Abstract
Induction of cytotoxic T lymphocytes (CTLs) by vaccination has been shown to protect against bacterial, viral, and tumoral challenge. The aim of this study was to identify CTL epitopes on the 38-kDa lipoglycoprotein from Mycobacterium tuberculosis. The identification of these CTL epitopes was based on synthesizing peptides designed from the 38-kDa lipoglycoprotein, with known major histocompatibility complex class I (MHC-I) binding motifs (H-2Db), and studying their ability to up-regulate and stabilize MHC-I molecules on the mouse lymphoma cell line RMA-S. To improve the capacity of the identified peptides to induce CTL responses in mice, palmitic acid with a cysteine-serine-serine spacer amino acid sequence was attached to the amino terminus of the peptide. Two of five peptides with H-2Db binding motifs and their corresponding lipopeptides up-regulated and stabilized the H-2Db molecules on RMA-S cells. Both lipopeptides, in combination with incomplete Freund’s adjuvant, induced CTL responses in C57BL/6 (H-2b) mice. Moreover, the lipopeptide induced stronger CTL responses than the peptide. The capacity of the various lipopeptides to induce CTL displayed a good relationship with the ability of the (lipo)peptide to up-regulate and to stabilize H-2Db molecules. The capacity of the peptides and lipopeptides to up-regulate and stabilize MHC-I expression can therefore be used to predict their potential to function as a CTL epitope. The newly identified CTL epitopes and their lipid derivatives provide us with important information for future M. tuberculosis vaccine design.
Mycobacterium tuberculosis is a facultatively intracellular bacterium that causes tuberculosis (TB). Between 1985 and 1991, TB increased 33% in Switzerland, 30% in Denmark, 20% in Norway, and 18% in both Ireland and the United States. Presently, TB affects 1.7 billion people worldwide. With 55 million cases of active disease, there are 8 million new cases and around 3 million deaths per year (2, 14). This situation, mainly in the developing countries, is directly related to a “weakness” of the immune system caused by human immunodeficiency virus infection, cancer, and the application of immunosuppressive drugs in chemotherapy (6). The situation is further complicated by the emergence of multi-drug-resistant strains (2, 6).
Mycobacterium bovis BCG is the currently used vaccine against tuberculosis, but its efficacy varies widely, from 0 to 90% (15). BCG mainly activates CD4+ T cells, but it fails to activate CD8+ T cells (14). Cytotoxicity mediated by CD4+ cells, however, is probably not sufficient to eradicate the mycobacterium, which has an excellent capacity to survive intracellularly. Several studies have demonstrated the importance of CD8+ cytotoxic T lymphocytes (CTLs) against M. tuberculosis infection in mouse (CD8 knockout) models (11) and humans (12, 19, 22). However, only a few studies have described the mycobacterial antigens and the epitopes recognized by CD8+ CTLs (4, 31, 32, 38). Induction of cellular immunity, covering both CD4+ and CD8+ T-cell activation, may therefore be required for providing protection against M. tuberculosis (13).
For the identification of CTL epitopes on viral, bacterial, and tumoral proteins, different strategies can be followed. One approach used is immunization with the whole protein to generate CTL responses or CTL clones and the subsequent mapping of the epitope by overlapping synthetic peptides (23). An alternative approach is to select 8- to 11-amino-acid (aa)-long peptides based on known major histocompatibility complex class I (MHC-I) binding motifs. These peptides are then studied for their ability to up-regulate and stabilize MHC-I expression (44). The advantage of this approach is that it limits the number of peptides to be synthesized and to be tested in animal experiments.
The 38-kDa lipoglycoprotein, one of the better-studied antigens of M. tuberculosis, is actively secreted but is also partly attached to the surface of mycobacteria by a lipid tail (43). The protein induces strong antibody and T-cell responses and provides partial protection against M. tuberculosis infection in mice when it is administered either entrapped in biodegradable microparticles or in the form of a DNA vaccine (37, 46). Epitopes on the 38-kDa lipoglycoprotein have been recognized by CD4+ T cells and studied extensively with both mice (34, 36) and humans (35, 42). Recently, a number of epitopes recognized by CD8+ T cells (CTL epitopes), with an H-2b restriction, have also been described (38, 46, 47).
This report describes the identification of CTL epitopes with an H-2Db restriction by synthesizing peptides with MHC-I binding motifs and studying their ability to up-regulate and stabilize MHC-I molecules. Positive peptides in these assays do not necessarily induce cytotoxic T-cell responses, even when they are injected with incomplete Freund’s adjuvant (IFA) (3, 9, 10, 38). The immunogenicity of these peptides, however, can be strongly improved by attachment of lipid tails, preferably with a spacer amino acid sequence between peptide and lipid (5, 18, 20, 26, 33). Previously, it has been demonstrated that the cysteine-serine-serine (CSS) spacer amino acid sequence was required for the efficient induction of CTLs against malaria epitopes (33). Therefore, palmitic acid with a CSS spacer amino acid sequence was linked to the amino terminus of the peptide, and mice were immunized with these lipopeptides in combination with IFA. Two new H-2Db CTL epitopes were identified on the 38-kDa mycobacterial lipoglycoprotein, which increases our knowledge for future M. tuberculosis vaccine design. The relationship between the ability to up-regulate and stabilize MHC-I molecules and the capability to induce CTLs by peptides and lipopeptides will be discussed.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 (H-2b) female mice were purchased from Iffo Credo (Someren, The Netherlands) and kept at the Central Animal Laboratory of the Utrecht University. Animals were used at ages of 8 to 12 weeks. Experiments were approved by the Ethics Committee on Animal Experimentation of the Utrecht University.
