Abstract
Two field trials, involving 66 cats (15 trial 1; 51 trial 2) were conducted to assess the efficacy of a psyllium-enriched diet for management of constipation in cats. After investigations and faecal evacuation (by enema if required), all cats were fed on a moderate fibre, psyllium-enriched, dry extruded diet. Additional therapy was either not used (trial 1), or initially allowed but was subsequently withdrawn if possible (trial 2). The diet was well tolerated, and palatability was excellent. Most cases improved after initial therapy (at 2 months; trial 1: 14/15 [93%]; trial 2: 42/51 [82%]), and faecal consistency improved significantly in both trials (P < 0.001). Use of cisapride and lactulose decreased significantly in trial 2 (P < 0.001 for both). The diets used in these pilot studies were efficient in the management of recurrent feline constipation. Randomised control trials are now recommended to examine whether a clinical benefit can be proven.
Constipation is defined as infrequent or difficult emission of hard, dry, faeces and is a common clinical complaint in cats. 1 The clinical signs can result from a number of different disorders including dietary (eg, ingestion of indigestible material), environmental disturbances (eg, hospitalisation, change in routine, inactivity), colonic obstruction (eg, strictures, neoplasia, foreign body, pelvic trauma), electrolyte imbalances (eg, dehydration, chronic kidney disease, hypokalaemia), iatrogenic (eg, drug therapy) and neuromuscular disease (eg, spinal cord disease, idiopathic megacolon). 1
Treatment involves identifying and eliminating the cause, if possible, together with medical or, in some cases, surgical management. 2 Medical therapy includes the use of laxatives, enemas and prokinetic agents such as cisapride. However, whilst expert reviews and textbook articles are available to advise clinicians on recommended therapy and management, 1–5 this is in areas where objective clinical evidence is lacking.
Dietary modification is commonly recommended for the management of feline constipation, and recommendations involve the incorporation of dietary fibre, either insoluble, soluble or both. 5 Dietary fibre sources with low solubility, eg, cellulose, act as bulk-forming laxatives, and improve intestinal motility by distending the colonic lumen. 5 However, the main disadvantages of adding insoluble fibre are lower nutrient digestibility and faecal moisture content, 6 and high levels of inclusion can lead to excessively dry stools (VB, unpublished observations). Other foods contain fibre of higher solubility and sources include pectins from carrots or fruits, canned pumpkin, and guar gum, and psyllium. 5 Some soluble fibres are fermented by intestinal bacteria, producing short-chain fatty acids (SCFA), thereby having a strong impact on the colonic micro-environment. The SCFA released by bacteria can be partly used by colonocytes, and in vitro studies have also shown that they have prokinetic effects on colonic smooth muscle. 7 However, over-supplementing with fibre sources that maximise SCFA production can lead to liquid stools, and decreased nutrient digestibility. 6 Psyllium is a soluble fibre with low fermentability, 8 with a husk containing both polysaccharide and non-polysaccharide components that, on lubrication, exudes a hydrophilic mucilaginous gel thereby increasing faecal bulk. 9 Stool bulk is also increased by additional, water-holding, properties of psyllium. Preparations containing psyllium increase stool frequency and consistency in human patients with idiopathic constipation. 9
Given the suggested benefits, addition of moderate amounts of insoluble and soluble dietary fibre is recommended by gastroenterology texts and review articles,1–5 and is common practice in the management of feline constipation. Fibre can either be added by switching the cat to a purpose-formulated ‘high-fibre’ diet, which usually incorporates an increased level of insoluble fibre (up to 28% total dietary fibre on an as-fed basis; crude fibre 8–15%). Alternatively, an existing diet can be supplemented with fibre, and this is most commonly done by adding psyllium, canned pumpkin, or coarse wheat bran to a wet diet.1,10 Wet food is recommended as the fibre supplement mixes in easily, and fluid intake is maximised, thereby avoiding dehydration which may exacerbate the constipation. However, as with medical management, the authors are not aware of previous studies that have objectively assessed the use of fibre supplementation in the management of feline constipation.
Recently, a new formulation of fibre-supplemented food for cats has become commercially available, and is designed for use in managing constipation. This diet differs from the traditional approach in that the principle fibre source, psyllium husk, is added to a highly digestible dry extruded formula, but the degree of supplementation is less marked (ie, 11.5% total dietary fibre, 2.9% crude fibre on an as-fed basis). Further, given the use of psyllium, most of the fibre is soluble rather than insoluble. However, there has been no critical assessment of its efficacy in cats. Therefore, the aim of the current study was to perform the first phase I clinical trials into the use of a psyllium-enriched dry expanded diet for management of feline constipation. Assuming a favourable response, we also wished to estimate the required sample size for future a prospective randomised controlled trial of efficacy.
