Abstract
Lactococcus lactis is a nonpathogenic and noncolonizing bacterium which is being developed as a vaccine delivery vehicle for immunization by mucosal routes. To determine whether lactococci can also deliver cytokines to the immune system, we have constructed novel constitutive expression strains of L. lactis which accumulate a test antigen, tetanus toxin fragment C (TTFC), within the cytoplasmic compartment and also secrete either murine interleukin-2 (IL-2) or IL-6. When mice were immunized intranasally with various different expression strains of L. lactis, the anti-TTFC antibody titers increased more rapidly and were substantially higher in mice immunized with the bacterial strains which secreted IL-2 or IL-6 in addition to their production of TTFC. This adjuvant effect was lost when the recombinant strains of L. lactis were killed by pretreatment with mitomycin C and could therefore be attributed to the secretion of IL-2 or IL-6 by the recombinant lactococci. These results provide the first example of the use of a cytokine-secreting, noninvasive experimental bacterial vaccine vector to enhance immune responses to a coexpressed heterologous antigen and point the way to experiments which will test the possible therapeutic efficacy of this mode of cytokine delivery.
The number of communicable diseases which might feasibly be controlled by vaccination or treated by immunotherapy is increasing rapidly, alongside advances in our understanding of cellular and molecular biology as applied to the study of infectious agents. However, virtually all of the numerous recombinant antigen delivery systems developed to date have been derived from attenuated pathogenic infectious agents, e.g., rationally attenuated Salmonella spp. (23, 38) or traditionally attenuated Mycobacterium bovis (14).
By contrast, the use of Lactococcus lactis as a vaccine vector is emerging as one of the most advanced prototypes of a possible new class of bacterial vaccines derived from noninvasive, nonpathogenic gram-positive bacteria (45). L. lactis is a gram-positive bacterium which is classified as “generally regarded as safe” following its long history of use for the production of fermented milk products. As a gram-positive nonpathogen, its closest functional relative is Streptococcus gordonii, with which it shares the capacity to serve as an antigen delivery vehicle for mucosal immunization (22). Yet unlike S. gordonii, which is an oral commensal bacterium, L. lactis lacks any known capacity to multiply in vivo, except in gnotobiotic mice (15). Studies on the feeding of live lactococci to animals and to human volunteers have shown that the passage of these bacteria through the enteric tract is transitory, without any evidence of colonization (15, 18).
The development of constitutive and inducible gene expression systems for L. lactis has recently made it possible to undertake systematic investigations of the immunological activity of experimental recombinant lactococcal vaccines (46). We have been able to show that despite its lack of invasiveness, L. lactis is able to deliver heterologous antigens to the systemic and mucosal immune systems via mucosal routes (46). A number of antigens of protozoal, bacterial, and viral origin have been efficiently expressed by us in L. lactis, but most of our work to date has focused on studies of immune responses to tetanus toxin fragment C (TTFC) and to the partially protective 28-kDa (glutathione S-transferase [SmGST]) immunogen from Schistosoma mansoni (5) used as test immunogens.
Intranasal and oral immunization of mice with recombinant L. lactis expressing TTFC or SmGST elicits significant serum antibody responses against these antigens. In the case of TTFC, these responses proved to be protective against lethal challenge with 5 to 20 50% lethal doses of tetanus toxin (25, 32). Additionally, oral inoculation of lactococci expressing TTFC significantly but transiently elevated the levels of anti-TTFC immunoglobulin A (IgA) antibodies detected in the gut secretions (32).
In the light of our previous results, the present study was carried out to determine whether lactococci can deliver biologically active molecules such as cytokines as well as heterologous antigens to the immune system. Cytokines produced by subpopulations of T cells critically influence the balance between humoral and cell-mediated types of immune responses and are potentially useful as immune response modulators for vaccines and immunotherapeutic agents (40). Recombinant strains of M. bovis BCG secreting functional mammalian cytokines have been shown to be more potent stimulators of cell-mediated immune responses than their nonrecombinant counterparts in mouse models of experimental infection (24). By contrast, antibody responses to whole bacterial cells, outer membrane proteins, or lipopolysaccharide antigens of attenuated Salmonella typhimurium were not augmented when these strains were engineered to express interleukin-6 (IL-6), IL-1, or IL-4 intracellularly (3, 7, 11). The influence of these cytokines on responses to heterologous antigens expressed by these bacteria has not subsequently been investigated. In viral vector systems, the coexpression of IL-6 has been shown to augment both systemic and mucosal antibody responses to the viral antigens (21, 30). In this study, murine IL-2 and IL-6 were chosen for expression in L. lactis, as they have been shown to enhance antibody titers to either vaccine antigens or inactivated viral vaccines when parenterally administered either exogenously or in liposomal formulations (10, 16, 20, 35). Studies both in vitro and in vivo have suggested a potential application for IL-6 in augmenting IgA antibody responses in mucosal B cells or at mucosal surfaces (26, 27, 30, 33). Additionally, it has been shown that serum levels of IL-6 correlate with the serum concentration of IgA in patients with alcoholic liver cirrhosis and children infected with human immunodeficiency virus (8, 31), implicating its role in augmenting IgA synthesis in the serum. Inclusion of IL-2 in liposomes containing bacterial polysaccharide has also been shown to enhance antibody titers of polysaccharide-specific secretory IgA and to increase the numbers of polysaccharide-specific pulmonary plasma cells more than 80-fold following intranasal immunization of mice (1).