Synthetic peptides and lipopeptides.
Peptides (8 to 11 aa) with H-2Db binding motifs were designed from the 38-kDa lipoglycoprotein by using binding motifs as described by Engelhard (8). These peptides and lipopeptides (Table 1) were either synthesized at the Peptide Center of the Research School of Infection and Immunity, Veterinary Faculty, Utrecht, or at the Core Facility of the Centers for Disease Control, Atlanta, Ga.
TABLE 1.
Peptides of the 38-kDa mycobacterial lipoglycoprotein and corresponding lipopeptides used
| Designation | Position (length in aa) | Amino acid sequencea | MHC-I restriction |
|---|---|---|---|
| Controls | |||
| Lymphocytic choriomeningitis virus | 118–126 (10) | RPQASGVYM | H-2Ld |
| Influenza virus nucleoprotein | 366–374 (9) | ASNENMETM | H-2Db |
| Peptidesb | |||
| P1 | 97–108 (12) | AGTVNIGASDAY | H-2Db |
| P2 | 142–150 (9) | HLKLNGKVL | H-2Db |
| P3 | 166–175 (10) | IAALNPGVNL | H-2Db |
| P4 | 309–318 (10) | YPIINYEYAI | H-2Db |
| P5 | 340–349 (10) | ITDGNKASFL | H-2Db |
| Lipopeptides | |||
| LP1 | 97–108 (12) | PA-CSS AGTVNIGASDAY | H-2Db |
| LP3 | 166–175 (10) | PA-CSS-IAALNPGVNL | H-2Db |
| LP4 | 309–318 (10) | PA-CSS-YPIINYEYAI | H-2Db |
Predicted anchor residues are underlined. PA, palmitic acid.
Based on the amino acid sequence of the 38-kDa lipoglycoprotein published by Vordermeier et al. (34).
Lipopeptides with a CSS spacer sequence were prepared by the same methodology used for the peptides. Palmitic acid was linked to the deprotected amino terminus of the resin-bound peptide by using the peptide synthesizer, employing the reaction conditions used for a standard amino acid coupling. Peptides and monopalmitic acid-peptide conjugates were cleaved from the resin, deprotected, and purified by standard procedures (30). The composition and purity of the peptides and lipopeptides were checked by amino acid analysis, reverse-phase (C8) high-pressure chromatography, and capillary electrophoresis. Traces of free peptide were detected in the lipopeptides LP1 and LP4, but not in LP3.
Cell lines and culture medium.
RMA-S (H-2b), a mouse lymphoma cell line transformed with Rauscher virus, was a gift from Jacques Neefjes (The Netherlands Cancer Institute, Antonie Van Leeuwenhoek Hospital, Amsterdam, The Netherlands). EL-4 (H-2b), a thymoma cell line, was a gift from Peter Van Koten (Department of Immunology, Veterinary Faculty, Utrecht University). EX-38 (H-2b) is an EL-4 cell line transfected with the gene encoding an M. tuberculosis 38-kDa lipoglycoprotein (38). Cells were grown in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum, 10 μg of gentamicin per ml, 2 mM glutamine, 1.7 g of NaHCO3, and 50 μM β-mercaptoethanol (IMDM-complete).
Flow cytometry analysis of MHC-I up-regulation.
The flow cytometry assay was performed as described by Zhou et al. (44). In short, RMA-S (H-2b) cells at log phase (106/ml) were collected, washed with IMDM-complete, and cultured overnight at 25°C at a concentration of 106/ml in IMDM-complete. The next morning, RMA-S cells (4 × 105 to 5 × 105/well) were washed with IMDM-complete, incubated with different concentrations of the synthetic peptides or lipopeptides at 25°C in 5% CO2 for 2 h, and then incubated for an additional 2 h at 37°C. After incubation, cells were washed once with ice-cold Hanks-bovine serum albumin-NaN3 and incubated with monoclonal antibody 28-14-8 (Pharmingen, San Diego, Calif.), which recognizes mouse H-2Ld, H-2Db, and H-2Dq, on ice for 30 min. Cells were then washed two times as before and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (Dako, Copenhagen, Denmark) on ice for 30 min. The stained cells were fixed with 1% formaldehyde and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Results are expressed as mean fluorescence intensity.
RMA-S stabilization assay.
In the RMA-S stabilization assay, the cells were incubated for 30 min at 25°C with various concentrations of peptide and then transferred to 37°C and incubated for the indicated times (0 to 180 min), followed by washes at 4°C in IMDM-complete. For a zero time point determination, cells were immediately washed at 4°C after the 25°C incubation. Next, the cells were stained for Db surface expression, as described above.
Immunization procedures.
Mice were immunized by one subcutaneous injection of 100 μg of peptide P3 or lipopeptides LP1, LP3, and LP4, emulsified in IFA 1:1 (vol/vol), at the base of the tail (total volume, 100 μl). Spleens were isolated between 1 and 2 weeks after immunization.
