Abstract
Purpose:
To discuss the features of an artifact on optical coherence tomography angiography (OCTA), termed “pupil vignetting artifact,” and describe how it may masquerade as true chorioretinal pathology.
Design:
This was a retrospective, observational case series.
Methods:
The authors studied 12 eyes at a vitreoretinal clinic in Eastern India, reviewing a dark shadow such as an artifact on OCTA images.
Results:
In all 12 eyes, there was an appearance of a dark shadow on OCTA imaging, located at the macula, superior, superotemporal, or superonasal to the fovea, which did not correspond to any ischemic area responsible for flow-void or any media opacity casting a posterior shadow. It was believed to be an artifact caused by the vignetting effect of the pupil as the incident OCT beam clips the iris during OCTA scanning, and therefore reduces the amount of total light incident on the retina. The variability in the size, shape, and location of the artifact is contributed by a few factors such as variable angle of incident light on the pupil, pupillary dynamics, and curvature of the retinal surface.
Conclusion:
Pupil vignetting artifact is a unique undescribed phenomenon appearing at the macula on OCTA imaging that can masquerade as numerous true chorioretinal pathologies. This article aims to describe this artifact to avoid misinterpretation and further confusion in real-life clinical practice.
Keywords: Flow-void, low OCT-signal artifact, optical coherence tomography, optical coherence tomography angiography, vignetting artifact
Optical coherence tomography angiography (OCTA) is a non-invasive, high-resolution imaging modality to visualize ocular blood vessels by detecting motion contrast from the flowing red blood cells in the bloodstream. It provides visualization of various layers of retinochoroidal vasculature including the superficial and deep retinal plexus, choriocapillaries, and choroidal vessels while allowing both qualitative and quantitative measurements.[1,2,3,4] OCTA has been considered to be valuable in evaluating vascular abnormalities in a variety of diseases, such as choriocapillary drop-out in retinal degeneration, retinal capillary non-perfusion in diabetic retinopathy (DR), retinal vascular occlusion (RVO), macular telangiectasia, and choroidal neovascularisation (CNV) in age-related macular degeneration.[5,6,7,8]
However, OCTA remains riddled with numerous artifacts that can affect both qualitative and quantitative outputs, and thereby make image interpretation even more challenging, despite various software iterations trying to control them constantly.[9,10,11] There is a multitude of OCTA artifacts mentioned in the literature, which can result from decentration, segmentation error, eye movements, defocus, blink, refractive-shift, z-offset, tilt, fringe wash-out, projection, and low-OCT signal. It is of utmost importance to distinguish those artifacts from true chorioretinal pathology, although it is a difficult job because these artifacts frequently mimic certain pathological conditions quite closely.[12,13]
In this article, we aim to highlight a shadow-like low-OCT signal artifact that can masquerade as true chorioretinal pathology. The low-OCT signal artifact we report here looks similar to a flow void that corresponds to a site of retinal ischemia and also to the posterior shadow cast by a media opacity. Recognition and minimizing or controlling such artifacts is crucial to avoid clinical misinterpretation.
Methods
This was a retrospective, observational case series of a total of 12 eyes of eight consecutive patients attending the vitreoretinal clinic of a tertiary eye-care center in Eastern India. The study was approved by the institutional review board. Data were extracted from an electronic medical records database. OCTA images were obtained using Spectralis–Heidelberg Retinal Tomograph (Heidelberg Engineering, Germany). A shadow-like dark area not corresponding to either any area of retinal ischemia or any media opacity was photographed in the OCTA images from superficial and deep retinal plexus, and avascular complex as well. It closely resembled flow voids caused by retinal ischemia, and dark shadows corresponding to media opacity. This dark spot was termed a “pupil vignetting artifact.” Age, lens status, location of the artifact with respect to the fovea (LOC), best-corrected visual acuity (BCVA), and the diagnosis suggested by OCT characteristics were the other parameters recorded for each patient.
