Abstract
Effects of bacterial direct-fed microbial (DFM) mixtures on intake, nutrient digestibility, feeding behavior, ruminal fermentation profile, and ruminal degradation kinetics of beef steers were evaluated. Crossbred Angus ruminally cannulated steers (n = 6; body weight [BW] = 520 ± 30 kg) were used in a duplicated 3 × 3 Latin square design and offered a steam-flaked corn-based finisher diet to ad libitum intake for 3, 28-d periods. Treatments were 1) Control (no DFM, lactose carrier only); 2) Treat-A (Lactobacillus animalis, Propionibacterium freudenreichii, Bacillus subtilis, and Bacillus licheniformis), at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum; and 3) Treat-B, the same DFM combination, but doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected. Data were analyzed using the GLIMMIX procedure of SAS with the model including the fixed effect of treatment and the random effects of square, period, and animal (square). For repeated measure variables, the fixed effects of treatment, time, and their interaction, and the random effects of square, period, animal (square), and animal (treatment) were used. Preplanned contrasts comparing Control × Treat-A or Treat-B were performed. Intake and major feeding behavior variables were not affected (P ≥ 0.17) by treatments. Steers offered Treat-A had an increased (P = 0.04) ADF digestibility compared with Control. Steers offered Treat-A experienced daily 300 min less (P = 0.04) time under ruminal pH 5.6, a greater (P = 0.04) ruminal pH average and NH3–N concentration (P = 0.05) and tended (P = 0.06) to have a lower ruminal temperature compared to Control. Ruminal VFA was not affected (P ≥ 0.38) by treatments. Steers offered Treat-A increased (P = 0.02) and tended (P = 0.08) to increase the ruminal effective degradable NDF and ADF fractions of the diet-substrate, respectively. When the forage-substrate (low quality) was incubated, steers offered Treat-A tended (P = 0.09) to increase the effective degradable hemicellulose fraction compared to Control. In this experiment, the bacterial combinations did not affect intake and feeding behavior, while the combination with a greater proportion of B. licheniformis (Treat-A) elicited an improved core-fiber digestibility and a healthier ruminal pH pattern, in which the ruminal environment showed to be more prone to induce the effective degradability of fiber fractions, while also releasing more NH3–N.
Keywords: B. subtilis, B. licheniformis, digestibility, L. animalis, P. freudenreichii, ruminal kinetics
Steers receiving a bacterial DFM combination containing Lactobacillus animalis, Propionibacterium freudenreichii, Bacillus subtilis, and Bacillus licheniformis at 1:1:1:3 ratio (6 × 109 CFU) showed improved core-fiber digestibility, and a safer ruminal pH pattern, enhancing ruminal effective degradability of fiber fractions, and increased NH3–N release.
Introduction
Advancements in beef cattle production are partly due to utilizing technology and feeding strategies that help cattle reach their genetic potential. During the finishing phase, high-energy diets are primarily given to improve feed efficiency, digestion, meat quality, and the sustainability of beef production. However, these diets can cause digestive issues like ruminal acidosis, leading to challenges such as ruminal parakeratosis, leaky gut, liver abscesses, and laminitis. These problems can impact animal welfare, growth, and potential economic returns (Buntyn et al., 2016; NASEM, 2016).
Feed additive technologies, like direct-fed microbials (DFM), which consist of live naturally occurring microorganisms, offer potential in achieving this goal (Yoon and Stern, 1995). In mature animals, the use of DFM seems to primarily affect ruminal function, as the rumen is the first major site of microbial action within the gastrointestinal tract of ruminants. Nutritional strategies involving the use of DFM can assist in overcoming physiological digestive disturbances in cattle, particularly during the finishing phase (McAllister et al., 2011).
In ruminant diets, bacterial DFM technologies commonly combine lactate-utilizing and lactate-producing bacteria for improved ruminal fermentation (Ban and Guan, 2021). For instance, Lactobacillus animalis, a lactic acid-producing bacterium, thrives in acidic conditions, aiding the ruminal microbiome’s adaptation. This facilitates the production of organic acids like lactic acid, fostering the growth of bacteria such as Propionibacterium freudenreichii (Yoon and Stern, 1995; Krehbiel et al., 2003; Wilson and Krehbiel, 2012). According to Retta (2016), the combination of L. animalis and P. freudenreichii induced the conversion of lactic acid to propionate, consequently maintaining a safer ruminal pH and positively affecting the ruminal microbiome.
Other bacterial DFM have been explored to enhance nutrient absorption in the host. For instance, spore-forming bacteria like Bacilli have shown growth capabilities in both rumen and post-rumen, impacting the entire gastrointestinal tract (Green et al., 1999). Bacilli licheniformis has demonstrated improved fiber digestibility in dairy cows (Qiao et al., 2010), and specific strains produce enzymes like amylases and proteases, aiding in ruminal and intestinal digestion (Springer-Verlag et al., 1994). Moreover, Bacilli subtilis has been observed to elevate ruminal NH3–N levels and enhance amylolytic and proteolytic activities (Sun et al., 2013).
Due to the potential beneficial effects, it was hypothesized that offering a unique combination of L. animalis, P. freudenreichii, B. licheniformis, and B. subtilis to beef cattle consuming energy-dense diets would positively impact ruminal fermentation, nutrient digestibility, and digestive physiology. Therefore, the effects of this bacterial DFM mixture on intake, nutrient digestibility, feeding behavior, ruminal fermentation, and degradation kinetics were evaluated.
Material and Methods
Texas Tech University Animal Care and Use Committee IACUC Protocol no. 20078-10 approved all procedures involving the use of live animals.
Animals, experimental design, and facility
Six crossbred steers (BW = 520 ± 30 kg) fitted with ruminal cannulae were stratified by BW, assigned to two squares and to one of three treatments following a duplicated 3 × 3 Latin Square design (three periods of 28 d each, with 21 d used for adaptation to treatments and 7 d for data collection). Prior to the experiment period, animals were processed with doramectin (0.5%) at 1 mL/50 kg (Dectomax Pour-on, Zoetis, Florham Park, NJ) and oral fenbendazole (10%) at 5 mg/kg (Safe-guard, Merck Animal Health, Madison, NJ). Individual steers (the experimental unit) were individually housed (3.5 × 3.5 m) at the Ruminant Nutrition Center located at the Texas Tech University Research and Education Center, Idalou, Texas. The indoor facility has a covered area, individual automatic water troughs, a turbine-exhaust/heater system, a drainage system, and a concrete floor with a 1.5 × 0.5 m rubber mat. The stalls were washed after feeding once daily.
Diets, treatments, and feeding strategy
Prior to experimental period, animals were individually adapted from a hay-based diet to a traditional stem-flaked corn-based finishing diet (Table 1) using a step-up program, which consisted of three steps of 7 d each until the finisher diet was achieved, and in which animals stayed for another 10 d prior to the study initiation and throughout the entire assessment.
Table 1.