In vitro expansion of antigen-specific CTLs.
After spleen isolations, cell suspensions were prepared as described previously (45), and the erythrocytes were lysed by hypotonic shock and washed twice with IMDM. Spleen cells (30 × 106) from in vivo-primed mice were cocultured with mitomycin (Sigma Chemical Co., St. Louis, Mo.)-treated EX-38 cells (1.5 × 106) in a 10-ml cell suspension in IMDM-complete for 5 to 6 days at 37°C at 5% CO2. Ten to 30 U of IL-2 (supernatant of EL-4 cells incubated with concanavalin A and phorbol myristate acetate for 24 h; the remaining concanavalin A was neutralized by mannose) was added after 48 h of culture.
51Chromium release assay.
Cells of the in vitro culture were collected and washed once with IMDM-complete. Viable cytotoxic effector cells were separated from dead cells by centrifugation through a Lympholyte-M density gradient (2,000 rpm for 10 min), washed two times, and resuspended in RPMI 1640 supplemented with 5% fetal calf serum (CTL medium) at a concentration of 4 × 106 cells/ml. EX-38 or EL-4 target cells (2 × 106) were incubated for 1 h at 37°C with 100 μCi of 51Cr (Amersham, Life Science). Cells were washed three times, and 5 × 103 target cells in 100 μl were added to 100 μl of various numbers of effector cells which had been plated in 96-well U-bottom plates. The plate was centrifuged for 1 to 2 min at 1,000 rpm and incubated for 4 to 6 h at 37°C in 5% CO2. After the incubation, 100 μl of supernatant was collected and counted in a gamma counter. The specific lysis (percentage of cytotoxicity) was calculated by the equation: % cytotoxicity = [(sample − spontaneous release)/(total release − spontaneous release)] × 100%.
Statistical analysis.
Differences between the up-regulation induced by various peptides and lipopeptides and their ability to induce CTLs were analyzed by Student’s t test. P < 0.05 was considered to be significant.
RESULTS
MHC-I (H-2Db) up-regulation by peptides and lipopeptides.
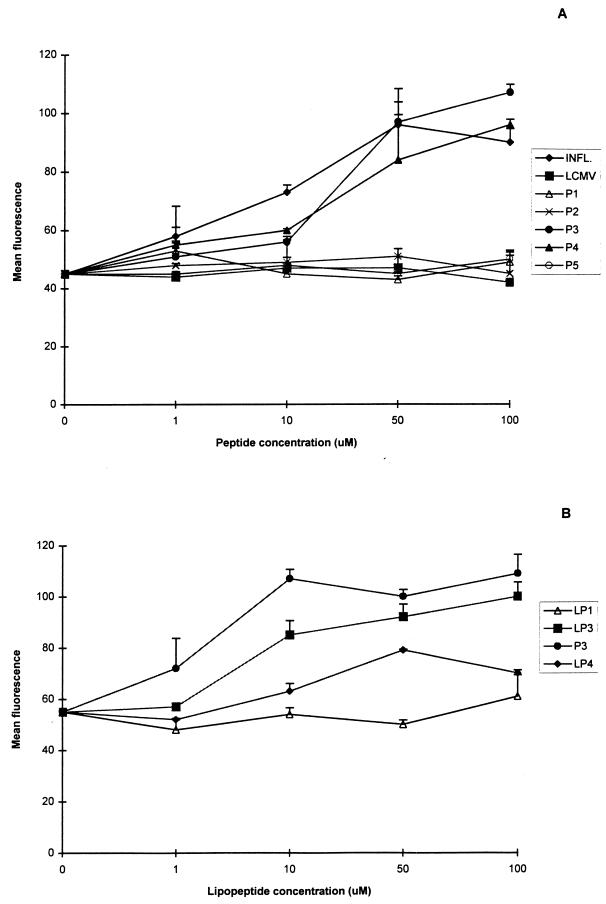
Peptides and lipopeptides from the 38-kDa mycobacterial lipoglycoprotein with known MHC-I mouse binding motifs were studied for their ability to up-regulate MHC-I molecules on RMA-S cells. A twofold increase in mean fluorescence was observed for peptides P3 and P4 at the highest peptide concentration, whereas for peptides P1, P2, and P5, the mean fluorescence showed no increase above the background (Fig. 1A). The increase in mean fluorescence was peptide concentration dependent, and peptides P3 and P4 had a similar capacity to up-regulate MHC-I molecules, as did the known CTL epitope of the influenza virus nucleoprotein (44), which also had the H-2Db MHC restriction (Fig. 1A).
FIG. 1.
MHC-I (H-2Db) up-regulation by peptides (A) and lipopeptides (B) of the 38-kDa mycobacterial lipoglycoprotein, with known binding motifs. INFL., influenza virus nucleoprotein; LCMV, lymphocytic choriomeningitis virus. The results are expressed as mean fluorescence of duplicate determinations ± standard deviation from one of two representative experiments. RMA-S cells were incubated with several concentrations of the peptides or lipopeptides. Up-regulation of the MHC-I molecules was detected with a specific monoclonal antibody directed against H-2Db molecules and a fluorescein-isothiocyanate-labeled goat anti-mouse immunoglobulin G conjugate.
