Abstract
The OxyR regulon is known to mediate protection against oxidizing agents in Salmonella typhimurium. We reported previously that ahp, one of the OxyR-regulated loci, is induced during macrophage interaction (K. P. Francis, P. D. Taylor, C. J. Inchley, and M. P. Gallagher, J. Bacteriol. 179:4046–4048, 1997). We now report on the effects of disrupting ahp or oxyR on virulence in a BALB/c mouse model. Surprisingly, insertion of a Mudlux derivative within ahpC was found to result in attenuation, while irreversible inactivation of the locus through insertion of a cml cassette did not. An SL1344 derivative carrying an oxyR::kan disruption was also found to be as virulent as the parental strain. Moreover, both cell-mediated and humoral responses to AhpC were found to develop during the course of infection, probably through T-helper-cell (type I) activation. These results indicate that, although not essential for virulence, AhpC is expressed by S. typhimurium during infection of BALB/c mice and constitutes a target for the immune system.
Typhoid fever remains the most serious of the diseases caused by serovars of Salmonella, with an estimated 16.6 million cases occurring per year in developing countries. In addition, however, nontyphoidal serovars are responsible for approximately 1.3 billion incidences of diarrheal disease per year (33). Salmonella infection of animals is also a common occurrence; such infection not only has economic importance in agriculture and animal husbandry but also is believed to be a major factor in the transmission of Salmonella to humans via the food chain (43). Our abilities to control Salmonella infections have become threatened by the spread of multidrug resistance, and there is a growing need to develop effective vaccine strategies to diminish reservoirs of this organism and thereby reduce the incidence of related illness.
One of the most promising approaches to vaccination against Salmonella is the development of live attenuated bacterial strains that carry defined genetic lesions in genes which are required for virulence in the host. A number of such lesions that reduce the ability of Salmonella typhimurium to cause disease in a mouse model have been identified. These include mutations in genes encoding metabolic enzymes, such as aroA (31), or encoding regulatory proteins, such as cya or crp (18) or phoPQ (42). In many cases, these same lesions have also proved valuable as the basis for developing vaccines against S. typhi (58).
Invasive Salmonella serovars are capable of breaching the intestinal epithelium and penetrating the underlying tissue (36). Here they enter macrophages and, shortly afterward, disseminate to the liver and spleen, where they are capable of replicating and causing serious disease. Normally, macrophages are capable of destroying bacteria by a respiratory burst that produces high levels of reactive oxygen species, such as superoxide, hydrogen peroxide, and nitric oxide, as well as through the use of antimicrobial factors associated with the lysosome (29, 36, 47). However, Salmonella cells are capable of surviving within macrophages. This ability forms part of the defensive strategy of the microbe and is important for virulence.
Oxidative stress is known to damage the bacterial cell at all macromolecular levels, and S. typhimurium has been shown to respond to such stress by the derepression of multigenic defense systems (reviewed in reference 21). The hydrogen peroxide stress response, for example, results in the induction of about 30 proteins (14), and a small subset of the genes encoding these proteins is regulated by a protein called OxyR. Genes of the OxyR regulon, which include katG (catalase), ahpCF (alkyl hydroperoxide reductase), and dps (DNA binding protein from starved cells), help to reduce the detrimental effects of hydrogen peroxide.
Indications that the genes which convey resistance to oxidative stress might be important for virulence have been provided in a number of studies. Previously, for example, we showed that a Mudlux fusion induced in S. typhimurium SL1344 by hydrogen peroxide was also induced upon interaction with macrophages (24). More recently, we reported that the Mudlux element lies within the ahpC gene (25). Moreover, in alternative studies, the dps gene of S. typhimurium was demonstrated to be induced within macrophages (61) and, in an earlier report, transposon mutants which exhibited reduced survival in macrophages and attenuation in mice were found to be hypersusceptible to hydrogen peroxide (23). In addition, disruption of the recA or htrA locus in S. typhimurium has been shown to result in attenuation and in enhanced susceptibility to oxidative stress (9, 35).
It seemed reasonable, then, that genes which encode proteins involved in protection against hydrogen peroxide damage might be important for the survival of S. typhimurium during the course of infection and, in particular, during interaction with macrophages or polymorphonuclear cells. Such genes, when disrupted, would also appear to be likely candidates for mediating attenuation and therefore are of potential interest for the development of live bacterial vaccines. In the present study, we report the effects of disrupting ahp or oxyR on the virulence of S. typhimurium in a BALB/c mouse model. Moreover, we provide evidence that an immune response is elicited against the AhpC polypeptide by both the humoral and the cell-mediated aspects of the immune system during the course of infection.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani medium or Spizizen minimal medium containing ampicillin (50 μg/ml), kanamycin (50 μg/ml), chloramphenicol (25 μg/ml), or tetracycline (10 μg/ml) as appropriate.
TABLE 1.
Bacterial strains and plasmids used in this studya
| Bacterial strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BL21(DE3) | F−omp+ rB− mB− (DE3 is a λ lysogen bearing lacI, lacUV5, and gene 1) pLysS | 57 |
| DH5α | F− φ80dlac ΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK−) supE44 thi-1 gyrA96 relA1 λ− | 28 |
| S. typhimurium | ||
| SL1344 | his; virulent mouse pathogen | 31 |
| SL1346 | hisG46 aroA554::Tn10 | 31 |
| MPG203 | ahpC::Mudlux derivative of SL1344 | 25 |
| MPG473 | ahp::cml derivative of SL1344 | This study |
| MPG484 | oxyR::kan derivative of SL1344 | This study |
| MPG479 | aroA554::Tn10 derivative of SL1344 | This study |
| MPG481 | BL21(DE3)(pLysS) containing pPDT14 | This study |
| Plasmids | ||
| pBR322 | Apr Tcr | 4 |
| pBR325 | Apr Tcr Cmr | 5 |
| pGEM-T | TA cloning vector; Apr | Promega |
| pUC4-K | Apr Kmr | Pharmacia |
| pET-19b | T7 expression vector; Apr | Novagen |
| pPDT5 | pBR322 ahpCFSt; Apr | This study |
| pPDT6 | pPDT5 ahp::cml Apr Cmr | This study |
| pPDT7 | pGEM-T oxyRSt Apr | This study |
| pPDT8 | pPDT7 oxyR::kan Apr Kmr | This study |
| pPDT14 | pET-19b ahpCSt Apr | This study |
Apr, ampicillin resistance; Tcr, tetracycline resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; St, S. typhimurium.
Recombinant DNA techniques.
General DNA manipulations were performed as described previously (51). DNA sequences were determined by the dideoxy chain termination procedure (52). For the PCR, samples were denatured for 1 min (94°C), followed by annealing at 55°C for 1 min and elongation at 72°C for 2 min. The process was completed by a final cycle of 72°C for 10 min. Southern blotting was carried out on Hybond-N (Amersham) as described previously (51). Probes were labelled by random priming essentially as described previously (22). The Genetics Computer Group suite of programs was used for sequence analysis.
Insertional inactivation of the S. typhimurium ahp and oxyR loci.
