Abstract
The ultrasonographic findings of kidneys, liver and urinary bladder of 288 Persian and 44 Exotic Shorthair clinically normal cats that underwent screening for polycystic kidney disease (PKD) between July 2003 and December 2005 were reviewed. Cats were divided into two groups, one including cats aged <9 months (group 1) and one cats aged ≥9 months (group 2). Cats were classified as PKD-positive when at least one renal cyst was found. One hundred and thirty-six cats (41.0%) had more than one cyst in at least one kidney. The prevalence of PKD was similar in both groups. Eight PKD-positive cats had cystic livers (5.9%). Other renal abnormalities included a pelvic calculus and a medullary rim sign (MRS). The difference in prevalence of an MRS in group 2 compared to group 1 and the difference between PKD-positive and -negative cats in group 2 were not significant. There was no difference in mean kidney length between PKD-positive and -negative cats in group 2. Urinary bladder anomalies were principally represented by urinary sediment, with prevalence significantly higher in group 2. No difference was detected in group 2 between PKD-positive and -negative cats. In conclusion feline PKD is common in Italy. The ultrasonographic findings of MRS and urinary bladder sediment did not correlate with feline PKD. Urinary bladder sediment is common in Persians and Exotic Shorthairs and more likely in adults.
Feline polycystic kidney disease (PKD) is an inherited autosomal dominant disease that has been identified in Persian cats and Persian related breeds (Biller et al 1990, 1996, Lyons et al 2004), such as the Exotic Shorthair cats. The disease has a mode of inheritance similar to human autosomal dominant PKD, is progressive and may lead to irreversible renal failure. Occasionally, cysts can be found in liver and pancreas (Biller et al 1990, 1996, Bosje and van den Ingh 1998, Barrs et al 2001, Cannon et al 2001, Barthez et al 2003, Testault 2003, Lyons et al 2004). Although a genetic test is now available (Lyons et al 2004), renal ultrasonography is currently the most practical non-invasive diagnostic method in Italy. Its sensitivity is 91% at 9 months of age, increasing with the age of the patient, while the specificity is said to be 100% (Biller et al 1996, Barrs et al 2001, Beck and Lavelle 2001, Cannon et al 2001, Barthez et al 2003).
Ultrasonographic screening programmes have indicated that the prevalence of this disease is about 49.2% in the UK, 43% in Germany and Australia, and 41.8% in France (Biller et al 1996, Barrs et al 2001, Beck and Lavelle 2001, Cannon et al 2001, Barthez et al 2003). There is only a single preliminary study on a small number of cases that assessed the prevalence of PKD in Italy (Bonazzi et al 2004).
The purpose of the present report is (1) to determine the prevalence of PKD in Persian and Exotic Shorthair cats in Italy, in order to justify the screening programme and to evaluate its success, and (2) to evaluate the prevalence of renal, hepatic and urinary bladder ultrasonographic abnormalities and their possible correlation with PKD.
Materials and methods
Medical records of clinically normal Persian and Exotic Shorthair cats that underwent ultrasonographic screening for PKD at the Veterinary Teaching Hospital of the University of Parma (Italy) from July 2003 to December 2005 were evaluated.
All cats underwent ultrasonographic scanning of liver and urinary tract with a 7.5–10 MHz linear transducer (Megas GPX, Esaote, Genova, Italy), set at 10 MHz. The reminder of the abdomen was not examined. Each kidney was measured in its greatest length in the sagittal plane (Reichle et al 2002). Ultrasonographic abnormalities of kidneys, liver and urinary bladder were recorded. According to the literature (Beck and Lavelle 2001, Cannon et al 2001, Barthez et al 2003), cats were classified as positive when at least one anechoic cavity was found in at least one kidney. This criterion has been used in other similar studies (Beck and Lavelle 2001, Cannon et al 2001). It is justified by the progressive nature of the disease (Biller et al 1996) and the low prevalence of solitary cysts not related to PKD in the cat (Barthez et al 2003). Cats were divided into two groups, the first (group 1) including cats aged <9 months and the second (group 2) cats aged ≥9 months in order to see if there was a significant difference between the prevalence of PKD in adult and immature cats. In fact, false-negative cats could be included in group 1.
