Abstract
BACKGROUND:
We examined the interplay of apolipoprotein B (apoB) and LDL particle size, approximated by the LDL-cholesterol (LDL-C)/apoB ratio, on the risk of new-onset coronary heart disease (CHD).
METHODS:
Participants without cardiovascular disease from the UK Biobank (UKB; n = 308 182), the Women’s Health Study (WHS; n = 26 204), and the Framingham Heart Study (FHS; n = 2839) were included. Multivariable Cox models were used to assess the relationship between apoB and LDL-C/apoB ratio and incidence of CHD (14 994 events). Our analyses were adjusted for age, sex (except WHS), HDL-cholesterol (HDL-C), systolic blood pressure, antihypertensive treatment, diabetes, and smoking.
RESULTS:
In all 3 studies, there was a strong positive correlation between apoB and LDL-C (correlation coefficients r = 0.80 or higher) and a weak inverse correlation of apoB with LDL-C/apoB ratio (−0.28 ≤ r ≤ −0.14). For all 3 cohorts, CHD risk was higher for higher levels of apoB. Upon multivariable adjustment, the association between apoB and new-onset CHD remained robust and statistically significant in all 3 cohorts with hazard ratios per 1 SD (95% CI): 1.24 (1.22–1.27), 1.33 (1.20–1.47), and 1.24 (1.09–1.42) for UKB, WHS, and FHS, respectively. However, the association between LDL-C/apoB and CHD was statistically significant only in the FHS cohort: 0.78 (0.64–0.94).
CONCLUSIONS:
Our analysis confirms that apoB is a strong risk factor for CHD. However, given the null association in 2 of the 3 studies, we cannot confirm that cholesterol-depleted LDL particles are substantially more atherogenic than cholesterol-replete particles. These results lend further support to routine measurement of apoB in clinical care.
Introduction
Except for intact chylomicrons, all the apolipoprotein B (apoB) particles, whether of intestinal or hepatic origin, can enter and be trapped within the arterial wall, depositing cholesterol within them and initiating and driving the progressive transformation of the arterial wall from normal to advanced complex atherosclerotic lesions, which cause myocardial infarctions and strokes (1).
Triglycerides have been the conventional marker to quantify VLDL apoB lipoprotein particles whereas cholesterol has been the conventional marker to quantify LDL apoB particles. However, the lipid content of VLDL and LDL particles can differ substantially, and there is increasing evidence that cardiovascular risk relates more closely to the total number of apoB-containing atherogenic lipoproteins than to the total circulating mass of triglyceride or cholesterol within them (2). This does not exclude the possibility that some apoB particles are more atherogenic than others. LDL particles almost always make up the great majority of total apoB particles (3), and LDL is, therefore, generally thought to account for most of the cardiovascular risk attributable to the apoB lipoproteins. However, some have proposed that larger VLDL particles may be particularly atherogenic and that risk may relate as much, or more, to VLDL particles as to LDL particles (4, 5).
The hypothesis of differential atherogenicity for different apoB particles extends to LDL particles, which may be subdivided into those that are larger because they contain more cholesterol and those that are smaller because they contain less. Indeed, multiple subclasses of LDL particles have been demonstrated by ultracentrifugation (6), gradient gel electrophoresis (7), nuclear magnetic resonance (8), and automated homogenous assays (9). There is general agreement that the larger, more buoyant LDL particles contain more cholesterol per particle whereas the smaller, denser LDL particles contain less. Several recent studies have reported that smaller cholesterol-depleted LDL particles are particularly atherogenic (10–14). This conclusion is supported by multiple lines of evidence describing several potential mechanisms for why smaller, denser LDL particles might be more atherogenic than the larger ones (15–20). Whether small cholesterol-depleted LDL particles are an independent factor increasing risk beyond total atherogenic particle number has not been settled, in part because differing methods have been applied to measure LDL particle subfractions and data from multiple large prospective studies using a single method are not available. Prior studies that have taken particle number into account have found that LDL particle size was no longer significant after adjusting for particle number (21, 22). Nevertheless, given the recent evidence that cholesterol-depleted LDL particles are more atherogenic than cholesterol-replete LDL particles (10–13), the issue remains unresolved.