Plasmid pPDT5 (Table 1) was constructed by PCR amplification of the ahpCF open reading frames (bases 198 to 2524) (59) with SL1344 genomic DNA as a template and primers 1 (5′-GCGGATCCCAAAAACCAGGCGTTCA-3′) and 2 (5′-CGAAGCTTGGTGCGAATCAGATAAT-3′), which contain BamHI or HindIII sites near their 5′ ends to facilitate cloning of the amplified product into the corresponding sites of pBR322. Plasmid pPDT6 was derived from plasmid pPDT5 by insertion of a cml cartridge within the natural MluI and HpaI sites of ahp (Fig. 1A). The cml cartridge was amplified from pBR325 (bases 5245 to 5316) (5) with primers 3 (5′-GCGATATCAAGCTTACGCGTCGTAGCACCAGGCGTTTAAGGGCAC-3′) and 4 (5′-GGGATATCAAGCTTGTTAACCGTCTAAGAAACCATTATTATCATG-3′), which carry the corresponding restriction sites to aid in subcloning into pPDT5.
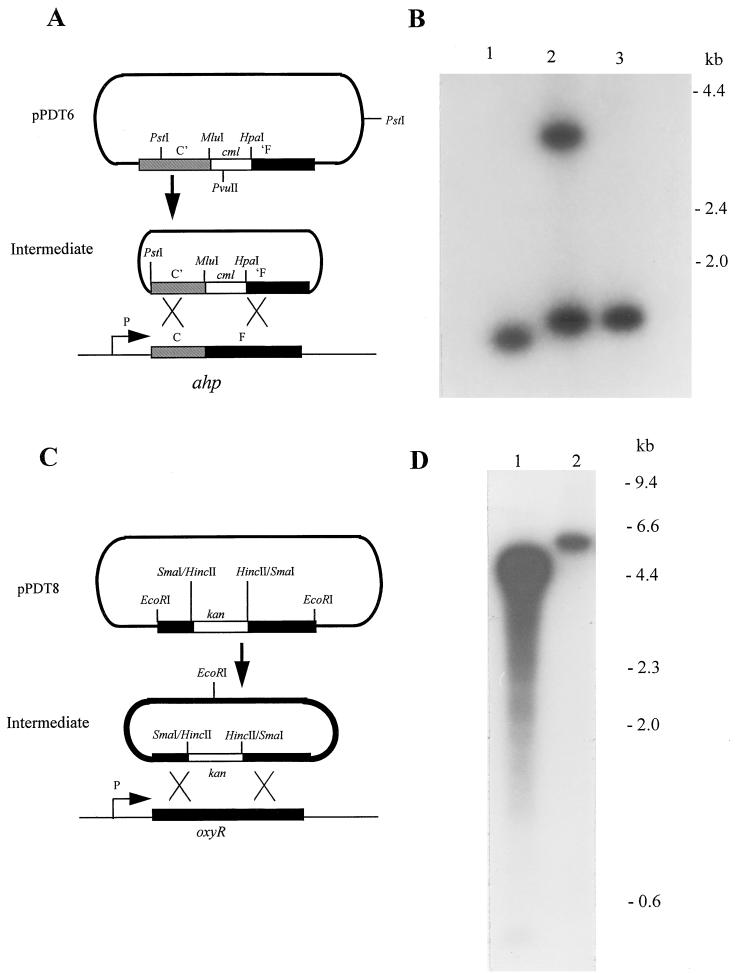
FIG. 1.
Disruption of the S. typhimurium ahp and oxyR loci. (A) The ahpCF locus of S. typhimurium was amplified by PCR (see Materials and Methods) and inserted into the BamHI and HindIII sites of pBR322, forming pPDT5. Subsequently, the PCR-amplified cml gene of pBR325 was inserted into the MluI and HpaI sites of the cloned ahp locus, resulting in the disruption of both ahpC and ahpF (pPDT6). The ahp locus was then liberated by PstI digestion, recircularized with DNA ligase (Intermediate), and electroporated into SL1344 in order to disrupt the corresponding chromosomal locus. Relevant restriction sites are shown. Plasmid DNA is shown as a thick line, and chromosomal DNA is shown as a thin line. C′ and ′F represent truncation of the 3′ and 5′ regions of ahpC and ahpF, respectively. (B) Southern blot of genomic DNAs from SL1344 and derivatives carrying an ahp::cml insertion. Samples of genomic DNA from SL1344 (lane 1) or from mutants that had undergone a single crossover event (lane 2) or a double crossover event (lane 3) (MPG473) are shown following digestion with HpaI. The ahpC gene, amplified by PCR from SL1344 with primers 7 and 8 (see Materials and Methods), was used as the probe. The positions of relevant DNA molecular size markers are indicated. (C) The oxyR locus of S. typhimurium was amplified by PCR (see Materials and Methods) and inserted into pGEM-T, forming pPDT7. Subsequently, a HincII fragment from pUC4-K, carrying the kanamycin resistance gene, was inserted into the SmaI site of the cloned oxyR locus, resulting in disruption (pPDT8). The oxyR locus was then liberated by EcoRI digestion, recircularized with DNA ligase (Intermediate), and electroporated into SL1344 in order to disrupt the corresponding chromosomal locus. Thick and thin lines are as in panel A. (D) Southern blot of genomic DNAs from SL1344 and a derivative carrying an oxyR::kan insertion. Samples of genomic DNA from SL1344 (lane 1) or from a mutant that had undergone a double crossover event (lane 2) (MPG484) are shown following digestion with HpaI. Probing was carried out with the oxyR gene, which was amplified by PCR from SL1344 with primers 5 and 6 (see Materials and Methods). The positions of relevant DNA molecular size markers are indicated.
Plasmid pPDT7 was constructed by PCR amplification of the complete oxyR open reading frame with SL1344 genomic DNA as a template and primers 5 (5′-CGGAATTCATCGCCATGACTATCG-3′) and 6 (5′-CCGAATTCATATCGGTCAGGCGATT-3′), which are based on the Escherichia coli oxyR sequence (bases 167 to 1238) (15) and which contain an artificial EcoRI site near their 5′ ends. In this case, the amplified product was cloned directly into pGEM-T without restriction. The kanamycin resistance cassette of pUC4-K, which carries its own promoter, was excised as a 1.3-kb HincII fragment and cloned into the unique SmaI site of plasmid pPDT7, forming pPDT8 (Fig. 1C).
The ahp::cml and oxyR::kan inserts were liberated with PstI and EcoRI, respectively, and then circularized with DNA ligase. The products were electroporated into SL1344 as previously described (45), and the transformants were subsequently screened for loss of ahp or oxyR function by disc inhibition assays which examined the sensitivity of isolates to hydrogen peroxide or cumene peroxide (14, 25). Genomic DNA was isolated from a selection of mutants which exhibited large zones of inhibition equivalent to that of MPG203 and, in the case of the oxyR mutants, grew as small colonies. This screen was used to determine by Southern blotting whether single or double crossover events had occurred.