Dietary ingredients and nutritional composition of step-up and finisher diets offered to beef steers with or without bacterial direct-fed microbial mixtures
| Item | Step 1 | Step 2 | Step 3 | Finisher |
|---|---|---|---|---|
| Diet inclusion, % DM | ||||
| Steam-flaked corn | 32.75 | 43.50 | 54.25 | 65 |
| Wet corn gluten feed | 45.67 | 37.25 | 28.62 | 20 |
| Low-quality alfalfa hay | 17 | 14 | 11 | 8 |
| Yellow grease | 0.75 | 1.5 | 2.25 | 3 |
| Mineral/vitamin supplement1 | 2.38 | 2.25 | 2.13 | 2 |
| Limestone | 1.45 | 1.5 | 1.55 | 1.6 |
| Urea | 0 | 0 | 0.2 | 0.4 |
| Lactose carrier or microbial mixtures2, g/hd | 0 | 0 | 0 | 2 |
| Analyzed nutritional composition | ||||
| Crude protein, % | 16.53 | 14.99 | 13.97 | 13.11 |
| Acid detergent fiber, % | 10.84 | 8.90 | 7.85 | 7.85 |
| Neutral detergent fiber, % | 27.06 | 22.96 | 21.22 | 20.01 |
| Crude fat, % | 4.18 | 4.84 | 5.48 | 5.76 |
| Starch content, % | 31.03 | 37.4 | 43.74 | 50.08 |
| NEm, Mcal/kg3 | 1.86 | 1.95 | 2.02 | 2.12 |
| NEg, Mcal/kg3 | 1.22 | 1.30 | 1.37 | 1.45 |
| Calcium, % | 0.87 | 0.87 | 0.89 | 0.86 |
| Phosphorus, % | 0.61 | 0.58 | 0.60 | 0.46 |
| Magnesium, % | 0.31 | 0.30 | 0.31 | 0.24 |
| Potassium, % | 1.24 | 1.22 | 1.24 | 0.92 |
| Sulfur, % | 0.27 | 0.26 | 0.25 | 0.19 |
1Supplement contained (DM basis) 68.2599% carrier (cottonseed meal), 0.5% antioxidant (Endox; Kemin Industries, Inc., Des Moines, IA), 3.76% urea, 10% potassium chloride, 15% sodium chloride, 0.0022% cobalt carbonate, 0.1965% copper sulfate, 0.0833% iron sulfate, 0.0031% ethylenediamine dihydroiodide, 0.167% manganous oxide, 0.125% selenium premix (0.2%), 0.9859% zinc sulfate, 0.0099% vitamin A (1,000,000 IU/g), 0.157% vitamin E (500 IU/g), and provided (dietary) 30 mg/kg of monensin (0.75% Rumensin-90 in supplement; Elanco Animal Health, Indianapolis, IN).
2Microbial mixtures (A and B) or carrier only (Control) were kept under refrigeration (−20 ºC) and top dressed to each diet bucket a few minutes prior to feeding.
3Calculated using the 2016 Beef Cattle Nutrient Requirement (BCNRM).
Dietary treatments were considered as follows: 1) Control (no DFM, lactose carrier only); 2) Treat-A (L. animalis, P. freudenreichii, B. subtilis; and B. licheniformis), at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (provided by Chr. Hansen Incorporated, Milwaukee, WI [Research Award # 80711]); and 3) Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected. The DFM diluted mixtures and carrier (Control) were preweighed and stored at −20 ºC until incorporated into the diet (2 g/animal-daily) 10 min before feeding. For treatment incorporation into the diet, ~1 kg (as-is) of the diet being offered was removed from the individual recipient, DFM was mixed by hand (using gloves to avoid cross-contamination among treatments), and the mixture was incorporated into the feed allocated for the day. Animals were offered to ad libitum intake (target 3% to 5% refusals), once daily at 0600 hours. Feed refusals were collected at 0550 hours, weighed, and subsampled for the determination of dry matter (DM) content and subsequent calculation of DM intake (DMI). The steam-flaked corn-based finisher diets (without treatments added) were prepared twice weekly using a horizontal Roto-Mix mixer (Roto-Mix, KS, USA) and stored under refrigeration (4 to 6 ºC).
Diet samples were collected twice a week, and daily feed refusals were dehydrated in a forced-air oven (Sheldon Manufacturing, Cornelius, OR) at 100 ºC for 24 h to calculate DMI. Additional diet samples were also collected twice a week and dehydrated (55 ºC for 48 h) for further nutrient analyses. The same procedure was conducted with refusals composite when exceeding the pre-established target of 5%.
Apparent total tract digestibility of nutrients
The digestibility assessment was conducted from days 24 to 28 of each period. Diet and refusal samples were collected immediately prior to feeding time (0550 hours), while feces (~100 g, as-is basis) were individually collected (directly from the rectum) twice daily at 0530 and 1730 hours from each steer. Samples were then frozen (−20 ºC) until further analysis. Diet and refusal sub-samples that were dehydrated at 55 ºC and ground to pass a 1-mm screen in a Wiley Mill (Thomas Scientific, Swedesboro, NJ) for further nutritional analysis. The fecal samples were composited within animal and period by taking ~20 g (as-is basis) from each homogenized sample and the entire 200 g of composite samples acquired were dehydrated in a forced-air oven set at 55 ºC for 96 h, and ground (1 mm) for further laboratorial analyses.
Total fecal output was estimated by using an internal dietary marker (288-h indigestible NDF [iNDF]) as described by Cole et al. (2011), Gregorini et al. (2008), and Krizsan and Huhtanen (2013). Briefly, Ankom F-57 bags (Ankom Technology Co., Fairport, NY) where 0.5 g of sample (diet and feces) were incubated in duplicate during a period of 288 h inside a ruminally cannulated beef steer that was offered a hay-based diet (‘WW-B Dahl’ Od World bluestem [Bothriochloa bladhii]). After the incubation period, bags were rinsed with tap water until the water was completely clear, followed by a deionized water final rinse, and processed for neutral detergent fiber analysis (Van Soest et al., 1991) with the inclusion of α-amylase, sodium sulfite, final acetone rise, dehydration (4 h, 100 ºC), and without discounting final residual ash. The apparent total tract nutrient digestibility (ATTND) was determined using the following equation:
Feeding behavior
On day 26 of each period, a continuous 24-h feeding behavior assessment was performed starting 30 min before feeding and ending at 30 min after feeding on the following day (1,440 min used only). Trained personnel observed and recorded individual animals’ behavior every 5 min intervals according to Nardi et al. (2023). Activities recorded consisted of eating, drinking, resting, ruminating, active (resting while performing other movements than those assessed), and chewing (time spent eating plus ruminating). The number of meals was calculated by counting the animal’s visits to the feed bunk (when animal was standing and with its head projected inside the feed bunk for at least 5 min), while the meal length was accounted by adding the time spent for each meal (minimum of 5 min). The recorded activities of each animal were assumed to persist throughout the entire 5-min period between each observation. Calculations of time (min) spent chewing, ruminating, eating, and drinking per kilogram of intake and digestible intake of DM, organic matter (OM), neutral detergent fiber (NDF), acid detergent fiber (ADF), and hemicellulose (HEM) were performed by combining the feeding behavior measurements with the intake and ATTND data analyzed.
Ruminal pH and temperature, NH3−N, and VFA analysis
Wireless intra-ruminal pH probes (LRCpH T5; Dascor, Escondido, CA) were used to continuously record the ruminal pH and temperature every 6 min throughout the entire 7 d collection-phase of each period, following procedures described by Penner et al. (2006). Probes were calibrated immediately prior to and after ruminal insertion/removal into the rumen via ruminal cannula. Data were tabulated by daily-hour for analysis purposes.
On day 28 at 0, 2, 4, 8, 16, and 23 hours after feeding, rumen fluid samples were collected through a rumen cannula. Ruminal content was collected from the ventral sac, filtered through four layers of cheesecloth, treated with 1% of an H2SO4 solution (20% vol/vol), and frozen (−20 °C) for further analyses. By the time of the analyses, samples were thawed overnight under refrigeration (4 °C) and centrifuged (10,000 × g; 10 min; 4 °C). Volatile fatty acid (VFA) profile total concentration and respective molar proportions were analyzed according to Vazant et al. (1996). Briefly, after centrifugation, 4 mL of the supernatant was deproteinized using 0.8 mL of 25% metaphosphoric acid. Subsequently, VFA molar proportions were determined using gas chromatography (Model 5890, Hewlett-Packard, Avondale, PA) with a flame ionization detector. The chromatograph was fitted with a 1.8-m, 4-mm i.d. glass column, packed with 10% SP1200/1% H3P04 on 80/100 WAW Chromosorb (Supelco, Bellefonte, PA). The column was maintained at 130 °C, the detector and injector were maintained at 250 °C, and carrier gas (N2) flow was 60 mL/min.