Materials and methods
Study outline
Two prospective, uncontrolled, open-label field trials were conducted, one in Europe (study 1) and one in North America (study 2). The aim of both trials was to investigate the efficacy of a psyllium-enriched diet in the management of feline constipation. Experimental protocols complied with European Union guidelines on animal welfare and were approved by the Royal Canin committee for animal ethics and welfare. This committee has been established in accordance with legislation regarding care and use of experimental animals, and comprises eight members including two lay members. All owners were informed as to the nature of the trial and consented to their cats being involved.
Trial 1
Cats
Trial 1 was a single-centre study based at a referral clinic (Clinique Vétérinaire Alliance, Bordeaux, France). A single experienced gastroenterologist (VF) performed all investigations. Fifteen cats participated, all referred for the investigation and management of recurrent constipation. All cases were enrolled between August 2009 and January 2010. For inclusion in the study, cats had to have signs consistent with constipation, such as infrequent emission of faeces, dry or firm faecal consistency (see below), straining, tenesmus and dyschezia. Each cat had to have had at least two episodes of constipation, each lasting at least 24 h, and signs had to be present for at least 14 days. Further, all cases had to have been refractory to previous therapy given by the primary veterinarian.
Patient assessment and initial therapy
Initially, a medical history was taken, the cat was weighed and a physical examination performed. In some cases, haematology, clinical biochemistry, and contrast radiography were performed in order to confirm the diagnosis and identify underlying causes. Cases with severe clinical signs (eg, dehydration, lethargy, depression, anorexia, vomiting, abnormal body temperature) were hospitalised and intravenous fluid therapy was administered. Colonic lavage was performed in cases with severe accumulation of faecal material in the colon, as long as their condition was deemed to be stable. Anaesthesia was induced with intravenous propofol; cats were then intubated and maintained with inhaled isoflurane. Colonic lavage involved the use of warm water enemas and manual fragmentation of stools using abdominal palpation.
Monitoring
Cats were reassessed 1 month (T1) and 2 months (T2) after initiating dietary management (see below). At each check, physical examination was performed to insure that the cat had remained healthy and body weight was measured using the same electronic weigh scales (Teraillon). Subjective response to therapy was assessed as follows: a ‘complete response’ was defined as a case where clinical signs had abated completely, and defecation was judged to be normal by the owner; a ‘partial response’ was defined as a case where noticeable improvement in clinical signs had occurred (ie, frequency and severity of signs declining by at least 1/3) but, in the opinion of the owner, the pattern of defecation had not completely returned to normal; cats that did not improve on therapy (including cases with marginal improvement, ie, less than 1/3 decrease in frequency and severity of signs) were defined as ‘non-responders’.
Faecal quality was assessed with a 5-point scale, as previously described. 11 Briefly, scores of 3 and 4 represented optimal faecal quality, a score of 5 represented hard dry faeces, and scores of 2 and 1 represented progressively looser faeces (score 1: completely liquid). Finally, owners were also asked to report on the appetite of their cat and to comment on palatability of the food.
Apart from the scheduled reassessments, telephone and email advice was provided as required, and other revisits were allowed based upon the needs and concerns of the owner. After the 2-month initial trial phase, diet therapy was continued if the cat remained asymptomatic. Periodic rechecks were scheduled, as dictated by the clinical case. All owners were contacted in May 2011 to generate information on long-term follow-up. The presence of clinical signs and requirement for both medical and dietary management were recorded at this stage.
Trial 2
Cats
Trial 2 was a multi-centre study, based at 14 different first-opinion clinics across Canada, and involving multiple investigators. The trial was co-ordinated by three veterinarians (DH, HW and ME) who liaised with all clinics, insured that the trial was conducted according to the guidelines, and collated the data. Inclusion criteria were similar to those used in trial 1, namely signs consistent with recurrent constipation (see above). However, refractoriness to previous therapy was not an inclusion criterion.
Patient assessment and initial therapy
Patient assessment was similar to trial 1, with all veterinarians taking amedical history, performing a physical examination, and measuring body weight on electronic scales. Further investigations (ie, haematology, clinical biochemistry, and radiography) were performed if deemed to be necessary by the attending veterinarian. Aggressive initial therapy (hospitalisation, intravenous fluid therapy and colonic evacuation) was again used when the veterinarian deemed it to be necessary.
