Abstract
This study investigated 339 cases of feline mycobacterial infection, with histopathology findings from 225 cases, and treatment and outcome information from 184 cases. Tissue samples from cats with cutaneous lesions or suspicious masses at exploratory laparotomy were submitted to the Veterinary Laboratories Agency for mycobacterial culture over a 4-year period to December 2008. The study reviewed the files for information about histopathology, treatment and outcome, and blindly reviewed histopathological changes (including staining for acid-fast bacteria [AFB]) in a sub-set of 45 cases. When a cat is suspected of having a mycobacterial infection, accurate identification of the species involved helps to determine possible treatment options and prognosis. The study confirmed that histopathology and the presence of AFB are useful tools in the recognition of mycobacterial infection. Unfortunately, they did little to help determine the species of mycobacteria involved. The study identified a group of cats that were negative for AFB at the primary laboratory, but from which mycobacteria could be cultured; commonly Mycobacterium bovis or Mycobacterium microti. The study also identified a group of cats which where culture negative, despite typical signs of mycobacterial infection and positive AFB staining. Many cases responded favourably to treatment (56% of the cases where information was available), and many cats gained complete remission (42%). However, relapses were common (64%) and often followed by pulmonary and/or systemic spread that may have resulted from treatment with short courses of single drugs. This study shows that the diagnosis and treatment of feline mycobacteriosis is complex and challenging.
Mycobacterial infections in humans and other animals are of international concern.1–3 One mammalian species that can be infected by a number of different mycobacteria is the domestic cat. Unfortunately, many aspects of feline mycobacteriosis remain unknown, and there have been few recently published research papers on this subject. 4
Diagnosis of feline mycobacterial disease can be challenging. It is usually made by finding suggestive histopathological changes in biopsies and identifying morphologically typical acid-fast bacteria (AFB),5–8 with confirmation by specialist culture of fresh tissue. 9 However, many samples fail to culture and those that do can take up to 3 months to grow. 10 Specific tests such as serology and intradermal testing have generally proved unhelpful in cats,7,9,11,12 although the interferon (IFN) gamma test and other immunoassays are showing promise in detecting and differentiating cats infected with Mycobacterium bovis, Mycobacterium microti and Mycobacterium avium.13–15 Molecular [polymerase chain reaction (PCR) and sequencing-based] tests have been developed and are now being used more commonly; however, they are expensive and have low sensitivity when only a few mycobacterial organisms are present.8,16–20
Treatment of mycobacterial infection in cats is complicated, and successful outcomes are more likely when the species of mycobacteria has been identified and the cat has been treated with a long course of multiple appropriate drugs, plus surgery (where indicated).4,9,21–26 To date, there are no drugs approved for the treatment of mycobacterial infections in animals and the ‘recommended’ treatment regimes for cats (under the United Kingdom's Cascade procedure) are based on clinical experience, rather than controlled clinical trials. In addition, deciding to treat a cat with mycobacteriosis can be contentious, particularly if M bovis, M microti or M avium are involved, as these infections are potentially zoonotic, particularly to humans with compromised immune systems.9,27 Treatment of a cat with an M bovis infection is particularly contentious, as under the Tuberculosis Orders in force in England, Wales, and Scotland the suspicion or identification of M bovis infection in a cat is notifiable (DEFRA 2008 28 ). Unfortunately, tubercle group bacteria and non-tubercle group mycobacteria (NTM) can cause similar clinical signs,4,10 and because successful culture may take many weeks, 10 many cats commence treatment in the interim, sometimes being given inappropriate drugs, risking the development of antibiotic resistant mycobacterial clones. 29
Given the paucity of our knowledge about feline mycobacterial disease in Great Britain (GB) the primary aim was to carry out a field survey to assess the histopathological changes caused by these infections; determine how these infections are currently treated; and assess their current prognosis. By knowing which bacteria are present, it is possible to determine which cases are appropriate to treat, which are more likely to respond to treatment and how best to tailor the treatment protocols. It is particularly important to identify cats infected with M bovis, M microti and M aviumas these have significant potential zoonotic risk. As culture can take up to 3 months 10 and access to molecular diagnostics is limited, the secondary aim of the study was to determine if the histopathological findings could enable prediction of which mycobacterial species is present.