A Student's t-test was used to compare mean kidney length of cats affected and not affected by PKD in group 2. Comparison of the prevalence of renal and urinary bladder incidental abnormalities was made by the χ 2 test or Fisher's test. Differences with P value <0.05 were considered significant.
Results
Three hundred and thirty-two cats were evaluated, including 288 Persians and 44 Exotic Shorthairs; 212 were females and 120 males. Four adult cats referred a clinical history of cystitis. Group 1 included 138 cats aged <9 months (median=3 months, range 1 month; 8 months) and group 2 included 194 cats aged ≥9 months (median=2.25 years, range 9 months; 15 years).
In 136 cats (41.0%) more than one cyst in at least one kidney were found (Table 1). One hundred and thirty-four (98.5%) of these cats had both kidneys affected and eight (5.9%) had at least one cyst in the liver. Among the cats with more than one renal cyst, 53 cats belonged to group 1 and 83 cats to group 2. The prevalence of PKD in group 1 was 38.4%, and in group 2 42.8%; this difference was not significant (Table 1).
Table 1.
Prevalence of PKD related to the age of cats
| Group | PKD+ | PKD− |
|---|---|---|
| Group 1 | 53 | 85 |
| Group 2 | 83 | 111 |
| χ 2=0.639; P=0.424 | ||
| Odds ratio=1.20 (CI 95% 0.52–1.33) | ||
In group 1, the positive cats were classified as 49 Persians (30 females, 19 males) and four Exotic Shorthairs (females). In group 2 the cats considered positive were classified as 70 Persians (45 females, 25 males) and 13 Exotic Shorthairs (nine females, four males). All the cats with cystic liver were Persians, females and belonged to group 2.
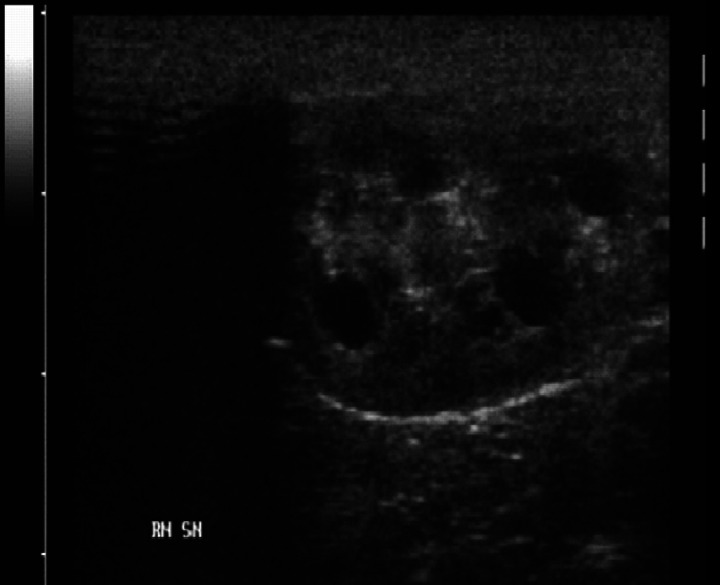
Ultrasonographically, the renal cysts appeared as round, anechoic or hypoechoic cavities (Fig 1). Acoustic enhancement was present in larger cysts, which in some cases showed also hyperechoic specks probably representing sediment. The greatest number of cysts was located in the cortex or between cortex and medulla, but several cysts could be also detected in the medullary area. When few cysts were present, the sonographic appearance of the kidneys was only slightly modified, while large cysts, found either in young or adult cats, completely altered renal contour and parenchyma.
Fig 1.
Sonographic features of a polycystic kidney.