Monitoring
Despite the multi-centre approach, a similar protocol for monitoring was used in trial 2. Two main follow-up assessments were conducted, at 1 month (T1) and 2 months (T2) after initiating dietary management (see below). Physical examination was performed at each examination, and faecal consistency was scored with the same 5-point scale. 11 Response to therapy was determined by changes in faecal scores and reported response to therapy, as judged by the owner. Given that numerous clinics and veterinarians were involved, the rigid criteria used for response to therapy in trial 1, was not used. Both appetite and diet palatability were subjectively assessed by owners. Body weight was measured at the start and end of the trial, using the electronic weigh scales of the practice. Apart from the scheduled reassessments, telephone advice was provided as and when required, and other revisits were allowed based upon the needs and concerns of the owner.
Study diets
Both trials used moderate fibre dry, expanded diets with a similar formulation (trial 1: Fibre Response FR31, Feline Veterinary Diet, Royal Canin; trial 2: Feline Gastrointestinal Fiber Response HF 29; Royal Canin; Table 1). However, the exact ingredients used and formulation differed due to differences in raw material availability in Europe and North America. Nevertheless, both diets used psylliumhusks and seeds as the principle source of fibre. Other fibre sources incorporated in both diets included chicory pulp, fructo-oligo-saccharides, mannan-oligo-saccharides, rice and corn.
Table 1.
Average analysis of the diets used in both trials
| Trial 1 diet | Trial 2 diet | |||
|---|---|---|---|---|
|
|
|
|||
| As fed (%) | g/1000 kcal | As fed (%) | g/1000 kcal | |
|
| ||||
| Moisture | 7.0 | 18.1 | 7.0 | 18.0 |
| Protein | 31.0 | 80.3 | 31.0 | 79.9 |
| Fat | 15.0 | 38.9 | 15.0 | 38.7 |
| Nitrogen-free extract | 36.3 | 94.0 | 36.5 | 94.1 |
| Total dietary fibre | 11.5 | 29.8 | 11.3 | 29.1 |
| Crude fibre | 2.9 | 7.5 | 2.7 | 7.0 |
| Calcium | 1.04 | 2.7 | 1.03 | 2.7 |
| Phosphorus | 0.99 | 2.6 | 0.99 | 2.6 |
| Sodium | 0.50 | 1.3 | 0.45 | 1.2 |
| Omega 6 | 2.58 | 6.7 | 3.37 | 8.7 |
| Omega 3 | 0.61 | 1.6 | 0.58 | 1.5 |
| EPA + DHA | 0.31 | 0.8 | 0.31 | 0.8 |
| Metabolisable energy * | 3860 kcal/1000 g as fed | 3879 kcal/1000 g as fed | ||
| Ingredients † | Dehydrated poultry meat, rice, maize, wheat gluten, animal fats, maize gluten, psyllium husks and seeds, hydrolysed animal proteins, chicory pulp, minerals, egg powder, fish oil, yeasts, soya oil, FOS, hydrolysed yeast (source of MOS), marigold extract (source of lutein) | Rice, chicken meal, chicken fat, ground corn, corn gluten meal, wheat gluten, psyllium seed husk, chicory pulp, dried egg product, natural flavours, potassium chloride, fish oil, calcium sulphate, brewers dried yeast, monosodium phosphate, monocalcium phosphate, FOS, salt, vegetable oil, mono-potassium phosphate, dl-methionine, hydrolysed yeast, choline chloride, l-lysine, taurine, Vitamins [dl-alpha tocopherol acetate (source of vitamin E), l-ascorbyl-2-polyphosphate (source of vitamin C), niacin supplement, biotin, riboflavin supplement (vitamin B2), d-calcium pantothenate, pyridoxine hydrochloride (vitamin B6), vitamin A acetate, thiamine mononitrate (vitamin B1), folic acid, vitamin B12 supplement, vitamin D3 supplement], marigold extract (Tagetes erecta L), trace minerals [zinc oxide, zinc proteinate, ferrous sulphate, manganese proteinate, copper sulphate, copper proteinate, manganous oxide, calcium iodate, sodium selenite], rosemary extract, preserved with natural mixed tocopherols, citric acid | ||
FOS = fructo-oligo-saccharides; MOS = mannan-oligo-saccharides.
Metabolisable energy measured according to the Association of American Feed Control Officials (AAFCO) metabolisable energy protocol. 15
Ingredients as listed by the manufacturer; nb differences in details reflect differences in labelling requirements for Europe and North America.