Materials and methods
Tissue samples
Between January 2005 and December 2008, 339 feline samples were submitted to the veterinary Laboratory Agency (VLA) Weybridge by veterinary surgeons in GB for mycobacterial culture. 30 Culture was performed free of charge following the introduction of Tuberculosis Orders in England, Wales, and Scotland, and was funded by DEFRA. The samples came from cats that had been found to have cutaneous lesions or suspicious masses at exploratory laparotomy, and when formalin-fixed samples were sent to private pathology laboratories for histopathology the tissue was found to have lesions suggestive of mycobacterial infection (typical granulomatous and/or pyogranulomatous inflammation consisting of multifocal to coalescent infiltration with large numbers of macrophages containing variable numbers of AFB). 6 The veterinary surgeons then took a second sample and submitted it without fixation to the VLA for mycobacterial culture. Depending on the availability of material, either swabs, impression smears or fixed material was stained with Ziehl Nielsen (ZN) for the detection of AFB. 31 Histopathology findings were available from 225 cats from the primary diagnostic laboratory and 93 from the VLA.
Veterinary surgeons that submitted the samples to the VLA were contacted by one of the authors (SMcF) and asked to provide information on histopathological changes found within the lesions, the course of disease progression (development of respiratory and/or systemic signs, radiographic or ultrasound changes), treatment details (surgery, drugs given, duration of treatment), and eventual outcome (remission, relapse, euthanasia or death). Data on where the cat lived (ie, the postcode of the owner's house), plus the cat's signalment (age, breed, gender), and clinical presentation are presented in the accompanying paper. 32 In many cases the information was incomplete or not available so where data were missing the number of samples included in the analysis has been noted.
To investigate the variation in histopathology between cats infected with different Mycobacterium species, samples from tissues of 45 animals were selected for detailed retrospective examination. This was not a random selection of cases as care was taken to include as many possible culture outcomes as possible (please see the sister paper for detailed information about which species of mycobacteria were cultured from the 339 cats and how commonly each species was identified). 32 However, with in each group of samples infected with a particular Mycobacterium species, samples were retrieved at random from the tissue archive. This selection process resulted in 15 M bovis samples (28% of M bovis samples in the study), 13 M microti (21%), five M avium (21%), one Mycobacterium malmoense and 11 samples with lesions where culture was negative (6%). The samples were examined without knowing the culture result by a single experienced histopathologist (author AS). Lesions were assessed for the presence of epithelioid cells, neutrophils, extent of necrosis, presence of multinucleated cells and semi-quantitatively for the number of AFB. The numbers of AFB were assessed using the following criteria: ‘0.5’ — AFB difficult to find, ‘1’ — one AFB every three to four high powered field (HPF), ‘2’ — AFB easy to find and ‘3’ — cells contain very large numbers of AFB. Necrosis was scored as ‘0’ — none present, ‘1’ — some necrosis, ‘1A’ — autolytic and therefore difficult to judge, ‘2’ — moderately extensive necrosis, and ‘3’ — multifocal to coalescent necrosis. If multiple samples were present for a single animal, the mean score was calculated for final evaluation.
Statistical analyses
Two groups of factors were considered for analyses: (i) diagnostics and (ii) treatment. For each group standard univariate logistic regression risk factor analysis was performed to see whether particular factors were associated with whether culture and classification of the sample was possible. In addition, odds ratios (OR) and associated 95% confidence intervals (CI) were calculated. The culture results were divided into four groups: (i) M bovis, (ii) M microti, (iii) NTM and (iv) no growth, and Fisher exact tests were carried out to identify any association with diagnostics and treatment factors. The NTM group was also divided further into M avium and non-M avium NTM for some of the comparisons. Fisher exact tests were also performed to look for association between measures of histopathology (inflammation, ZN-staining). To test for differences in AFB numbers in the 45 sample sub-set a Kruskal—Wallis test was carried out in StatsDirect (1990–2010 StatsDirect), with the non-parametric post-hoc pairwise comparison test (Dwass—Steele—Chritchlow—Fligner) also performed. 33 In all cases statistical significance was set at P < 0.05.