To increase cytotoxic T-cell induction by the identified peptides, palmitic acid was coupled to peptides with the CSS spacer amino acid sequence. Three different lipopeptides were prepared: LP3 and LP4, of the two identified positive peptides, and LP1 as a negative control. The ability of these lipopeptides to up-regulate MHC-I molecules was also studied. LP3 showed up-regulation of MHC-I molecules similar to that of peptide P3 (Fig. 1B). A much lower level of up-regulation was observed for LP4, whereas for LP1, like for the peptide, no up-regulation was detected.
Stabilization of MHC-I (H-2Db) molecules.
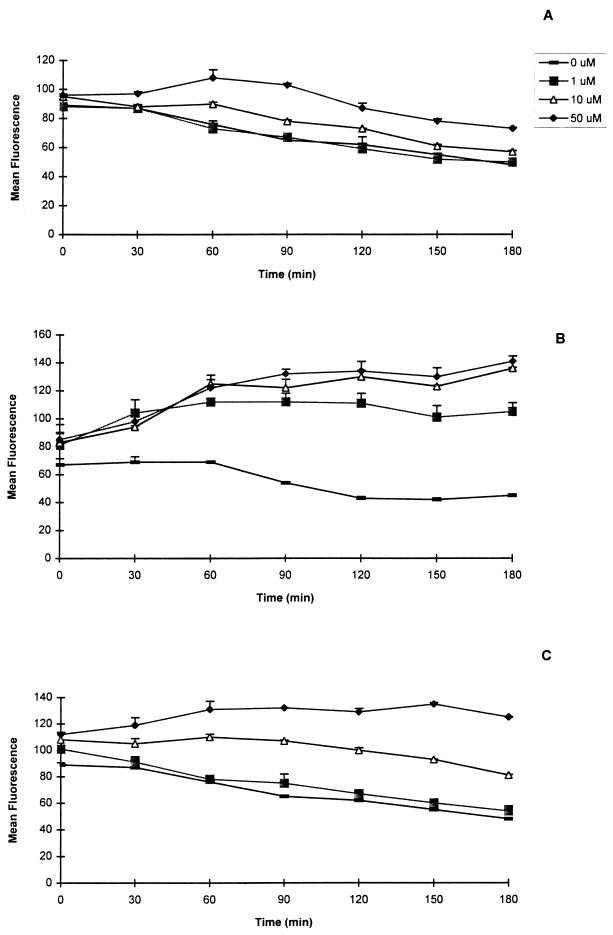
It has been shown previously that the capacity of a peptide to bind and stabilize MHC-I molecules is directly correlated with the peptide’s ability to induce specific CTL responses (9, 16, 28, 44). Therefore, the binding and stabilization of H-2Db molecules by peptides P1, P3, and P4 and their corresponding lipopeptides were investigated. Peptides P3 and P4 up-regulated and stabilized the MHC-I molecules for a period of up to 3 h (Fig. 2B and C). This stabilization was peptide concentration dependent. However, P3 was much better able to stabilize H-2Db molecules than P4 was. The minimal concentrations required for stabilization of P3 and P4 were 1 and 50 μM, respectively. In contrast, no stabilization was seen for peptide P1 with any of the concentrations used (Fig. 2A).
FIG. 2.
Stability of peptide binding to MHC-I 2Db molecules. RMA-S cells were incubated at 25°C with various concentrations of peptides and then transferred to 37°C and incubated for the indicated times. The cells were then washed at 4°C and stained as described in the legend to Fig. 1. The results are expressed as mean fluorescence of duplicate determinations ± standard deviation from one of two representative experiments (P1 [A], P3 [B], and P4 [C]).
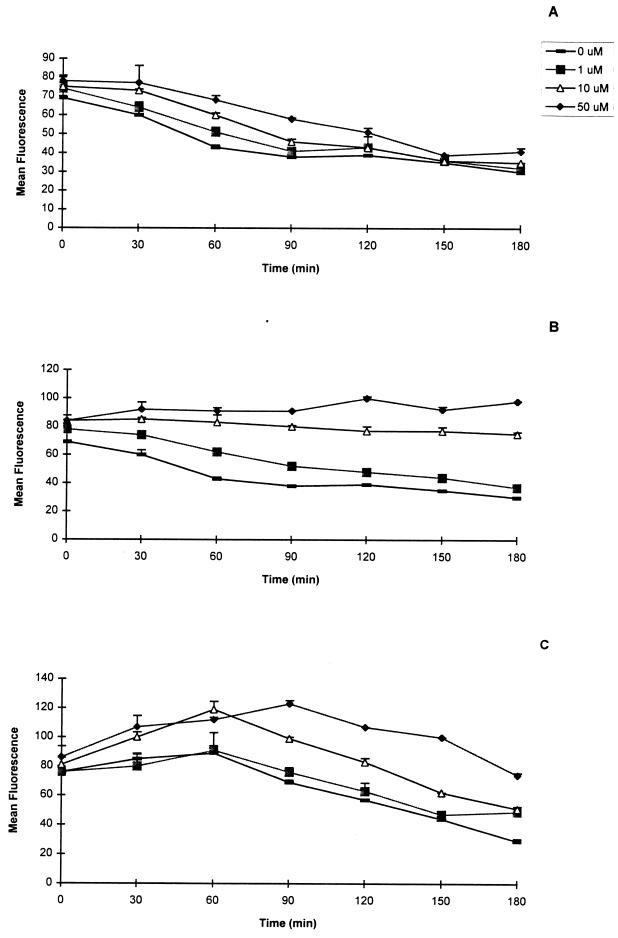
The ability of the lipopeptides to stabilize MHC-I molecules was also investigated. The lipopeptides gave results similar to those of the corresponding peptides (Fig. 3). Lipopeptides LP3 and LP4 stabilized the MHC-I molecules, whereas for lipopeptide LP1, no stabilization was detected for any of the concentrations tested. The minimal concentrations of lipopeptide required for stabilization during the 3-h period were 10 μM for LP3 and more than 50 μM for LP4. Thus, two of five peptides and the corresponding lipopeptides were identified by the peptide binding assay.