For cats in both trials, the diet was fed to supply maintenance energy requirements, and a sliding scale was used based upon body condition (eg, overweight cats: 45 kcal/kg/day; ideal weight cats: 55 kcal/kg/ day; lean cats: 70 kcal/kg/day). Owners were instructed to introduce the new diet gradually, over 7 days, by substituting it for increasing proportions of their current diet. Once the transition to the study diet was complete, owners fed this exclusively, and were asked to avoid feeding any other food unless the diet proved to be unpalatable when fed exclusively. Fresh drinking water was available at all times.
Additional therapies
Prior to enrolment, most cats were treated with a variety of medications, and this was recorded for each cat. For trial 1, no other therapies were used unless the owner was reluctant to withdraw them immediately. However, an attempt was made to withdraw them or reduce the dose if response was favourable. For trial 2, the medical therapy used at the start of the trial was initially continued but, if response to therapy was favourable, attempts were then made either to discontinue medication or to reduce the doses of other therapies.
Statistical analysis
For both trials, the primary outcomes of interest were faecal score, and subjective improvement in clinical signs, as judged by the owner and veterinarian. Secondary outcome measures included change in body weight, and requirement for other therapy. In both trials, cases were enrolled on an intention to treat basis. Therefore, for all analyses, any cats not responding remained in the analyses, and post-treatment data (for faecal scores, body weight, and use of concurrent therapy) were assumed not to have changed. In addition to comparing differences amongst time points, comparisons were also made between cats diagnosed with idiopathic constipation, and those with other causes. Given the small number of cases, the latter were grouped.
Statistical analysis was performed using computer software (Stats Direct version 2.6.2; Stats Direct). The level of statistical significance was set at P < 0.05, and two-sided P-values are reported throughout. Given that most data sets were categorical rather than continuous, non-parametric statistical analyses were used throughout, and results reported as median (range). Tests used included the Fisher's exact test, signed ranks test, the Mann—Whitney test, the Friedman test (non-parametric analysis of variance), Cusick's trend test and the χ2 test for trend.
Results
Trial 1
Study animals
Fifteen cats were included in the study, and their details are given in Table 2. Haematology and serum biochemistry were unremarkable in all cases in which it was assessed. Causes of constipation were variable and included idiopathic (10), pelvic malunion fracture (one), pelvic canal stenosis (one), idiopathic megacolon (one), and faecolith (one). One of the cats with intermittent idiopathic constipation had a concurrent rectal prolapse at the time of presentation. The remaining cat presented with severe obstipation, although the history revealed signs of recurrent constipation and also large intestinal diarrhoea. However, constipation and obstipation were reported to be the predominant signs. Further investigations, including large intestinal biopsy and histopathological examination, suggested lymphoplasmacytic colitis.
Table 2.
Characteristics of cats in the study
| Parameters | Trial 1 | Trial 2 |
|---|---|---|
|
| ||
| Breed | Domestic * (8), Siamese (2), Persian (2), Burmese (1), Chartreux (1) | Domestic * (42), Siamese (3), British shorthair (2), Maine Coon (2), Manx cross (2) |
| Sex | Male (1), female (1) | Neutered male (36) |
| Neutered male (6) | Neutered female (15) | |
| Neutered female (7) | ||
| Age | 3 years (9 month—10 years) | 8 years (9 months—16 years) |
| Toileting habits | Litter tray exclusively (13) | Not available |
| Litter tray and outdoors (2) | ||
| Duration of signs | 8 months (3–60 months) | 18 months (24 h—60 months) |
| Weight | ||
| T0 | 3.9 kg (3.1–5.4 kg) | 6.3 (3.0–10.8 kg) |
| T1 | 4.0 kg (3.1–5.2 kg) | — |
| T2 | 4.1 kg (3.3–5.4 kg) | 6.7 (3.2–9.2 kg) |
Numerical data are expressed as median (range).
Domestic = domestic shorthair, medium hair or longhair cats.
In one cat, the primary veterinarian had suggested surgery (subtotal colectomy) given the severity and persistence of signs. However, the owner had refused and was instead considering euthanasia; referral had been suggested as a last resort.