Analysis of the sequence of the ahp locus (59) revealed that an HpaI site exists within ahpF such that restriction of DNA from the wild-type locus liberates the entire ahpC gene as a 1.38-kb fragment. In addition, a single crossover event should produce two fragments, a 1.45-kb fragment containing the ahpC′::cml region and a 2.93-kb fragment carrying the truncated ahpF′ gene, together with a fragment of pBR322 and an intact copy of ahpC. In contrast, a double crossover event should liberate a single, 1.45-kb ahpC::cml fragment following digestion. Genomic DNA was therefore restricted with HpaI and probed with the PCR-amplified ahpC gene. The results from representative examples of mutants are shown in Fig. 1B. An ahp-70 strain was found to produce a fragment of the correct size, indicating that it had arisen through a double crossover event. This strain was redesignated MPG473 and was used for subsequent attenuation studies.
For oxyR, sequence analysis revealed a unique HpaI restriction site at the 3′ end (data not shown), and this provided a means to distinguish cells that contained two copies of the oxyR gene (as a result of a single crossover event) from those that contained a single copy of the gene (as occurs in the wild-type situation or as a result of a double crossover event). However, because the location of the nearest upstream HpaI site on the genome is not known, the sizes of the HpaI fragments liberated from the genomes of wild-type cells or cells which have undergone a double crossover event cannot be predicted, although in the latter case the fragment should be 1.3 kb larger than that from wild-type DNA due to the kanamycin resistance cassette. A single recombination event, on the other hand, should produce two bands, one of the same size as that produced from a double recombination event and the other of 1.1 kb (because two copies of the oxyR gene are present). DNAs from SL1344 and from selected mutants were therefore digested with HpaI and examined by Southern blotting with the PCR-amplified S. typhimurium oxyR gene as a probe. DNA from MPG484 was found to produce a single band of approximately 6.0 kb, while DNA from SL1344 produced a band of approximately 4.7 kb (Fig. 1D). A parallel sample was also probed with the kanamycin resistance cassette obtained from pUC4-K and produced bands of predicted sizes (data not shown).
Overexpression and purification of AhpC.
Expression vector pPDT14 was constructed by subcloning the PCR-amplified complete open reading frame of ahpC (bases 156 to 761) (59) from SL1344. Primers 7 and 8 (5′-GGAATTCCATATGTCCTTAATTAA-3′ and 5′-GCGGATCCAACGCAGCTATGGC-3′, respectively) were designed such that the amplified product contained an NdeI site (overlapping the ATG of the translation initiation codon) and a BamHI site at the 5′ and 3′ ends, respectively. The 0.6-kb fragment was cloned into the corresponding sites of pET-19b (Table 1) such that an in-frame fusion protein would be formed with a polyhistidine tag at the N terminus when expressed from the upstream T7 promoter. E. coli BL21(DE3)(pLysS), which carries on the chromosome the T7 RNA polymerase gene under the control of the lac promoter, was used for expression studies.
For overproduction, cells carrying pPDT14 (designated MPG481) were cultured overnight at 37°C with shaking in Luria-Bertani medium containing chloramphenicol and ampicillin. The bacteria were then centrifuged, resuspended in fresh Luria-Bertani medium, diluted 50-fold in Spizizen minimal medium containing the appropriate antibiotics, and grown until the culture reached an optical density at 600 nm of 0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG) (0.5 mM final concentration) was added to elicit protein expression, and the culture was incubated for a further 3 h. Cells were then harvested, resuspended in buffer A (10 mM Tris-Cl [pH 7.9] containing 10% [vol/vol] glycerol, 0.5 M NaCl, 0.1% [vol/vol] Nonidet P-40, 5 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]), and sonicated.
The soluble fraction, obtained after low-speed centrifugation, was subjected to affinity chromatography on Ni2+-nitrilotriacetic acid affinity resin (Qiagen) that had been preequilibrated with buffer A and was then washed extensively with buffer B (20 mM Tris-Cl [pH 7.9] containing 20% [vol/vol] glycerol, 100 mM KCl, 5 mM dithiothreitol, and 0.5 mM PMSF) containing 20 mM imidazole. The AhpC fusion protein was subsequently eluted with buffer B containing 80 mM imidazole. The protein was rechromatographed through the Ni2+ column to maximize purity prior to dialysis against phosphate-buffered saline (PBS), concentration, and analysis by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
SDS-PAGE and immunoblotting.
Protein samples were electrophoresed in the presence of SDS as described previously (39). Following electrophoresis, proteins were either stained with Coomassie brilliant blue or transferred by electroblotting to a nitrocellulose membrane (60). Immunoblotting procedures were carried out with antisera (equal amounts pooled from mice in the group) and alkaline phosphatase-conjugated anti-mouse immunoglobulin G (whole molecule; Sigma Corporation, Poole, United Kingdom). The detection of bound antisera by alkaline phosphatase staining was carried out as described previously (38).
Attenuation studies.
Bacterial strains used for infection were grown overnight at 37°C in Luria-Bertani medium containing antibiotics as appropriate, and the cells were washed twice with PBS prior to serial dilution. Bacterial cell numbers were determined by viability plating. Groups of six female BALB/c mice (8 to 10 weeks old) were used for infection studies. Each mouse was inoculated with 0.1 ml of PBS containing between 101 and 106 cells/ml (groups differed in the numbers of bacterial cells that they received [approximately 10-fold]). Survival of the mice was monitored for at least 28 days, and the 50% lethal dose (LD50) was calculated as described previously (49).
Determination of CMI and humoral immunity.
An inflammatory (delayed-type hypersensitivity) reaction, based on footpad swelling, was used as a measure of cell-mediated immunity (CMI). For this purpose, groups of six female BALB/c mice (8 to 10 weeks old) were injected intraperitoneally with 0.1 ml of PBS containing approximately 105 CFU of attenuated S. typhimurium MPG479 or with PBS alone. On day 33 or 104 postinfection, mice were challenged by footpad injection with 0.05 ml of PBS containing 0.04 mg of purified, heat-aggregated, His-tagged AhpC or mouse albumin (Sigma) or with PBS alone or were left uninjected (see legends to figures). At 24 and 48 h after injection, the thickness of each hind footpad was determined as the mean ± standard error of the mean of three measurements, in thousandths of an inch, taken in different vertical planes with dial-type calipers. Data is presented as the footpad thickness ratio, defined as [thickness of right footpad/thickness of left (control) footpad] × 100. Statistical comparisons were made after appropriate arcsine transformation of individual data, according to the formula y = arcsin (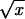 + k), where x is the incremental enlargement of the right footpad [(footpad thickness ratio − 100)/100], k is 0.1, and y is the transformed product, expressed in degrees. Mean values for y were calculated and compared with the Student t test.
+ k), where x is the incremental enlargement of the right footpad [(footpad thickness ratio − 100)/100], k is 0.1, and y is the transformed product, expressed in degrees. Mean values for y were calculated and compared with the Student t test.
Humoral immunity was determined by exploring whether pooled sera obtained from infected or uninfected mice at appropriate intervals were capable of binding to purified His-tagged AhpC, as determined by immunoblotting. For this purpose, equal volumes of blood were taken from the mice in the group and pooled.