Results
A total of 339 records were reviewed, but in many cases the information was incomplete or not available. Histopathological details were available for 225 (66.4%) of these 339 cases, and treatment and outcome details for 184 (54.3%) of the 339 cases. Both histopathological and treatment and outcome details were only available for 126 cats.
Histopathology
The primary diagnostic laboratories reported granulomatous inflammation in 57% of the samples (120/212 cats; where the nature of the inflammation was recorded), pyogranulomatous inflammation in 35% (74/209 cats), and pyogranulomatous and granulomatous in different areas of the sample for six cases. The primary diagnostic laboratories found AFB in 64% (125/195 cats) of the cases tested, and they found 36% (70/195) to be AFB negative. Despite this, 41% (29/70) of these samples that were AFB negative were successfully cultured by the VLA. Of these 29 samples, 34% (n = 10) were found to be M bovis, 31% (nine) M microti, 17% (five) M avium, 10% (three) unclassified mycobacteria, and 7% (two) Mycobacterium fortuitum.
Histopathology results were available for 93 samples submitted to the VLA because of suspect mycobacterial infection by the primary laboratory. Interestingly, the VLA identified lesions consistent with mycobacterial infection in only 67 of these cases (72%); but found AFB in 66 (99%) of these 67 cases.
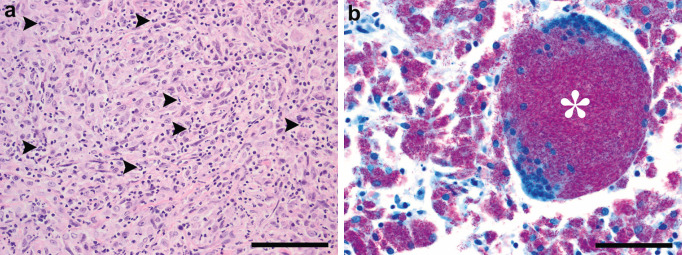
The blind retrospective histopathology study on 45 of samples showed that lesions were characterised by multifocal to coalescent infiltration of the tissue with large numbers of macrophages associated with variable numbers of AFB. Macrophages frequently were fusiform to polygonal (Fig 1a). At least one AFB was detected in all sections, but they were very difficult to find in 21 cases (47%). There were statistically significant differences between the number of AFB detected between the Mycobacterium species involved (P = 0.011, Table 1), with post-hoc analysis revealing that M bovis samples were associated with more AFB than M microti (P = 0.007), but there was no difference in AFB numbers between samples where there was no growth and both M bovis and M microti (P > 0.163). Neutrophil infiltration consistent with pyogranulomatous inflammation was present in 36 cats (80%) independent of the aetiological agent (P = 0.945). Multinucleated giant cells were present in only three cats: a cat with M avium infection (Fig 1b), a cat with M malmoense infection and a cat with M microti infection. In 15 cats (33%), no necrosis could be observed, and there was no difference in levels of necrosis between the groups (P = 0.091). Mineralisation was observed in one cat.
Fig 1.
(a) Typical histopathology of a cat with a mycobacterial infection. Note the densely packed polygonal to fusiform macrophages associated with a sparse neutrophilic infiltrate (arrow heads) in the lymph node of a M microti infected cat (H&E, scale bar = 100 μm). (b) Unusual histopathology of a cat with a mycobacterial infection. Note the multinucleated cell (asterisk) and very large numbers of fuchsin coloured acid-fast bacteria (AFB) in the multinucleated cell and the surrounding macrophages in the lymph node of a M avium infected cat (ZN, scale bar = 50 μ).
Table 1.
Semi-quantitative assessment of the number of acid-fast bacteria (AFB) in 45 cats. Number of cats and percentage of animals per Mycobacterium species in parentheses expressed as ‘<1’ e one AFB in four HPF; ‘2’ e AFB present in every field and ‘3’ e very large numbers of AFB per isolate.