After the centrifugation process, ruminal ammonia−N (NH3−N) was quantified according to the procedure described by Broderick and Kang (1980). Briefly, duplicate 50-µL aliquots of rumen fluid samples supernatant and ammonia standards (0, 0.5, 1, 2, 3, 4, 6, and 8 mM) were diluted using 2.0 mL of hypochlorite and 2.5 mL of phenol reagent following a gentle vortex mixing process. Samples were then incubated in a water bath set at 95 °C for 5 min and allowed to cool down for 5 min. The following analytes and the subsequent ammonia standards were read using a spectrophotometer at 630 nm (U-V 1800 Shimadzu Spectrophotometer, Tokyo).
In situ ruminal degradability of nutrients
The ruminal degradability of a diet and a forage-based substrate was assessed. For the diet-substrate, major ingredients of the diet offered throughout the project (steam flaked corn [70%], alfalfa hay [10%], and wet corn gluten feed [20%], on a DM basis) were dehydrated (55 °C for 72 h), ground to pass a 2-mm screen using a Wiley Mill (Thomas Scientific), mixed and separated using quantile sample splitter (Humboldt, Chicago, IL). For the forage-substrate, a sample of (‘WW-B Dahl’ Old World bluestem [Bothriochloa bladhii]) was dehydrated at 55 °C using the forced-air oven for 72 h, ground, and homogenized as aforementioned. Approximately 5 g of diet and 8 g of forage (predehydrated) were placed into separate nylon bags (10 × 20 cm; pore size 28 µm; Ankom Technology Co.) in duplicates. Bags with samples and blanks (no content) were then placed within nylon mesh bags with weights within the ruminal ventral sac starting on day 22 by using a reverse sequential order (removal of all bags at the same time) to account for the following 11 incubation times: 0, 2, 4, 8, 12, 20, 32, 48, 64, 72, and 96 h. Immediately after the bags being removed from the rumen, specimens were delicately rinsed under running tap water until water through the bags was transparent, as described by Caton et al. (1988). Following, bags were dehydrated at 55 °C using the forced-air oven (Sheldon Manufacturing, Cornelius, OR) for 72 h. The residue from each bag was composited within duplicates, and homogenized, while subsamples were analyzed for DM (100 °C 4 h), OM, NDF, ADF, and HEM as further described.
The residues from processed in situ incubations were fitted into a first-order kinetic model according to Ørskov and Mcdonald (1970) using the nonlinear procedure of SAS (SAS Inst. Inc.), so then estimates for lag time (T0), rate of degradation (kd), and the fractions, as follows: Residue (t) = Fraction C + Fraction B × e—Kd × (t—T0), where Residue (t) = residue at each given incubation time (%, DM basis); Fraction C = non-degradable fraction (%, DM basis) represented by the proportion of residue at time 96-h; Fraction B = potentially degradable fraction (%, DM basis) represented by the percentage remained at each incubation time minus the “Fraction A” [soluble fraction (%, DM basis), proportion of material that washed out from the bags at time 0-h] and the “Fraction C”; e = 2.71828, kd = degradation rate of Fraction B (%/h); t = time incubated in the rumen (h); and T0 = lag time (h).
The effective degradability (ED) of DM, OM, NDF, ADF, and hemicellulose was calculated according to the following equation: EDi = Fraction A + Fraction B × kd/(kd + kp), where i = nutrient evaluated; Fraction A = soluble fraction (as previously described); Fraction B = potentially degradable fraction (as previously described); kd = degradation rate (%/h) of Fraction B; and kp = rate of passage, which was set at 4, 5, and 6%/h (some communal approach used when small particle samples is offered to beef steers to ad libitum intake; McCollum and Galyean, 1985).
Laboratory analyses
Except for diet and ort samples dehydrated at 100 °C in a forced-air oven for 24 h used to calculate the DM content only, diet samples collected daily during the last 7 d of each period were composited within batch and period (2 batches of each diet per week of collection), and dehydrated at 55 °C using the forced-air oven (Sheldon Manufacturing) for 72 h. Samples were then ground to pass a 1-mm screen using a Wiley Mill (Thomas Scientific) for nutrient analyses. A similar procedure was used for feces that were composited within animals and periods. The method 950.01 (AOAC, 1990) was used to adjust for the laboratory DM (100 °C for 4 h). The OM was determined by subtracting the ash residue from the DM sample analyzed, which was done using a muffle furnace (Cole-Parmer Stable Temp, Vernon Hills, IL) set for 600 ºC during 4 h, according to the methodology 942.05 (AOAC, 2005). The fiber content (NDF, ADF, and hemicellulose) were analyzed in sequence (Ankom 200, Macedon, New York) where the NDF technique included thermo-stable amylase, sodium sulfite and followed by an acetone rinse, and finally discounting residual ash (Van Soest et al., 1991). The hemicellulose content was calculated by accounting for the difference between NDF and ADF. A commercial lab (Servitech, Amarillo, TX) analyzed other dietary nutrient analyses used for dietary description purposes only.
Statistical analysis
Data were analyzed using the GLIMMIX procedure of SAS (SAS Inst., Inc., Cary, NC) in a 3 × 3 Latin square design (three periods and three treatments) and the steer served as the experimental unit. For analysis of intake, feeding behavior, and apparent total tract digestibility, the model included the fixed effects of treatment and random effects of square, period, and animal (square). For repeated measure variables (ruminal pH, temperature, NH3–N, and VFA) the model included treatment, time, and treatment × time interaction as fixed effects, while random effects were square, period, animal within square, and animal within treatment. The covariance structure for repeated measures was selected based on the smallest Akaike information criterion (AIC). The general degrees of freedom procedure Kenward–Rogers was used to adjust for any bias on standard errors caused by multiple terms in the random statement. To compare Control vs. Treat-A and Control vs. Treat-B, F-test protected pre-planned orthogonal contrasts were used. The differences were considered significant at P < 0.05 and meaningful tendencies discussed when 0.05 < P ≤ 0.15.
Results
Intake, digestibility, and feeding behavior
The DMI (average 18.58 kg), OMI (average 17.63 kg), NDF intake (average 3.56 kg), ADF intake (average 1.19 kg), and HEM intake (average 2.37 kg) were not affected (P ≥ 0.17) by treatments (Table 2). No difference (P ≥ 0.35) was observed for the apparent total tract nutrient digestibility of DM (average 75.98 %), OM (average 79.31 %), NDF (average 49.85 %), or HEM (average 50.86 %). However, steers offered Treat-A increased (P = 0.04) ADF digestibility by 26% compared to Control, while steers offered Treat-B tended (P = 0.08) to increase ADF digestibility by 17% (Table 2).
Table 2.
Intake and apparent total tract nutrient digestibility of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item | Control | Treat-A | Treat-B | SEM3 | Treatment | CTL vs. A | CTL vs. B |
| Intake, kg | |||||||
| DM | 18.68 | 19.03 | 18.03 | 1.51 | 0.61 | 0.74 | 0.51 |
| OM | 17.73 | 18.07 | 17.11 | 1.47 | 0.61 | 0.73 | 0.51 |
| NDF | 3.29 | 3.87 | 3.53 | 0.40 | 0.18 | 0.07 | 0.39 |
| ADF | 1.09 | 1.29 | 1.18 | 0.15 | 0.22 | 0.09 | 0.37 |
| HEM | 2.20 | 2.58 | 2.33 | 0.26 | 0.17 | 0.07 | 0.41 |
| Apparent total tract nutrient digestibility 4 , % | |||||||
| DM | 74.72 | 75.51 | 77.70 | 3.01 | 0.35 | 0.72 | 0.17 |
| OM | 78.18 | 78.74 | 81.02 | 2.82 | 0.38 | 0.80 | 0.20 |
| NDF | 45.06 | 52.73 | 51.77 | 10.37 | 0.17 | 0.10 | 0.12 |
| ADF | 39.81 | 54.00 | 48.19 | 12.06 | 0.04 | 0.02 | 0.08 |
| HEM5 | 47.41 | 51.62 | 53.55 | 9.80 | 0.39 | 0.40 | 0.20 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3SEM = standard error of the mean (n = 6 steers/treatment).