In this report, we present results which indicate that it is possible to prepare genetic constructs in L. lactis which confer on this organism the capacity to deliver physiologically active quantities of murine IL-2 and IL-6 in vivo.
MATERIALS AND METHODS
Recombinant DNA techniques.
PCR amplification of DNA was performed with Vent polymerase and using conditions recommended by the manufacturer. DNA-modifying enzymes and restriction endonucleases were used under standard conditions and in the buffers recommended by the manufacturers. General molecular cloning techniques and the electrophoresis of DNA and proteins were carried out essentially as described previously (34). L. lactis was transformed by electroporation of cells grown in the presence of glycine (47), and Escherichia coli was transformed by the electroporation method of Dower et al. (9).
Fractionation of lactococci and immunoblotting.
Total-cell protein extracts of L. lactis cells were prepared by the method of Wells et al. (48). To recover proteins from the cell wall of lactococci, the cell wall was enzymatically digested with mutanolysin and lysozyme in the presence of an osmotically stabilizing buffer. Bacteria (approximately 2.5 × 109 CFU) were pelleted by centrifugation, washed three times in Tris-buffered saline (0.15 M NaCl, 0.02 M Tris-HCl [pH 7.5]), resuspended in 200 μl of 20 mM Tris-HCl (pH 7.5)–10% (wt/vol) sucrose containing mutanolysin (100 U/ml) and lysozyme (5 mg/ml), and incubated for 1 h at 37°C. After treatment with mutanolysin and lysozyme, the cells were separated from enzymatically released cell wall material by centrifugation. Enzymatically digested cell wall fractions and cell pellets were boiled for 3 min in Laemmli sample buffer. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose. The transfer of protein was checked by reversibly staining the filter with Ponceau S, after which TTFC and or murine cytokines were detected by immunoblotting as previously described (36, 48).
Preparation of bacterial cells for immunization.
L. lactis strains carrying pTREX1, pT1TT, pT1TT-IL2, and pT1TT-IL6 were cultured at 30°C in M17 broth (Difco Ltd.) supplemented with 0.5% (wt/vol) glucose and 5 μg of erythromycin per ml to mid-logarithmic growth phase. The cells were washed with sterile phosphate-buffered saline (PBS) before resuspension in a solution of 0.2 M sodium bicarbonate (Sigma, Poole, Dorset, United Kingdom), 5% casein hydrolysate [GIBCO Ltd., Paisley, United Kingdom), and 0.5% (wt/vol) glucose (Sigma) at 5 × 1010 CFU/ml.
Mitomycin C pretreatment of bacteria.
Cells from a fresh overnight culture at 2 × 108 CFU/ml were treated with 50 μg of mitomycin C (Sigma) per ml for 2 h at 30°C. Aliquots of 1 ml were taken after incubation, and the concentration of cells was determined by nephelometry. The cells were washed three times in sterile PBS before spreading over GM17 agar plates containing the relevant selective antibiotics in order to calculate the efficiency of killing. In each case, fewer than 1 in 104 cells remained viable after treatment with mitomycin C. All cells used for immunization were washed three times in a large excess of PBS before resuspension at 5 × 1010 CFU/ml as described above.
Immunization protocol.
Groups of six specific-pathogen-free female C57BL/6 mice (Harlan UK Ltd. Bicester, Oxon, United Kingdom), aged 6 to 8 weeks at day 0, were immunized intranasally with recombinant L. lactis. Intranasal doses of 109 cells in 20 μl were applied to lightly anesthetized animals, using a micropipette. Serum samples were taken at intervals of 14 days and were stored at −20°C until required.
ELISA for detection of TTFC-specific serum antibody.
Using a method based on that of Wells et al. (48), enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with recombinant purified TTFC (50 ng/well; Boehringer Mannheim, East Sussex, United Kingdom) in carbonate-bicarbonate buffer (pH 9.6). Wells were blocked for 1 h at room temperature, using 3% bovine serum albumin (BSA) (Sigma). Primary antisera were tested in duplicate wells, using a twofold dilution series, including replicate wells of a 1/50-diluted preimmune serum on every plate. After 90 min, secondary anti-mouse immunoglobulin-alkaline phosphatase conjugates (Southern Biotechnology Associates, Inc., Birmingham, Ala.) were applied before development, using n-nitrophenyl phosphate (Sigma) as the substrate. Dilution curves were drawn for each sample, and the endpoint titer was calculated as the dilution producing the same optical density as the 1/50 dilution of a pooled preimmune serum. Statistical comparisons between groups were made by the Mann-Whitney U test. A P value of >0.05 was considered nonsignificant.
ELISA for detection of antilactococcal serum antibody.
An extract of soluble lactococcal protein was prepared from the L. lactis control strain carrying pTREX1 as previously described (48). The protein concentration of the cell extract was determined by using a Bradford assay (Bio-Rad Laboratories, Hertfordshire, United Kingdom). ELISA plates were coated overnight with protein extract in carbonate-bicarbonate buffer (pH 9.6) (50 ng/well) and then blocked for 1 h at room temperature with 3% BSA (Sigma). Dilutions of the primary antisera were tested in duplicate for antilactococcal antibody as described above for the TTFC ELISA.
Assay of IgA in fecal material.