| <1 | 2 | 3 | Total | |
|---|---|---|---|---|
|
| ||||
| M bovis | 2 (13.3%) | 4 (26.7%) | 9 (60.0%) | 15 |
| M microti | 11 (84.6%) | 2 (15.4%) | 13 | |
| M avium | 2 (40.0%) | 3 (60.0%) | 5 | |
| M malmoense | 1 (100%) | 1 | ||
| Negative culture | 6 (54.5%) | 2 (18.2%) | 3 (27.3%) | 11 |
Treatment and outcome
Some form of treatment was attempted in 184 cats (87%) (where the treatment regime was recorded) (Table 2). Thirty-eight percent of 177 cats underwent surgery and 90% were treated medically. The percentage of cats reported as being treated with a particular drug varied from 5% for tetracycline drugs (eg, doxycycline) to 53% for fluoroquinolones. Triple therapy (macrolide/azalide, fluoroquinolone and rifampicin) was used in only 16% of the cats (Table 2). Most cats were treated for less than 1 month (61%). In 56% of cats there was a positive response to treatment, with 42% gaining a complete remission. Of the 33 cats that achieved apparent complete remission 18 were no growth, five M bovis, four M microti, three unclassified, two M malmoense, and one M avium.
Table 2.
Results on the treatment and outcome of cats with mycobacterial disease. The tissue samples had histopathological changes suggestive of mycobacterial infection and were submitted to the VLA for mycobacterial culture between January 2005 and December 2008. Results shown with prevalence and 95% exact binomial CI, the statistical significance (Wald P-value) and the OR (and 95% CI) for these factors associated with mycobacterium being grown from the samples.
| n | Positive | % | P-value | OR (95% CI) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Received some treatment | 212 | 184 | 86.8 | (81.4–91.0) | 0.148 | 0.54 (0.24–1.24) |
| Underwent surgery | 177 | 67 | 37.9 | (30.6–45.4) | 0.321 | 0.73 (0.40–1.35) |
| Treated with a drug | 187 | 168 | 89.8 | (84.5–93.8) | 0.112 | 0.44 (0.16–1.21) |
| Macrolide/azalide (eg, clarithromycin/azithromycin) | 169 | 42 | 24.9 | (18.5–32.1) | 0.563 | 1.23 (0.61–2.48) |
| Lincosamide (eg, lincomycin, clindamycin) | 172 | 23 | 13.4 | (8.6–19.4) | 0.122 | 0.49 (0.19–1.21) |
| β-lactam (eg, penicillins, cephalosporins) | 177 | 85 | 48 | (40.4–55.6) | 0.327 | 0.74 (0.41–1.34) |
| Fluoroquinolone (eg, enrofloxacin, marbofloxacin) | 175 | 93 | 53.1 | (45.4–60.7) | 0.943 | 1.02 (0.56–1.85) |
| Tetracycline (eg, doxycycline) | 169 | 9 | 5.3 | (2.4–9.9) | 0.305 | 0.48 (0.11–1.97) |
| Rifampicin | 170 | 35 | 20.6 | (14.7–27.5) | 0.385 | 1.39 (0.66–2.95) |
| Triple therapy (ie, macrolide/azalide + fluoroquinolone + rifampicin) | 168 | 27 | 16.1 | (10.8–22.5) | 0.296 | 1.56 (0.68–3.60) |
| Corticosteroid | 173 | 25 | 14.5 | (9.5–20.6) | 0.243 | 0.60 (0.25–1.42) |
| Duration of treatment | 140 | |||||
| <1 month | 85 | 60.7 | (52.1–68.9) | — | Reference level | |
| 1–6 months | 40 | 28.6 | (21.2–36.8) | 0.184 | 1.67 (0.78–3.57) | |
| ≥6 months | 15 | 10.7 | (6.1–17.1) | 0.538 | 1.41 (0.47–4.25) | |
| Positive response to treatment | 152 | 85 | 55.9 | (47.6–64.0) | 0.337 | 0.73 (0.38–1.39) |
| Complete remission | 78 | 33 | 42.3 | (31.1–54.0) | 0.630 | 1.25 (0.50–1.31) |
| Recurrence of lesion/lump or other clinical signs after treatment (surgery and/or medical management) | 159 | 101 | 63.5 | (55.5–71.0) | 0.674 | 0.87 (0.46–1.66) |
| Progression to respiratory disease, regardless of whether treated or not | 165 | 52 | 0.359 | 0.73 (0.38–1.42) | ||
| Progression to systemic disease, regardless of whether treated or not | 193 | 100 | 31.5 | (24.5–39.2) | 0.364 | 1.30 (0.74–2.29) |
There was a recurrence of clinical signs in 63% of the cats post-treatment (Table 2). Given the nature of this retrospective study, where only limited data are available, it was not possible to determine if one treatment protocol was better than another. However, some observations can be made, such as all the cats that gained complete remission had a fluoroquinolone in their treatment, and 45% of the cats that received triple therapy went into complete remission, compared to 41% of the cats that did not receive triple therapy. Of the 15 cats that received treatment for 6 months or more (two with M microti, two with M bovis, four with NTM and seven with no growth), all responded positively, with six (40%) gaining complete remission.