Results
In all the 12 eyes that were included, there was the appearance of a dark shadow-like artifact on all three OCTA images depicting the superficial and deep capillary plexus and the avascular complex. The shadow, located at the macula, did not correspond to any apparent area of retinal ischemia or media opacity on multicolor and infrared reflectance imaging but did correspond to a hyporeflective zone spanning the whole inner and outer retina, and the underlying choroid on spectral domain-optical coherence tomography (SD-OCT) imaging in all those eyes. The artifacts showed large inter-individual variability in size and shape between different eyes. Its appearance ranged from a small, well-circumscribed, circular, or oval dark shadow spot to a larger, more diffuse dark area with indistinct borders. Out of the total 12 eyes, the artifact was located superior, superotemporal, and superonasal to the fovea in 6 (50%), 5 (41.66%), and 1 (8.33%) eyes, respectively.
Table 1 enumerates the clinical and imaging characteristics of the 12 studied eyes. The mean age of the eight patients with the aforesaid artifact was 50.5 years (37–64 years), 50% were male and the rest 50% were female. Out of 12, 5 (41.66%) were observed in the right, and 7 (58.4%) were observed in the left eye. The majority of the eyes (9 out of 12) demonstrating the artifact were phakic (75%), and in the rest (3 out of 12), the presence of posterior-chamber intraocular lens (PCIOL) (25%) was noted. Among them, 6 (50%), 2 (16.66%), and 4 (33.33%) belonged to the groups having BCVA of 20/16–20/32 (Snellen visual acuity chart), <20/32–20/100, and <20/100–20/200, respectively. Figs. 1–4 illustrate the size, shape, location, and other imaging characteristics of the artifacts in multicolor, OCT, and OCTA images of four eyes having no pathology at all.
Table 1.
Clinical and imaging characteristics of study eyes
| L | A | G | LS | LOC | BCVA | OCT |
|---|---|---|---|---|---|---|
| OD | 64 | F | PS | S | 20/126 | Foveal thinning |
| OS | 64 | F | PS | ST | 20/160 | Foveal thinning |
| OD | 45 | F | P | SN | 20/20 | Compact Fovea |
| OS | 45 | F | P | ST | 20/32 | Compact Fovea |
| OS | 67 | F | PS | ST | 20/126 | Scarred CNVM |
| OD | 48 | F | P | S | 20/32 | Intraretinal fluid |
| OS | 52 | M | P | ST | 20/20 | CSCR |
| OS | 41 | M | P | ST | 20/20 | Intraretinal fluid |
| OD | 37 | M | P | S | 20/16 | Compact fovea |
| OS | 37 | M | P | S | 20/16 | Compact fovea |
| OD | 53 | M | P | S | 20/200 | Intraretinal fluid |
| OS | 53 | M | P | S | 20/20 | Compact fovea |
L- Laterality; A- Age in years; G- Gender; F- Female; M- Male; LS- Lens status; PS- Pseudophakic; P- Phakic; LOC- Location of the artifact with respect to fovea; S- Superior; ST- Superotemporal; SN- Superonasal; BCVA- Best-corrected visual acuity; OCT- Optical coherence Tomography; CNVM- Choroidal neovascular membrane; CSCR- Central serous chorioretinopathy
Figure 1.
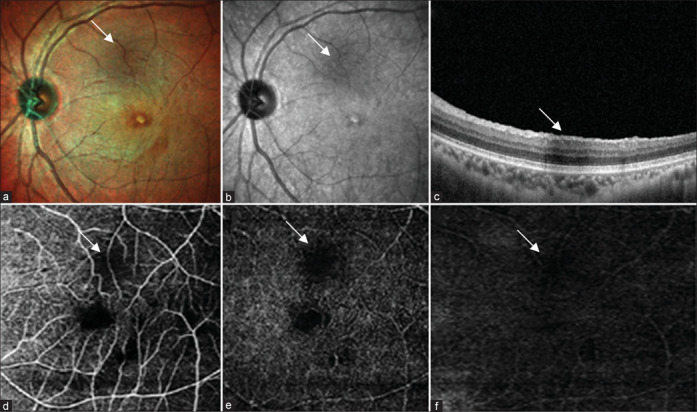
(a) Multicolor composite image of the right eye shows hyperreflective area at the posterior pole. (b) IR reflectance shows an area of hyporeflectance. (c) Structural OCT shows area of shadowing corresponding to the area of hyporeflectance. (d-f) OCT angiography shows an area of flow void in the superficial plexus, choroidal complex, and avascular zone
Figure 4.