Fresh fecal pellets were collected from each group of mice and frozen at −20°C until the end of the experiment. Soluble fecal extracts were prepared in 2-ml microcentrifuge tubes by adding 1 ml of PBS containing 1% BSA and 1 mM freshly added phenylmethylsulfonyl fluoride per 0.1 mg of fecal material. The tubes were incubated overnight at 4°C to soften the pellets and then vigorously mixed by vortexing to disrupt and suspend all solid matter. The samples were then centrifuged at full speed in a microcentrifuge for 5 min to pellet the insoluble material. The supernatants were removed and assayed for total IgA concentration, using a commercially available radial immunodiffusion test kit (The Binding Site, Birmingham, United Kingdom) according to the manufacturer’s instructions.
RESULTS
Coexpression of TTFC and murine IL-2 or IL-6 in L. lactis.
To coexpress the test antigen (TTFC) together with a cytokine, artificial operons were constructed within the lactococcal expression plasmid pTREX1 (Fig. 1) (46). This was achieved by modifying a strain which expresses and accumulates the model immunogen TTFC intracellularly so that it could additionally coexpress and secrete murine IL-2 or IL-6.
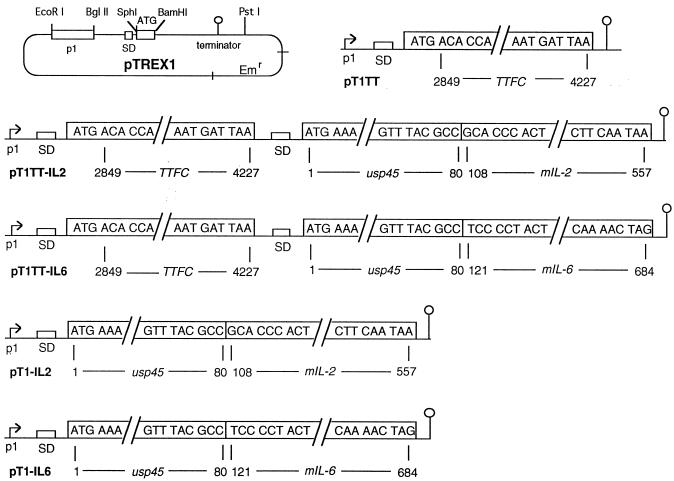
FIG. 1.
Schematic representation of pTREX1 (46) and the expression plasmids constructed for use in L. lactis. The location of unique restriction endonuclease sites, promoter (p1), Shine-Dalgarno motif (SD), translation initiation start codon (ATG), and transcription terminator ( ) present in the expression cassette of pTREX1 are indicated. The nucleotides encoding TTFC are numbered as previously described (12). In plasmids pT1TT-IL2, pT1TT-IL6, pT1-IL2, and pT1-IL6, the cDNA fragments encoding mature forms of murine IL-2 and IL-6 (nucleotide numbering as in references 6 and 17) were fused to the secretion leader of L. lactis usp45 (nucleotide numbering as in reference 42).
Briefly, the DNA fragment encoding TTFC was amplified by PCR and cloned between blunted SphI and BamHI sites in pTREX1 to generate plasmid pT1TT, which expressed TTFC intracellularly in L. lactis. To obtain secretion of the mammalian cytokines by L. lactis, the eukaryotic secretion signal sequences present in the cDNAs of IL-2 and IL-6 were replaced with the prokaryotic signal sequence of the lactococcal usp45 gene. Previous studies had shown that such fusions resulted in the secretion of fully active cytokines into L. lactis culture supernatants (36, 37). Initially the DNA fragments encoding the mature processed form of murine IL-2 or IL-6 were cloned into the lactococcal expression plasmid pLET2N (25). DNA fragments encoding the lactococcal usp45 secretion signal-cytokine fusion product and its upstream Shine-Dalgarno sequence were then cloned downstream of the TTFC gene in pT1TT to generate pT1TT-IL2 and pT1TT-IL6 (Fig. 1). For use as controls, we constructed two strains of L. lactis which expressed only IL-2 or IL-6. These were constructed by cloning PCR-amplified fragments encoding the L. lactis usp45 secretion signal-cytokine fusion products and their upstream translation initiation region (initially constructed in pLET-based plasmids) between the BamHI and BglII sites in pTREX1 (Fig. 1). The characteristics of the various different strains of recombinant L. lactis constructed are shown in Table 1.
TABLE 1.