Irrespective of treatment, where reported, apparent progression to involve the lungs occurred in 32% of cases, and apparent progression to cause systemic signs (such as weight loss) was seen in 52%, with all but 2/50 cats with a respiratory recurrence also having a recurrence of systematic signs (96%). None of the treatment or outcome factors were associated with being able to culture mycobacteria from the samples (P > 0.111, Table 2). A similar lack of difference was seen when the results were divided into whether the infections were M bovis, M microti, NTM, or no growth (P > 0.131). However, when looking at the 30 samples where data was available in the NTM group, 15/17 M avium samples came from cats that had either systemic and/or pulmonary involvement, as did 5/6 unclassified samples. The one Mycobacterium celatum case had systemic, but no pulmonary changes, and the one Mycobacterium intercellulare case had no systemic signs (there was no information about pulmonary involvement); the three M fortuitum and two M malmoense cases had neither systemic nor pulmonary signs.
Discussion
This paper (and the accompanying paper focusing on the geographic distribution, culture results, signalment and clinical presentation of the 339 cats 32 ) details the largest study of feline mycobacterial disease reported to date. Analysis of this unique data set has generated some interesting results relating to the importance of histopathology, possible responses to treatment, and overall prognosis.
The data in the current study was divided into four groups so that the two most important infections (M microti and M bovis) could be defined, and then compared to the heterogeneous NTM and the no growth group. This was undertaken because one aim of the study was to determine if it was possible to predict which mycobacterial species was present based on the nature of histopathological changes in the primary lesions, and from this to predict the likely response to treatment.
Veterinary surgeons rely on primary pathology laboratories for the recognition of histopathology changes suggestive of mycobacterial infection. However, this study shows that interpretation of samples varied between the primary and specialist laboratories. Samples were assessed by pathologists at the primary laboratories (225 samples) and the VLA (93 samples) (with the information in both cases being retrieved from the case files). The primary laboratories reported granulomatous and/or pyogranulomatous inflammation suggestive of mycobacterial infection in all cases, but found AFB in only 64% of the samples tested. The more specialist pathologists in the VLA identified lesions consistent with mycobacterial infection in only 72% of these cases; and found AFB in 99% of the cases they selected as having typical histopathological changes. These findings may suggest that some of the cases diagnosed by the primary laboratory were miss-diagnosed; however, the VLA were able to grow mycobacteria from 36% of samples that were negative for AFB at the primary diagnostic laboratory (with M bovis and M microti being grown most commonly), so this is not necessarily the case.
When 45 cases were reviewed by a pathologist experienced in interpreting mycobacterial infection the histopathology revealed multifocal to coalescent, granulomatous and/or pyogranulomatous inflammation, which was frequently associated with AFB. This is consistent with previous reports.5–8 Given the nature and scale of the study, the exact stage of the mycobacterial infection could not be determined for individual cases. Taking this into consideration, the typical histopathology in naturally occurring mycobacterial infections in cats from the UK was characterised by groups of macrophages variably associated with neutrophil infiltration (80% cases) and necrosis (67% cases). AFB were frequently very difficult to find (47% of cases), which may be why they were missed by the primary laboratories. Mineralisation and multinucleated cells were an uncommon finding. A lack of multinucleate giant cells in lesions from cats with M microti infections has been reported previously.22,34,35 The study identified a tendency of increased numbers of AFB in cases of M bovis. If AFB were detected in less than one HPF, either M microti or negative culture results is likely. Based on the currently available data, relatively few false negatives (2/21 = 9.5%) would be expected. However, these results have to be interpreted cautiously, because the semi-quantitative scoring was performed by a single experienced pathologist on a relatively small number of cases under experimental conditions rather than in a commercial environment. A study based on a large number of field samples from various stages of disease including a larger variety of Mycobacterium species read by multiple pathologists would be necessary to investigate this hypothesis before it could be considered as a diagnostic tool.