FIG. 3.
Stability of lipopeptide binding to MHC-I 2Db molecules. RMA-S cells were incubated at 25°C with various concentrations of lipopeptides and then transferred to 37°C and incubated for the indicated times. The cells were then washed at 4°C and stained as described in the legend to Fig. 1. The results are expressed as mean fluorescence of duplicate determinations ± standard deviation from one of two representative experiments (LP1 [A], LP3 [B], and LP4 [C]).
Induction of cytotoxic T cells.
To measure the CTL responses against the selected peptides identified by the peptide binding assay, a classical 51Cr release assay was used. The ability of the peptide and corresponding lipopeptide to induce CTL was also investigated. C57BL/6 mice were injected subcutaneously either with peptide P3 or with lipopeptide LP3 emulsified in IFA. Cytotoxic activity was determined, after an in vitro restimulation, against the target cell line EX-38, which expresses the 38-kDa lipoglycoprotein. EL-4 was used as a negative control. A strong specific CTL response against target cell line EX-38 was seen only after immunization with the lipopeptide in IFA and not with the peptide in IFA (Table 2). This was statistically significant at a target/effector (T/E) ratio of 1:20 (P < 0.005). With the EL-4 cell line as the negative control, no significant lysis was observed. In addition, statistical differences were found between the target cell lines EX-38 and EL-4 for the lipopeptide (T/E ratios of 1:40 [P < 0.0025] and 1:20 [P < 0.025]) and for the peptide (T/E ratio of 1:40 [P < 0.025]) (Table 2).
TABLE 2.
CTL responses to peptide and lipopeptide P3
| Combination | % Cytotoxicity at T/E ratio givena
|
|||
|---|---|---|---|---|
| EX-38b
|
EL-4c
|
|||
| 1:40 | 1:20 | 1:40 | 1:20 | |
| Lipopeptide-IFA | 28 ± 14 | 34 ± 10 | 3 ± 5 | 1 ± 2 |
| Peptide-IFA | 10 ± 6 | 3 ± 3 | 0 ± 1 | 0 ± 0 |
CTL responses (percentage of cytotoxicity) measured after an in vitro restimulation of the in vivo-primed spleen cells with mitomycin-treated EX-38 cells. Results are presented as means ± standard deviations of three experiments. Statistical differences were found between lipopeptide and peptide at a T/E ratio of 1:20 with target cell EX-38 (P < 0.005), between target cells EX-38 and EL-4 for lipopeptide at T/E ratios of 1:40 and 1:20 (P < 0.0025 and P < 0.025, respectively), and for peptide at a T/E ratio of 1:40 (P < 0.025).
Target cell line transfected with the gene encoding the 38-kDa lipoglycoprotein.
Target cell line used as a negative control.
The ability of (lipo)peptides to induce CTLs is directly correlated with their capacity to up-regulate and stabilize MHC-I molecules in vitro (16). C57BL/6 mice were immunized subcutaneously with either lipopeptide LP1, LP3, or LP4 in IFA, and the cytotoxic activity was evaluated. LP3 induced the strongest CTL response, followed by LP4 and LP1 (Table 3). These differences were statistically significant between lipopeptides LP3 and LP1 (P < 0.05) and between LP4 and LP1 (P < 0.05) (Table 3). The differences in CTL induction displayed a good relationship with the different abilities of the (lipo)peptides to up-regulate and stabilize MHC-I molecules (Table 3). Lipopeptide LP3 was the most immunogenic and gave the highest up-regulation and best stabilization of the MHC-I molecules. The differences in up-regulation were statistically significant between LP3 and the background (P < 0.005), LP3 and LP4 (P < 0.01), and LP3 and LP1 (P < 0.025). In contrast, LP1 was shown to be weak in inducing CTLs and up-regulating and stabilizing the MHC-I molecules. LP4 displayed an intermediate behavior; the difference in up-regulation was statistically significant between LP4 and the background (P < 0.005).
TABLE 3.
MHC-I up-regulation and CTL in vivo responses
| Lipo- peptidea | Class I H-2Db up-regulation (mean fluorescence ± SD at 100 μM lipopeptide)b | CTL response (mean % cytotoxicity ± SD at T/E ratio of 1:80)c |
|---|---|---|
| LP3 | 100 ± 6 | 32 ± 13 |
| LP4 | 70 ± 1 | 29 ± 11 |
| LP1 | 61 ± 8 | 8 ± 11 |
| None | 54 ± 3 |
Immunization with lipopeptide-IFA.