4Determined by a procedure using indigestible NDF.
5Calculated as NDF minus ADF.
Feeding behavior variables were not affected by treatments (P ≥ 0.19), except by a tendency (P = 0.08) observed for steers offered Treat-B to spend less time ruminating per kilogram of digestible DM and digestible OM, when compared to Control (Table 3). Regardless of treatments, in general, animals spent ~14, 8, 1, and 77% of their daily time ruminating, eating, drinking, and resting (active + resting), respectively.
Table 3.
Feeding behavior of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item | Control | Treat-A | Treat-B | SEM3 | Treatment | CTL vs. A | CTL vs. B |
| Ruminating min/d | 215 | 189 | 191 | 26.5 | 0.61 | 0.38 | 0.41 |
| Eating min/d | 122 | 100 | 128 | 20.8 | 0.31 | 0.25 | 0.75 |
| Chewing min/d4 | 337 | 289 | 318 | 33.0 | 0.53 | 0.27 | 0.66 |
| Drinking min/d | 20 | 23 | 23 | 3.3 | 0.76 | 0.48 | 0.60 |
| Active min/d | 163 | 162 | 198 | 27.4 | 0.24 | 0.94 | 0.16 |
| Resting min/d | 920 | 966 | 902 | 48.5 | 0.48 | 0.40 | 0.73 |
| Meals, n/d | 17 | 15 | 16 | 1.9 | 0.70 | 0.41 | 0.68 |
| Meal length min/meal | 8 | 7 | 8 | 0.9 | 0.54 | 0.33 | 0.95 |
| Rumination min/kg of intake | |||||||
| DM | 11.55 | 12.05 | 10.62 | 1.46 | 0.30 | 0.58 | 0.29 |
| OM | 12.18 | 12.72 | 11.19 | 1.54 | 0.29 | 0.58 | 0.28 |
| NDF | 65.85 | 60.37 | 54.41 | 7.86 | 0.19 | 0.39 | 0.08 |
| ADF | 199.75 | 181.47 | 162.7 | 23.71 | 0.23 | 0.42 | 0.10 |
| HEM | 98.4 | 90.63 | 81.87 | 11.93 | 0.17 | 0.39 | 0.07 |
| Digestible DM | 15.5 | 16.31 | 13.54 | 2.12 | 0.08 | 0.48 | 0.09 |
| Digestible OM | 15.61 | 16.42 | 13.69 | 2.11 | 0.08 | 0.48 | 0.09 |
| Digestible NDF | 155.93 | 173.23 | 112.25 | 53.81 | 0.35 | 0.69 | 0.29 |
| Digestible ADF | 653.63 | 493.9 | 367.11 | 211.45 | 0.25 | 0.38 | 0.11 |
| Digestible HEM | 215.65 | 264.16 | 164.24 | 78.25 | 0.33 | 0.46 | 0.40 |
| Eating min/kg of intake | |||||||
| DM | 6.48 | 5.93 | 7.18 | 1.37 | 0.34 | 0.51 | 0.38 |
| OM | 6.83 | 6.26 | 7.57 | 1.45 | 0.34 | 0.52 | 0.37 |
| NDF | 36.48 | 29.6 | 37.16 | 6.88 | 0.21 | 0.14 | 0.87 |
| ADF | 109.25 | 90.36 | 111.73 | 21.39 | 0.24 | 0.17 | 0.84 |
| HEM | 54.84 | 44.03 | 55.77 | 10.17 | 0.21 | 0.13 | 0.88 |
| Digestible DM | 8.63 | 7.9 | 9.21 | 1.81 | 0.45 | 0.48 | 0.55 |
| Digestible OM | 8.7 | 7.98 | 9.3 | 1.81 | 0.44 | 0.48 | 0.52 |
| Digestible NDF | 82.12 | 78.13 | 80.15 | 28.97 | 0.98 | 0.83 | 0.91 |
| Digestible ADF | 311.2 | 231.24 | 286.62 | 111.89 | 0.56 | 0.30 | 0.73 |
| Digestible HEM | 117.08 | 117.15 | 113.66 | 40.57 | 0.99 | 1.00 | 0.90 |
| Chewing min/kg of intake 4 | |||||||
| DM | 18.03 | 17.97 | 17.81 | 1.77 | 0.98 | 0.97 | 0.86 |
| OM | 19.02 | 18.97 | 18.76 | 1.87 | 0.98 | 0.98 | 0.84 |
| NDF | 102.33 | 89.88 | 91.57 | 9.3 | 0.27 | 0.16 | 0.18 |
| ADF | 309 | 271.28 | 274.43 | 28.22 | 0.32 | 0.21 | 0.20 |
| HEM | 153.24 | 134.67 | 137.64 | 14.21 | 0.26 | 0.15 | 0.18 |
| Digestible DM | 24.13 | 24.32 | 22.75 | 2.52 | 0.52 | 0.91 | 0.35 |
| Digestible OM | 24.3 | 24.51 | 22.99 | 2.49 | 0.52 | 0.89 | 0.35 |
| Digestible NDF | 238.06 | 247.98 | 192.4 | 75.88 | 0.57 | 0.87 | 0.41 |
| Digestible ADF | 964.85 | 710.79 | 653.74 | 301.2 | 0.33 | 0.28 | 0.17 |
| Digestible HEM | 332.72 | 377.12 | 277.89 | 109.79 | 0.55 | 0.63 | 0.52 |
| Drinking min/kg of intake | |||||||
| DM | 1.09 | 1.36 | 1.27 | 0.14 | 0.43 | 0.22 | 0.39 |
| OM | 1.15 | 1.44 | 1.33 | 0.15 | 0.64 | 0.22 | 0.39 |
| NDF | 6.12 | 6.91 | 6.69 | 0.86 | 0.81 | 0.55 | 0.65 |
| ADF | 18.43 | 21.03 | 19.85 | 2.57 | 0.79 | 0.51 | 0.70 |
| HEM | 9.17 | 10.29 | 10.1 | 1.3 | 0.82 | 0.58 | 0.62 |
| Digestible DM | 1.46 | 1.84 | 1.64 | 0.2 | 0.47 | 0.23 | 0.53 |
| Digestible OM | 1.47 | 1.85 | 1.66 | 0.2 | 0.64 | 0.22 | 0.52 |
| Digestible NDF | 14.14 | 17.84 | 15.19 | 5.09 | 0.66 | 0.38 | 0.78 |
| Digestible ADF | 54.89 | 53.92 | 53.32 | 19.72 | 0.99 | 0.95 | 0.92 |
| Digestible HEM | 19.89 | 26.84 | 21.72 | 7.29 | 0.50 | 0.26 | 0.74 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.3 SEM = standard error of the mean (n = 6 steers/treatment).
3SEM = standard error of the mean (n = 6 steers/treatment).
4Chewing activity calculated by adding time spent eating and time spent ruminating.