Histopathology is useful at establishing the initial diagnosis of a mycobacterial infection and differentiating lesions from other conditions such as other infections or neoplasia. This study confirms that histopathology and ZN-staining provide addition information when trying to make a diagnosis of mycobacterial infection.6,20 However, as many cases are paucibacilliary, a negative smear does not rule out a mycobacterial infection.10,20 Unfortunately, the slow-growing nature of some of the Mycobacterium species (especially M microti), low numbers of AFB, multifocality of the lesions, use of different staining methods, using fixed tissue rather than fresh tissue, need to perform multiple tests (eg, histology and culture, particularly when only small biopsies are submitted), experience of the histopathologist, and pressures of workload can all lead to false negatives or discordant histopathology results, especially when assessing different sections from the same biopsy or different samples from the same cat.
This study shows just how difficult it can be to make a diagnosis of feline mycobacteriosis. If a veterinary surgeon has any suspicion of mycobacterial infection, ie, there is an unusual mass in any tissue, we strongly recommend that histopathology and ZN-staining be performed. If there is granulomatous and/or pyogranulomatous inflammation (even in the absence of multinucleated cells, necrosis, mineralisation or AFB), a sample should be considered suggestive of a mycobacterial infection and sent for specialist culture and/or molecular diagnostics.
Where recorded, treatment was attempted in 87% of cases, involving either surgery and/or medical therapy. A positive response was seen in 56% of these cases, with 42% gaining a complete remission. Treatment most typically consisted of a fluoroquinolone (53%) or a b-lactam antibiotic (48%), which was given for less than a month (61%). This was despite the recommendation that feline mycobacterial infections, particularly those involving members of the tuberculosis complex or Mycobacterium avium-intercellularie complex (MAC) organisms, should be treated with double or triple therapy (typically involving a macrolide or an azalide with a fluoroquinolone and rifampicin), which should be given for up to 6 months.9,21,22,24 However, it is important to realise that there are no drugs approved for the treatment of mycobacterial infections in animals and the ‘recommended’ treatment regimes for cats (under the UK Cascade procedure) are based on clinical experience, rather than controlled clinical trials. Given that most cats received short course of single drugs it is perhaps not surprising that 64% of the cases relapsed.
Unfortunately, because the number of cases with complete treatment records was limited, the study does not allow for good comparison of the different treatment protocols, either in the whole group or for the different infections. Only a few observations can be made: for example, all the cats that gained complete remissions had a fluoroquinolone in their treatment, and 45% of the cats that received triple therapy went into complete remission (compared to 41% of the cats that did not). However, the role of confounding factors is unclear as cats receiving a fluoroquinolone and/or triple therapy could have been more mildly affected. Similarly, only 42% of the 14 cats that received treatment for at least 6 months gained a complete remission, which could have been confounded by these cats being more severely affected. A prospective study is needed to elucidate this further. It was probably because of the poor treatment regimes that disease appeared to progress to involve the lungs in 32% of cases and systemic signs in 52%. Interestingly, no particular group of mycobacteria appeared to respond more favourably to treatment or to have a better prognosis. This unexpected finding is believed to result from the retrospective nature of this study and incomplete data sets.
Cats with systemic involvement are believed to have a poorer prognosis and may be more resistant to treatment.9,18–25 Unfortunately, when reviewing the overall treatment and prognosis data, no group of cats could be found to have a better prognosis, and 32–52% of the cats infected with different mycobacteria species progressed to develop either pulmonary and/or systemic involvement. As above, this unhelpful finding probably resulted from lack of data.
Clearly, there are a number of caveats to this study. Most importantly, this is a retrospective study and it relied on reviewing case records for detail on treatment and prognosis, not all of which were complete. In addition, the accompanying paper 32 has already presented the large number of steps that have to be taken before a sample is submitted to the VLA for testing. Prospective studies are needed to investigate the different treatment options in feline mycobacterial infections, to determine if different species of mycobacteria respond differently to particular treatments, and to find out whether systemic infections carry a poorer prognosis. Nevertheless, this study represents the largest study of its type and generates some fascinating results.