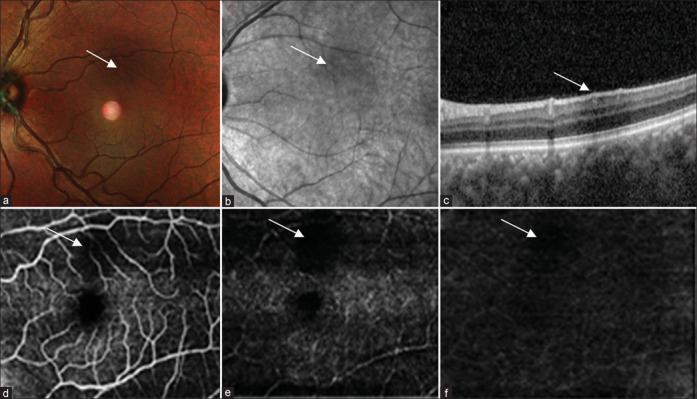
(a) Multicolor composite image of the left eye shows hyporeflective area at the posterior pole. (b) IR reflectance shows an area of hyporeflectance. (c) Structural OCT shows an area of shadowing corresponding to an area of hyporeflectance. (d-f) OCT angiography shows an area of flow void in the superficial plexus, choroidal complex, and avascular zone
Figure 2.
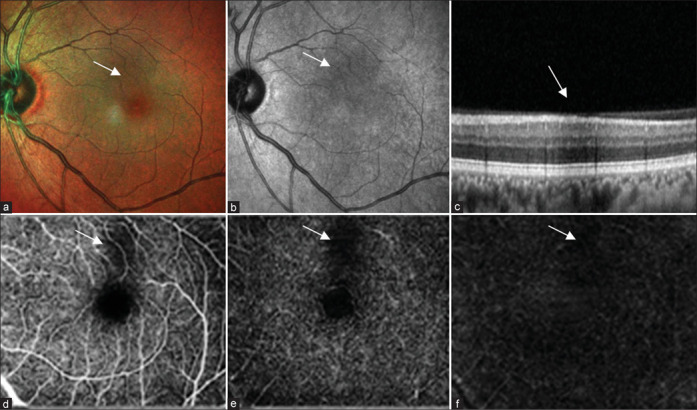
(a) Multicolor composite image of the left eye shows a hyporeflective area at the posterior pole. (b) IR reflectance shows an area of hyporeflectance. (c) Structural OCT shows the area of shadowing corresponding to the area of hyporeflectance. (d-f) OCT angiography shows an area of flow void in the superficial plexus, choroidal complex, and avascular zone
Figure 3.
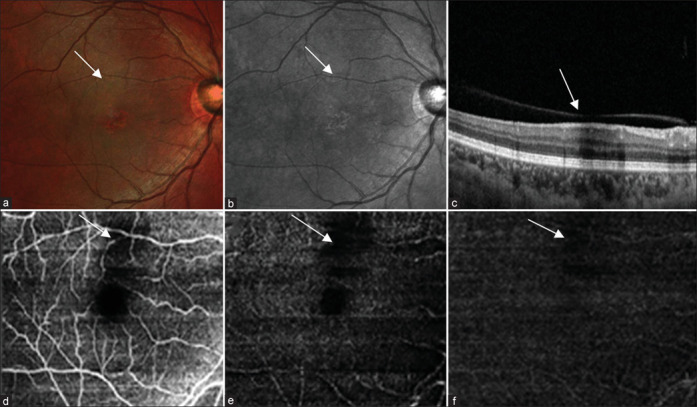
(a) Multicolor composite image of the left eye shows a hyporeflective area at the posterior pole. (b) IR reflectance shows an area of hyporeflectance. (c) Structural OCT shows an area of shadowing corresponding to an area of hyporeflectance. (d-f) OCT angiography shows an area of flow void in the superficial plexus, choroidal complex, and avascular zone
Although the fovea was absolutely normal in five eyes (41.66%), the rest of them had various pathological associations, such as intraretinal fluid in three (25%), foveal thinning in two (16.66%), scarred CNV in one (8.33%), and CSCR in one (8.33%) eyes. However, none of the eyes had any ischemic lesions or opacity in the ocular media, which could have culminated in the appearance of a dark shadow-like area in the OCTA images.