MHC-I up-regulation was measured as described in the legend to Fig. 1, and CTL induction was measured as described in Table 2. The estimated minimal concentrations of lipopeptide required for stabilization were 10 μM for LP3, >50 μM for LP4, and none for LP1. The results of class I H-2Db up-regulation are from one of two representative experiments. Statistical differences were found between lipopeptides LP3 and LP4 (P < 0.01), LP3 and LP1 (P < 0.025), LP3 and none (P < 0.005), and LP4 and none (P < 0.005).
Results from CTL responses are means of four or five experiments. Statistical differences were found between LP3 and LP1 (P < 0.05) and LP4 and LP1 (P < 0.05).
DISCUSSION
Peptides designed from the 38-kDa protein of M. tuberculosis, with known MHC-I (H-2Db) mouse binding motifs, were studied for their ability to up-regulate and stabilize MHC-I molecules on the RMA-S cells. CTL induction by the identified peptides and corresponding lipopeptides was investigated in vivo.
Of the five peptides selected, two peptides up-regulated and stabilized MHC-I (H-2Db) molecules on RMA-S cells (P3 and P4) (Fig. 1A and B). Although all five peptides contained the required anchoring amino acid residues (asparagine on position 5 and either tyrosine, leucine, or isoleucine at the C terminus), this does not necessarily mean that they bind to the empty 2Db molecules. In general, 30 to 75% of the peptides with known binding motifs will bind to class I molecules (3, 9, 10, 28, 38). Moreover, the binding affinity of peptides to class I molecules is not determined only by the presence of anchoring residues forming the binding motif: factors such as the length of the peptide, spacing between required anchoring residues, the other amino acids present in the peptide (secondary anchoring residues), and the amino acids flanking the CTL epitope also play an important role (7, 25, 44). The absence of binding of peptides P1, P2, and P5 is therefore not easy to explain.
The ability of the lipopeptides to bind and to stabilize the H-2Db molecules was also investigated. Remarkably, the lipopeptides were, just as their corresponding peptides, capable of up-regulating and stabilizing the MHC-I molecules (Fig. 1B and 3). This could not be attributed to the presence of free peptide, since no or only trace amounts of free peptide were detected in the lipopeptide preparations by high-pressure liquid chromatography analysis (data not shown). Schirmbeck et al. (27) demonstrated that processing of particulate protein antigens by cell lines and the subsequent presentation of CTL epitopes is a fast process (30 min to 2 h). Therefore, it is very likely that the lipopeptides are (partially) processed to the free peptide during the assays. This processing, however, takes place within the cell, and since the RMA-S cell line has a defect in the transporter for antigen presentation (TAP) (17), the free peptide will not be transported into the lumen of the endoplasmic reticulum, and therefore it cannot bind to the MHC-I molecules. Recently, an alternative MHC-I processing pathway for particulate antigens was described. This pathway was resistant to brefeldin A and is TAP independent (1, 21, 27, 29). Particulate antigens are phagocytosed and processed, and the generated peptides may subsequently bind to the MHC-I molecules in the post-Golgi vesicular compartments (e.g., phagosomes or phagolysosomes) or on the cell surface following peptide regurgitation (21, 29). However, as a consequence of their amphipathic nature, lipopeptides form vesicle-like structures in solution. These antigens will therefore probably resemble particulate antigens, which might explain their ability to up-regulate and stabilize MHC-I molecules on RMA-S cells. An alternative hypothesis is that the lipid tail is inserted into the cellular membrane and that just the peptide part interacts with the MHC-I molecules on the surface of the cells. Experiments to determine whether the alternative class I pathway is involved are in progress in our laboratory.
Binding and stabilization of MHC-I molecules by peptides are directly correlated to their ability to induce cytotoxic T cells in vivo (9, 16, 28, 44). Indeed, also in our experiments, a good correlation was observed between the peptide or lipopeptide binding and stabilization of MHC-I molecules in vitro and their CTL-inducing capacity in vivo. The peptide P3 and its corresponding lipopeptide, LP3, excelled in their capacity to up-regulate and stabilize H-2Db molecules compared to peptide P4 and lipopeptide LP4, whereas peptide P1 and lipopeptide LP1 were unable to do so (Fig. 2 and 3). These properties were reflected in their ability to induce CTLs in vivo (Table 3).
Efficient induction of CTL responses in animals against soluble proteins or peptides is difficult to achieve. Linkage of lipid tails to peptides is a good method for inducing CTLs. Coupling of peptides to a complex tripalmitoyl-cysteine derivative induced strong CTL responses against pathogens such as human immunodeficiency virus, foot-and-mouth disease virus, influenza virus, and malaria parasites (5, 24, 26, 39–41). We and others demonstrated that similar results can be obtained with monopalmitic acid-peptide constructs (18, 26). We compared the immunogenic properties of peptide P3 and lipopeptide LP3 in combination with IFA (Table 2). Only the lipopeptide induced a good CTL response. A possible explanation for this effect is that coupling of lipid tails to peptides will keep the conjugate attached to the oil droplet. As a consequence, adjuvant and antigen will end up in the same compartment of the immune system, which facilitates the induction of a good immune response. In addition, this formulation might protect the peptide against degradation by proteases and will introduce the epitope into the class I pathway more efficiently. Other peptides in combination with IFA might induce CTLs, but their interaction with oil will probably depend on the hydrophobic properties of the peptide (3, 9, 10, 38). Most other groups who are using the same general approach for identifying CTL epitopes evaluate the in vivo capability of the identified peptides by immunization with peptides in combination with IFA. Had we used only the peptide in combination with IFA and not its lipid derivate, we would have failed to identify peptide P3 as a CTL epitope. Our results therefore indicate that it is better to use lipopeptides for the evaluation of the in vivo CTL-inducing capacity of peptides, especially since the monopalmitic acid lipid-peptide conjugates can be easily prepared during standard peptide synthesis.