To overcome the limitations of sample size, a simpler estimate, the LDL-cholesterol (LDL-C)/apoB ratio, which can be easily derived from established validated databases, can be used as a surrogate for average LDL particle cholesterol content and size. LDL-C is the mass of cholesterol within LDL particles and lipoprotein (a) particles. Because there is one molecule of apoB per particle, apoB equals the total number of LDL particles of which LDL particles, with few exceptions, account for 85% to 90% of total apoB particles. Almost all the rest are VLDL and lipoprotein (a); only 1% or fewer are chylomicrons or chylomicron remnants (3). Because of the preponderance of LDL particles, a low LDL-C/apoB identifies individuals with a preponderance of small cholesterol-depleted LDL particles (23, 24). Accordingly, we assembled data from 3 prospective cohorts: the UK Biobank (UKB), the Women’s Health Study (WHS), and the Framingham Heart Study (FHS) to examine the interplay of atherogenic particle number, measured by apoB, and LDL particle size, approximated by the LDL-C/apoB ratio, on the 10-year risk of incident coronary heart disease (CHD).
Methods
OUTCOME DEFINITIONS
The primary outcome was defined as time to 10-year incidence of CHD, comprised of coronary death or nonfatal myocardial infarction. A history of myocardial infarction, angina, coronary insufficiency, stroke, transient ischemic attack, intermittent claudication, or congestive heart failure excluded participation.
STUDY SAMPLE
UKB is a large cohort of over 500 000 women and men ages 37 to 73 years recruited in the UK between 2006 and 2010 from primary-care lists. Collection of the data and methods used for lipids assays were described elsewhere (25, 26). Participants with prevalent cardiovascular disease (CVD) at baseline examination were excluded from all analyses. Of the 502 452 participants enrolled, 40 to 73 years of age at baseline, 308 182 individuals were analyzed. A total of 32 143 patients were excluded due to a prior CVD event; 69 326 were on lipid-lowering therapy; 60 583 had missing records at baseline for lipids measurements; 25 877 had missing records for sex, systolic blood pressure, hypertension treatment, diabetes, body mass index, and smoking status. Participants with LDL-C ≥ 250 mg/dL and triglycerides ≥ 400 mg/dl were excluded because LDL-C could not be calculated using the Friedewald equation; out of assay range apoB levels (>400 mg/dL or < 20 mg/dL) were also excluded from the sample (n = 6341). The study protocol was approved by the McGill University Health Center Institutional Review Board.
The WHS is a primary prevention, randomized trial of vitamin E, β-carotene, or aspirin vs placebo (27). Participants were enrolled from April 1993 through January 1996. Of the 39 876 women enrolled in this trial, 26 204, ages 45 to 79, who were free of self-reported CVD or cancer at study entry, were followed for up to 23 years. Participants with missing data for lipid levels, who were on lipid-lowering treatment, and whose triglycerides ≥ 400 mg/dL and LDL-C ≥ 250 mg/dL were excluded from the analyses. The study was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
The FHS Offspring cohort started recruitment in 1971. In this study, out of 3203 women and men ages 40 to 75 who attended examination 4 (1987–1991) of the Offspring cohort and did not have prevalent CVD at baseline, 2839 were analyzed with follow-up censored at 12 years. One hundred three participants on lipid-lowering therapy were excluded, 204 did not have records for lipids measurements, and 41 did not have records for covariates (systolic blood pressure, blood pressure treatment, diabetes, and smoking). In addition, 26 people with out-of-range apoB levels (<20 mg/dL or >400 mg/dL), triglycerides ≥ 400 mg/dL, or LDL-C ≥ 250 mg/dL were excluded from the sample. All participants in the FHS gave written informed consent and the study protocol was approved by the McGill University Health Center Institutional Review Board.
DATA ANALYSIS
Demographic and baseline characteristics of participants were presented separately for each study. Values were expressed as median and interquartile range for continuous variables and numbers and percentages for categorical data. The magnitude of the association between lipid levels and clinical outcome was assessed using Pearson correlation coefficients. To aid assessment of the relationship between apoB and the LDL-C/apoB ratio with new-onset CHD, we created heat maps that plot the 10-year risk of CHD from a Cox proportional hazards model as a point on a 2-dimensional plane defined by apoB and LDL-C/apoB ratio, the 2 predictors included in the model. Each point was color-coded, with color moving from blue to red as risk increased.
Multivariable Cox proportional hazards models were fit to assess the relationship between apoB and LDL-C/apoB ratio and the incidence of CHD. In the WHS cohort, follow-up for CHD was censored at initiation of lipid-lowering therapy. The hazard ratio (HR) estimates, and the corresponding 95% CIs, were expressed per 1 SD for lipid and blood pressure variables. Models were adjusted for age, sex (except WHS), HDL-C, systolic blood pressure, antihypertensive treatment, diabetes, and smoking. In addition, Cox regression was adjusted for treatment assignment in the WHS cohort. Hypotheses were tested at the 2-sided alpha level of 0.05. Analyses were performed on SAS v.9.4 (SAS Institute) and R software version 9.2.4 (www.r-project.org).
