Abstract
Asingle dose of a rapidly-absorbed non-steroidal anti-inflammatory drug (NSAID) was injected into the subcutaneous tissue of the interscapular region of a 12.5-year-old cat. A mild swelling was noticed at the injection site 6 weeks later. This progressed into a 5 cm diameter mass which was removed 6 months after the injection had been given. An injection site sarcoma (ISS) was diagnosed histologically. As the cat had not been vaccinated for at least 12 years, the previous NSAID injection was considered to be a possible cause of the ISS. Inflammation is thought to be important in the development of ISS. If injection of a rapidly-absorbed NSAID can stimulate sufficient inflammation to promote the development of an ISS, other non-vaccine injections may also have the potential to influence ISS development. This suggests that injection of both vaccines and non-vaccine medications should be minimised to reduce the risk of ISS development.
A 12.5-year-old male domestic shorthair cat presented with a 3-month-history of gradually-worsening stiff gait and occasional limping. A presumptive diagnosis of osteoarthritis was made on clinical examination and a subcutaneous injection of 0.3 mg/kg meloxicam (Metacam; Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany) was given in the interscapular area. Six weeks later, a small soft mass was observed at the injection site. This mass continued to enlarge and a 5 cm diameter firm mass was palpable when the cat represented to the veterinarian 6 months after the injection had been given. Due to concerns of possible neoplasia, it was decided to surgically excise the mass. A preanesthetic blood chemistry panel did not reveal significant findings. Anaesthesia was induced using a combination of 1 mg butorphanol (Torbugesic; Pfizer Australia, West Ryde, Australia), 0.2 mg medetomidine (Domitor; Pfizer Australia, West Ryde, Australia) and 13 mg diazepam (Pamalin; Parnell Laboratories New Zealand, Auckland, New Zealand) given intravenously followed by maintenance using inhaled isoflurane. During surgery, the mass was observed to be penetrating deeply within the interscapular region. The mass was dissected from the surrounding tissue and fixed in 10% neutral buffered formalin.
Formalin-fixed samples were embedded in paraffin. Sections were cut at 5 μm and stained with haematoxylin and eosin. Histological examination revealed a nodular proliferation of neoplastic spindle shaped cells within the hypodermis. The cells were arranged in poorly-defined bundles that were supported by a marked fibrous stroma (Fig 1). Neoplastic cells had multifocally infiltrated the panniculus muscle. A central area of necrosis was visible within the neoplasm. The cells were a pleomorphic population that ranged from elongate with an ovoid central nucleus, indistinct nucleoli, and moderate quantities of eosinophilic finely granular cytoplasm to large plump cells with round peripheral nuclei that contained prominent, often multiple nucleoli, and large quantities of deeply eosinophilic cytoplasm. Numerous giant cells and 29 mitoses/10 hpfs were visible within the neoplasm. Lymphocyte aggregates were visible surrounding the neoplasm.
Fig 1.
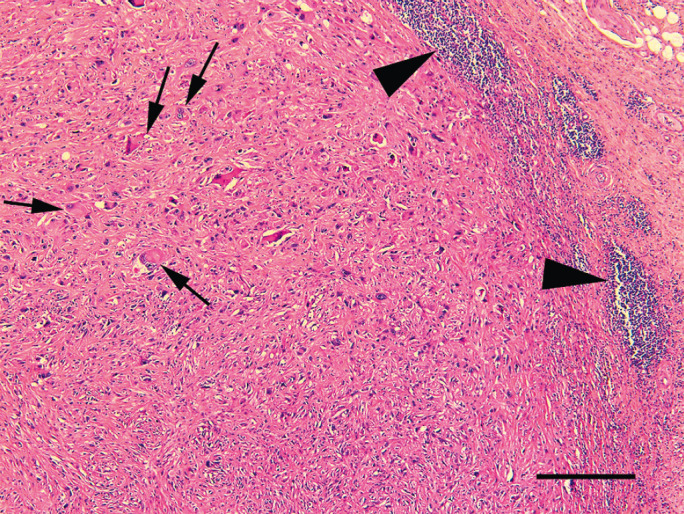
Photomicrograph of a vaccine associated sarcoma removed 6 months after meloxicam injection. Neoplastic cells are arranged within poorly-defined bundles within the hypodermis. Note the presence of scattered giant cells (arrows) and the nodular accumulations of lymphocytes at the periphery of the neoplasm (arrowheads). Haematoxylin and eosin. Bar = 240 μm.