Other renal abnormalities detected were a pelvic calculus in a single cat from group 1 and a medullary rim sign (MRS). A MRS was present in 41 cats (12.3%). The prevalence of a MRS in group 1 was 10.9%, in group 2 13.4%; the difference was not significant (Table 2).
Table 2.
Prevalence of a medullary rim sign (MRS) related to the age of cats
| Group | MRS+ | MRS− |
| Group 1 | 15 | 123 |
| Group 2 | 26 | 168 |
| χ 2=0.478; P=0.489 | ||
| Odds ratio=1.27 (CI 95% 0.37–1.62) | ||
Of the 41 cats with a MRS, 14 had PKD and 27 did not. The prevalence of a MRS in PKD-positive cats was 10.3%, while in negative 13.7%; the difference was not significant (Table 3). In all cases the MRS was present in both kidneys and was considered as an unspecific finding (Nyland et al 2002).
Table 3.
Medullary rim sign (MRS) related to PKD in group 1 and group 2
| Group 1 | Group 2 | |||
| PKD+ | PKD− | PKD+ | PKD− | |
| MRS+ | 5 | 10 | 9 | 17 |
| MRS− | 48 | 75 | 74 | 94 |
| Fisher test P=0.783 | χ 2=0.818; P=0.366 | |||
| Odds ratio=1.28 (CI 95% 0.20–2.70) | Odds ratio=1.49 (CI 95% 0.25–1.71) | |||
In group 2 the mean kidney length was 3.7 cm±0.26 in PKD-positive cats and 3.6 cm±0.47 in PKD-negative cats.
Hepatic cysts showed the same sonographic features of renal cysts. No other abnormalities were detected in liver parenchyma.
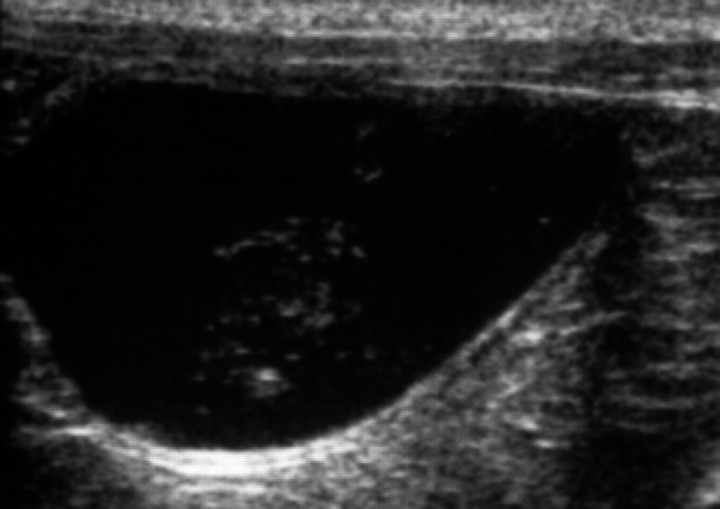
Urinary bladder anomalies included urinary bladder sediment, calculi and wall thickening compatible with chronic cystitis, and were detected in 98 cats (29.5%). Among these, three belonged to group 1 and 95 to group 2. All of them (54 females and 44 males) had urinary bladder sediment (Fig 2), which appeared as hyperechoic, non-shadowing specks in the urine, suspending easily with bladder agitation, as described in the literature (Luerssen et al 2001, Nyland et al 2002).
Fig 2.
Sonographic features of urinary bladder sediment, suspending easily after bladder agitation.
Two cats from group 2 with a history of urinary tract disease had a concurrent mildly thickened and irregular urinary bladder wall, indicating chronic cystitis. A small urinary bladder calculus was detected concurrently with urinary sediment in two cats, one from group 1 and one from group 2. The prevalence of urinary bladder sediment in group 1 was 2.1%, while in group 2 was 48.9%. This difference was statistically significant (Table 4).