Characteristics of recombinant strains of L. lactis
| Strain description | Plasmid |
|---|---|
| Nonexpressor control strain harboring expression plasmid | pTREX1 |
| Expresses and accumulates TTFC intracellularly | pT1TT |
| Secretes murine IL-2 and accumulates TTFC intracellularly | pT1TT-IL2 |
| Secretes murine IL-6 and accumulates TTFC intracellularly | pT1TT-IL6 |
| Secretes murine IL-2 | pT1-IL2 |
| Secretes murine IL-6 | pT1-IL6 |
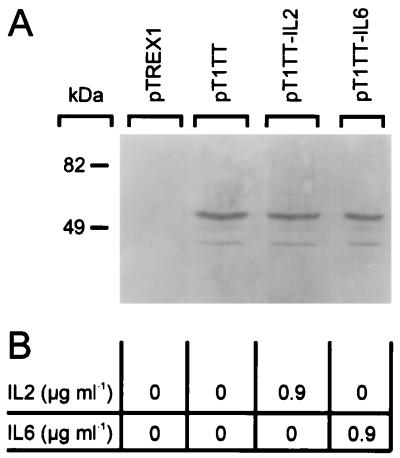
Immunoblotting of equal amounts of cell extract prepared from L. lactis strains carrying plasmid pT1TT, pT1TT-IL2, or pT1TT-IL6 revealed that all three strains produced similar amounts of TTFC (Fig. 2A). Cytokine bioassays carried out on culture supernatants harvested from exponentially growing cultures showed that biologically active IL-2 and IL-6 were secreted into the growth medium at levels of approximately 0.9 μg/ml by the L. lactis strains harboring pT1TT-IL2 and pT1TT-IL6, respectively (Fig. 2B). Immunoblotting with anti-IL-2 and anti-IL-6 antisera revealed that small quantities of IL-2 and IL-6 were present in total cell extracts prepared from the strains carrying plasmids pT1TT-IL2 and pT1TT-IL-6, respectively (not shown). The IL-2 and IL-6 detected in these total cell extracts were probably in their unprocessed forms (signal sequence plus cytokine) since the proteins detected were consistently found to be larger than purified IL-2 and IL-6, respectively. The mature processed forms of these cytokines were not detected in whole bacterial cell extracts or in enzymatically degraded cell wall fractions of protoplasted bacteria, indicating that cytokines secreted by recombinant strains of L. lactis were not being trapped in the cell wall.
FIG. 2.
(A) Immunoblotting of whole-cell extracts from approximately 5 × 108 CFU of L. lactis MG1363 transformed with plasmid pTREX1, pT1TT, pT1TT-IL2, or pT1TT-IL6 (as indicated) with rabbit polyclonal antiserum to TTFC. (B) Amount of biologically active IL-2 or IL-6 detected in the culture supernatant of the L. lactis strains based on the mean values of four separate titrations as described previously for IL-2 and IL-6 (13, 44).
Immunization of mice with live recombinant strains of L. lactis secreting IL-2 or IL-6 potentiated antibody responses to the coexpressed antigen TTFC.
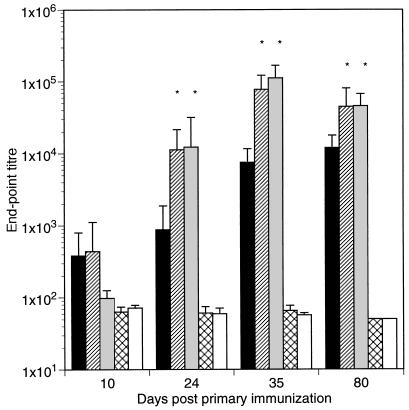
Groups of six C57BL/6 mice were immunized intranasally with three doses of 109 CFU of the various strains of recombinant lactococci on days 0, 14, and 28. The TTFC-specific serum antibody titers reached mean endpoint titers of 104 to 105 in all mice which had been immunized with lactococcal strains expressing TTFC compared to mean endpoint titers of approximately 50 for naive mice and mice inoculated with a nonexpressor control strain carrying only the expression vector pTREX1. Strikingly, the anti-TTFC antibody titers increased more rapidly and were substantially (10- to 15-fold by day 35) higher in the groups of mice immunized with the lactococcal strains coexpressing IL-2 (P = 0.001) or IL-6 (P = 0.001) than in the group immunized with the strain expressing only TTFC (Fig. 3). On day 80 following immunization, the mean TTFC antibody titers were still significantly higher in the groups of mice immunized with the recombinant L. lactis expressing IL-2 (P = 0.040) or IL-6 (P = 0.001) than in the group immunized with L. lactis expressing only TTFC.
FIG. 3.
Mean serum anti-TTFC IgG levels of groups of six mice intranasally inoculated on days 0, 14, and 28 with 109 recombinant L. lactis as follows: ▪, expressing TTFC (pT1TT); ▨, expressing TTFC and cosecreting IL-2 (pT1TT-IL2); , expressing TTFC and cosecreting IL-6 (pT1TT-IL6); ▧▨, control nonexpressor strain (pTREX1). Sera from a naive, nonvaccinated control group (□) were also assayed. The endpoint titer was calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum included in six replicate wells on each plate. ∗, the endpoint titer was significantly higher than those of the group inoculated with the pT1TT strain (P > 0.05).
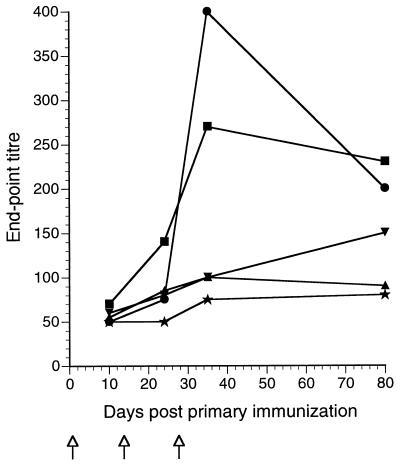
Both IgG1 and IgG2a subclasses of anti-TTFC antibody were found in the sera of mice immunized with TTFC-expressing lactococci. The relative proportions of these antibody subclass-specific responses were not significantly different in mice inoculated with the cytokine-secreting lactococci. As in previous studies (32), the serum antibody responses directed against antigens of L. lactis were low (maximum endpoint titers between 50 and 500) in comparison with the anti-TTFC responses (Fig. 4). Interestingly, antibody responses directed against antigenic components of L. lactis were not detectably raised by coexpression of the cytokines. The substantial potentiation of the anti-TTFC response in mice inoculated with the IL-2- and IL-6-secreting lactococci appeared to be restricted to the expressed heterologous protein.