Discussion
We have termed the dark shadow-like spot observed on OCTA imaging in all the 12 eyes “pupil vignetting effect artifact.” Almost every artifact produced by an optical imaging system can be explained with the help of a proper understanding of the imaging strategies. These are caused by multiple technical and clinical factors which include 1) how OCT data are acquired and generated, 2) the intrinsic properties of the eye and ocular pathology, 3) eye movements, and finally, 4) how OCT data are processed and displayed as angiographic images.[11,12,13] Low-OCT signal artifacts can be contributed to numerous factors including vignetting, ocular and systemic aberrations, angle-dependent back-scattering, retina moving out of the focus, signal roll-off, any kind of ocular media opacity, intra- or sub-retinal fluid or blood, vascular shadowing, and RPE pigment clumping. Vignetting is one of the principal causes of low-OCT signal artifacts. It is exacerbated by small pupil size, slow pupillary dynamics as observed in sluggish pupillary reaction to light, and increased field size.[14,15,16,17]
The low signal artifact we noticed here is believed to be a result of partial or complete blockage of the light beam by the iris at the pupillary margin, thereby reducing the total amount of light incident on the retina, creating a vignetting effect. It was observed in our study that the artifact could vary from a well-circumscribed dark spot to a more diffuse dark area with less-defined borders and was present superior, superotemporal, and superonasal to the fovea. We postulate that this inter-individual variability in size, shape, and location of the artifact is caused mostly by the variable angle of the incident OCT light beam that is passing through the pupil in addition to a few other factors such as curvature of the retinal surface, and pupillary dynamics in reaction to light.[18,19] Pang and Freund[20] have described an artifact on multicolor and infrared reflectance imaging, which they have termed “ghost maculopathy artifact” and they hypothesized that it was caused by reflections from posterior chamber intraocular lenses.
The OCTA artifact can be easily mistaken as a flow void caused by an area of retinal ischemia, or a posterior shadow cast by any media opacity; however, it did not correspond to either of them in multicolor and infrared reflectance imaging. A similar image on OCTA imaging can be observed in acute macular neuroretinopathy (AMN) where flow voids are observed at ischemic areas on the retina. However, those flow voids correspond to the wedge-shaped areas of retinal ischemia observed both clinically and on multicolor infrared reflectance imaging. They also correspond to an area of hyper-reflectivity on OCT images.[21,22,23] On the contrary, the pupil vignetting artifact on OCTA corresponds to a hyperreflective area on OCT imaging. This feature differentiates the pupil vignetting artifact from the ischemic lesions of an important pathology such as AMN having a similar appearance on OCTA. Hence, interpretation of OCTA images requires correlation with multicolor, red-free fundus photography, and OCT imaging to differentiate pupil vignetting artifacts from true chorioretinal pathology. Identification of the artifacts is crucial as they can simulate numerous pathological low-flow situations in retinal ischemia frequently observed in RVOs, DR, AMN, etc. To conclude, pupil vignetting artifacts on OCTA imaging can easily be mistaken by the observer as true chorioretinal pathology, and this study intends to promote a greater understanding of this phenomenon. However, the very small sample size, retrospective study design, and therefore inability to image the same eye by changing the angle of the incident light while performing OCTA were the limitations of our study. This should be taken into account in future studies to check whether or not the size, shape, and intensity of the artifact changes with changing the angle of the incident light on the retina. This might further show us the way to finally eliminate the artifact and thereby spare the clinician of the diagnostic dilemma.
Conclusion
Pupil vignetting artifacts on OCTA imaging can easily be mistaken by the observer as true chorioretinal pathology. This study intends to promote a greater understanding of this phenomenon.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge Debalina Maitra, an optometrist and imaging technician working at our institution, who has captured all the images we provided with the paper.