Prior therapy and initial management
Prior to enrolment, previous therapies given included lactulose (two), liquid paraffin (one), fibre supplementation (psyllium [six]; addition of green vegetables [two]), eserine salicylate (Felipurgatyl; TVM laboratories, one: Feligastryl; Virbac, one), flubendazole (Flubenol; Janssen; one), Lactobacillus boucardii tyndallises probiotic supplement (Bacteol; Virbac, Carros, France, one), neomycin sulphate (Neomydiar; Novartis), prednisolone (Microsolone; Merial Laboratories), and spiramycin/metronidazole (Stomorgyl; Merial). At the time of presentation, 10 cats (67%) were bright, alert, and able to pass faeces, albeit of smaller volume and hard in character. The remaining five cats (33%) had more severe clinical signs: these cats were unable to pass faeces and required more intensive medical care, which included the use of intravenous fluids and colonic lavage under general anaesthesia. A colopexy was performed in the cat with the concurrent rectal prolapse.
Response to therapy
Fourteen cats ate the prescribed diet readily, whilst one cat (with a diagnosis of idiopathic constipation) ate the diet when also tempted with other food. There was no difference in response between the cats with idiopathic constipation and those diagnosed with other causes (P = 1.000). One cat (with a diagnosis of idiopathic megacolon) vomited after dietary transition, whist the remaining cats had no dietary disturbances. In 10/15 cases (67%), owners reported improvement in clinical signs within 7 days of starting the new diet. At the first re-evaluation (T1), 14 cats (93%) were classified as complete responders, whilst the final cat (with a diagnosis of idiopathic constipation) was classified as a partial responder: clinical signs had improved and faecal consistency returned to normal, but the owner reported the duration of defecation to be excessively long. No additional therapy was required in 14 cats (93%). In the remaining cat, liquid paraffin was used initially, but this had been successfully stopped by the time of re-evaluation (T1).
At T2, no recurrence had occurred in any of the cats, and no drug therapy was required. Again, 14 cats (93%) were classified as complete responders, and one cat classified as a partial responder. In this final cat, faecal consistency was normal, but the owners reported defecation to be slower than before onset of signs.
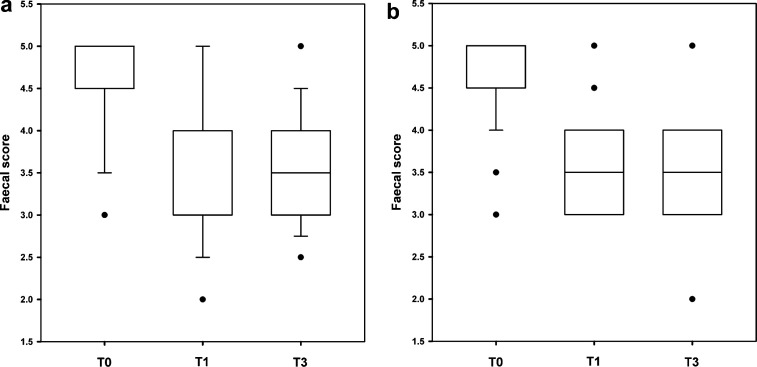
Median faecal score improved significantly throughout the course of the study (T0: 5 [3–5]; T1: 4 [2–5]; T2: 3.5 [3–5]; Cusick's test for trend P < 0.001; Fig 1a), but there was no change in body weight (Friedman test P = 0.553; Table 3). There were no differences in response between cats diagnosed with idiopathic constipation and those with other diseases, both for faecal score (P = 0.653) and body weight (P = 0.730).
Fig 1.
Faecal scores from cats enrolled in trials from France (a) and Canada (b) assessing the efficacy of a psyllium-enriched diet for management of feline constipation. The boxes depict interquartile range (top and bottom of box), the vertical lines show the range, median is represented by the bold horizontal line, and outliers are shown as separate points. T0: start of the trial; T1: recheck at 1 month; T2: recheck at 2 months. Vertical range and median lines are not shown when they coincide with the limits of the interquartile range boxes. In both trials, median faecal score improved significantly throughout the course of the study (Cusick's test for trend P < 0.001).
Table 3.
Concurrent medical therapies used in the cats in trial 2
| Drug | Time | ||
|---|---|---|---|
|
|
|||
| T0 (n = 50) | T1 (n = 40) | T2 (n = 40) | |
|
| |||
| Cisapride | 36 | 20 | 14 |
| Decrease * 4 | Decrease 5 | ||
| Lactulose | 35 | 18 | 12 |
| Decrease 4 | Decrease 5 | ||
| White petrolatum/mineral oil | 11 | 6 | 6 |
| Polyethylene glycol | 3 | 1 | 1 |
| Decrease 1 | Decrease 1 | ||
| Psyllium supplement | 3 | 1 | 1 |
| Other | 2 | 2 | 2 |
| Meloxicam | Meloxicam | Meloxicam | |
| Gabapentin | Amitriptyline | Amitriptyline | |
Decrease: drug continued but at a lower dose.