Results
SUBJECT CHARACTERISTICS
The demographic and baseline characteristics of the study population by cohort are presented in Table 1. Women comprised the entire WHS cohort, 58.1% of the UKB, and 52.8% of the FHS. Median and interquartile ranges of baseline apoB levels were 104.7 mg/dL (90.0, 120.2), 99.0 mg/dL (83.2, 119.6), and 98.0 mg/dL (83.0, 114.0) in the UKB, WHS, and FHS, respectively. The corresponding levels of LDL-C were 141.5 mg/dL (121.6, 163.0), 120.9 mg/dL (100.3, 143.6). and 131.0 mg/dL (108.0, 155.0). Baseline levels of the LDL-C/apoB ratio were lower in WHS than in the other 2 cohorts. Triglycerides and HDL-C were lowest among the FHS participants. In total, 14 994 events occurred during follow-up (14 088, 702, and 204, for UKB, WHS, and FHS cohorts, respectively). Even though they were much smaller than UKB, including 2 studies from the United States should ensure greater generalizability of our analyses.
Table 1.
Baseline characteristics of study participants by study.
| Characteristic | UKB n = 308182 |
WHS n = 26 204 |
FHS n = 2839 |
|---|---|---|---|
| Age, median (25th–75th; years) | 56.00 (49.00; 62.00) | 52.7 (48.9; 58.7) | 52.0 (46.0; 59.0) |
| Female sex, n (%) | 178917 (58.1) | 26 204 (100) | 1498 (52.8) |
| Non-white race, n (%) | 49675 (16.1) | 1435 (5.5) | 0 (0) |
| BMI, median (25th–75th; kg/m 2 ) | 26.3 (23.8; 29.3) | 24.8 (22.3; 28.3) | 26.13 (23.5; 29.1) |
| Systolic blood pressure, median (25th–75th; mmHg) | 135.0 (123.5; 148.0) | 125.0 (115.0; 135.0) | 126.0 (115.0; 138.0) |
| Use of blood pressure-lowering medication, n (%) | 40 367 (13.1) | 3191 (12.2) | 447 (15.7) |
| Use of LDL-C-lowering medication, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Smoking, n (%) | 179986 (58.4)a | 12623 (48.2)a | 659 (23.2)b |
| Diabetes, n (%) | 4570 (1.5) | 597 (2.3) | 134 (4.7) |
| Total cholesterol, median (25th–75th; mg/dl) | 225.2 (199.4; 252.8) | 207.0 (183.0; 235.0) | 205.0 (181.0; 229.0) |
| HDL-C, median (25th–75th; mg/dl) | 55.8 (47.0; 66.4) | 52.0 (43.3; 62.4) | 48.0 (40.0; 59.0) |
| Triglycerides, median (25th–75th; mg/dl) | 125.2 (89.2; 178.7) | 116.0 (82.0; 168.0) | 98.0 (68.0; 146.0) |
| Non-HDL-C, median (25th–75th; mg/dl) | 167.4 (142.5; 194.2) | 152.5 (128.0; 179.4) | 154.0 (128.0; 181.0) |
| LDL-C, median, (25th–75th; mg/dl) | 141.5 (121.6; 163.0) | 120.9 (100.3; 143.6) | 131.0 (108.0; 155.0) |
| ApoB, median (25th–75th; mg/dl) | 104.7 (90.0; 120.2) | 99.0 (83.2; 119.6) | 98.0 (83.0; 114.0) |
| LDL-C/ApoB, median (25th–75th; mg/dl) | 1.35 (1.30; 1.41) | 1.21 (1.10; 1.33) | 1.35 (1.22; 1.46) |
| ASCVDc 10-year risk, median (25th–75th; %) | 4.56 (1.91; 9.55) | 2.05 (1.12; 4.28) | 4.0 (1.5, 9.2) |
Median and interquartile ranges for continuous variables and numbers and percentages for categorical data.
Current or former.
Current smoker.
Atherosclerotic cardiovascular disease.
ASSOCIATIONS BETWEEN LIPID PARAMETERS
The Pearson correlation coefficients for continuous lipids levels, log-transformed triglycerides, and LDL-C/apoB ratio are presented in Table 2. In all 3 studies, there was a high positive correlation between apoB and LDL-C and non-HDL-C values (correlation coefficients r = 0.80 or higher). The association between apoB and the LDL-C/apoB ratio was negative and weak in each study population (−0.28 ≤ r ≤ −0.14). HDL-C was weakly negatively correlated with apoB, non-HDL-C, and LDL-C. The correlation between apoB and log-transformed triglycerides was moderate and positive for all 3 studies. Furthermore, the LDL-C/apoB ratio was weakly positively correlated with non-HDL-C and negatively correlated with log-transformed triglycerides and only weakly to moderately positively correlated with LDL-C and HDL-C.