FIG. 4.
Antilactococcal serum immunoglobulin responses of groups of six mice intranasally inoculated on days 0, 14, and 28 ( ) with 109 recombinant L. lactis as follows: •, expressing TTFC (pT1TT); ▴, expressing TTFC and cosecreting IL-2 (pT1TT-IL2); ▾, expressing TTFC and cosecreting IL-6 (pT1TT-IL6); ▪, control nonexpressor strain (pTREX1). Sera from a naive, nonvaccinated group (★) were also assayed. The endpoint titer was calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum included in six replicate wells on each plate.
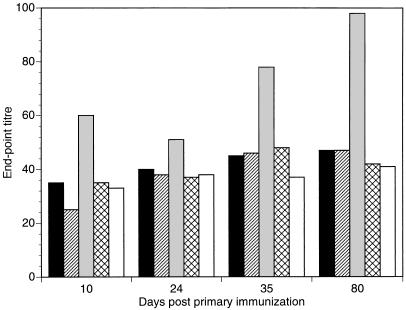
Influence of cytokine coexpression by recombinant L. lactis on IgA responses.
To determine whether immunization with recombinant lactococci coexpressing IL-6 or IL-2 would specifically enhance IgA responses, we assayed for TTFC-specific IgA in the pooled serum from groups of animals immunized intranasally with the different strains of L. lactis (Table 1). Mice immunized with lactococci coexpressing IL-6 had serum IgA antibodies to TTFC substantially higher than those of the naive nonimmunized group of animals (Fig. 5). To determine whether the cytokines secreted by recombinant L. lactis would also influence the production of IgA in mucosal tissues, the feces collected from groups of mice immunized intranasally with different doses of recombinant L. lactis and a naive control group of mice were assayed for total IgA content by using a commercially available radial immunodiffusion kit. At 35 days postimmunization (7 days after the final boost), the concentrations of IgA measured in the soluble extracts prepared from fecal material were similar for both immunized and naive groups of mice (results not shown). These results suggest that intranasal immunization with IL-6- or IL-2-secreting L. lactis had no measurable effect on total IgA production in the gastrointestinal tract.
FIG. 5.
Anti-TTFC IgA levels in pooled sera from groups of six mice intranasally inoculated on days 0, 14, and 28 with 109 recombinant L. lactis as follows: ▪, expressing TTFC (pT1TT); ▨, expressing TTFC and cosecreting IL-2 (pT1TT-IL2); , expressing TTFC and cosecreting IL-6 (pT1TT-IL6); ▧▨, control nonexpressor strain (pTREX1). Sera from a naive, nonvaccinated control group (□) were also assayed. The endpoint titer in the ELISA was calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum included in six replicate wells on each plate.
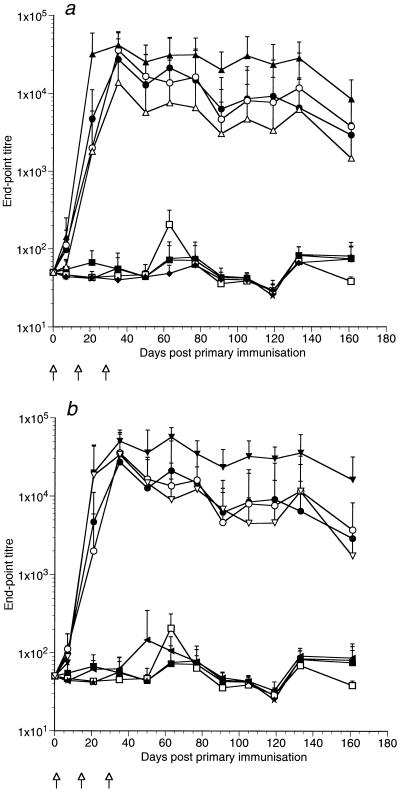
Influence of lactococcal cell viability on immune responses elicited by recombinant L. lactis.
Groups of six mice were immunized with live or mitomycin C-killed strains of L. lactis to determine whether effective cytokine delivery, as determined by the adjuvant effect of the anti-TTFC antibody response, was dependent on the production of active cytokine in vivo (rather than being due to the possible release of preformed, bacterially associated cytokine in the animal). All mice immunized with the TTFC-expressing strains of L. lactis elicited serum antibody responses to TTFC with peak endpoint titers of 2 × 103 to 3 × 104, compared to mean endpoint titers of approximately 50 for naive mice and control mice inoculated with a nonexpressor control strain (Fig. 6). The mean antibody titers elicited by live and killed L. lactis expressing only TTFC were not significantly different on any of the days on which serum was sampled (Fig. 6), showing (as have our previous results [25, 32]) that antigen expression in vivo is not required to elicit an immune response to TTFC expressed intracellularly in lactococci. However, when the cytokine-secreting strains of L. lactis were treated with mitomycin C, the titers of anti-TTFC antibodies were not significantly different from those elicited by bacteria expressing TTFC alone, indicating that cytokine-secreting strains of lactococci need to be viable in vivo for effective cytokine delivery to occur (Fig. 6).