Long-term follow-up
After the end of the trial, cats continued to be fed the diet, with no other therapy, and were monitored remotely by phone or email. The median duration of follow-up was 597 days (range 486–645 days). At the time of final assessment in May 2011, one cat had developed a fibrosarcoma, and was euthanased 486 days after initial assessment. Of the 14 cats still alive, 13 were in clinical remission at this stage, and one cat had intermittent vomiting but no signs of constipation. Ten of these cats were still receiving the test diet as sole therapy, whilst the remaining cats were receiving either a combination of the test diet and a diet designed for the management of urinary tract disease (Feline Urinary S/O LP34, Royal Canin: n = 1), or had been successfully switched to an alternative diet. Other diets used included commercial maintenance diets (Digestive Comfort 38, Royal Canin; Feline neutered female mature cat, Royal Canin) or a low-phosphorus low-protein prescription diet (Feline Renal RF23, Royal Canin). No medical therapies were required in any cats. Finally, intermittent rectal prolapse had been noted in the cat that had originally presented with this problem, but there were no signs of constipation.
Trial 2
Study animals
Fifty-one cats were initially included in the second trial, from 14 different clinics (Table 2). The distribution of cases by province was as follows: Ontario 36, Quebec seven, Alberta five, British Columbia one, Nova Scotia one, News Brunswick one. Of these, three cats were withdrawn: one cat was euthanased for an unrelated condition, one cat developed diarrhoea upon starting the food, whilst signs of constipation deteriorated in the remaining cat. A further six cases were reported to have improved and completed the trial, but the primary care veterinarian did not return any follow-up paperwork. Hence, despite a subjective improvement, these cases were classified as ‘lost to follow-up’ and their data was not included in further analysis. The remaining 42 cats (82%) completed the 2-month trial, and clinical signs had improved in all cases. In nine cats, a neurological cause was suspected, and this included megacolon (six cats), lower motor neurone disease (one) and undefined neurological disease (two). In a further three cats, concurrent orthopaedic disease was present, and included a sacral deformity (one), bridging spondylosis (L7—S1; one) and osteoarthritis (one). In the remaining 30 cases, an underlying cause was either not known or not reported. Prior to enrolment the primary veterinarian had considered performing surgery (subtotal colectomy) in four cats, given poor response to therapy, and the owners of a further two cats were considering euthanasia. There was no difference in response between the cats with idiopathic constipation and those diagnosed with other causes (P = 0.409).
Initial presentation and concurrent medical therapy
Prior to enrolment, clinical signs were present for a median of 18 months (24 h—60 months). As with trial 1, a number of cats required initial therapy: fluid therapy was used in 14 cases (28%; intravenous 13: subcutaneous one), 29 (58%) cats required enemas and manual evacuation of faeces was required in one cat (2%). At the start of the trial, a variety of concurrent medications were used, including cisapride, laxatives (including lactulose, polyethyl glycol, and white petrolatum/liquid paraffin), and psyllium supplementation (Table 3). There was a significant reduction in the use of both cisapride (χ2 for trend, P = 0.014) and lactulose (χ2 test for trend, P = 0.009), but not for use of other laxatives (χ2 test for trend, P = 0.072). Nonetheless, in some of the cats where drugs were continued, the dose was successfully reduced (Table 3). There was no difference in the reduction in use of the therapies between cats diagnosed with idiopathic constipation and those with other diseases (P > 0.18 for all) treatments.
Reassessment
All cats ate the prescribed diet readily and, apart from the case that developed diarrhoea and was withdrawn (see above), no other refusals or gastrointestinal disturbances were reported. Median faecal score improved significantly throughout the course of the study (T0: 5 [3–5]; T1: 4 [2–5]; T2: 4 [2–5]; Cusick's test for trend P < 0.001; Fig 1b), but there was no change in body weight (Wilcoxon signed ranks test, P = 0.168; Table 3). There was no difference in response between cats diagnosed with idiopathic constipation and those with other diseases, for both faecal score (P = 0.113) and body weight (P = 0.113).