RESULTS
S. typhimurium MPG203 (ahp::Mudlux) is attenuated in vivo.
Previously we showed that the S. typhimurium SL1344 derivative MPG203 contained a Mudlux element within the ahpC gene and that this locus was induced during interaction with macrophages of the mouse cell line J774.2 (24). The availability of this strain provided an opportunity to test whether the ahp locus was required for the virulence of S. typhimurium in a BALB/c mouse model. In order to address this issue, cultures of MPG203 or the virulent parental strain SL1344 were grown overnight in Luria-Bertani medium and then serially diluted 10-fold in PBS to give a range of bacterial concentrations of approximately 101 to 106 organisms per ml. Groups of six female BALB/c mice (8 to 10 weeks old) were injected by the intraperitoneal route with 100 μl from one of the six cultures of bacteria, and their survival was monitored over 28 days. From Table 2 it can be seen that SL1344 killed almost all of the mice in the study, with an LD50 of 1.0, in agreement with other studies (31). In contrast, the LD50 of MPG203 was found to be 4.8, suggesting that the disruption of ahp by insertion of the Mudlux element results in a substantial loss of virulence.
TABLE 2.
Attenuation of SL1344 derivatives carrying genetic disruptions in ahpC or oxyRa
| Log10 no. of bacterial cells injected | No. of mice surviving at 28 days postinfection with:
|
|||
|---|---|---|---|---|
| SL1344 | MPG203 (ahp::Mudlux) | MPG473 (ahp::cml) | MPG484 (oxyR::kan) | |
| 0–1 | 3 | 6 | 2 | 2 |
| 1–2 | 1 | 6 | 0 | 1 |
| 2–3 | 0 | 5 | 0 | 1 |
| 3–4 | 0 | 5 | 0 | 0 |
| 4–5 | 0 | 3 | 0 | 0 |
| 5–6 | 0 | 0 | 0 | 0 |
| LD50 (log10 no. of bacterial cells) | 1.0 | 4.8 | <1.0 | 1.0 |
Attenuation studies were carried out with groups of six female BALB/c mice (8 to 10 weeks old). Bacterial strains were grown overnight in Luria-Bertani medium, pelleted, and washed in PBS before dilution. Mice were injected intraperitoneally with between 101 and 106 bacteria (confirmed by plating) in 100 μl of PBS, each group differing by approximately 1 log unit of bacteria. Survival was monitored over a period of 28 days after infection, and the LD50 was calculated as described previously (49).
ahp::cml or oxyR::kan mutants are not attenuated in vivo.
In order to confirm the above observations and to rule out the possibility that attenuation was a consequence of the Mudlux element per se, we decided to irreversibly disrupt the chromosomal ahp locus by an alternative approach by inserting the cml gene (which conveys chloramphenicol resistance) from pBR325 (5). The ahp locus from S. typhimurium is a bicistronic locus which encodes ahpC and ahpF and has been cloned and sequenced (59). Primers with BamHI or HindIII restriction sites at their 5′ ends were used to amplify the ahp locus from the SL1344 genome by PCR, and the product was cloned into the corresponding sites of pBR322 (4), forming pPDT5. A 0.98-kb chloramphenicol cassette was then amplified from pBR325 with primers which incorporated MluI or HpaI sites at their 5′ ends, and the product was inserted into the corresponding natural sites in ahp to produce pPDT6.
When restricted with PstI, pPDT6 liberated a fragment of approximately 3 kb carrying the disrupted ahp locus (Fig. 1A). This fragment was circularized with DNA ligase and then used to transform SL1344 by electroporation as previously described (45). Transformants in which the cml gene had been inserted within the chromosomal ahp locus were selected on Luria-Bertani agar containing chloramphenicol and then screened for ampicillin sensitivity by replica plating. Allelic exchange as a result of a double crossover event was confirmed by Southern blotting (see Materials and Methods and Fig. 1B). The resulting strain, designated MPG473, was used for subsequent attenuation studies.
A culture of MPG473 was prepared as previously described, and the cells were serially diluted in PBS to approximately 101 to 106 organisms per ml. Groups of six female BALB/c mice (8 to 10 weeks old) were injected intraperitoneally with 0.1 ml of one of the dilutions of bacteria, as before, and the mice were monitored for survival over 28 days. From Table 2 it can be seen that virtually all of the mice injected with MPG473 were killed by fewer than 10 organisms, with an LD50 of less than 1 (49). Thus, it seems that this mutant is as virulent as SL1344 and that the ahpCF locus has little effect on the gross virulence of S. typhimurium. These results suggest that the attenuation observed with MPG203 results from some property of the Mudlux element, rather than a loss of Ahp function.
The above study indicated that the ahpCF locus of S. typhimurium plays little or no discernible role in virulence, as determined by LD50s. It seemed likely, though, that other genes of the OxyR regulon might be of more significance or perhaps might compensate for the disruption of ahp. This view is suggested by the demonstration of Fields et al. (23) that a number of mutants which were unable to survive in macrophages and which were attenuated in mice were sensitive to oxidizing agents, such as hydrogen peroxide. We therefore decided to examine the importance of the oxyR gene per se and thereby the OxyR regulon by using a similar strategy of gene disruption and LD50 trials.
The sequence of the oxyR gene is known for E. coli but has not been reported for S. typhimurium (15). To overcome this problem, we exploited the fact that the genomes and commonly the coding regions of these two organisms are highly related. Primers which were based on the E. coli oxyR sequence and which incorporated EcoRI sites at their 5′ ends were designed (see Materials and Methods), and these were used to amplify the S. typhimurium oxyR gene from SL1344 by PCR. The resulting product (approximately 1.1 kb) was cloned into pGEM-T, forming plasmid pPDT7, and the insert was partially sequenced to confirm that it encoded the S. typhimurium oxyR gene. The insert was found to exhibit 82.3% sequence identity at the DNA level to the E. coli oxyR gene over a 400-bp region (data not shown).
Restriction analysis and sequencing revealed that a unique SmaI site existed within the insert approximately 100 bp upstream of the 3′ end of the oxyR coding region. This site was chosen for insertion of the kanamycin resistance cassette of pUC4-K, which carries its own promoter. The cassette was excised as a 1.3-kb HincII fragment and cloned into the SmaI site of oxyR to form pPDT8 (Fig. 1C). The disrupted oxyR gene was then liberated by restriction with EcoRI, recircularized by treatment with DNA ligase, and electroporated into SL1344, as before. Kanamycin-resistant colonies were selected and screened for ampicillin and cumene peroxide sensitivities. Allelic exchange, whereby the kanamycin resistance gene was inserted within the chromosomal oxyR gene by a double crossover event, was confirmed by Southern blotting (see Materials and Methods and Fig. 1D). This process gave rise to MPG484.
In order to assess the virulence of MPG484, cells were prepared as before and injected into mice, which were then monitored for survival over 28 days. From Table 2 it can be seen that the LD50 for MPG484 was 1, indicating that the oxyR mutant is as virulent as S. typhimurium SL1344. Moreover, when the livers and spleens of infected animals were homogenized, kanamycin-resistant organisms which exhibited cumene peroxide hypersensitivity in disc inhibition assays were obtained, indicating that reversion was unlikely to have occurred. From these results it seems that neither ahp nor oxyR plays an essential role in S. typhimurium virulence.