FIG. 6.
Influence of lactococcal cell viability on the immune responses elicited by IL-2-secreting (a) and IL-6-secreting (b) L. lactis. Shown are mean anti-TTFC serum antibody responses of groups of six mice following intranasal administration of 109 live (closed symbols) or mitomycin C-killed (open symbols) recombinant L. lactis strains on days 0, 14, and 28 ( ) as follows: •, expressing TTFC (pT1TT); ▴, expressing TTFC and cosecreting IL-2 (pT1TT-IL2); ▾, expressing TTFC and cosecreting IL-6 (pT1TT-IL6); ▪, control nonexpressor strain (pTREX1); ⧫, secreting IL-2 (pT1-IL2); ◂, secreting IL-6 (pT1-IL6). Sera from a naive, nonvaccinated group (★) were also assayed. The endpoint titer was calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum included in six replicate wells on each plate.
DISCUSSION
The results presented here indicate that it is possible to construct physiologically active antigen- and cytokine-expressing recombinant strains of L. lactis and that these strains appear to constitute a simple and effective means of delivering murine IL-2 and murine IL-6 across mucosal surfaces to the immune system.
This study was made possible by constructing an artificial bicistronic operon within the constitutive expression plasmid pTREX1 to yield strains of L. lactis which secrete IL-2 or IL-6 and also coexpress TTFC and accumulate this protein intracellularly. These bacteria secreted biologically active IL-2 or IL-6 and elicited significantly (10- to 15-fold) higher TTFC antibody titers than did the strains expressing only TTFC (Fig. 3). As the amounts of TTFC produced by the different strains were similar, and since we have found (unpublished results) that the TTFC antibody titers elicited by recombinant strains of L. lactis expressing 10-fold-higher levels of TTFC antigen are not substantially different, the enhanced anti-TTFC immune responses must be directly attributable to the coexpression of IL-2 or IL-6.
The influence of cytokine coexpression by recombinant lactococci on IgA responses was investigated by measuring the serum levels of anti-TTFC IgA and total IgA recovered in the feces of mice immunized with the various strains of L. lactis. Elevated serum anti-TTFC IgA responses were seen only in mice immunized with recombinant lactococci secreting IL-6. These TTFC-specific IgA serum responses were elevated even after the primary dose but increased over time for at least 80 days postimmunization (Fig. 5). Although the differences between the IgA levels measured in the IL-6 group of animals and those of the naive and control groups were small (up to twofold), these differences are significant since we have never previously been able to measure serum IgA responses to TTFC following immunization with recombinant TTFC-expressing lactococci which differed from those of the naive or nonexpressor control groups. The amounts of total IgA measured in fecal material were not increased in the groups immunized with the cytokine-secreting strains of L. lactis. However, further experiments aimed at measuring IgA levels in mucosal secretions at various times after immunization with cytokine-secreting L. lactis by different routes will be needed before any firm conclusions can be drawn as to their possible effects on IgA production in mucosal tissues.
By comparison with the marked enhancement of the serum anti-TTFC IgG antibody titers seen here, it was striking that the antibody responses to lactococci were not elevated in mice inoculated with the strains coexpressing IL-2 or IL-6 (maximum ELISA titers of 400 in all groups of mice [Fig. 4]). We have previously observed that lactococci appear to have a low innate antigenicity. This property may be advantageous if the use of repeated administration of recombinant lactococci is required.
The mode of action of the cytokine-secreting strains on the immune system requires further investigation. Lactococci are sufficiently small (about 1 μm in diameter) to be taken up by microfold cells present in the follicular epithelium overlaying the mucosa-associated lymphoid tissue. We have shown in this report and previously that these bacteria resemble inert microparticles to the extent that effective presentation of TTFC by lactococci to the immune system does not require bacterial viability (25, 32). By contrast, the adjuvant effect on the immune responses to TTFC observed with the live cytokine-secreting strains was lost when the bacteria were treated with lethal doses of mitomycin C prior to immunization (Fig. 6). This result constitutes the most direct evidence that lactococci can deliver cytokines in vivo and that the adjuvant effect of cytokine coexpression and secretion by lactococci is due to a level of continued physiological activity by the lactococci in vivo. An alternative explanation would be that the adjuvant effects observed here were due to the release of preformed, bacterially associated cytokines by live but not by mitomycin-killed L. lactis. We cannot completely preclude this possibility but consider it unlikely, in view of the fact that we could not detect processed cytokines in the digested cell wall fractions of live protoplasted cytokine-expressing bacteria.
The finding that L. lactis can be used to deliver biologically active cytokines to the immune system in vivo is surprising because L. lactis is not a commensal bacterium and is not known to replicate in vivo. The fate of intranasally inoculated L. lactis is also not known, although it is likely that a proportion of any inoculum given intranasally may be inhaled and then enter the lungs or be swallowed and enter the gastrointestinal tract. Physiological studies of the murine nasal-associated mucosal immune system indicate that antigens and pathogens encountered at the nasal epithelium will be sampled by microfold cells and transported to the underlying lymphoid tissue, where they can induce an immune response (2, 19). Here the local production of IL-2 or IL-6 by L. lactis may stimulate early proliferation of mucosal T cells or augment B-cell growth and/or differentiation and immunoglobulin production (5, 28, 39, 41, 43). The results presented here suggest that it may be possible to tailor the type of immune response elicited to antigens delivered by lactococci through the expression of appropriate cytokines and in such way lead to an appropriate vaccination strategy against a particular pathogen. The striking fact that a pulse of cytokines can be delivered by recombinant lactococci in this way also raises interesting and testable questions concerning the possible therapeutic uses of such recombinant bacteria.