Discussion
The current study has assessed the use of a highly digestible formula, with added psyllium, in cats with constipation. This approach differs from conventional recommendations of adding insoluble fibre to provide bulk, and limiting soluble fibre sources to 5% of total food. 5 Evidence-based medicine principles are gaining widespread acceptance in veterinary science, 12 and with it a growing need to generate objective evidence on therapeutic efficacy in common clinical conditions. Whilst the study is limited by the fact that it was open-label in design and uncontrolled, it does still represent the first attempt to generate objective data to help clinicians determine the most appropriate therapy for constipated cats. Nonetheless, randomised clinical trials are now needed to confirm what therapy is indeed most appropriate.
With these caveats in mind, the main conclusions from both trials were that the test diet was palatable and well tolerated, that clinical remission was noted in the majority of cats on the study, and that other symptomatic therapy was either not required in management or could often be discontinued or reduced without recurrence of clinical signs. Palatability is always a concern with any prescription diet as, without it, patient compliance is likely to be poor. Indeed palatability is a particular issue when fibre is incorporated in to diets for cats. 13 Therefore, the fact that the majority of cats readily ate both formulations used was encouraging.
Given that that a contemporaneous control group was not studied, we cannot be certain that the clinical improvement and withdrawal of other drugs were solely the result of the dietary management. Indeed, it is feasible that resolution may have been the result of a placebo effect or change in another unmeasured parameter. Further, apparent improvement can also be seen through ‘regression to the mean’ whereby cases with waxing-waning clinical signs tend to be presented when at their worst, and subsequent improvement is coincidental. Finally, in human studies, enrolment in a trial can mean improved compliance with other strategies or other changes that are implemented by the patient. 14 Such concerns would arguably be a greater concern in study 2, as both duration of signs before the trial and post-intervention follow-up were shorter. Nonetheless, the >80% response rate seen in both of the current trials is perhaps of a greater magnitude than that expected from either the placebo effect or from the other reasons for spontaneous improvements. 14 The fact that many of the cats in trial 1 had not previously responded to other therapy and the fact that remission was maintained over a relatively long period lends further support to this being a genuine effect rather than a chance finding. It was also noteworthy that both surgical management and euthanasia were being considered in a small number of cats, but were not subsequently required.
As mentioned previously, the diet formulation was a novel formulation, whereby psyllium husks and seeds were added at a moderate level to a highly digestible formula. Psyllium is commonly used as a supplement in the management of idiopathic constipation in humans, and also for feline constipation and megacolon.1,3,9 Any clinical benefit of the diet might be directly related to increased faecal moisture and improved faecal consistency, but may also have been due to prokinetic effects from the effect of SCFA on colonic myocytes. 6 There may also be beneficial effects from the fact that the formulation is highly digestible, which may have had other effects on faecal composition. Comparative trials of different formulations would help to determine the mechanism of action. For instance, direct comparisons could be made between diets with different types of fibre, or different levels of inclusion of the same fibre. Not only would this provide more objective evidence of efficacy, but parallel studies of digestive tract physiology and faecal composition could be performed, which might help to determine the mechanism of action. Based upon the findings of the current study, such a prospective trial should aim to include 30–40 cases and a similar number of controls in order to maximise the chances of avoiding type II error.
As mentioned above, the trials performed are preliminary in nature and a number of limitations must be taken into account when interpreting the results. With regard to trial 1, this study was relatively small and was based at a referral centre meaning that the cases seen were not typical of those seen in first-opinion practice. However, the fact that all cases were managed at a single centre by an experienced gastroenterologist (VF) was advantageous, and ensured that inclusion criteria and approach were consistent. Further, the high response rate is particularly encouraging in light of the use of referral cases which are likely to be more complicated and problematic than the typical first-opinion caseload. Nonetheless, 10 of the cases had a final diagnosis of idiopathic constipation. Whilst these cases might be typical of many seen in practice, they still fitted the inclusion criteria of having been deemed refractory to therapy. As we have previously stated, findings should be cautiously interpreted in light of the unblinded and uncontrolled study design.
Some of the limitations of trial 1, namely the single-centre approach and use of referral cases, were overcome by performing a second clinical trial using cases recruited from many centres across Canada. This allowed recruitment of a larger study population, all of which were from first-opinion practices. Nonetheless, this study design has other limitations in that there was likely some heterogeneity in approach and management even though all centres followed a single study protocol. The multi-centre nature may also have contributed to a relatively greater rate study drop-out in this trial. Further, the presence of recurrent clinical signs was not an inclusion criterion of trial 2, and some cases may have resolved spontaneously.