For immunohistochemistry, sections were cut at a thickness of 4 mm, deparaffinised in xylene, rehydrated in graded ethanol, and rinsed in distilled water. Antigen retrieval for the anti-desmin antibody was achieved by incubating slides in epitope retrieval solution 2 (Leica Microsystems GmBH, Wetzlar, Germany) for 20 min. No antigen retrieval was necessary for the anti-smooth muscle actin immunohistochemistry. The slides were then incubated with antibodies against desmin (1:200 dilution, Dako, Carpinteria, CA) and smooth muscle actin (1:1000 dilation, Dako, Carpinteria, CA). A Bond Refine Detection staining kit (Leica Microsystems GmBH, Wetzlar, Germany) using3,3'-diaminobenzidine substrate was used to visualise the immunoreaction and sections were counterstained with haematoxylin. Immunohistochemistry revealed the presence of desmin and smooth muscle actin in neoplastic cells predominantly within the periphery of the neoplasm (Fig 2).
Fig 2.
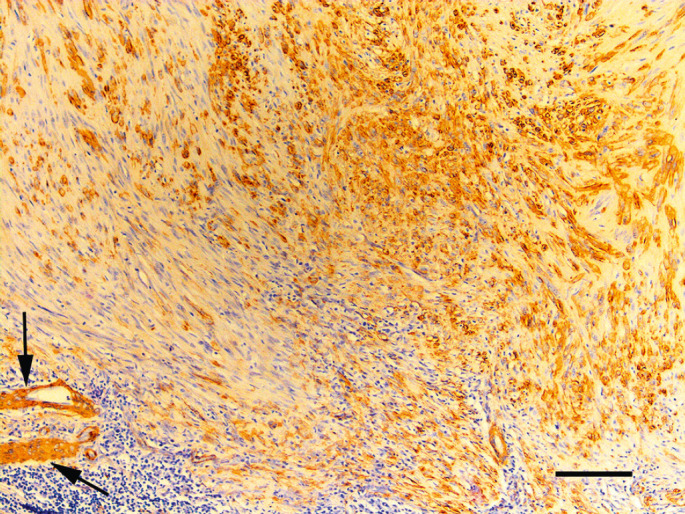
Photomicrograph of a vaccine associated sarcoma removed 6 months after meloxicam injection. Neoplastic cells within the neoplasm express smooth muscle actin. Expression is most prominent by cells within the periphery of the neoplasm. Lymphoid nodules are visible. Vascular smooth muscle cells provide an internal positive control (arrows). Immunoperoxidase using antibodies against smooth muscle actin with haematoxylin counterstain. Bar = 100 μm.
The histological and immunohistological appearance was considered consistent with an injection site sarcoma (ISS). The cat had been adopted from an animal rescue organisation when 4 months old and had not been vaccinated after adoption. Prior to developing osteoarthritis, the only veterinary attention that the cat had received was at 6 months of age when he was neutered.
ISSs (also referred to as vaccine associated sarcomas) are a well-recognised feline neoplasm that have been associated with the administration of adjuvanted vaccines and, to a lesser extent, non-adjuvanted vaccines.1–3 Epidemiological evidence suggests that injection of long-acting penicillin and corticosteroid formulations may also cause ISS development. 4 In the present case, the ISS developed soon after injection of meloxicam, a non-steroidal anti-inflammatory drug (NSAID) that is rapidly absorbed from the subcutaneous tissue. 5 To the authors' knowledge, this is the first time that an ISS has been associated with the injection of a NSAID. Additionally, this is the first evidence to suggest that the injection of rapidly-absorbed medications could be a possible cause of ISS development. However, as this is only a single case, the nature of the association between the NSAID injection and ISS development remains uncertain and it cannot be proven that the injection of the NSAID caused the ISS in this cat.