AhpC is recognized as part of the CMI to Salmonella infection.
Having determined that the OxyR-regulated ahp locus is not essential for virulence, we decided to address whether the AhpC polypeptide is recognized by the host during the course of infection. We approached this issue by examining whether mice which had been infected previously with an attenuated strain of S. typhimurium generated a specific inflammatory response, an in vivo manifestation of CMI, in response to subcutaneous injection of purified AhpC. In previous studies, Hoiseth and Stocker (31) constructed an aroA mutant of pathogenic S. typhimurium SL1344 and showed that injection of this mutant into susceptible mice resulted in protection against a subsequent challenge by a lethal dose of pathogenic organisms. Moreover, this protection was shown to require both antibody and CMI responses in BALB/c mice (41). An aroA554::Tn10 mutation was therefore transduced with phage P22 HT int4 (50) from SL1346 (31) into SL1344 to generate MPG479 for use in the present study.
In order to determine whether the recognition of AhpC occurred as part of the CMI which developed during infection, a source of purified polypeptide was required for inoculation. An AhpC chimera which contained a histidine tag at the N terminus of the S. typhimurium AhpC protein to aid in purification was therefore constructed, and this protein was overproduced and purified (see Materials and Methods). Groups of six female BALB/c mice were injected intraperitoneally either with approximately 105 CFU of MPG479 (prepared as described above) in 100 μl of PBS or with 100 μl of PBS alone. (The bacterial inoculum of ∼105 CFU had been shown previously to result in no visible signs of illness in mice but to confer substantial protection to challenge by SL1344 [31]). The capacity to mount an inflammatory response to purified AhpC was examined 33 days after infection by subcutaneously injecting the right hind footpad (RHFP) of each mouse with 50 μl of PBS containing 40 μg of either heat-aggregated AhpC or purified mouse albumin. As a control to show that any increase in footpad size was a specific response to the injection of the proteins, the left hind footpad (LHFP) of each mouse was injected with 50 μl of PBS alone. An additional treatment in which mice were initially infected with bacteria and then challenged on day 33 by the injection of 50 μl of PBS into the RHFP alone was also carried out.
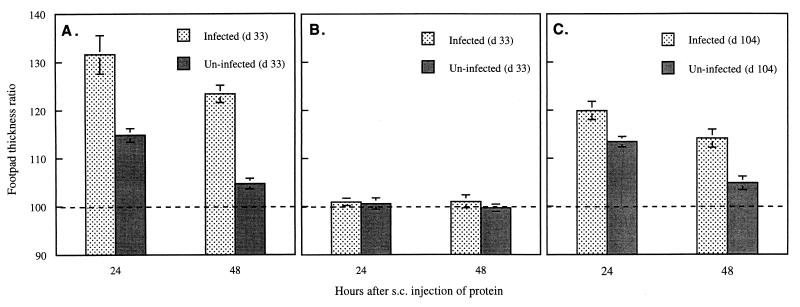
After 24 and 48 h, the thicknesses of the RHFPs and LHFPs were measured, and the ratio of the thickness of the RHFP to that of the LHFP was calculated. The ability of AhpC or albumin to elicit an inflammatory response was determined by comparing the footpad thickness ratios of infected and uninfected mice. For both infected and uninfected mice, there was a considerable increase in thickness of the RHFP over that of the LHFP (32 and 15%, P < 0.01 and P < 0.001, respectively) at 24 h with AhpC (Fig. 2A). However, the swelling seen in infected mice was twice that seen in uninfected mice. Moreover, the increase in footpad thickness was maintained at 48 h in infected animals, although the overall thickness ratio declined from that seen at 24 h. In contrast, the effect in uninfected mice was short lived, and the increase in footpad thickness was not found to be significant at 48 h. The swelling induced by AhpC at 24 h in both infected mice (0.52 ± 0.06 mm) and uninfected mice (0.22 ± 0.02 mm) was equivalent to that previously reported for the OxyR-induced enzyme catalase II (37). Notably, injection of the RHFP with mouse albumin did not result in a significant increase in thickness over the thickness of the PBS-injected LHFP (Fig. 2B) at either 24 or 48 h, independently of the infection status of the mice. A control group in which infected mice received PBS in the RHFP alone showed a small increase in footpad thickness (5%; P < 0.05) at 48 h, compared with the uninjected LHFP, but not at 24 h (data not shown). Overall, the results indicate that specific immunity to AhpC is established during the course of S. typhimurium infection of mice and that epitopes of AhpC are specifically recognized by a subpopulation of the T-cell repertoire. It is also interesting that the increase in footpad thickness in uninfected mice injected with AhpC appeared greater than might be expected for animals that were theoretically naive to this antigen, suggesting that some previous immunological exposure to AhpC or a homologous antigen might have occurred.
FIG. 2.
Measurement of CMI against AhpC during the course of infection of BALB/c mice with S. typhimurium. Groups of six female BALB/c mice (8 to 10 weeks old) were injected intraperitoneally with approximately 105 CFU of attenuated S. typhimurium MPG479 in 100 μl of PBS or with 100 μl of PBS alone. The capacity for CMI was measured by subcutaneous (s.c.) injection of the RHFP with 40 μg (in 50 μl of PBS) of purified His-tagged AhpC 33 days (d) (A) or 104 days (C) postinfection or with mouse albumin 33 days postinfection (B). In each case, the LHFP was also injected with 50 μl of PBS. The thicknesses of the RHFP and LHFP were measured from three different orientations after 24 and 48 h, and the increase in the footpad thickness was expressed as a ratio [(RHFP/LHFP) × 100)] (mean ± standard error of the mean). LHFP = 100% (dashed line).
Long-lived T-cell memory of AhpC develops in infected BALB/c mice.
Having determined that AhpC was recognized as part of the CMI which developed during infection of BALB/c mice by MPG479, we explored whether an immunological memory of this protein persists over a more protracted period of time postinfection. To examine this situation, a protocol similar to that described for the antigen challenge at day 33 was applied, but the challenge was carried out at 104 days postinfection, a time when the mice would have cleared their bacterial load (32, 41). Groups of six BALB/c mice were injected intraperitoneally either with 105 CFU of MPG479 in 100 μl of PBS or with 100 μl of PBS alone, as before; at 104 days postinfection, the RHFP or LHFP of each mouse was injected subcutaneously with 40 μg of AhpC in 50 μl of PBS or with 50 μl of PBS alone, respectively. Footpad thickness ratios were determined after 24 and 48 h.
From the results (Fig. 2C), it can be seen that the footpad thickness of infected mice injected with AhpC was substantially greater than that of uninfected animals, although both showed a significant increase at 24 h (P < 0.05 and P < 0.01, respectively). For example, the AhpC-injected RHFPs were 20% thicker at this point than the PBS-injected LHFPs in infected mice but only 13% thicker in uninfected mice. As with the antigen challenge experiment at day 33, the inflammatory responses were still apparent 48 h postchallenge in the mice which were previously infected (P < 0.01, compared with LHFPs) but had diminished below a significant level by this time in uninfected mice. Furthermore, the overall increase in footpad thickness at 104 days postinfection was smaller than that at 33 days postinfection.