Ruminal fermentation characteristics
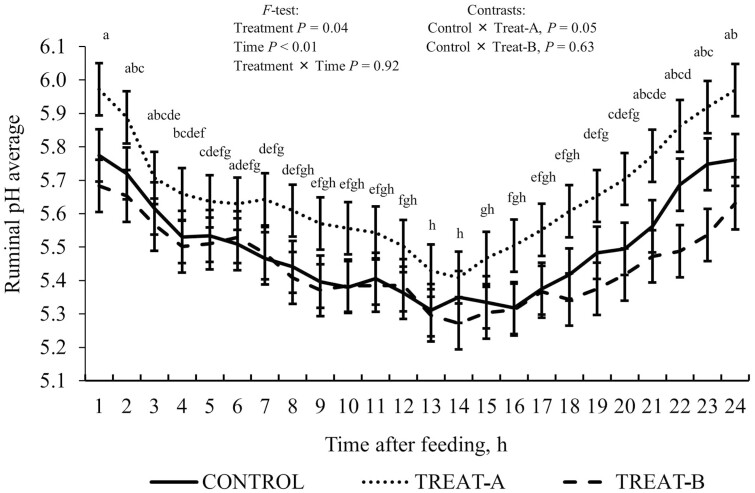
No treatment × time interactions (P ≥ 0.20) for pH variables (average, maximum, minimum, and magnitude) were observed (Table 4). Steers offered Treat-A had a daily reduction of ~300 min (P = 0.04) spent under ruminal pH 5.6 compared to Control, while steers offered Treat-B were not different (P = 0.78) from Control. Steers offered Treat-A tended (P = 0.13) to have a lesser ruminal pH area below pH 5.6 (178 vs. 261) and showed a greater (P = 0.04) ruminal pH average (5.67 vs. 5.50) compared to Control (Figure 1). No difference for ruminal pH area and time below pH 5.0 (P ≥ 0.37) among treatments were observed, in which animals spent ~75 min-daily below such threshold.
Table 4.
Ruminal pH, temperature, ammonia-N, and volatile fatty acid profile of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | Treat-A | Treat-B | SEM3 | Treatment | Time | Treatment × Time | CTL vs. A | CTL vs. B |
| Ruminal pH variables | |||||||||
| pH average | 5.5 | 5.66 | 5.47 | 0.078 | 0.04 | <0.01 | 0.92 | 0.05 | 0.63 |
| pH area below 5.6 | 260 | 178 | 348 | 124 | 0.13 | 0.06 | 0.38 | 0.30 | 0.28 |
| Time below 5.6, min | 928 | 627 | 965 | 220.24 | 0.04 | 0.23 | 0.66 | 0.04 | 0.78 |
| pH maximum | 6.15 | 6.32 | 6.04 | 0.1 | 0.05 | 0.12 | 0.24 | 0.13 | 0.28 |
| pH minimum | 5.09 | 5.21 | 5.06 | 0.12 | 0.08 | 0.70 | 0.92 | 0.08 | 0.68 |
| pH magnitude | 1.06 | 1.1 | 0.98 | 0.09 | 0.40 | 0.23 | 0.20 | 0.66 | 0.37 |
| pH area below 5.0 | 8 | 1 | 10 | 5.5 | 0.47 | 0.22 | 0.67 | 0.35 | 0.83 |
| Time below 5.0, min | 65 | 31 | 129 | 53.7 | 0.37 | 0.25 | 0.69 | 0.62 | 0.36 |
| Ruminal volatile fatty acid profile | |||||||||
| Total, mM/L | 106.21 | 105.14 | 103.92 | 7.217 | 0.88 | 0.38 | 0.21 | 0.81 | 0.61 |
| Acetate:propionate | 1.53 | 1.48 | 1.7 | 0.333 | 0.59 | 0.05 | 0.50 | 0.82 | 0.46 |
| mM/100 mM total VFA | |||||||||
| Acetate | 45.64 | 44.69 | 46.27 | 1.763 | 0.71 | <0.01 | 0.33 | 0.62 | 0.75 |
| Propionate | 32.6 | 31.66 | 31.43 | 3.86 | 0.80 | 0.52 | 0.22 | 0.61 | 0.54 |
| Butyrate | 13.87 | 14.98 | 14.67 | 2.762 | 0.76 | 0.73 | 0.33 | 0.48 | 0.61 |
| Valerate | 5.89 | 6.44 | 5.35 | 1.022 | 0.63 | <0.01 | 0.14 | 0.63 | 0.64 |
| Isobutyrate | 0.74 | 0.69 | 0.65 | 0.1 | 0.80 | 0.11 | 0.19 | 0.72 | 0.51 |
| Isovalerate | 1.13 | 1.36 | 1.65 | 0.341 | 0.38 | 0.02 | 0.23 | 0.53 | 0.17 |
| Branched chain | 1.98 | 2.22 | 2.33 | 0.338 | 0.68 | 0.01 | 0.45 | 0.57 | 0.42 |
| Others | |||||||||
| Ruminal NH3–N, mg/dL | 4.35 | 10.78 | 9.11 | 1.813 | 0.05 | 0.63 | 0.47 | 0.02 | 0.07 |
| Ruminal temperature, o C | 39.35 | 39.19 | 39.33 | 0.192 | 0.06 | <0.01 | 0.77 | 0.02 | 0.62 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3SEM = standard error of the mean (n = 6 steers/treatment).
Figure 1.
a,b,c,d,e,f,g,hMeans with different superscripts differ by P ≤ 0.05. Continuous ruminal pH average (intra-ruminal wireless probe) of beef steer offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures. Control (no DFM, lactose carrier only); Treat-A (Lactobacillus animalis, Propionibacterium freudenreichii, Bacillus subtilis, and Bacillus licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. No treatment × Time interaction was observed (P = 0.92). Ruminal pH increased (P = 0.04) when Treat-A was offered compared to the control. Regardless of treatment, the ruminal pH average was greater (P < 0.01) at the initial and final 8 h after feeding than during between 8 and 16 h.