Table 2.
Pearson correlation coefficients between lipids levels among participants in the 3 studies.
| Study | ApoB | LDL-C | HDL-C | Non-HDL-C | Log Trigs | LDL-C/ApoB | |
|---|---|---|---|---|---|---|---|
| ApoB | UKB | 0.96 | −0.11 | 0.96 | 0.42 | −0.14 | |
| WHS | 0.80 | −0.30 | 0.85 | 0.52 | −0.28 | ||
| FHS | 0.81 | −0.33 | 0.88 | 0.55 | −0.20 | ||
| LDL-C | UKB | 0.01 | 0.98 | 0.38 | 0.14 | ||
| WHS | −0.04 | 0.94 | 0.30 | 0.33 | |||
| FHS | −0.18 | 0.94 | 0.25 | 0.32 | |||
| HDL-C | UKB | −0.07 | −0.46 | 0.44 | |||
| WHS | −0.13 | −0.36 | 0.41 | ||||
| FHS | −0.32 | −0.50 | 0.17 | ||||
| Non-HDL-C | UKB | 0.50 | 0.08 | ||||
| WHS | 0.54 | 0.17 | |||||
| FHS | 0.55 | 0.16 | |||||
| Log Trigs | UKB | −0.13 | |||||
| WHS | −0.32 | ||||||
| FHS | −0.33 |
HEAT MAPS OF ASSOCIATION BETWEEN APOB AND DL-C/APOB WITH CHD
Supplemental Figs. 1–3 plot incidence of CHD as a function of LDL-C/apoB ratio on the x axis and apoB on the y axis. Every point on the plot represents a person with a combination of apoB and LDL-C/apoB ratio that corresponds to a 10-year CHD risk, which is color-coded depending on its severity (with blue representing low risk and red representing high risk according to the scale at the bottom of the plot). We observe that, for all 3 cohorts, the top of the graph appears redder than the bluer bottom, consistent with CHD risk increasing as a function of increasing apoB. We also note that the red color seems more concentrated in the top left quadrant, suggesting that higher apoB as well as lower LDL-C/apoB ratio is associated with the highest risk. This effect appears the most pronounced in the UKB and FHS. Finally, we note the range of values for the LDL-C/apoB ratio appears greater at lower levels of apoB in all 3 cohorts.
MULTIVARIABLE-ADJUSTED ASSOCIATION OF APOB AND LDL-C/APOB WITH CHD
In the multivariable Cox proportional hazards regression models, standard risk factors displayed consistent association with 10-year CHD (Table 3). The association between apoB and new-onset CHD remained robust and statistically significant in all 3 cohorts upon multivariable adjustment (Table 3, Fig. 1). The HRs for apoB, expressed per 1 SDs, were: HR=1.24 (95% CI: 1.22–1.27), HR=1.33 (95% CI: 1.20–1.47), and HR=1.24 (95% CI: 1.09–1.42) for UKB, WHS, and FHS, respectively. However, the association between LDL-C/apoB and CHD was statistically significant only in the FHS cohort, with respective HRs of HR=1.00 (95% CI: 0.98–1.02), HR=0.97 (95% CI: 0.87–1.08), and HR=0.78 (95% CI: 0.64–0.94).
Table 3.
Association by study between apoB, LDL-C/apoB with CHD outcomes, per 1 SD increments adjusting for standard risk factors.
| UKB | WHS | FHS | ||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Sex (male) | 1.96 (1.89, 2.04) | <0.001 | — | — | 2.28 (1.64, 3.17) | <0.001 |
| Age (per year) | 1.07 (1.06, 1.07) | <0.001 | 1.09 (1.08, 1.10) | <0.001 | 1.05 (1.03, 1.07) | <0.001 |
| ApoB | 1.24 (1.22, 1.27) | <0.001 | 1.33 (1.20, 1.47) | <0.001 | 1.24 (1.09, 1.42) | 0.002 |
| LDL-C/ApoB | 1.00 (0.98, 1.02) | 0.842 | 0.97 (0.87, 1.08) | 0.540 | 0.78 (0.64, 0.94) | 0.010 |
| HDL-C | 0.79 (0.77, 0.81) | <0.001 | 0.90 (0.81, 1.01) | 0.061 | 0.79 (0.65, 0.97) | 0.022 |
| Systolic Blood Pressure | 1.19 (1.17, 1.21) | <0.001 | 1.35 (1.23, 1.48) | <0.001 | 1.17 (1.01, 1.36) | 0.037 |
| Blood pressure-lowering medications | 1.53 (1.47, 1.59) | <0.001 | 1.19 (0.93, 1.51) | 0.160 | 1.17a (0.84, 1.63) | 0.361 |
| Diabetes | 2.04 (1.87, 2.23) | <0.001 | 4.23 (3.08, 5.82) | <0.001 | 2.53 (1.70, 3.76) | <0.001 |
| Smoking | 1.22 (1.18, 1.26) | <0.001 | 1.77 (1.47, 2.13) | <0.001 | 1.49 (1.09, 2.05) | 0.014 |
Estimates for ApoB, LDL-C/ApoB, HDL-C, and systolic blood pressure are expressed per 1 SD.