Bacterial clearance from the tissues of infected mice was investigated at intervals because of its relevance to the development of immunity. In general agreement with the data for the SL1344 (aroA) derivative SL3261, reported by Hormaeche and colleagues (32), the recovery of MPG479 cells declined from approximately 105 organisms per liver or spleen at days 7 and 14 to fewer than 200 organisms by day 35; by day 104, no viable bacteria could be detected (data not shown). These results suggest that the reduction in response at day 104 is linked to the decline in antigenic stimulation following elimination of the bacteria. Nevertheless, our results provide a strong indication that AhpC plays a role both in the initial stimulation of T cells during infection and in the generation of long-lived immunological memory.
AhpC is recognized as part of the humoral response that develops against Salmonella during infection of BALB/c mice.
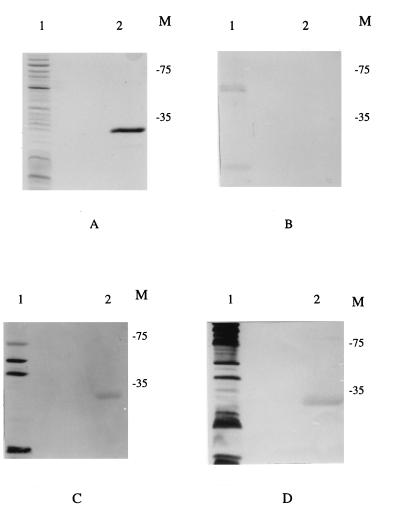
Previous studies showed that both CMI and humoral immunity are required in BALB/c mice for effective protection against Salmonella infection (41). Having determined that AhpC is recognized by BALB/c mice during the course of infection by Salmonella, we also examined whether an antibody response to AhpC was elicited in the animals at various times postinfection and prior to their use for the inflammation studies. An SDS-polyacrylamide gel (Fig. 3A) was loaded with approximately 1 μg of purified His-tagged AhpC (lane 2) and subjected to electrophoresis. A whole-cell extract of S. typhimurium (Fig. 3A, lane 1) was included as a control to assess the background level of anti-Salmonella antibody in the uninfected mice. Equivalent gels (Fig. 3B to D) were blotted onto nitrocellulose, and the filters were incubated in the presence of 1:200 dilutions of pooled sera taken at different times from the group of mice which was used for infection and analysis of CMI against AhpC at 33 days (Fig. 2A). The presence of specifically bound antibody was then determined by incubation with a rabbit anti-mouse alkaline phosphatase-conjugated antibody.
FIG. 3.
BALB/C mice develop humoral immunity to AhpC during the course of infection with S. typhimurium. Western blot analysis was performed on pooled sera from groups of mice prior to infection with MPG479 (B) or at 14 (C) or 28 (D) days postinfection. (A) Standard SDS-PAGE (12.5% [vol/vol] acrylamide) loaded with a whole-cell lysate from SL1344 (lane 1) or with 2 μg of purified His-tagged AhpC (lane 2). The order of sample loading is maintained throughout the panels. Bound antibody was detected with a rabbit anti-mouse antibody conjugated to alkaline phosphatase as previously described (38). The migration positions of prestained molecular mass markers (in kilodaltons) are indicated (M).
The preinfection sera from the mice did not show any response to AhpC, although a number of other proteins were detected in the whole-cell lysate with these sera (Fig. 3B). In contrast, by day 14 postinfection, a band that corresponded to AhpC (Fig. 3C) was clearly visible, and this was also detected at day 28 postinfection (Fig. 3D), indicating that this protein was recognized as part of the developing humoral response. As might be expected, the detection of other S. typhimurium polypeptides also increased over the 28-day interval (Fig. 3B to D, lanes 1).
DISCUSSION
The ability of Salmonella serovars to infect and cause invasive disease in humans and animals has been attributed to intracellular survival in phagocytic cells (7, 16). Macrophages play an integral role in the prevention of bacterial infection through their phagocytic capabilities and antimicrobial mechanisms; these include the production of detrimental reactive oxygen intermediates, such as superoxide and hydrogen peroxide (29) or, alternatively, nitric oxide (47). S. typhimurium has been shown to respond to oxidative stress by the induction of multigenic responses under the control of regulators such as OxyR and SoxRS. These regulators are activated by peroxide stress and superoxide stress, respectively, and both have been demonstrated to respond to the production of nitric oxide (30, 46).
In previous studies (24, 25, 61), OxyR-regulated genes ahpC and dps were shown to be induced by S. typhimurium during interaction with macrophage cells. In the present study, we specifically examined the importance of ahp and oxyR in virulence. Analysis of the SL1344 derivative MPG203, which carries a Mudlux element within ahpC (25), indicated that this strain was significantly attenuated when injected into mice by the intraperitoneal route (Table 2). In contrast, however, irreversible inactivation of the chromosomal ahp locus through specific insertion of a cml cartridge resulted in a strain that was essentially as virulent as the parental strain, SL1344. Thus, it appears that the attenuation observed with MPG203 was unlikely to have occurred as a direct result of the loss of Ahp function but rather was a consequence of the use of the Mudlux element.
The reason for attenuation in MPG203 remains unclear. Mudlux encodes luciferase, the enzyme responsible for catalyzing the bioluminescence reaction, but also carries luxC, luxD, and luxE, which produce the aldehyde substrate (19); the bioluminescence reaction also utilizes reduced flavin mononucleotide and NAD(P)H. We have not observed any differences in growth rates between MPG203 and MPG473 treated with 100 μM hydrogen peroxide. However, we have found that anomalous high-level expression of ahp may occur under certain circumstances as a consequence of the Mudlux element (59a). It remains feasible that such high-level expression may arise during the course of infection when MPG203 cells are exposed to combinations of host stresses; this exposure may impose a detrimental metabolic burden on the bacteria either through depletion of NAD(P)H and reduced flavin mononucleotide or through toxic overproduction of intracellular aldehyde. Alternatively, it is conceivable that an additional, as-yet-unidentified open reading frame may lie downstream of ahpF and that insertion of the Mudlux element within ahpC may have a polar effect on the expression of such a gene. No other gene has been reported to lie downstream of the E. coli ahpF operon. However, it is noteworthy that previous studies reported that the ahpF gene of S. typhimurium but not of E. coli is induced by heat shock (56); therefore, differences may well exist between these two enteric bacteria in the structure or regulation of the ahp operon.
Examination of S. typhimurium MPG484, which carries an insertionally inactivated oxyR gene, also revealed no significant difference in virulence from SL1344 (Table 2). This finding may suggest that the OxyR regulon is unlikely to provide adequate protection against the respiratory burst of phagocytic cells during the course of infection. Such an interpretation is supported to a degree by the finding that S. typhimurium cells deficient in OxyR were no more sensitive to killing by tissue-cultured human neutrophils than were wild-type cells during the first few hours of infection (48). However, it must be remembered that during the early stages of macrophage infection, the viability of S. typhimurium is known to diminish by several orders of magnitude (7), so the influence of OxyR might be difficult to assess accurately under such circumstances.