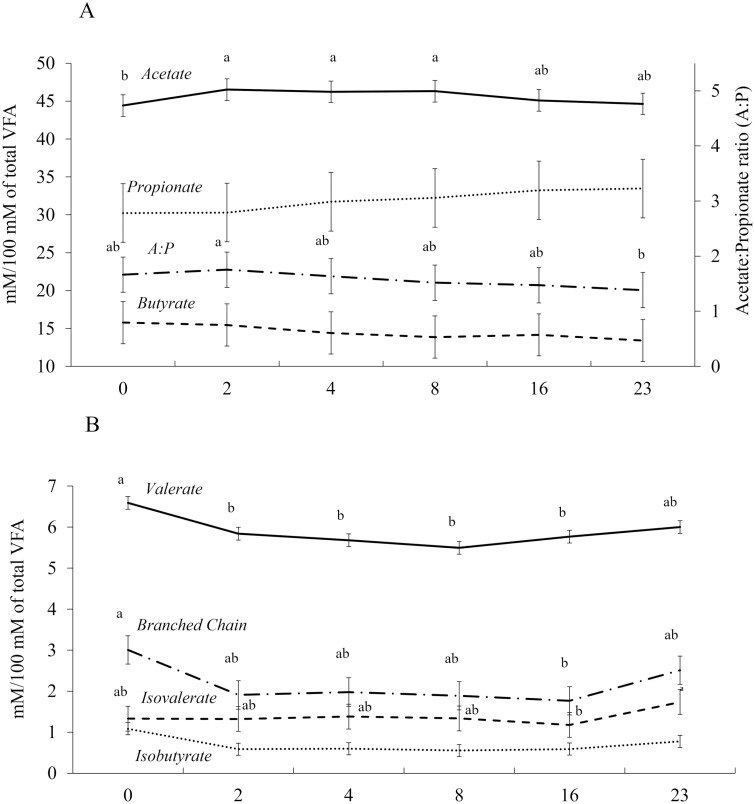
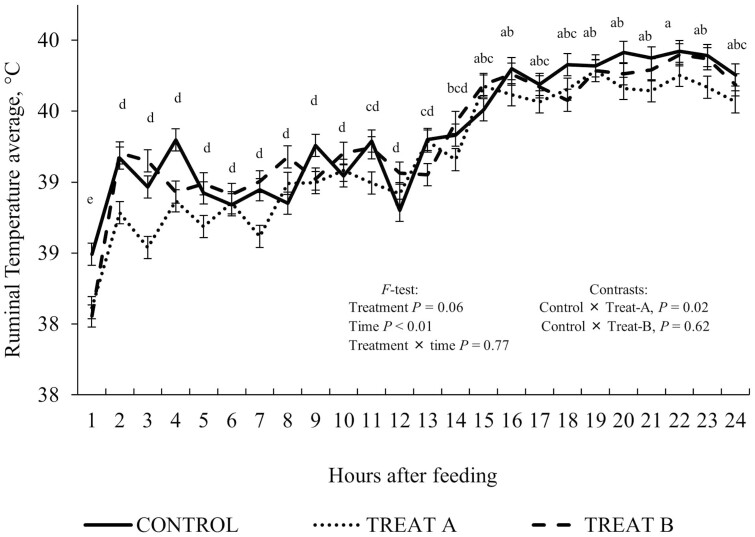
No treatment × time interactions (P = 0.19) for VFA variables were observed. The individual VFA profile molar proportions (P ≥ 0.38) and acetate: propionate ratio (P = 0.59) did not differ among treatments (Table 4). Regardless of treatment, the main effect of collection time influenced VFA responses, in which acetate molar proportion was greater (P < 0.01) at 2, 4, and 8 h postfeeding compared to 0 h (at feeding time), while 23 h postfeeding was intermediate. Propionate (P = 0.52) and butyrate (P = 0.73) molar proportions were not affected by time postfeeding. The acetate:propionate ratio peaked (P = 0.05) at 2 h and the lowest ratio was observed at 23 h postfeeding, while other times were intermediate (Figure 2A). Valerate molar proportion was greater (P < 0.01) at feeding time (0 h) and the least at 2, 4, 8, and 16 h postfeeding, while intermediate at 23 h. Total branched chain VFA molar proportion was greater (P = 0.01) at feeding time (0 h) and the least at 16 h postfeeding, while intermediate at 2, 4, 8, and 23 h were intermediate. Isovalerate molar proportion was greater (P = 0.02) at 23 h postfeeding and the least at 16 h, while 0, 2, 4, and 8 h were intermediate (Figure 2B). No treatment × time interaction (P ≥ 0.47) was observed for ruminal NH3−N and ruminal temperature (Table 4). The ruminal concentration of NH3−N was greater (P = 0.02) for steers offered Treat-A compared to Control (10.78 vs. 4.35 mg/dL), while animals offered Treat-B tended (P = 0.07) to have a greater (9.11 mg/dL) concentration compared to Control. A tendency (P = 0.06) was observed for steers offered Treat-A to have a lower ruminal environment temperature than Control (Table 4). Regardless of treatments, the ruminal temperature was less (P < 0.01) during the initial 12 h after feeding compared to the last 12 h (Figure 3).
Figure 2.
a,bMeans with different superscripts differ by P ≤ 0.05.The main effect of time postfeeding for ruminal volatile fatty acid (VFA) variables of beef steers offered steam-flaked corn-based finishing diets. A) Acetate, propionate, butyrate molar proportions, and Acetate:Propionate (A:P) ratio. Acetate molar proportion was greater (P < 0.01) at 2, 4, and 8 h postfeeding compared to 0 h (at feeding time), while 23 h postfeeding was intermediate. Propionate (P = 0.52) and butyrate (P = 0.73) molar proportions were not affected by time postfeeding. The A:P peaked (P = 0.05) at 2 h and the lowest ratio was observed at 23 h postfeeding, while other times were intermediate. B) Valerate, branched chain VFA, Isovalerate, and Isobutyrate. Valerate molar proportion was greater (P < 0.01) at feeding time (0 h) and the least at 2, 4, 8, and 16 h postfeeding, while intermediate at 23 h. Total branched-chain VFA molar proportion was greater (P = 0.01) at feeding time (0 h) and the least at 16 h postfeeding, while intermediate at 2, 4, 8, and 23 h were intermediate. Isovalerate molar proportion was greater (P = 0.02) at 23 h postfeeding and the least at 16 h, while 0, 2, 4, and 8 h were intermediate. Isobutyrate molar proportion showed an F-test tendency (P = 0.11) for the main effect of time but means within each time were not able to be separated after Tukey adjustment.
Figure 3.
a,b,c,dMeans with different superscripts differ by P ≤ 0.05. Continuous ruminal temperature average (intra-ruminal wireless probe) of beef steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures. Control (no DFM, lactose carrier only); Treat-A (Lactobacillus animalis, Propionibacterium freudenreichii, Bacillus subtilis, and Bacillus licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were approximately 30% greater than the minimum expected. A tendency (P = 0.06) was observed for steers offered Treat-A to have a numerically colder rumen than control. Regardless of treatments, rumen was colder (P < 0.01) during the initial 12 h after feeding compared to the last 12 h.
Ruminal in situ degradability
When the in situ bags containing the diet-substrate were incubated, animals offered Treat-A tended to have a lower rate of degradation of DM (P = 0.06) and OM (P = 0.09) when compared to Control. On the other hand, steers offered Treat-A tended to show an increase of the NDF (P = 0.15) and ADF (P = 0.10) rate of degradation compared to Control (Table 5). The potentially degradable fraction (B) of NDF (P = 0.13) and ADF (P = 0.06) tended to increase when animals were offered the Treat-A compared to Control, while the undegradable fraction (C) of NDF and ADF tended (P = 0.08) to decrease when compared to Control. Ruminal degradation variables for HEM dramatically improved when steers were offered Treat-A compared to Control, in which the fraction B increased (68.52% vs 23.22%; P < 0.01) and the fraction C decreased (14.37% vs 57.79%; P < 0.01) as shown in Table 5. The effective degradable fraction of NDF at pre-established rate of passages of 4%, 5%, and 6% increased (P = 0.02) for steers offered Treat-A compared to Control, while tending (P = 0.08) to increase for the ADF fraction (Table 6). Other ruminal degradation in situ variables for the incubated diet-substrate were not affected (P ≥ 0.26) by treatments (Tables 5 and 6).
Table 5.