Currently, the influence of non-vaccine injections on ISS development is uncertain. Part of the uncertainly is because most cats that receive non-vaccine injections have also previously been vaccinated. 6 In contrast, if the presently described cat had ever been vaccinated, this occurred over 12 years before ISS development. ISSs are reported to develop between 2 months and 3.5 years after vaccination.1,2 Therefore, a previous vaccination is extremely unlikely to be the cause of the ISS in the presently described cat. If the cat in the present case had been regularly vaccinated, the ISS would have been assumed to be due to previous vaccination. This case was only further investigated because the ISS was diagnosed in a cat that was known to have not been recently vaccinated. This suggests that it is possible that some feline ISSs that are attributed to previous vaccination could have actually been caused by injection of other non-vaccine medications.
Vaccines are thought to cause ISS by inducing inflammation within the subcutaneous tissue.7,8 In the present case, it appears unlikely that a small volume of rapidly-absorbed NSAID would induce significant inflammation. Therefore, if the present ISS was caused by inflammation, this inflammation appears unlikely to have been due to the injected meloxicam. Therefore, it may have been the process of injection that resulted in inflammation rather than the injected medication. Injection causes inflammation due to the needle penetrating the tissue. This inflammation can be increased if hair or other material is inadvertently introduced into the subcutaneous tissue. If the process of injection does sometimes induce enough inflammation to increase the chance of ISS development, every injection that is given would, therefore, carry a small risk of neoplasia. This suggests that injection of a vaccine could cause neoplasia even if the injected vaccine does not directly cause ISS development.
The purpose of this report is not to discourage the use of injectable medications or NSAIDs in feline practice. As this is the first report of a possible association between an injected NSAID and ISS development, ISSs appear to infrequently develop after non-vaccine injections. Therefore, as with vaccination, the benefits of the prudent use of injectable non-vaccine medications outweigh the possible risk of ISS development. Many injections are administered to cats while ISSs are comparatively rare neoplasms and it remains possible that the presently reported cat was uniquely susceptible to ISS development. However, these results suggest that non-vaccine injections, as well as the currently recommended vaccines, 9 should be minimised when possible in cats.
While inflammation due to the injection of a vaccine is considered to be the most common cause of an ISS, these neoplasms have also developed close to non-absorbable sutures 10 and an implanted microchip, 6 suggesting other causes of inflammation can influence ISS development. In the present case, it cannot be excluded that another cause of inflammation, unrelated to the NSAID injection, resulted in ISS development. The cat was a farm cat and so inflammation from injuries sustained from hunting or fighting was possible, although such injuries would be unusual in the interscapular area. 11 If a previous traumatic injury induced ISS development, evidence of such an injury would have been unlikely to be histologically detectable within the subsequent neoplasm. Therefore, cause and effect cannot be proven in this case and it remains possible that the ISS developed coincidentally to NSAID injection.
Although the feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) status of this cat was unknown, previous studies suggest neither virus influences ISS development.12,13 Therefore, if the presently described cat was infected by FIV or FeLV, these viruses would not be expected to have influenced ISS development.
The sarcoma in the present case was diagnosed histologically as an ISS. Histological features used to make this diagnosis included the subcutaneous location of the neoplasm, the central necrosis, peritumoral lymphocytes, scattered giant cells, marked cellular pleomorphism, and myoblastic differentiation.3,14–16 The histological diagnosis was supported by the clinical observation that the neoplasm developed at a site that had been recently used for an injection. However, it cannot be excluded that an unusual fibrosarcoma variant was misdiagnosed as an ISS in this case.