ACKNOWLEDGMENTS
We thank the Underwood Fund of the Biotechnology and Biological Sciences Research Council (BBSRC) for providing a travel and subsistence grant to L.S. This work was supported by grants from the BBSRC to J.M.W., K.M.S., and R.L.P., The Wellcome Trust (to K.R.), and the EU (to L.C.). J.M.W. also gratefully acknowledges the award of an advanced research fellowship from the BBSRC.
We thank J. Halpern for providing the gene encoding TTFC.
REFERENCES
- 1.Abraham E, Shah S. Intranasal immunization with liposomes containing IL-2 enhances bacterial polysaccharide antigen-specific pulmonary secretory antibody response. J Immunol. 1992;149:3719–3726. [PubMed] [Google Scholar]
- 2.Borghesi C, Regoli M, Bertelli E, Nicoletti C. Modifications of the follicle-associated epithelium by short-term exposure to a non-intestinal bacterium. J Pathol. 1996;180:326–332. doi: 10.1002/(SICI)1096-9896(199611)180:3<326::AID-PATH656>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Carrier M J, Chatfield S N, Dougan G, Nowicka U T, O’Callaghan D, Beesley J E, Milano S, Cillari E, Liew F Y. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148:1176–1181. [PubMed] [Google Scholar]
- 4.Ceuppens J L, Bloemmen F J, van Wauwe J P. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J Immunol. 1985;135:3882–3886. [PubMed] [Google Scholar]
- 5.Chamberlain L, Wells J M, Robinson K, Schofield K, Le Page R W F. Mucosal immunization with recombinant Lactococcus lactis. In: Pozzi G, Wells J M, editors. Gram-positive bacteria as vaccine vehicles for mucosal immunization. Austin, Tex: Landes Bioscience; 1997. pp. 83–106. [Google Scholar]
- 6.Chiu C-P, Moulds C, Coffman R L, Rennick D, Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci USA. 1988;86:7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denich K, Borlin P, O’Hanley P D, Howard M, Health A W. Expression of the murine interleukin-4 gene in an attenuated aroA strain of Salmonella typhimurium: persistence and immune response in BALB/c mice and susceptibility to macrophage killing. Infect Immun. 1993;61:4818–4827. doi: 10.1128/iai.61.11.4818-4827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devier J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokine production. Clin Exp Immunol. 1989;77:221–225. [PMC free article] [PubMed] [Google Scholar]
- 9.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coliby high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duits A J, van Puijenbroek A, Vermeulen H, Hofhuis F M, van de Winkel J G, Capel P J. Immunological activity of a liposomal IL-6 formulation. Vaccine. 1993;11:77–781. doi: 10.1016/0264-410x(93)90265-y. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan S J, Ramsay A J, Strugnell R A. Studies of immunity and bacterial invasiveness in mice given a recombinant salmonella vector encoding murine interleukin-6. Infect Immun. 1996;64:2730–2736. doi: 10.1128/iai.64.7.2730-2736.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisel U, Jarausch W, Goretzki K, Henschen A, Engels J, Weller U, Hudel M, Habermann E, Niemann H. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986;5:2495–2502. doi: 10.1002/j.1460-2075.1986.tb04527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis S, Fern M M, Ou W, Smith K A. T cell growth factor: parameters of production and quantitative microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 14.Grange J M, Gibson J, Osborn T W, Collins C H, Yates M D. What is BCG? Tubercle. 1983;64:129. doi: 10.1016/0041-3879(83)90038-7. [DOI] [PubMed] [Google Scholar]
- 15.Gruzza M, Fons M, Ouriet M F, Duval-Iflah Y, Ducluzeau R. Study of gene transfer in vitro and in the digestive tract of gnotobiotic mice from Lactococcus lactisstrains to various strains belonging to human intestinal flora. Microb Releases. 1994;2:183–189. [PubMed] [Google Scholar]
- 16.Heath A W, Playfair J H. Cytokines as immunological adjuvants. Vaccine. 1992;10:427–434. doi: 10.1016/0264-410x(92)90389-2. [DOI] [PubMed] [Google Scholar]
- 17.Kashima N, Nishi-Takaoka C, Fujita T, Taki S, Yamada G, Hamuro J, Taniguchi T. Unique structure of murine interleukin-2 as deduced from cloned cDNAs. Nature. 1985;313:402–404. doi: 10.1038/313402a0. [DOI] [PubMed] [Google Scholar]
- 18.Klijn N, Weerkamp A H, de Vos W M. Genetic marking of Lactococcus lactisshows its survival in the human gastrointestinal tract. Appl Environ Microbiol. 1995;61:2771–2774. doi: 10.1128/aem.61.7.2771-2774.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraehenbuhl J-P, Neutra M R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:53–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 20.Lachman L B, Ozpolat B, Rao X M. Cytokine-containing liposomes as vaccine adjuvants. Eur Cytokine Netw. 1996;7:693–698. [PubMed] [Google Scholar]