Another limitation of both studies is the fact that the study period was short, and may not prove long-term remission. However, longer term follow-up was assessed in trial 1 and suggested that clinical remission was maintained with the use of the diet. Further, response was assessed with both subjective measures (response to treatment as determined by owner and clinician) and semi-quantitative (faecal consistency). Lack of objectivity could lead to a greater potential for bias. For this reason, it would be preferable for any future randomised controlled trial to be double-blinded in some way, if at all possible. A final limitation was the fact that information on toileting habits was not available for the cats in trial 2, and it is possible that some results may be unreliable. For instance, response would be difficult to judge reliably in cats that defecate outside exclusively. This is yet another reason to consider performing a controlled trial in the future.
In conclusion, the current trials have provided the first evidence that dietary modification, through the use of a psyllium-enriched dry expanded diet, may be beneficial in the management of chronic constipation in cats. Controlled clinical trials are now warranted, to confirm these findings, to improve the strength of available evidence and to determine the mechanism of action of this dietary formulation.
Conflict of interest
Royal Canin manufactured the diets used in this study and financially supported both trials. Doreen Houston, Heather Weese and Michelle Evason, are employees of Medi-Cal Royal Canin Canada, whilst Géraldine Deswarte, Gérald Ettinger, Yannick Soulard, and Vincent Biourge are all employees of Royal Canin. Royal Canin also financially supports the post of AJ German, at the University of Liverpool. All of these authors were involved in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Acknowledgements
The authors would like to acknowledge all veterinary clinics and owners for participating in the trials. AJG's senior lectureship is funded by Royal Canin.
References
- 1.Washabau RJ, Holt D. Feline constipation and idiopathic megacolon. In: Bonagura JD, ed. Kirk's current veterinary therapy. 13th edn. Philadelphia: WB Saunders, 2000: 648–52. [Google Scholar]
- 2.Byers C, Leasure C, Sanders N, Colahan P. Feline idiopathic megacolon. Comp Contin Edu Pract Vet 2006; 28: 658–65. [Google Scholar]
- 3.Washabau RJ. Gastrointestinal motility disorders and gastrointestinal prokinetic therapy. Vet Clin N Am 2003; 33: 1007–28. [DOI] [PubMed] [Google Scholar]
- 4.Washabau RJ. Feline megacolon. In: Steiner JM, ed. Small animal gastroenterology. Hannover: Schlutersche, 2008. 230–6. [Google Scholar]
- 5.Davenport DJ. Constipation/obstipation/megacolon. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Novotny BJ, eds. Small animal clinical nutrition. 5th edn. Topeka: Mark Morris Institute, 2010: 1117–26. [Google Scholar]
- 6.Sunvold GD, Fahey GC, Merchen NR, et al. Dietary fiber for cats: in vitro fermentation of selected fiber sources by cat fecal inoculum and in vivo utilization of diets containing selected fiber sources and their blends. J Anim Sci 1995; 73: 2329–30. [DOI] [PubMed] [Google Scholar]
- 7.Rondeau MP, Meltzer K, Michel KE, McManus CM, Washabau RJ. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg 2003; 5: 167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunvold GD, Fahey GC, Merchen NR, Reinhart GA. in vitro fermentation of selected fibrous substrates by dog and cat fecal inoculum: influence of diet composition on substrate organic matter disappearance and short-chain fatty acid production. J Anim Sci 1995; 73: 1110–22. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf W, Park F, Lof J, Quigley MM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Therap 1995; 9: 639–47. [DOI] [PubMed] [Google Scholar]
- 10.Washabau RJ, Holt D. Pathogenesis, diagnosis, and therapy of feline idiopathic megacolon. Vet Clin N Am Small Anim Pract 1999; 29: 589–603. [PubMed] [Google Scholar]
- 11.Bissot T, Servet E, Vidal S, et al. Novel dietary strategies can improve the outcome of weight loss programmes in obese client-owned cats. J Feline Med Surg 2009; 12: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor AM, Sargeant JM, Gardner IA, et al. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. J Vet Intern Med 2010; 24: 57–64. [DOI] [PubMed] [Google Scholar]
- 13.Servet E, Soulard Y, Venet C, Biourge V. Evaluation of diets for the ability to generate ‘satiety’ in cats. J Vet Intern Med 2008; 22: 808. [Google Scholar]
- 14.Bausell RB. Snake oil science: the truth about complementary and alternative medicine. New York: Oxford University Press, 2007. [Google Scholar]
- 15.Association of American Feed Control Officials. Dogs and cats nutrient profiles. AAFCO Official Publication. Washington: AAFCO, 2010: 169–83. [Google Scholar]