In conclusion, this large study looking at 339 cases of feline mycobacterial infection was able to report on histopathological changes, response to treatment and overall prognosis. It clearly demonstrated that diagnosis of mycobacteriosis in cats is difficult. Histopathology is an important step to reach the initial diagnosis of mycobacterial infection; however, it should not be considered diagnostic for any particular species of Mycobacterium. Cases may be seen that have histopathological changes consistent with mycobacterial infection and are positive for AFB, but no mycobacteria will grow; while in other cases mycobacteria can be grown, despite the tissue being negative for AFB. Although many cases may respond favourably to treatment, short courses of single drugs are commonly associated with relapse and systemic spread. This study shows that the diagnosis and treatment of feline mycobacteriosis is complex and challenging.
Acknowledgements
The authors wish to thank all of the veterinary surgeons, nurses and reception staff who helped in this study. We also thank Robert Higgins for providing constructive criticism as well as Martin Wilson and the rest of the team at VSD Lasswade for preparing the histology. The VLA cultures and histopathology were funded by DEFRA under the project SB4510.
References
- 1.Glaziou P, Floyd K, Raviglione M. Global burden and epidemiology of tuberculosis. Clin Chest Med 2009; 30: 621–36. [DOI] [PubMed] [Google Scholar]
- 2.Lobue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis 2010; 14: 1075–8. [PubMed] [Google Scholar]
- 3.Shiloh MU, DiGiuseppe Champion PA. To catch a killer. What can mycobacterial models teach us about Mycobacterium tuberculosis pathogenesis? Curr Opin Microbiol 2010; 13: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn-Moore DA. Mycobacterial infections in cats and dogs. In: Ettinger S, Feldman E, eds. Textbook of veterinary internal medicine. 7th edn. Philadelphia: WB Saunders, 2010: 875–81. [Google Scholar]
- 5.Snider WR. Tuberculosis in canine and feline populations. Review of the literature. Am Rev Respir Dis 1971; 104: 877–87. [DOI] [PubMed] [Google Scholar]
- 6.Ginn PE, Mansell J, Rakich PM. Mycobacterial infections. In: Maxie G, ed. Jubb, Kennedy and Palmer's pathology of domestic animals. 5th edn. Edinburgh: Saunders Elsevier, 2007: 687–91. [Google Scholar]
- 7.Kaneene JB, Bruning-Fann CS, Dunn J, et al. Epidemiologic investigation of Mycobacterium bovis in a population of cats. Am J Vet Res 2002; 63: 1507–11. [DOI] [PubMed] [Google Scholar]
- 8.Kipar A, Schiller I, Baumgärtner W. Immunopathological studies on feline cutaneous and (muco)cutaneous mycobacteriosis. Vet Immunol Immunopathol 2003; 91: 169–82. [DOI] [PubMed] [Google Scholar]
- 9.Greene CE, Gunn-Moore DA. Infections with slow-growing mycobacteria. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis, Missouri: Saunders, Elsevier, 2006: 462–77. [Google Scholar]
- 10.Smith NH, Crawshaw T, Parry J, Birtles RJ. Mycobacterium microti: more diverse than previously thought. J Clin Microbiol 2009; 47: 2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawthorne VM, Lauder IM. Tuberculosis in man, dog and cat. Am Rev Respir Dis 1962; 85: 858–69. [DOI] [PubMed] [Google Scholar]
- 12.Snider WR, Cohen D, Reif JS, Stein SC, Prier JE. Tuberculosis in canine and feline populations. Study of high risk populations in Pennsylvania, 1966–1968. Am Rev Respir Dis 1971; 104: 866–76. [DOI] [PubMed] [Google Scholar]
- 13.Fenton KA, Fitzgerald SD, Kaneene JB, Kruger JM, Greenwald R, Lyashchenko KP. Comparison of three immunodiagnostic assays for antemortem detection of Mycobacterium bovis stimulation in domestic cats. J Vet Diagn Invest 2010; 22: 724–9. [DOI] [PubMed] [Google Scholar]