Recently, Zhu et al. (47) also demonstrated that P3 was able to up-regulate class I expression, but they were unable to identify this peptide as a CTL epitope, nor was it recognized by effector CTLs, which were induced either by infection or by immunization. However, immunization of mice was carried out by them with the transfected cell line EX-38 or the DNA construct pXJ38.
In another report (46), the DNA vaccine gave a level of protection similar to that of the BCG vaccine. Interestingly, striking differences in the CTL epitopes identified were observed in mice vaccinated with the 38-kDa DNA vaccine compared to those in mice which were infected with M. tuberculosis. The CTL epitopes described in this report gave only background lysis in their studies. Induction of strong CTL responses against these epitopes, however, might improve the protection provided by the 38-kDa lipoglycoprotein. The use of lipopeptides will enable us to define which CTL epitopes of the 38-kDa lipoglycoprotein should be included in future M. tuberculosis vaccines.
ACKNOWLEDGMENTS
This work was supported by grant PRAXIS XXI BD/2698/94, JNICT, Portugal.
We thank B. Benaissa-Trouw and T. Harmsen for excellent technical assistance and C. A. Kraaijeveld for careful reading of the manuscript.
REFERENCES
- 1.Bachmann M F, Oxenius A, Pircher H, Hengartner H, Ashton-Richardt P A, Tonegawa S, Zinkernagel R M. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739–1743. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 2.Bloom R B, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Catipovic B, Dal Porto J, Mage M, Johansen T E, Schneck J P. Major histocompatibility complex conformational epitopes are peptide specific. J Exp Med. 1992;176:1611–1618. doi: 10.1084/jem.176.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delibero G, Flesh I, Kauffman S H E. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 5.Deres K, Schild H, Weismuller K-H, Jung G, Rammensee H-G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 6.Earnest M, Sbarbaro J A. A plague returns. Sciences. 1993;Sept./Oct.:14–19. [Google Scholar]
- 7.Eberl G, Sabbatini A, Servis C, Romero P, Maryanski J L, Corradin G. MHC class I H-2kd-restricted antigenic peptides: additional constraints for the binding motif. Int Immunol. 1993;5:1489–1492. doi: 10.1093/intimm/5.11.1489. [DOI] [PubMed] [Google Scholar]
- 8.Engelhard V H. Structure of peptides associated with MHC class I molecules. Curr Opin Immunol. 1994;6:13–23. doi: 10.1016/0952-7915(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 9.Feltkamp M C W, Smits H L, Vierboom M P M, Minnaar R P, de Jongh B M, Drijfhout J W, der Schegget J, Melief C J M, Kast W M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 10.Feltkamp M C W, Vierboom M P M, Kast W M, Melief C J M. Efficient MHC class I-peptide binding is required but does not ensure MHC class I-restricted immunogenicity. Mol Immunol. 1994;31:1391–1401. doi: 10.1016/0161-5890(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 11.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaleab B, Ottenoff T, Converse P, Halapi E, Tadesse G, Rottenberg M, Kiessling R. Mycobacterial-induced cytoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651–2659. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 13.Kauffman S H E. CD8+ lymphocytes in intracellular microbial infections. Immunol Today. 1988;9:168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- 14.Kauffman S H E. Tuberculosis, the role of the immune system. Immunologist. 1993;1:109–114. [Google Scholar]
- 15.Koch-Weser D. BCG vaccination, can it contribute to tuberculosis control? Chest. 1993;103:1641–1642. doi: 10.1378/chest.103.6.1641. [DOI] [PubMed] [Google Scholar]
- 16.Lipford G B, Bauer S, Wagner H, Heeg K. In vivo CTL induction with point-substituted ovalbumin peptides: immunogenicity correlates with peptide-induced MHC class I stability. Vaccine. 1995;13:313–320. doi: 10.1016/0264-410x(95)93320-9. [DOI] [PubMed] [Google Scholar]
- 17.Ljunggren H-G, Stam N J, Ohlen C, Neefjes J J, Hoglund P, Heemels M-T, Bastin J, Schumacher T N M, Towsend A, Karre K, Ploegh H L. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Gras-Mass H, Boutillon C, Chirat F, Deprez B, Guillet J-G, Gomard E, Tartar A, Levy J-P. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. J Immunol. 1992;149:3416–3422. [PubMed] [Google Scholar]
- 19.Ottenhoff T H M, Mutis T. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur J Clin Invest. 1995;25:371–377. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 20.Pancre V, Gras-Mass H, Delanoye A, Hernot J, Capron A, Auriault C. Induction of cytotoxic T-cell activity by the protective antigen of Schistosoma mansoni Sm28GST or its derived C-terminal lipopeptide. Scand J Immunol. 1996;44:485–492. doi: 10.1046/j.1365-3083.1996.d01-340.x. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Hardig C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 22.Rees A, Scoging A, Mehlert A, Young D B, Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988;18:1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- 23.Romero P, Maryanski J L, Corradin G, Nussenzweig R S, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 24.Romero P, Eberl G, Casanova J, Cordey A, Widmann C, Luescher I F, Corradin G, Maryanski J L. Immunization with synthetic peptides containing a defined malaria epitope induces highly diverse cytotoxic T lymphocyte responses. J Immunol. 1992;148:1871–1878. [PubMed] [Google Scholar]