- 21.Leong K H, Ramsay A J, Boyle D B, Ramshaw I A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medaglini D, Pozzi G, King T P, Fischetti V A. Mucosal and systemic responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordoniiafter oral colonisation. Proc Natl Acad Sci USA. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller S I. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence. Mol Microbiol. 1991;5:2073–2078. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray P J, Aldovini A, Young R A. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norton P M, Wells J M, Brown H W G, Macpherson A M, Le Page R W F. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactisexpressing tetanus toxin fragment C. Vaccine. 1997;15:616–619. doi: 10.1016/s0264-410x(96)00241-1. [DOI] [PubMed] [Google Scholar]
- 26.Oka Y, Rolink A G, Suematsu S, Kishimoto T, Melchers F. An interleukin-6 transgene expressed in B lymphocyte lineage cells overcomes the T cell-dependent establishment of normal levels of switched immunoglobulin isotypes. Eur J Immunol. 1995;25:1332–1337. doi: 10.1002/eji.1830250530. [DOI] [PubMed] [Google Scholar]
- 27.Pockley A G, Montgomery P C. In vivoadjuvant effect of interleukins 5 and 6 on rat tear IgA antibody responses. Immunology. 1991;73:19–23. [PMC free article] [PubMed] [Google Scholar]
- 28.Poupart P, Vandenabeele P, Cayphas S, Van Snick J, Haegeman G, Kruys V, Fiers W, Content J. B cell growth modulating and differentiating activity of recombinant human 26-kDa protein (BSF-2, HuIFN-beta 2, HPGF) EMBO J. 1987;6:1219–1224. doi: 10.1002/j.1460-2075.1987.tb02357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafferty D E, Montgomery P C. The effects of transforming growth factor-beta and interleukins 2, 5 and 6 on immunoglobulin production in cultured rat salivary gland tissues. Oral Microbiol Immunol. 1995;10:81–86. doi: 10.1111/j.1399-302x.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay A J, Husband A J, Ramshaw I A, Bao S, Matthaei K I, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 31.Rautonen J, Rautonen N, Martin N L, Philip R, Wara D W. Serum interleukin-6 concentrations are elevated and associated with elevated tumour necrosis factor-alpha and immunoglobulin G and A concentrations in children with HIV infection. AIDS. 1991;5:1319–1325. doi: 10.1097/00002030-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Robinson K, Chamberlain L M, Schofield K M, Wells J M, Le Page R W F. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657. doi: 10.1038/nbt0797-653. [DOI] [PubMed] [Google Scholar]
- 33.Saito S, Maruyama M, Kato Y, Moriyama I, Ichijo M. Detection of IL-6 in human milk and its involvement in IgA production. J Reprod Immunol. 1991;20:267–276. doi: 10.1016/0165-0378(91)90051-q. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- 35.Schijns V E, Claassen I J, Vermeulen A A, Horzinek M C, Osterhaus A D. Modulation of antiviral immune responses by exogenous cytokines: effects of tumour necrosis factor-alpha, interleukin-1 alpha, interleukin-2 and interferon gamma on the immunogenicity of an inactivated rabies vaccine. J Gen Virol. 1994;75:55–63. doi: 10.1099/0022-1317-75-1-55. [DOI] [PubMed] [Google Scholar]
- 36.Steidler J, Fiers W, Remaut E. Expression of human and murine interleukins in Lactococcus lactis. NATO ASI Ser H. 1996;98:63–79. [Google Scholar]
- 37.Steidler L, Wells J M, Raeymaekers A, Vandekerckhove J, Fiers W, Remaut E. Secretion of biologically active murine interleukin-2 by Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1995;61:1627–1629. doi: 10.1128/aem.61.4.1627-1629.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroDand immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taga T, Kawanishi Y, Hardy R R, Hirano T, Kishimoto T. Receptors for B cell stimulatory factor 2. Quantitative, specificity, distribution, and regulation of their expression. J Exp Med. 1987;166:967–981. doi: 10.1084/jem.166.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor C E. Cytokines as adjuvants for vaccines: antigen-specific responses differ from polyclonal responses. Infect Immun. 1995;63:3241–3244. doi: 10.1128/iai.63.9.3241-3244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theze J, Alzari P M, Bertoglio J. Interleukin-2 and its receptors—recent advances and new immunological functions. Immunol Today. 1996;17:481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- 42.Van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactisMG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 43.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 44.Van Snick J, Cayphas S, Vink A, Coulieand P G, Simpson R G. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA. 1986;83:9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells J M, Robinson K, Chamberlain L M, Schofield K M, Le Page R W F. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek. 1996;70:317–330. doi: 10.1007/BF00395939. [DOI] [PubMed] [Google Scholar]
- 46.Wells J M, Schofield K M. Cloning and expression vectors for lactococci. NATO ASI Ser H. 1996;98:37–63. [Google Scholar]
- 47.Wells J M, Wilson P W, Le Page R W F. Improved cloning vectors and transformation procedure for Lactococcus lactis. J Appl Bacteriol. 1993;74:629–636. doi: 10.1111/j.1365-2672.1993.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 48.Wells J M, Wilson P W, Norton P M, Gasson M J, Le Page R W F. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–1162. doi: 10.1111/j.1365-2958.1993.tb01660.x. [DOI] [PubMed] [Google Scholar]