The possibility that genes of the OxyR regulon play a role in the virulence of S. typhimurium cannot be fully excluded, however, because in the genetically related organism E. coli, at least four of these genes, katG, dps, gorA, and stiA, have also been shown to be regulated by a second mechanism independently of OxyR (1, 2, 34, 54). In several of these cases, regulation and the mechanism for resistance to oxidative agents are mediated by the stationary-phase sigma factor RpoS. Moreover, rpoS per se has been shown to be maximally induced in S. typhimurium within 1 to 3 h of macrophage entry (12), and rpoS null mutants of S. typhimurium are known to be attenuated (17, 20). It is noteworthy, however, that a katG katE double mutant of Salmonella was found to be extremely susceptible to hydrogen peroxide in cultures but displayed no increased sensitivity to killing by macrophages (8), suggesting that these genes may also be nonessential for virulence.
Interestingly, S. typhimurium oxyR mutants show a high frequency of spontaneous mutation after treatment with chemical oxidants (55), and this occurrence can be alleviated by multicopy expression of katG or ahpCF. Moreover, evidence from E. coli has shown that one OxyR-regulated gene, dps, can form protective complexes with DNA (40). Thus, although the OxyR regulon appears not to have a critical role in virulence, it may well act to maintain genomic fidelity in bacteria which survive the respiratory burst during infection. Moreover, there is some evidence that macrophage entry by a number of Salmonella serovars may also occur by a nonclassical route with the aid of a specialized invasion-protein-export system (13). Entry by such a route may well reduce the requirement for antioxidant defense systems and may explain why the OxyR regulon appears to play a supportive, rather than an essential, role in virulence.
It is noteworthy that a number of mutants which were isolated on the basis of a reduced capacity for intracellular survival have shown hypersensitivity to oxidizing agents (23), suggesting that inevitably some degree of exposure to these agents must still occur within the macrophage environment. The recent findings that slyA, which is essential for virulence, is expressed by Salmonella within macrophages and is required for resistance to oxidative stress (6) strongly support this interpretation. Indeed, the latter study also showed that the pattern of protein synthesis of slyA mutants within J774 macrophages differed from that of the wild-type Salmonella parent, and evidence was presented for both SlyA-dependent synthesis and repression. When considered together with our data, which show unaltered attenuation in MPG484, these findings suggest that SlyA may well regulate the induction of proteins which are involved in the oxidative protection of Salmonella in an OxyR-independent manner. It is also noteworthy that RpoS is not required for the expression of slyA (6).
Over the last decade, research into the development of vaccines against Salmonella has focused largely on the use of live attenuated vaccines (reviewed in reference 11). Such vaccines provide greater immunological protection in mice than do dead-cell vaccines, and this fact has been attributed to both the development of a strong cell-mediated response (16, 41) and the recognition of bacterial proteins which are induced in response to the host environment. In the present study, we observed a substantial inflammatory reaction in mice injected with AhpC at 33 days postinfection (Fig. 2A). This finding provides strong evidence that the ahp operon and possibly other OxyR-regulated genes are expressed by SL1344 during the course of infection, in agreement with the results of our previous studies on macrophages (24, 25). Moreover, detection of a significant reaction upon challenge at day 104 postinfection (Fig. 2C), a time at which the bacteria have been cleared from the host (41), indicates that part of the long-term T-cell memory which develops following infection by Salmonella is elicited against AhpC.
Purified AhpC, but not mouse albumin, was found to elicit a significant inflammatory reaction in both uninfected and infected mice, although in the latter the response was much more substantial and was sustained for a longer time (Fig. 2). In uninfected mice, this indicated that a subpopulation of T cells was present, cells which recognized the polypeptide and so may have encountered this or a related antigen prior to inoculation. The nature of such preexposure is uncertain. However, since the mice were not germ free, it is possible that related antigens may have arisen from commensal microorganisms which colonize the body surfaces. Indeed, highly related homologs of AhpC are known to be widespread in bacteria. Moreover, preexisting immunity could also be the consequence of cross-reaction with self-antigens because, although less well conserved, eukaryotic homologs of AhpC have also been identified (10).
A humoral response to AhpC was also seen to develop in mice, resulting in positive serum responses at 14 and 28 days postinfection (Fig. 3C and D) but with no detectable signal against this polypeptide being observed prior to infection (Fig. 3B) or in uninfected mice (data not shown). These results indicate that a pool of B cells with specificity for this antigen was activated during the course of infection. The fact that no antibody against AhpC was observed in sera from uninfected or preinfection mice, while an inflammatory reaction was demonstrated (Fig. 2), suggests that natural exposure to an AhpC homolog occurs relatively infrequently or at very low levels. CD4+ T-helper cells are known to be central for immunity to Salmonella infection because the bacteria enter into an endocytic compartment of macrophages and are processed and presented in the context of the class II major histocompatibility complex proteins. The development of strong CMI in response to Salmonella antigens such as AhpC indicates the activation of type 1 T-helper (Th1) cells during the response to attenuated bacteria. Th1 cells are also capable of stimulating antibody production, and most pathogens induce some antibody formation even when a strongly polarized Th1 response develops (44, 53). However, further analysis of antibody isotype or cytokine production will be necessary to determine whether the anti-AhpC antibodies detected in these studies were promoted by Th1 or Th2 cells.
Our studies of AhpC have shown that virulence and immunity are not necessarily connected, and similar observations have been made for the major secretory protein of Legionella pneumophila, which results in strong protective immunity but is apparently nonessential for virulence (3). Currently, little information exists about the ability of individual S. typhimurium proteins to induce full protective immunity. Theoretically, however, a subunit vaccine against Salmonella would provide widespread benefits in terms of disease control. The full importance of AhpC in immunity against SL1344 is unclear at present; on balance, it must be remembered that responses to a wide range of antigens were seen to develop during the course of infection (Fig. 3B to D). Nevertheless, it is worthy of mention that a small but growing number of Salmonella antigens have shown promise in their ability to stimulate CMI or in their ability to elicit some level of immunological defense in preliminary vaccination studies with mice (26, 27, 37). A combination of such proteins may well prove highly effective as a basis for a multisubunit vaccine, if appropriately delivered.
ACKNOWLEDGMENTS
We thank many members of the Institute of Cell and Molecular Biology for helpful discussions.
This work was supported by grants to M.P.G. from the Royal Society of Great Britain and by the BBSRC. P.D.T. was the recipient of a BBSRC postgraduate studentship award.