Fig. 1.
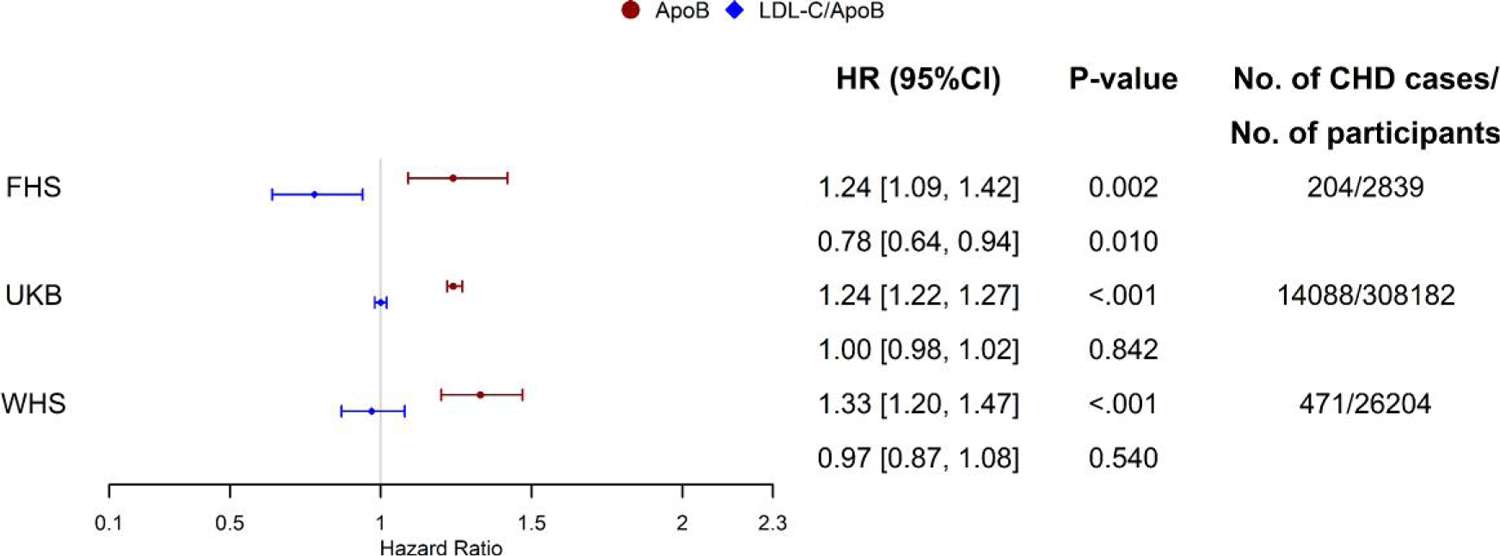
Forest plot for adjusted hazard ratios (95% CI) estimates for CHD incidence for a 1 SD higher apoB (red) and LDL-c/apoB ratio (blue) by study. Legend: Hazard ratios’ estimates and P values extracted from the Cox proportional hazards model adjusted for standard cardiovascular risk factors: age, sex (except WHS cohort), HDL-C, systolic blood pressure, blood pressure-lowering medication, diabetes, and smoking.
Discussion
In this study, we assessed the interplay between atherogenic particle number, estimated by apoB, and smaller, cholesterol-depleted LDL particles, estimated by the LDL-C/apoB ratio, as risk factors for new-onset CHD in 3 independent, epidemiological cohorts: the UKB, the WHS, and the FHS. Across all 3 studies we confirmed prior reports of a robust, positive association between apoB and CHD risk, of similar magnitude (HRs per 1 SD ranging from 1.24–1.33). Graphical inspection of the heat maps jointly relating apoB and LDL-C/apoB to new-onset CHD revealed that the highest risk appeared concentrated among participants with high apoB but low LDL-C/apoB (i.e., increased number of small, cholesterol-depleted LDL particles). However, after multivariable adjustment, apoB remained a significant predictor of CHD events, whereas particle size (as measured by the LDL-C/apoB ratio) remained significant only in the FHS but not in the more contemporary UKB or WHS cohorts. Accordingly, the present analysis confirms across 3 cohorts that particle concentration, as measured by apoB, is a powerful predictor of CHD events. By contrast, the LDL-C/apoB ratio showed no association with cardiovascular risk in 2 studies, UKB and WHS, whereas there was a significant negative relation in the FHS.