Table 4.
Prevalence of sediment related to the age of cats
| Group | Sediment+ | Sediment− |
| Group 1 | 3 | 135 |
| Group 2 | 95 | 99 |
| Fisher test P<0.0001 | ||
| Odds ratio=43.2 (CI 95% 14.3–99.9) | ||
The prevalence of urinary sediment in cats affected by PKD was 30.1%, while in PKD-negative cats was 29.1% and the difference was not significant (Table 5).
Table 5.
Sediment related to PKD in group 1 and group 2
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| PKD+ | PKD− | PKD+ | PKD− | |
| Sediment+ | 1 | 2 | 40 | 55 |
| Sediment− | 52 | 83 | 43 | 56 |
| Fisher test P=1.000 | χ 2=0.035; P=0.852 | |||
| Odds ratio=1.25 (CI 95% 0.01–15.71) | Odds ratio=1.06 (CI 95% 0.52–1.74) | |||
Discussion
Overall, the resulting prevalence of feline PKD in this study is 41.0% (CI 95% 35.6–46.5). This datum is similar to those reported elsewhere. Many authors (Biller et al 1996, Barrs et al 2001, Cannon et al 2001, Barthez et al 2003, Testault 2003) reported that the sensitivity of ultrasound for feline PKD increases with the age of the patient and reaches 91% at 9 months. A double ultrasonographic examination is also recommended to achieve a certain diagnosis. The first examination should be performed when cats are 9 months old, and the second 6 months after the first (Testault 2003). No solitary renal cyst was found in this series of cases and so false positives can be excluded. Conversely, the resulting prevalence of feline PKD could be underestimated, as a number of false-negatives could occur (small cysts may not be resolved by the scanner, some may become visible only after 9 months of age, examination is prone to error, etc.).
The hyperechoic band at the cortico-medullary junction (MRS) did not correlate with PKD nor with the age of the patient. An MRS has been associated with several pathological conditions (Nyland et al 2002) and is also in cats with normal deposition of mineral in the tubular epithelium of the outer zone of renal medulla (Yeager and Anderson 1989), as considered in this study.
No statistically significant difference for mean kidney length was found in group 2 between cats affected and not affected by PKD. This datum is not supported by the literature (Reichle et al 2002). PKD is a progressive disease in which the renal cysts tend to enlarge slowly in time, possibly causing an irreversible renal failure by compressing normal parenchyma (Biller et al 1990, 1996, Bosje and van den Ingh 1998, Barrs et al 2001, Testault 2003). This discrepancy could be explained by the fact that the median age of the cats in group 2 is only 2.25 years. It is possible that the mean dimensions of the renal cysts at this age may not be so conspicuous. Furthermore, renal length revealed by means of ultrasound is strongly dependant on the scanning plane, in which some large cysts may not have been included.
No correlation has been detected between urinary bladder sediment and feline PKD in adult Persian and Exotic Shorthair cats. A significant higher prevalence of urinary bladder sediment was seen in group 2, indicating that adult Persian and Exotic Shorthair cats have a higher risk of showing this anomaly than young ones.
In almost half (48.9%) of the adult cats examined, urinary bladder sediment was detected, and this may be a relevant and interesting datum. However, it is not possible to compare this finding and to assess its role in feline lower urinary tract disease (FLUTD) pathogenesis in Persians, without knowledge of the prevalence of urinary sediment in other breeds and its nature.
In conclusion, the resulting prevalence of 41.0% suggests that feline PKD is common in Italy. Estimating the prevalence is essential before starting a screening programme, in order to be able to document the efficiency of the eradication process (Barthez et al 2003). Eradication of the disease should be achieved by screening all cats used for breeding and retain only unaffected cats for future breeding programmes (Barrs et al 2001).
In this report a MRS and ultrasound-detectable urinary bladder sediment resulted to be incidental abnormalities not correlated with PKD. Urinary bladder sediment is a common finding more likely in adult cats, but further investigations are needed to establish a possible correlation with FLUTD in Persians and Exotic Shorthairs.