References
- 1.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey AM, Hutton EK, Combe L, Bhindi P, Gertig D, Constable PA. Optical coherence tomography angiography in primary eye care. Clin Exp Optom. 2021;104:3–13. doi: 10.1111/cxo.13068. [DOI] [PubMed] [Google Scholar]
- 4.Ang M, Tan ACS, Cheung CMG, Keane PA, Dolz-Marco R, Sng CCA, et al. Optical coherence tomography angiography: A review of current and future clinical applications. Graefes Arch Clin Exp Ophthalmol. 2018;256:237–45. doi: 10.1007/s00417-017-3896-2. [DOI] [PubMed] [Google Scholar]
- 5.Coscas G, Lupidi M, Coscas F, Chhablani J, Cagini C. Optical coherence tomography angiography in healthy subjects and diabetic patients. Ophthalmologica. 2018;239:61–73. doi: 10.1159/000485323. [DOI] [PubMed] [Google Scholar]
- 6.Choi WJ. Imaging motion: A comprehensive review of optical coherence tomography angiography. Adv Exp Med Biol. 2021;1310:343–65. doi: 10.1007/978-981-33-6064-8_12. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Miller JB. Optical coherence tomography angiography: Review of current technical aspects and applications in chorioretinal disease. Semin Ophthalmol. 2019;34:211–7. doi: 10.1080/08820538.2019.1620797. [DOI] [PubMed] [Google Scholar]
- 8.Coscas G, Lupidi M, Coscas F. Optical coherence tomography angiography in healthy subjects. Dev Ophthalmol. 2016;56:37–44. doi: 10.1159/000442775. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei X, Camino A, Pi S, Hormel TT, Cepurna W, Huang D, et al. Real-time cross-sectional and en face OCT angiography guiding high-quality scan acquisition. Opt Lett. 2019;44:1431–4. doi: 10.1364/OL.44.001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Yaghi NE, Obiedat AF, AlNawaiseh TI, Hamad AM, Bani Ata BA, Quzli AA, et al. Optical coherence tomography angiography in healthy adult subjects: Normative values, frequency, and impact of artifacts. Biomed Res Int. 2022;2022:7286252. doi: 10.1155/2022/7286252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen FK, Viljoen RD, Bukowska DM. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin Exp Ophthalmol. 2016;44:388–99. doi: 10.1111/ceo.12683. [DOI] [PubMed] [Google Scholar]
- 13.Hormel TT, Huang D, Jia Y. Artifacts and artifact removal in optical coherence tomographic angiography. Quant Imaging Med Surg. 2021;11:1120–33. doi: 10.21037/qims-20-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anvari P, Ashrafkhorasani M, Habibi A, Falavarjani KG. Artifacts in optical coherence tomography angiography. J Ophthalmic Vis Res. 2021;16:271–86. doi: 10.18502/jovr.v16i2.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmen IC, Konda SM, Pak JW, McDaniel KW, Blodi B, Stepien KE, et al. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol. 2020;138:119–26. doi: 10.1001/jamaophthalmol.2019.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Tang J. Blood vessel tail artifacts suppression in optical coherence tomography angiography. Neurophotonics. 2022;9:021906. doi: 10.1117/1.NPh.9.2.021906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Hormel TT, Xiong H, Wang B, Camino A, Wang J, et al. Development and validation of a deep learning algorithm for distinguishing the nonperfusion area from signal reduction artifacts on OCT angiography. Biomed Opt Express. 2019;10:3257–68. doi: 10.1364/BOE.10.003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco-Zevallos O, Nankivil D, Keller B, Viehland C, Lujan BJ, Izatt JA. Pupil tracking optical coherence tomography for precise control of pupil entry position. Biomed Opt Express. 2015;6:3405–19. doi: 10.1364/BOE.6.003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enders C, Lang GE, Dreyhaupt J, Loidl M, Lang GK, Werner JU. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS One. 2019;14:e0210505. doi: 10.1371/journal.pone.0210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang CE, Freund KB. Ghost maculopathy: An artifact on near-infrared reflectance and multicolor imaging masquerading as chorioretinal pathology. Am J Ophthalmol. 2014;158:171–8.e2. doi: 10.1016/j.ajo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, et al. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–65. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Bellot L, Laurent C, Arcade PE, Mouriaux F. Neurorétinopathie maculaire aiguë: Description et intérêt de l'OCT en face, série de cas [Acute macular neuroretinopathy: En face OCT description, case series] J Fr Ophtalmol. 2022;45:159–65. doi: 10.1016/j.jfo.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Liu JC, Nesper PL, Fawzi AA, Gill MK. Acute macular neuroretinopathy associated with influenza vaccination with decreased flow at the deep capillary plexus on OCT angiography. Am J Ophthalmol Case Rep. 2018;10:96–100. doi: 10.1016/j.ajoc.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