Ruminal in situ degradation kinetics of a diet-substrate incubated into the rumen of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item3 | Control | Treat-A | Treat-B | SEM4 | Treatment | CTL vs. A | CTL vs. B |
| Dry matter | |||||||
| kd, %/h | 7.30 | 4.64 | 5.43 | 0.706 | 0.06 | 0.02 | 0.09 |
| Lag time, h | 0 | 0 | 0.14 | 0.083 | 0.39 | 0.24 | 1.00 |
| A, % | 34.12 | 33.62 | 33.79 | 1.921 | 0.84 | 0.70 | 0.85 |
| B, % | 38.63 | 42.4 | 40.11 | 4.38 | 0.49 | 0.25 | 0.64 |
| C, % | 27.58 | 23.48 | 26.27 | 2.646 | 0.26 | 0.11 | 0.59 |
| Organic matter | |||||||
| kd, %/h | 7.09 | 4.24 | 6.43 | 0.89 | 0.09 | 0.04 | 0.61 |
| Lag time, h | 0 | 0.15 | 0 | 0.089 | 0.39 | 0.24 | 1.00 |
| A, % | 29.53 | 28.83 | 25.66 | 2.765 | 0.41 | 0.82 | 0.22 |
| B, % | 44.6 | 46.28 | 50.2 | 3.689 | 0.31 | 0.64 | 0.14 |
| C, % | 25.87 | 24.89 | 24.13 | 1.999 | 0.66 | 0.61 | 0.38 |
| NDF | |||||||
| kd, %/h | 1.38 | 2.68 | 1.42 | 0.626 | 0.15 | 0.09 | 0.95 |
| Lag time, h | 0 | 0.31 | 0.25 | 0.289 | 0.53 | 0.29 | 0.39 |
| A, % | 11.32 | 12.58 | 11.88 | 0.97 | 0.41 | 0.19 | 0.55 |
| B, % | 16.28 | 24.43 | 15.28 | 4.633 | 0.13 | 0.10 | 0.82 |
| C, % | 72.4 | 62.99 | 72.84 | 4.368 | 0.08 | 0.06 | 0.92 |
| ADF | |||||||
| kd, %/h | 2.32 | 3.75 | 1.72 | 0.745 | 0.10 | 0.13 | 0.50 |
| Lag time, h | 0.57 | 0 | 0 | 0.33 | 0.39 | 0.24 | 0.24 |
| A, % | 8.89 | 8.27 | 8.16 | 0.474 | 0.45 | 0.33 | 0.25 |
| B, % | 11.71 | 20.55 | 13.32 | 2.791 | 0.06 | 0.03 | 0.65 |
| C, % | 79.4 | 71.18 | 78.53 | 2.767 | 0.08 | 0.04 | 0.81 |
| Hemicellulose 5 | |||||||
| kd, %/h | 1.95 | 0.6 | 2.84 | 0.505 | <0.01 | 0.03 | 0.13 |
| Lag time, h | 0.08 | 0.22 | 0.11 | 0.17 | 0.73 | 0.46 | 0.85 |
| A, % | 19 | 17.11 | 15.48 | 0.782 | 0.02 | 0.10 | 0.01 |
| B, % | 23.22 | 68.52 | 25.41 | 4.86 | <0.01 | <0.01 | 0.75 |
| C, % | 57.79 | 14.37 | 59.1 | 4.834 | <0.01 | <0.01 | 0.85 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3kd, rate of degradation; A, soluble fraction; B, potentially degradable fraction; C, undegradable fraction.
4SEM = standard error of the mean (n = 6 steers/treatment).
Table 6.
Ruminal in situ effective degradable DM, OM, NDF, ADF, and hemicellulose of a diet-substrate incubated into the rumen of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item4 | Control | Treat-A | Treat-B | SEM3 | Treatment | CTL vs. A | CTL vs. B |
| Effective degradable fraction at 4% kp | |||||||
| DM | 58.50 | 55.74 | 55.67 | 0.981 | 0.11 | 0.07 | 0.07 |
| OM | 57.72 | 51.44 | 54.76 | 1.993 | 0.11 | 0.04 | 0.30 |
| NDF | 15.78 | 20.69 | 15.73 | 2.373 | 0.02 | 0.02 | 0.98 |
| ADF | 12.54 | 17.81 | 12.33 | 1.82 | 0.08 | 0.05 | 0.93 |
| Hemicellulose | 25.22 | 25.83 | 24.70 | 1.79 | 0.81 | 0.73 | 0.77 |
| Effective degradable fraction at 5% kp | |||||||
| DM | 56.48 | 53.47 | 53.52 | 1.141 | 0.09 | 0.06 | 0.06 |
| OM | 55.37 | 49.04 | 52.16 | 2.089 | 0.12 | 0.04 | 0.28 |
| NDF | 15.12 | 19.68 | 15.13 | 2.166 | 0.02 | 0.01 | 0.99 |
| ADF | 12.07 | 16.72 | 11.08 | 1.663 | 0.06 | 0.05 | 0.90 |
| Hemicellulose | 27.32 | 24.28 | 23.46 | 1.543 | 0.81 | 0.98 | 0.57 |
| Effective degradable fraction at 6% kp | |||||||
| DM | 54.76 | 51.64 | 51.76 | 1.256 | 0.10 | 0.06 | 0.07 |
| OM | 53.38 | 47.11 | 50.01 | 2.149 | 0.14 | 0.05 | 0.27 |
| NDF | 14.63 | 18.90 | 14.70 | 2.004 | 0.02 | 0.01 | 0.96 |
| ADF | 11.71 | 15.86 | 11.39 | 1.534 | 0.07 | 0.05 | 0.87 |
| Hemicellulose | 23.65 | 23.20 | 22.52 | 1.365 | 0.70 | 0.74 | 0.41 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3SEM = standard error of the mean (n = 6 steers/treatment).
4Effective degradable fraction calculated as: ED(y) = A + B × kd/(kd + kp), where y is the nutrient (DM, OM, NDF, ADF, or hemicellulose); A is the soluble fraction (%); B is the potentially degradable fraction (%); and kd is the rate of degradation (%).
5Calculated as NDF minus ADF.
When the in situ bags containing the forage-substrate were incubated, both Treat-A and Treat-B tended (P = 0.11) to increase for soluble fraction (A) of DM and OM compared to Control, by approximately one percentage unit only (Table 7). Steers offered Treat-A showed an increased (P < 0.01) lag time (T0) for HEM compared to Control, while those offered Treat-B had a decreased (P = 0.02) HEM potentially degradable fraction (B) and an increased (P < 0.01) HEM undegradable fraction (C) compared to Control. The effective degradable fraction of HEM at pre-established rate of passages of 4%, 5%, and 6% tended (P = 0.09) to numerically increase for steers offered Treat-A compared to Control (Table 8). Other ruminal degradation in situ variables for the incubated forage-substrate were not affected (P ≥ 0.18) by treatments (Tables 7 and 8).
Table 7.
Ruminal in situ degradation kinetics of a forage-substrate (‘WW-B Dahl’ Old World bluestem [Bothriochloa bladhii]) of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item3 | Control | Treat-A | Treat-B | SEM4 | Treatment | CTL vs. A | CTL vs. B |
| Dry matter | |||||||
| kd, %/h | 2.34 | 1.73 | 1.79 | 0.791 | 0.76 | 0.51 | 0.55 |
| Lag time, h | 0 | 0.13 | 0 | 0.074 | 0.39 | 0.24 | 1.00 |
| A, % | 17.62 | 16.66 | 16.68 | 0.421 | 0.11 | 0.07 | 0.07 |
| B, % | 25.65 | 22.31 | 22.71 | 2.48 | 0.53 | 0.32 | 0.37 |
| C, % | 56.73 | 61.03 | 60.61 | 2.554 | 0.43 | 0.25 | 0.29 |
| Organic matter | |||||||
| kd, %/h | 2.12 | 1.13 | 1.54 | 0.552 | 0.34 | 0.15 | 0.38 |
| Lag time, h | 0 | 0.18 | 0 | 0.104 | 0.39 | 0.24 | 1.00 |
| A, % | 11.55 | 10.62 | 10.55 | 0.43 | 0.10 | 0.07 | 0.05 |
| B, % | 16.41 | 13.09 | 13.85 | 1.514 | 0.18 | 0.08 | 0.17 |
| C, % | 72.04 | 76.29 | 75.61 | 1.813 | 0.15 | 0.07 | 0.12 |
| NDF | |||||||
| kd, %/h | 0.75 | 1.01 | 0.63 | 0.292 | 0.65 | 0.53 | 0.79 |
| Lag time, h | 0.16 | 1.61 | 1.4 | 0.729 | 0.33 | 0.17 | 0.24 |
| A, % | 4.64 | 3.83 | 4.33 | 1.003 | 0.83 | 0.55 | 0.82 |
| B, % | 5.55 | 4.75 | 4.93 | 1.31 | 0.90 | 0.66 | 0.74 |
| C, % | 89.81 | 91.42 | 90.73 | 2.288 | 0.87 | 0.61 | 0.77 |
| ADF | |||||||
| kd, %/h | 0.52 | 0.64 | 1.66 | 0.569 | 0.27 | 0.87 | 0.15 |
| Lag time, h | 1.21 | 0.61 | 0.95 | 0.938 | 0.62 | 0.34 | 0.67 |
| A, % | 2.94 | 3.32 | 3.91 | 1.302 | 0.79 | 0.79 | 0.51 |
| B, % | 3.31 | 3.8 | 5.44 | 1.746 | 0.38 | 0.75 | 0.19 |
| C, % | 93.75 | 92.88 | 90.65 | 3.02 | 0.551 | 0.76 | 0.30 |
| Hemicellulose 5 | |||||||
| kd, %/h | 2.42 | 5.04 | 4.4 | 2.413 | 0.73 | 0.45 | 0.57 |
| Lag time, h | 0 | 0.85 | 0.16 | 0.149 | <0.01 | <0.01 | 0.46 |
| A, % | 4.68 | 4.09 | 5.79 | 1.312 | 0.64 | 0.55 | 0.75 |
| B, % | 15.58 | 13.64 | 6.56 | 2.081 | 0.02 | 0.52 | 0.01 |
| C, % | 79.74 | 80.56 | 89.35 | 1.445 | <0.01 | 0.69 | <0.01 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc., Milwaukee, WI); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were approximately 30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3kd, rate of degradation; A, soluble fraction; B, potentially degradable fraction; C, undegradable fraction.