Acknowledgments
The authors thank Dr Marco Tartaglia and Mr Antonio Belicchi for technical assistance.
References
- Barrs V.R., Gunew M., Foster S.F., Beatty J.A., Malik R. Prevalence of autosomal dominant polycystic kidney disease in Persian cats and related-breeds in Sidney and Brisbane, Australian Veterinary Journal 79, 2001, 257–259. [DOI] [PubMed] [Google Scholar]
- Barthez P.Y., Rivier P., Begon D. Prevalence of polycystic kidney disease in Persian related cats in France, Journal of Feline Medicine and Surgery 5, 2003, 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C., Lavelle R.B. Feline polycystic kidney disease in Persian and other cats: a prospective study using ultrasonography, Australian Veterinary Journal 79 (3), 2001, 181–184. [DOI] [PubMed] [Google Scholar]
- Biller D.S., Chew D.J., Di Bartola S.P. Polycystic kidney disease in a family of Persian cats, Journal of the American Veterinary Medical Association 196 (8), 1990, 1288–1290. [PubMed] [Google Scholar]
- Biller D.S., Di Bartola S.P., Eaton K.A., Pflueger S., Wellman M.L., Radin M.J. Inheritance of polycystic kidney disease in Persian cats, Journal of Heredity 87, 1996, 1–5. [DOI] [PubMed] [Google Scholar]
- Bonazzi M., Gazzola M., Volta A., Ferrabini G., Gnudi G., Bertoni G. Incidenza della malattia del rene policistico nel gatto: indagine preliminare su 84 animali, Atti Società Italiana di Chirurgia Veterinaria SICV 11, 2004, 180–182. [Google Scholar]
- Bosje J.T., van den Ingh T.S., van der Linde-Sipman J.S. Polycystic kidney and liver disease in cats, Veterinary Quarterly 20, 1998, 136–139. [DOI] [PubMed] [Google Scholar]
- Cannon M.J., MacKay A.D., Barr F.J., Rudorf H., Bradley K.J., Gruffydd-Jones T.J. Prevalence of polycystic kidney disease in Persian cats in the UK, Veterinary Record 149, 2001, 409–411. [DOI] [PubMed] [Google Scholar]
- Luerssen D., Prufer A., Janthur M. Urinary bladder and urethra. Nautrup C.P., Tobias R. An Atlas and Textbook of Diagnostic Ultrasonography of the Dog and Cat, 2nd edn, 2001, Manson Publishing, 228–240. [Google Scholar]
- Lyons L.A., Biller D.S., Erdman C.A., Lipinski M.J., Young A.E., Roe B.A., Qin B., Grahn R.A. Feline polycystic kidney disease mutation identified in PKD1, Journal of the American Society of Nephrology 15, 2004, 2548–2555. [DOI] [PubMed] [Google Scholar]
- Nyland G.T., Mattoon J.S., Herrgesell E.J., Wisner E.R. Urinary tract. Nyland G.T., Mattoon J.S. Small Animal Diagnostic Ultrasound, 2nd edn, 2002, WB Saunders: Philadelphia, 158–191. [Google Scholar]
- Reichle J.K., DiBartola S.P., Leveille R. Renal ultrasonographic and computed tomographic appearance, volume, and function of cats with autosomal dominant polycystic kidney disease, Veterinary Radiology and Ultrasound 43, 2002, 368–373. [DOI] [PubMed] [Google Scholar]
- Testault I. La maladie polykystique du chat Persian, Le Point Vétérinaire, Numéro Spécial 32, 2003, 88–90. [Google Scholar]
- Yeager A.E., Anderson W.I. Study of association between histologic features and echogenicity of architecturally normal cat kidneys, American Journal of Veterinary Research 50, 1989, 860–863. [PubMed] [Google Scholar]