There are several possible explanations for these findings. It is possible that there exists a weak inverse association between LDL-C/apoB and cardiovascular risk that manifested itself in one of the 3 studies; conversely, it is also possible that there is no association and the effect observed in the FHS, our smallest cohort, represents the play of chance. Furthermore, the observed inconsistency can be driven by confounding lipid-lowering therapy. The FHS sample follow-up started in the late 1980s, before the widespread use of statins. The other 2 cohorts are more contemporary, with follow-up starting in the 1990s and 2000s, necessitating the exclusion of individuals who receive lipid-lowering treatment. Still, the consistency of the observed effect for apoB across the 3 cohorts makes this explanation less likely. Finally, it is possible that the lipid composition in the FHS participants, who had the lowest levels of triglycerides, contributed to the observed effect.
It is well established that increased numbers of smaller, cholesterol-depleted particles are common in patients with established CVD as well as in patients with conditions predisposing to high risk of CVD such as type 2 diabetes mellitus and abdominal obesity (14). In these groups of patients, hypertriglyceridemia in association with elevated apoB-hypertriglyceridemic hyperapobetalipoproteinemia is the most frequent atherogenic dyslipoproteinemia (28). This phenotype is characterized by elevated VLDL secretion, which results in increased production of LDL particles and, therefore, both hypertriglyceridemia and elevated apoB. This is consistent with the appearance of our heat maps, which illustrate that higher levels of apoB are associated with lower levels of the LDL-C/apoB ratio.
The pathophysiological mechanisms that generate cholesterol-depleted LDL particles have been worked out in detail. In the presence of hypertriglyceridemia, core lipid exchanges of cholesterol ester and triglyceride, mediated by cholesterol ester transfer protein, produce cholesterol-enriched VLDL particles and cholesterol-depleted LDL particles (29). However, as we and others have shown here and in prior work (1, 2), it appears to be the number of atherogenic particles, rather than their composition, that initiates and propagates the atherosclerotic process that culminates in clinical events.
Smaller LDL particles have been reported to be more atherogenic than larger LDL particles (14). However, smaller LDL cholesterol-depleted particles are generated from larger cholesterol-enriched ones, which creates an inverse relation between the concentrations of these 2 subgroups of LDL particles (21, 30, 31). Accordingly, studies that compare the atherogenic risk associated with different LDL particles must adjust the calculated risk for the differences in particle number of the different LDL subfractions. When this was done, no significant differences in atherogenic risk have been documented (21, 30, 31). Balling et al. demonstrated the hazard ratio for fatal and nonfatal myocardial infarction and stroke per mmol/LDL-C of small dense LDL was numerically greater than large buoyant LDL-C, but the difference was not statistically significant (10). However, the HRs per SD small, dense LDL-C, large, buoyant LDL-C, and apoB were identical. Since a SD is the same relative increase for all markers in a population, this finding is consistent with the risk posed by small and large LDL particles being identical when particle number is taken into account.
Our results also differ from those obtained by Drexel and his colleagues, who reported LDL-C/apoB ratio was a strong, independent risk factor for a clinical event (12). However, their study recruited patients with established coronary artery disease, whereas the subjects in the 3 studies we examined were all free from CVD at baseline. Statin use in the 3 tertiles of LDL-C/apoB ratio in their study varied between 43.9% and 59.3%, but statin use, which would have substantially reduced apoB during follow-up and therefore the cardiovascular risk associated with apoB measured at baseline, was not considered in multivariable analysis. Chang and Chen reported that a lower LDL-C/apoB ratio increased the risk of coronary artery calcification (11). However, a substantial increase in the odds ratio for the LDL-C/apoB ratio was only observed in those with severe coronary artery calcium compared to zero coronary artery calcium. Unfortunately, clinical data from the different groups were not included. The study by Ikezaki (13), also done with the Framingham cohort, did report small, dense LDL cholesterol to be particularly atherogenic but did not take apoB into account as the present study does. On the other hand, our results agree with previous work that takes particle number as well as composition into account (21, 30, 31).
STUDY LIMITATIONS
An important limitation of our study is that the LDL-C/apoB ratio is a surrogate for LDL size, not an exact measure. While this allows large numbers of subjects to be analyzed, it is an imperfect estimate of LDL particle size, as higher triglyceride content may compensate, in part, for a lower cholesterol content. Moreover, total apoB is an overestimate of the number of LDL particles since LDL apoB particles account for only 90% of total apoB particles (3). Nevertheless, the overall relation between this ratio and measured LDL size is strong enough (23, 24) that substantial differences in atherogenic risk among LDL particles with different cholesterol content should be detectable.
