunosuppressive therapy?
How could these two possibilities be differentiated?
Before making a commitment one way or the other, based on speculation or theoretical considerations, it would be wise to obtain some additional hard data. Accordingly, we re-aspirated the subcutaneous tissues medial to the right hock and determined the latex cryptococcal antigen agglutination (LCAT) titre (at the time of diagnosis) (Malik et al 1996b).
The needle aspirate from the lesion on the medial side of the right hock contained abundant cryptococcal organisms. The LCAT titre was 16,384. This is an extremely high titre, and likely indicative of disseminated disease with widespread release of capsular polysaccharide into the circulation (Malik et al 1996b). Had we stored serum from when the cat was originally presented, we would have been able to determine with certainty whether the cat had cryptococcosis from the outset. Unfortunately, no initial serum specimen had been preserved.
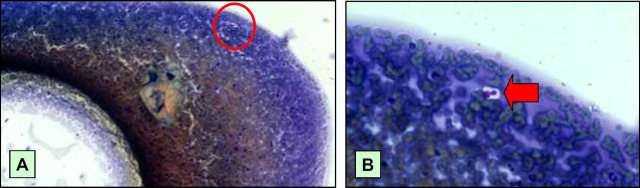
The cytopathologist subsequently reviewed the original smears taken from aspirates of this site which fortunately had been preserved in accord with good laboratory practice. Importantly, two yeast cells were detected in the original smear of the subcutaneous aspirate from the swollen hock, after a long and diligent search (Fig 7). Because this specimen had been collected prior to immunosuppressive therapy, organisms were sparse. In contrast, aspirates from the same site following immunosuppressive therapy, and the passage of time, yielded abundant cryptococcal cells.
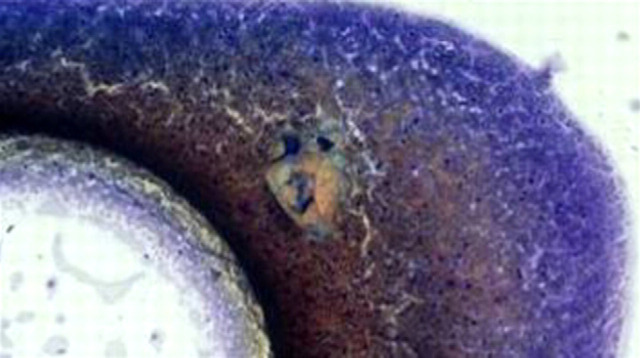
Fig 7.
Original aspirate from the swelling in the subcutaneous tissues of the medial hock of the right pelvic limb, at low power (A) and high power (B). At high power, two cryptococcal cells (arrow) are evident, highlighted by their prominent capsule. The low power micrograph, which is essentially Fig 3 repeated, shows the location of the organisms (red circle) which can just be discerned even at low power.
On the basis of these findings, it can be stated without doubt that disseminated cryptococcosis had been present from the outset. The subcutaneous lesions surrounding both hocks were, in actual fact, the first manifestation of widely disseminated cryptococcosis. It is of interest that some cats with the so-called ‘lung-digit syndrome’ can develop metastasis of primary pulmonary tumours at this site (Schmitz et al 1978, Gottfried et al 2000), suggesting that both infective and tumour emboli may be preferentially deposited in this location. The upper respiratory tract is the most common primary site for the development of cryptococcosis in the cat (Malik et al 1992, O'Brien et al 2004). The location of two ridge-like subcutaneous prominences in the tissues overlying the frontal sinus suggests that the sinonasal region had indeed been the primary site of infection in this case, with subsequent extension through the overlying nasal or frontal bones. For some reason, in this particular patient, signs of nasal cavity involvement (such as oculonasal discharge, stertor or sneezing) were absent and rather than causing more pronounced local disease at the primary site, the infection disseminated haematogenously. Although we favour this scenario, we have only circumstantial evidence to support our contention. But there is no doubt that the cat had disseminated disease at the time of presentation, with involvement of the subcutaneous tissues of both back legs and likely also the head. Presumably, the cat subsequently developed immune-mediated polyarthritis, either due to antigen/antibody complex deposition due to abundant anti-cryptococcal antibodies and antigen (Malik et al 1999), or more likely, some local effect of the infection in the subcutaneous tissues adjacent to articular and peri-articular structures.
Could we have determined what was happening earlier?