In conclusion, a sarcoma that was histologically consistent with an ISS was removed from the interscapular region of a cat. The cat had not been vaccinated for at least 12 years; however, a NSAID injection had recently been given at the site of ISS development. This suggests that the NSAID injection could have influenced the development of the ISS in this cat. Although ISS development has been epidemiologically associated with injection of long-acting penicillin and corticosteroids, to the authors' knowledge this is the first time an ISS has been associated with the injection of a rapidly-absorbed NSAID. Inflammation is thought to be important in ISS development. As the NSAID itself appears unlikely to cause significant inflammation, if the NSAID injection did promote ISS development, this may have been due to inflammation caused by the physical process of injection. While the results of this case suggest it is possible that any injection could cause ISS development, additional cases are required to confirm a causative relationship between injection and sarcoma development. Most cats that receive veterinary attention have been previously vaccinated. This often makes it impossible to determine whether an ISS has developed due to previous vaccination or due to a non-vaccine injection.
References
- 1.Hendrick MJ, Goldschmidt MH, Shofer FS, Wang YY, Somlyo AP. Postvaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res 1992; 52: 5391–4. [PubMed] [Google Scholar]
- 2.Esplin DG, McGill LD, Meininger AC, Wilson SR. Postvaccination sarcomas in cats. J Am Vet Med Assoc 1993; 202: 1245–7. [PubMed] [Google Scholar]
- 3.Doddy FD, Glickman LT, Glickman NW, Janovitz EB. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol 1996; 114: 165–74. [DOI] [PubMed] [Google Scholar]
- 4.Kass PH, Spangler WL, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc 2003; 223: 1283–92. [DOI] [PubMed] [Google Scholar]
- 5.Boehringer Ingelheim Vetmedica GmbH. Metacam package insert for cats.
- 6.Daly MK, Saba CF, Crochik SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg 2008; 10: 202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macy DW, Hendrick MJ. The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin North Am Small Anim Pract 1996; 26: 103–9. [DOI] [PubMed] [Google Scholar]
- 8.Kidney BA. Role of inflammation/wound healing in feline oncogenesis: a commentary. J Feline Med Surg 2008; 10: 107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Association of Feline Practitioners Feline Vaccine Advisory Panel Report. J Am Vet Med Assoc 2006; 229: 1405–41. [DOI] [PubMed] [Google Scholar]
- 10.Buracco P, Martano M, Morello E, Ratto A. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet J 2002; 163: 105–7. [DOI] [PubMed] [Google Scholar]
- 11.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin diseases of the dog and cat: clinical and histopathologic diagnosis; 2nd edition. Oxford, United Kingdom: Blackwell Science, 2005. [Google Scholar]
- 12.Ellis JA, Jackson ML, Bartsh RC, et al. Use of immunohistochemistry and polymerase chain-reaction for detection of oncornaviruses in formalin-fixed paraffin-embedded fibrosarcoma from cats. J Am Vet Med Assoc 1996; 209: 767–71. [PubMed] [Google Scholar]
- 13.Kidney BA, Ellis JA, Haines DM, Jackson ML. Comparison of endogenous feline leukemia virus RNA content in feline vaccine and non-vaccine site-associated sarcomas. Am J Vet Res 2001; 62: 1990–4. [DOI] [PubMed] [Google Scholar]
- 14.Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol 1994; 31: 126–9. [DOI] [PubMed] [Google Scholar]
- 15.Couto SS, Griffey SM, Duarte PC, Madewell BR. Feline vaccine-associated fibrosarcoma: morphologic distinctions. Vet Pathol 2002; 39: 33–41. [DOI] [PubMed] [Google Scholar]
- 16.Aberdein D, Munday JS, Dyer CB, Knight CG, French AF, Gibson IR. Comparison of the histology and immunohistochemistry of vaccination-site and non-vaccination-site sarcomas from cats in New Zealand. N Z Vet J 2007; 55: 203–7. [DOI] [PubMed] [Google Scholar]