- 14.Rhodes SG, Gruffydd-Jones T, Gunn-Moore D, Jahans K. Interferon-gamma test for feline tuberculosis. Vet Rec 2008; 162: 453–5. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes SG, Gruffydd-Jones T, Gunn-Moore D, Jahans K. Adaptation of IFN-gamma ELISA and ELISPOT tests for feline tuberculosis. Vet Immunol Immunopathol 2008; 124: 379–84. [DOI] [PubMed] [Google Scholar]
- 16.Aranaz A, Liébana E, Pickering X, Novoa C, Mateos A, Domínguez L. Use of polymerase chain reaction in the diagnosis of tuberculosis in cats and dogs. Vet Rec 1996; 138: 276–80. [DOI] [PubMed] [Google Scholar]
- 17.Brodin P, Eiglmeier K, Marmiesse M, et al. Bacterial artificial chromosome-based comparative genomic analysis identifies Mycobacterium microti as a natural ESAT-6 deletion mutant. Infect Immun 2002; 70: 5568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik R, Hughes MS, Martin P, Wigney D. Feline leprosy syndromes. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis, Missouri: Saunders, Elsevier, 2006: 477–80. [Google Scholar]
- 19.Malik R, Martin P, Wigney D, Foster S. Infections caused by rapidly growing mycobacteria. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd edn. St Louis, Missouri: Saunders, Elsevier, 2006: 482–8. [Google Scholar]
- 20.Mishra A, Singhal A, Chauhan DS, et al. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: correlation with conventional techniques. J Clin Microbiol 2005; 43: 5670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh DW. Feline leprosy: a review of forty-four cases from Western Canada. Can Vet J 1982; 23: 291–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn-Moore DA, Jenkins PA, Lucke VM. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant. Vet Rec 1996; 138: 53–8. [DOI] [PubMed] [Google Scholar]
- 23.Malik R, Hughes MS, James G, et al. Feline leprosy: two different clinical syndromes. J Feline Med Surg 2002; 4: 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Infectious nodular and diffuse granulomatous and pyogranulomatous diseases of the dermis. Skin disease of dog and cat: clinical and histopathological diagnosis. 2nd edn. St Louis, Missouri: Mosby Year Book, 2005: 272–340. [Google Scholar]
- 25.Baral RM, Metcalfe SS, Krockenberger MB, et al. Disseminated Mycobacterium avium infection in young cats: over-representation of Abyssinian cats. J Feline Med Surg 2006; 8: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horne KS, Kunkle GA. Clinical outcome of cutaneous rapidly growing mycobacterial infections in cats in the south-eastern United States: a review of 10 cases (1996–2006). J Feline Med Surg 2009; 11: 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 1996; 9: 177–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DEFRA. For DEFRA guidance notes on tuberculosis in cats go to: CatsTBbriefing (VIPER23 App Y5)_March 08 update.doc.
- 29.Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. Public health service task force on prophylaxis and therapy for Mycobacterium avium complex. N Engl J Med 1993; 329: 898–904. [DOI] [PubMed] [Google Scholar]
- 30.Jahans K, Worth D. The laboratory diagnosis of bovine tuberculosis. Gov Vet J 2006; 16: 53–7. [Google Scholar]
- 31.Twomey DF, Crawshaw TR, Anscombe JE, et al. Assessment of antemortem tests used in the control of an outbreak of tuberculosis in llamas (Lama glama). Vet Rec 2010; 167: 475–80. [DOI] [PubMed] [Google Scholar]
- 32.Gunn-Moore DA, McFarland S, Brewer J, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J Feline Med Surg 2011; 13: 934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollander M, Wolfe DA. Nonparametric statistical methods, 2nd edn. Wiley-Interscience Publications, 1999. [Google Scholar]
- 34.Rüfenacht S, Bögli-Stuber K, Bodmer T, Bornand Jaunin V, Gonin Jmaa DC, Gunn-Moore DA. Feline Mycobacterium microti infection: a case report and literature review. J Feline Med Surg 2011; 13: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunn-Moore DA, Dean R, Shaw S. Mycobacterial infections in cats and dogs. In Pract 2010; 32: 444–52. [Google Scholar]