The strengths of our study are that we examined 3 well-conducted prospective observational studies in which the baseline status and clinical course were clearly documented and which used uniform definitions of clinical events. Although in the FHS a lower LDL-C/apoB ratio was associated with a higher risk of cardiovascular events, this was not confirmed in the larger and more contemporaneous WHS and UKB cohorts. Therefore, we cannot entirely exclude an impact of smaller LDL particles on clinical outcome. Nevertheless, if this is the case, it would not seem to be substantial. Our study did not address whether certain subclasses of VLDL particles might be particularly atherogenic.
In summary, 3 prospective observational studies—FHS, WHS, and UKB—all demonstrate that increasing levels of apoB significantly increase CHD risk. Two of these studies, the WHS and UKB, do not support the hypothesis that cholesterol-depleted LDL particles are associated with increased CHD risk whereas one, the FHS, did. These mixed results suggest that cholesterol-depleted LDL particles are unlikely to contribute substantially to increased cardiovascular risk. Our analysis therefore confirms the significance of particle number as measured by apoB as a risk factor for CHD and suggests a limited impact of particle size and/or composition. Clinical guidelines should consider focusing on apoB, as a measure of atherogenic particle number, rather than LDL-C, as the primary lipid parameter.
Supplementary Material
Employment or Leadership:
S. Mora, Treasurer, International Atherosclerosis Society.
Consultant or Advisory Role:
M. Pencina reports past advisory board for Boehringer Ingelheim. P.M Ridker has served as a consultant to Corvidia, Novartis, Flame, Agepha, Alynlam, IQVIA, R-Pharma, Horizon Therapeutics, Inflazome, AstraZeneca, Jannsen, Civi Biopharm, SOCAR, Novo Nordisk, Uptton, Health Outlook, Omeicos, the Baim Institute, Boehringer-Ingelheim, Montai Health, and Cardiol Therapeutics and served on an academic advisory board for Peter Munk Institute, University of Toronto, and Foundation Leducq, Paris FR. G. Thanassoulis has participated in advisory boards for Amgen, Regeneron/Sanofi, HLS Therapeutics, Ionis, Servier, and Novartis and has consulted for Silence. S. Mora has served as consultant to Pfizer for work outside the current study.
Stock Ownership:
None declared.
Honoraria:
G. Thanassoulis has participated in a speaker bureau for Amgen, Regeneron/Sanofi, HLS Therapeutics, Ionis, Servier, Novartis.
Research Funding:
The Women’s Health Study was supported by the National Institutes of Health (CA-047988, HL-043851, HL-080467, HL-099355, and UM1CA182913). S. Mora was supported by a research grant from the National Heart, Lung, and Blood Institute (K24 HL136852). This research was also funded by an unrestricted grant from the Doggone Foundation. K.M. Pencina reports funding from the nonprofit Doggone Foundation. M.J. Pencina reports funding from the nonprofit Doggone Foundation and past grants to Duke from Regeneron/Sanofi and Amgen. P.R. Lawler is supported by a National New Investigator award from the Heart and Stroke Foundation of Canada. P.M Ridker has received research grant support from Novartis, Kowa, Amarin, Pfizer, Esperion, Operation Warp Speed, NHLBI, and NCI; drug supply for federal COVID-19 trials from Pfizer Bristol Myers Squibb Alliance; and testing devices for federal COVID-19 trial from Quidel, Inc. G. Thanassoulis has received grant funding from Servier and Ionis.
Expert Testimony:
None declared.
Patents:
S. Mora has a patent regarding the use of an NMR biomarker in relation to colorectal cancer risk.
Role of Sponsor:
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Nonstandard Abbreviations:
- apoB
apolipoprotein B
- LDL-C
LDL-cholesterol
- UKB
UK Biobank
- WHS
Women’s Health Study
- FHS
Framingham Heart Study
- HDL-C
HDL-cholesterol
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HR
hazard ratio
Footnotes
Supplementary Material
Supplementary material is available at Clinical Chemistry online.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
References
- 1.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019;4:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marston NA, Giugliano RP, Melloni GEM, Park J-G, Morrill V, Blazing MA, et al. Apolipoprotein-B-containing lipoproteins and risk of myocardial infarction: distinguishing between particle concentration, type and content. JAMA Cardiol 2022;7:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sniderman AD, Couture P, Martin SS, DeGraaf J, Lawler PR, Cromwell EC, et al. Hypertriglyceridemia and cardiovascular risk: a cautionary note about metabolic confounding. J. Lipid Res 2018;59:1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen MØ, Vedel-Krogh S, Nielsen SF, Afzal S, Davey Smith G, Nordestgaard BG. Per-particle triglyceride-rich lipoproteins imply higher myocardial infarction risk than low-density lipoproteins: Copenhagen General Population Study. Arterioscler Thromb Vasc Biol 2021;41: 2063–75. [DOI] [PubMed] [Google Scholar]
- 5.Balling M, Afzal S, Varbo A, Langsted A, Davey Smith G, Nordestgaard BG. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J Am Coll Cardiol 2020;76:2725–35. [DOI] [PubMed] [Google Scholar]
- 6.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. ATH 1994;106:241–53. [DOI] [PubMed] [Google Scholar]
- 7.Williams PT, Vranizan KM, Krauss RM. Correlations of plasma lipoproteins with LDL subfractions by particle size in men and women. J Lipid Res 1992;33:765–74. [PubMed] [Google Scholar]
- 8.Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem 1991;37:377–86. [PubMed] [Google Scholar]
- 9.Hirano T, Ito Y, Saegusa H, Yoshino G. A novel and simple method for quantification of small, dense LDL. J Lipid Res 2003;44: 2193–201. [DOI] [PubMed] [Google Scholar]
- 10.Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S. Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol 2020;75:2873–5. [DOI] [PubMed] [Google Scholar]
- 11.Chang T-Y, Chen J-D. Low-density lipoprotein cholesterol/apolipoprotein B ratio is superior to apolipoprotein B alone in the diagnosis of coronary artery calcification. Coron Artery Dis 2021;32:561–6. [DOI] [PubMed] [Google Scholar]
- 12.Drexel H, Larcher B, Mader A, Vonbank A, Heinzle CF, Moser B, et al. The LDL-C/ApoB ratio predicts major cardiovascular events in patients with established atherosclerotic cardiovascular disease. Atherosclerosis 2021;329:44–9. [DOI] [PubMed] [Google Scholar]
- 13.Ikezaki H, Lim E, Cupples LA, Liu C-T, Asztalos BF, Schaefer EJ. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham Offspring Study. J Am Heart Assoc 2021;10:e019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou L, Kaptoge S. Association of small, dense LDL-cholesterol concentration and lipoprotein particle characteristics with coronary heart disease: a systematic review and meta-analysis. PLoS One 2020; 15:e0241993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordestgaard BG, Zilversmit DB. Comparison of arterial intimal clearances of LDL from diabetic and nondiabetic cholesterol-fed rabbits. Differences in intimal clearance explained by size differences. Arteriosclerosis 1989;9:176–83. [DOI] [PubMed] [Google Scholar]
- 16.Björnheden T, Babyi A, Bondjers G, Wiklund O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. ATH 1996;123:43–56. [DOI] [PubMed] [Google Scholar]
- 17.Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Wiklund O, Bondjers G. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J Lipid Res 1990;31:1387–98. [PubMed] [Google Scholar]
- 18.Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. ATH 1996;124:261–71. [DOI] [PubMed] [Google Scholar]
- 19.de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb 1991;11:298–306. [DOI] [PubMed] [Google Scholar]
- 20.Chancharme L, Thérond P, Nigon F, Lepage S, Couturier M, Chapman MJ. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler Thromb Vasc Biol 1999;19:810–20. [DOI] [PubMed] [Google Scholar]
- 21.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, et al. LDL Particle subclasses, LDL particle size, and carotid atherosclerosis in the multi-ethnic study of atherosclerosis (MESA). ATH 2007;192:211–7. [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard P-M, Després J-P, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol 2005;25:553–9. [DOI] [PubMed] [Google Scholar]
- 24.Kaneva AM, Potolitsyna NN, Bojko ER. Usefulness of the LDL-C/apoB ratio in the overall evaluation of atherogenicity of lipid profile. Arch Physiol Biochem 2017; 123:16–22. [DOI] [PubMed] [Google Scholar]
- 25.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott P, Peakman TC, Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol 2008;37:234–44. [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Bassuk SS, Lee I-M, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sniderman AD, Scantlebury T, Cianflone K. Hypertriglyceridemic hyperapob: the un-appreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus. Ann Intern Med 2001;135:447–59. [DOI] [PubMed] [Google Scholar]
- 29.Nurmohamed NS, Ditmarsch M, Kastelein JJP. CETP-inhibitors: from HDL-C to LDL-C lowering agents? [Epub ahead of print] Cardiovasc Res November 26, 2021 as doi: 10.1093/cvr/cvab350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams PT, Zhao X-Q, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014;233: 713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, et al. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation 2012;125:2469–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