With the benefit of hindsight, there were a number of missed opportunities to diagnose cryptococcosis in a more timely manner, prior to implementing immunosuppressive therapy. Cats with disseminated cryptococcosis sometimes have cryptococcal organisms present in urine specimens, so obtaining a urinalysis (and culture) as part of a ‘minimum database’ may have been helpful. Obtaining a more substantial portion of material (eg, using a Keyes biopsy punch, or a Jamshidi needle) from the subcutaneous swelling on the medial hock may have provided a better opportunity for detecting cryptococcal organisms in tissue sections, although the modern tendency to obtain diagnostic specimens with minimal invasiveness and morbidity mitigates against this suggestion. On the other hand, culturing a portion of the aspirate that had been collected would have likely provided the diagnosis, as it is well known that culture is generally more sensitive than cytology in establishing the presence of infection. Finally, a case could be made, at least in Australia and British Columbia (Canada), where cryptococcosis is relatively common (but not always easy to diagnose), for the determination of the cryptococcal antigen titre in any cat with unexplained or atypical disease, especially when neurological signs (including pain or altered behaviour) or unusual bizarre signs are evident. To emphasise this point further, one of the authors can recall a case of cryptococcal meningitis which was initially presented for sitting in the cupboard for excessive periods! An LCAT determination would certainly have been diagnostic in this instance, although the titre would probably not have been as high as it was after a month of combination immunosuppressive therapy.
Even with the benefit of hindsight, the failure to initially diagnose cryptococcosis from the outset was understandable. However, our preliminary diagnosis of ‘periosteal proliferative polyarthritis’ should have been questioned when the cat failed to improve, as expected, in response to prednisolone therapy. It would have been prudent to have revisited the ‘work-up’ at this stage, most likely by repeating aspirates from the joints or from the increasingly evident soft tissue swelling medial to the right hock. This would likely have provided a diagnosis of cryptococcosis in a timelier manner.
There is often reluctance for veterinary internists to repeat diagnostic procedures that have already been conducted, probably as the client perceives this as a ‘waste of money’, and the veterinarian is loathe to subject the patient to additional painful interventions that have been performed previously without benefit. The present case emphasises that this ‘mind set’ can sometimes be detrimental to the patient.
Outcome
All immunosuppressive agents were stopped immediately. Cryptococcosis was treated using triple therapy consisting of fluconazole (32 mg orally every 8 h), flucytosine (250 mg orally every 8 h) and amphotericin B (6.4 mg in 375 ml of 0.45% NaCl and 2.5% dextrose subcutaneously twice weekly) (Malik et al 1996a, O'Brien et al 2006). We also provided a wide range of tasty canned cat foods (Friskies Feast varieties) to ensure a positive anabolic state during hospitalisation and when the cat was sent home.
Antifungal therapy commenced on June 18, 2001. After 1 week, the hocks were less swollen, the cat could move more ‘freely’ and with less pain, and the temperature was down to 39.2°C. After a further week of therapy the left hock was near normal, and the temperature was further reduced (39.0°C). Triple therapy was continued until November 9, 2001, when amphotericin B was discontinued. The LCAT declined to 32,768 on September 20, 2001, to 8 on July 25, 2001 and finally to 1 on December 4, 2002. The cat's clinical signs resolved and its weight at discontinuation of antifungal therapy was 4.2 kg.
Acknowledgments
Dr Robert Nichol initially reported on the radiographs. The authors wish to thank the cat's owner, Ms Melanie Robson, for her trust and diligence in medicating and supporting the patient. Professor Paul Canfield offered helpful comments on the manuscript.
References
- Gottfried S.D., Popovitch C.A., Goldschmidt M.H., Schelling C. Metastatic digital carcinoma in the cat: a retrospective study of 36 cats (1992–1998), Journal of the American Animal Hospital Association 36, 2000, 501–509. [DOI] [PubMed] [Google Scholar]
- Malik R., Craig A.J., Martin P., Wigney D.I., Love D.N. Combination chemotherapy of cryptococcosis using subcutaneously administered amphotericin B, Australian Veterinary Journal 73, 1996a, 124–128. [DOI] [PubMed] [Google Scholar]
- Malik R., McPetrie R., Wigney D.I., Love D.N. Use of the cryptococcal latex agglutination antigen test for diagnosis and monitoring of therapy in veterinary patients with cryptococcosis, Australian Veterinary Journal 74, 1996b, 358–364. [DOI] [PubMed] [Google Scholar]
- Malik R., Speed B., Kaldor J., Pergor X., Wigney D.I., Love D.N. Serum antibody response to Cryptococcus neoformans in cats, dogs and koalas with and without active infection, Medical Mycology 37, 1999, 43–51. [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Muir D.B., Gregory D.J., Love D.N. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole, Journal of Medical and Veterinary Mycology 30, 1992, 133–144. [DOI] [PubMed] [Google Scholar]
- O'Brien C.R., Krockenberger M.B., Wigney D.I., Martin P., Malik A retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases, Medical Mycology 42, 2004, 449–460. [DOI] [PubMed] [Google Scholar]
- O'Brien C.R., Krockenberger M.B., Martin P., Wigney D.I., Malik R. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis, Australian Veterinary Journal 84, 2006, 384–392. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Pool R.R., O'Brien T. Feline chronic progressive polyarthritis, American Journal of Veterinary Research 41, 1980, 522–535. [PubMed] [Google Scholar]
- Schmitz J.A., Bailey D.E., Bailey R.E. Bronchogenic carcinoma in a cat presenting as a rear leg lameness, Feline Practice 8, 1978, 18–21. [Google Scholar]