- 25.Ruppert J, Sidney J, Celis E, Kubo R T, Grey H M, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 26.Schild H, Deres K, Weismuller K-H, Jung G, Rammensee H-G. Efficiency of peptides and lipopeptides for in vivo priming of virus-specific cytotoxic T cells. Eur J Immunol. 1991;21:2649–2654. doi: 10.1002/eji.1830211102. [DOI] [PubMed] [Google Scholar]
- 27.Schirmbeck R, Melber K, Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995;25:1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 28.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J M, Oseroff C, Yuan L, Ruppert J, Sidney J, del Guercio M-F, Southwood S, Kubo R T, Chesnut R W, Grey H M, Chisari F V. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 29.Song R, Harding C V. Roles of proteasomes, transporter for antigen presentation (TAP), and β2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–4190. [PubMed] [Google Scholar]
- 30.Stewart G M, Young J D. Solid-phase peptide synthesis. 2nd ed. Rockford, Ill: Pierce Chemical Co.; 1984. [Google Scholar]
- 31.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 32.Turner J, Dockrell H M. Stimulation of human peripheral blood mononuclear cells with live M. bovis-BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheul A F M, Udhayakumar V, Jue D L, Wohlhueter R M, Lal A A. Monopalmitic acid-peptide conjugates induce cytotoxic T cell responses against malarial epitopes: importance of spacer amino acids. J Immunol Methods. 1995;182:219–226. doi: 10.1016/0022-1759(95)00052-c. [DOI] [PubMed] [Google Scholar]
- 34.Vordermeier H M, Harris D P, Román E, Lathigra R, Moreno C, Ivanyi J. Identification of T cell stimulatory peptides from the 38-kDa protein of Mycobacterium tuberculosis. J Immunol. 1991;147:1023–1029. [PubMed] [Google Scholar]
- 35.Vordermeier H M, Harris D P, Friscia G, Román E, Surcel H M, Moreno C, Pasvol G, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 36.Vordermeier H M, Harris D P, Moreno C, Singh M, Ivanyi J. The nature of the immunogen determines the specificity of antibodies and T cells to selected peptides of the 38-kDa mycobacterial antigen. Int Immunol. 1995;4:559–566. doi: 10.1093/intimm/7.4.559. [DOI] [PubMed] [Google Scholar]
- 37.Vordermeier H M, Coombes A G A, Jenkins P, McGee J P, O’Hagan D T, Davis S S, Singh M. Synthetic delivery system for tuberculosis vaccines: immunological evaluation of the M. tuberculosis 38 kDa protein entrapped in biodegradable PLG microparticles. Vaccine. 1995;13:1576–1582. doi: 10.1016/0264-410x(95)00084-e. [DOI] [PubMed] [Google Scholar]
- 38.Vordermeier H M, Zhu X, Harris D P. Induction of CD8+ CTL recognizing mycobacterial peptides. Scand J Immunol. 1997;45:521–526. doi: 10.1046/j.1365-3083.1997.d01-432.x. [DOI] [PubMed] [Google Scholar]
- 39.Weismuller K-H, Jung G, Hess G. Novel low-molecular-weight synthetic vaccine against foot-and-mouth disease containing a potent B-cell and macrophage activator. Vaccine. 1989;7:29–33. doi: 10.1016/0264-410x(89)90007-8. [DOI] [PubMed] [Google Scholar]
- 40.Weismuller K-H, Jung G, Gillessen L C, Bessler W G, Boltz W G. The antibody response in BALB/c mice to the Plasmodium falciparum circumsporozoite repetitive epitope covalently coupled to synthetic lipopeptide adjuvant. Immunology. 1991;72:109–113. [PMC free article] [PubMed] [Google Scholar]
- 41.Weismuller K-H, Bessler W G, Jung G. Solid-phase peptide synthesis of lipopeptide vaccines eliciting epitope-specific B-, T-helper and T-killer cell response. Int J Pept Protein Res. 1992;40:255–260. doi: 10.1111/j.1399-3011.1992.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 42.Young D, Kent L, Rees A, Lamb J, Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986;54:177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young D B, Garbe T R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142:55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Abdel Motal U M, Berg L, Jondal M. In vivo priming of cytotoxic T lymphocyte responses in relation to in vitro up-regulation of major histocompatibility complex class I molecules by short synthetic peptides. Eur J Immunol. 1992;22:3085–3090. doi: 10.1002/eji.1830221209. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Berg L, Abdel Motal U M, Jondal M. In vivo primary induction of virus-specific CTL by immunization with 9-mer synthetic peptides. J Immunol Methods. 1992;153:193–200. doi: 10.1016/0022-1759(92)90322-k. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]
- 47.Zhu X, Stauss H J, Ivanyi J, Vordermeier H M. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognizing the 38 kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997;9:1669–1676. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]