REFERENCES
- 1.Altuvia S, Almiron M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςs in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 2.Becker-Hapak M, Eisenstark A. Role of RpoS in the regulation of glutathione reductase (Gor) in Escherichia coli. FEMS Microbiol Lett. 1995;134:39–44. doi: 10.1111/j.1574-6968.1995.tb07911.x. [DOI] [PubMed] [Google Scholar]
- 3.Blander S J, Szeto L, Shuman H A, Horwitz M A. An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires’ disease. J Clin Invest. 1990;86:817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar F, Rodriguez R L, Betlach M C, Boyer H W. Construction and characterisation of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2:75–81. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar F. Construction and characterisation of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique EcoRI sites for selection of EcoRI generated recombinant DNA molecules. Gene. 1978;4:121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier N, Bossie S, Chen C Y, Fang F C, Guiney D G, Libby S J. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect Immun. 1997;65:3725–3730. doi: 10.1128/iai.65.9.3725-3730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier N A, Libby S J, Xu Y, Loewen P C, Switala J, Guiney D G, Fang F C. DNA repair is more important than catalase for Salmonella virulence. J Clin Invest. 1995;95:1047–1053. doi: 10.1172/JCI117750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchmeier N A, Lipps C J, So M Y H, Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to oxidative burst of macrophages. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 10.Chae H Z, Robson K, Poole L B, Church G, Storz G, Rhee S G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatfield S, Li J L, Sydenham M, Douce G, Dougan G. Salmonella genetics and vaccine development. In: Hormaeche C E, Penn C W, Smyth C J, editors. Molecular biology of bacterial infection. Society for General Microbiology 49th Symposium. Cambridge, England: Cambridge University Press; 1992. pp. 299–312. [Google Scholar]
- 12.Chen C-Y, Eckmann L, Libby S J, Fang F C, Okamoto S, Kagnoff M F, Fierer J, Guiney D G. Expression of Salmonella typhimurium rpoS and rpoS-dependent genes in the intracellular environment of eukaryotic cells. Infect Immun. 1996;64:4739–4743. doi: 10.1128/iai.64.11.4739-4743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L M, Kaniga K, Galan J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 14.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defence against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 15.Christman M F, Storz G, Ames B N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci USA. 1989;86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins F M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in expression of the RpoS (ςs) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 18.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor proteins are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engebrecht J, Simon M, Silverman M. Measuring gene expression with light. Science. 1985;227:1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- 20.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D. The alternative ς factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farr S B, Kogoma T. Oxidative responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinberg A P, Vogelstein B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:3–6. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 23.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis K P, Gallagher M P. Light emission from a Mudlux transcriptional fusion in Salmonella typhimurium is stimulated by hydrogen peroxide and by interaction with the mouse macrophage line J774.2. Infect Immun. 1993;61:640–649. doi: 10.1128/iai.61.2.640-649.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis K P, Taylor P D, Inchley C J, Gallagher M P. Identification of the ahp operon of Salmonella typhimurium as a macrophage-induced locus. J Bacteriol. 1997;179:4046–4048. doi: 10.1128/jb.179.12.4046-4048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galdiero F, Tufano M A, Galdiero M, Masiello S, Rosa M D. Inflammatory effects of Salmonella typhimurium porins. Infect Immun. 1990;58:3183–3186. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Vohra H, Saha B, Nain C K, Ganguly N K. Macrophage-T cell interaction in murine salmonellosis: selective down-regulation of ICAM-1 and B7 molecules in infected macrophages and its probable role in cell mediated immunity. Eur J Immunol. 1996;26:563–570. doi: 10.1002/eji.1830260310. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 29.Hassett D J, Cohen M S. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989;3:2574–2582. doi: 10.1096/fasebj.3.14.2556311. [DOI] [PubMed] [Google Scholar]
- 30.Hausladen A, Privalle C T, Keng T, DeAngelo J, Stamler J S. Nitrosative stress: activation of the transcription factor OxyR. Cell. 1996;86:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 31.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 32.Hormaeche C E, Joysey H S, Desilva L, Izhar M, Stocker B A D. Immunity conferred by Aro− salmonella live vaccines. Microb Pathog. 1991;10:149–158. doi: 10.1016/0882-4010(91)90075-l. [DOI] [PubMed] [Google Scholar]
- 33.Ivanoff B, Levine M N, Lambert P H. Vaccination against typhoid fever: present status. Bull W H O. 1994;72:957–971. [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanova A, Miller C, Glinsky G, Eisenstark A. The role of rpoS (KatF) in oxyR-dependent regulation of hydroperoxidase I in Escherichia coli. Mol Microbiol. 1994;12:571–578. doi: 10.1111/j.1365-2958.1994.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 36.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 37.Kagaya K, Miyakawa Y, Watanabe K, Fukazawa Y. Antigenic role of stress-induced catalase of Salmonella typhimurium in cell-mediated immunity. Infect Immun. 1992;60:1820–1825. doi: 10.1128/iai.60.5.1820-1825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knecht D A, Diamond R L. Visualisation of antigenic proteins on Western blots. Anal Biochem. 1984;136:180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Martinez A, Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller S I, Loomis W P, Alpuche-Aranda C, Behlau I, Hohmann E. The PhoP virulence regulator and live oral Salmonella vaccines. Vaccine. 1993;11:122–125. doi: 10.1016/0264-410x(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 43.Morris J G. Current trends in human-diseases associated with foods of animal origin. J Am Vet Med Assoc. 1997;209:2045–2047. [PubMed] [Google Scholar]
- 44.Mossman T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 45.Nohmi T, Yamada M, Watanabe M, Murayama S Y, Sofuni T. Roles of Salmonella typhimurium umuDC and samAB in UV mutagenesis and UV sensitivity. J Bacteriol. 1992;174:6948–6955. doi: 10.1128/jb.174.21.6948-6955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunoshiba T, DeRojas-Walker T, Wishnok J S, Tannenbaum S R, Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc Natl Acad Sci USA. 1993;90:9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacelli R, Wink D A, Cook J A, Krishna M C, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell J B. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papp-Szabo E, Firtel M, Josephy P D. Comparison of the sensitivities of Salmonella typhimurium oxyR and katG mutants to killing by human neutrophils. Infect Immun. 1994;62:2662–2668. doi: 10.1128/iai.62.7.2662-2668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 50.Roth J. Genetic techniques in study of bacterial metabolism. Methods Enzymol. 1970;17A:3–35. [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seder R A, Paul W E. Acquisition of a lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 54.Seymour R L, Mishra P V, Khan M A, Spector M P. Essential roles of core starvation-stress response loci in carbon-starvation-inducible cross-resistance and hydrogen-inducible adaptive resistance to oxidative challenge in Salmonella typhimurium. Mol Microbiol. 1996;20:497–505. doi: 10.1046/j.1365-2958.1996.5451068.x. [DOI] [PubMed] [Google Scholar]
- 55.Storz G, Christman M F, Sies H E, Ames B N. Spontaneous mutagenesis and oxidative damage to DNA in S. typhimurium. Proc Natl Acad Sci USA. 1987;84:8917–8921. doi: 10.1073/pnas.84.24.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli—genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Studier F W, Rosenberg A H, Dunn J J, Dubenorf J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 58.Tacket C O, Hone D M, Losansky G A, Guers L, Edelman R, Levine M N. Clinical acceptability and immunogenicity of CVD908 Salmonella typhi vaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 59.Tartaglia L A, Storz G, Brodsky M H, Lai A, Ames B N. Alkyl hydroperoxide reductase from Salmonella typhimurium. J Biol Chem. 1990;265:10535–10540. [PubMed] [Google Scholar]
- 59a.Taylor, P. D., C. J. Inchley, and M. P. Gallagher. Unpublished data.
- 60.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdivia R H, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:367–378. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]