4SEM = standard error of the mean (n = 6 steers/treatment).
5Calculated as NDF minus ADF.
Table 8.
Ruminal in situ effective degradable DM, OM, NDF, ADF, and hemicellulose of a forage-substrate (‘WW-B Dahl’ Old World bluestem [Bothriochloa bladhii]) incubated into the rumen of steers offered a steam-flaked corn-based finishing diet with or without bacterial direct-fed microbial mixtures1
| F-test | Contrasts2 | ||||||
|---|---|---|---|---|---|---|---|
| Item4 | Control | Treat-A | Treat-B | SEM3 | Treatment | CTL vs. A | CTL vs. B |
| Effective degradable fraction at 4% kp | |||||||
| DM | 26.86 | 23.19 | 23.68 | 2.813 | 0.55 | 0.32 | 0.39 |
| OM | 17.21 | 13.5 | 14.41 | 1.711 | 0.19 | 0.09 | 0.18 |
| NDF | 5.7 | 4.9 | 5.03 | 1.36 | 0.90 | 0.68 | 0.73 |
| ADF | 3.38 | 3.88 | 5.55 | 1.789 | 0.38 | 0.75 | 0.19 |
| Hemicellulose | 7.96 | 11.76 | 6.15 | 1.567 | 0.07 | 0.11 | 0.41 |
| Effective degradable fraction at 5% kp | |||||||
| DM | 25.72 | 22.4 | 22.68 | 2.577 | 0.55 | 0.34 | 0.37 |
| OM | 16.48 | 13.08 | 13.84 | 1.559 | 0.19 | 0.09 | 0.17 |
| NDF | 5.54 | 4.73 | 4.92 | 1.305 | 0.89 | 0.66 | 0.73 |
| ADF | 3.3 | 3.78 | 5.36 | 1.731 | 0.40 | 0.75 | 0.20 |
| Hemicellulose | 4.51 | 11.23 | 5.94 | 1.544 | 0.08 | 0.11 | 0.47 |
| Effective degradable fraction at 6% kp | |||||||
| DM | 24.84 | 21.8 | 21.93 | 2.377 | 0.55 | 0.34 | 0.36 |
| OM | 15.92 | 12.76 | 13.42 | 1.436 | 0.18 | 0.09 | 0.16 |
| NDF | 5.42 | 4.61 | 4.84 | 1.262 | 0.89 | 0.65 | 0.74 |
| ADF | 3.24 | 3.72 | 5.22 | 1.685 | 0.41 | 0.76 | 0.22 |
| Hemicellulose | 7.18 | 10.8 | 5.79 | 1.519 | 0.09 | 0.11 | 0.51 |
1Control (no DFM, lactose carrier only); Treat-A (L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, at 1:1:1:3 ratio, respectively; totaling 6 × 109 CFU (50 mg)/animal-daily minimum (Chr. Hansen Inc.); and Treat-B, the same DFM combination, but with doses at 1:1:3:1 ratio. Bacterial counts were ~30% greater than the minimum expected.
2 F-test protected pre-planned orthogonal contrasts were used to compare Control treatment vs. Treat-A and Control vs. Treat-B.
3SEM = standard error of the mean (n = 6 steers/treatment).
4Effective degradable fraction calculated as: ED(y) = A + B × kd/(kd + kp), where y is the nutrient (DM, OM, NDF, ADF, or hemicellulose); A is the soluble fraction (%); B is the potentially degradable fraction (%); and kd is the rate of degradation (%).
Discussion
The objective of current project was to evaluate the effects of bacterial direct-fed microbial mixtures containing L. animalis, P. freudenreichii, B. subtilis, and B. licheniformis, with a distinct ratio for the Bacilli offered to beef cattle consuming a steam-flaked corn-based finishing diet on intake, nutrient digestibility, feeding behavior, ruminal fermentation profile, and ruminal degradation kinetics.
Intake, digestibility, and feeding behavior
The absence of effects for currently offered bacterial DFM on cattle nutrient intake closely aligns with the findings observed by Lawrence et al. (2020), which conducted research involving the inclusion of L. animalis and P. freudenreichii as a top-dressed supplement to lactating dairy cows. The DFM was offered twice daily at a concentration of (1 × 109 CFU/d) for L. animalis and (2 × 109 CFU/d) for P. freudenreichii. Similarly, the research conducted by Kenney et al. (2015) demonstrated comparable findings, as they observed no discernible disparities in the intake variables when comparing treatment groups to control. The authors also offered a high-concentrate basal diet supplemented with lactic acid-producing and utilizing bacteria, containing L. acidophilus and P. freudenreichii at a dosage of 1 × 109 CFU/d.
The lack of effects on the apparent total tract digestibility of DM, OM, and HEM was partially corroborated by the finding observed by Qiao et al. (2010), which offered B. subtilis, and B. licheniformis at 2 × 1011 to Holstein cows. On the other hand, ADF digestibility increased for steers offered Treat-A by 26%, while a tendency for a 17% increase was observed for those offered the Treat-B compared to Control. The greater digestibility of fiber could be attributed to the inclusion of Bacillus species. Certain strains of Bacilli produce enzymes, such as amylases, proteases, and cellulases, that aid in the digestion of complex carbohydrates, proteins, and fiber (Ferrari et al., 1993). In the research conducted by Qiao et al. (2010), the dietary inclusion of B. licheniformis offered to Chinese Holstein, which consisted of a 40:60 concentrate-to-forage ratio, an enhancement of ~8% in fiber digestion was also observed. Such an improvement was attributed to the stimulation of cellulolytic bacteria growth in the rumen. Nevertheless, in a project conducted by Fuerniss et al. (2022), the impact of a combination of four Bacilli species (B. amyloliquefaciens, B. subtilis, B. pumilus, and B. licheniformis) at a dosage of 2 × 109 CFU/animal-daily was evaluated with regards to the hindgut microbiota in cattle. The authors revealed that the administration of such Bacilli species induced changes in the composition of microbial taxa responsible for fiber digestion in the hindgut. Such an increase in fibrolytic bacteria in the gastrointestinal tract suggests a potential ability for an enhanced degradation of the dietary fiber fractions. Improvements in the ability to degrade fiber fractions may have a meaningful implication on nutrient assimilation for ruminants, regardless of whether offered a finisher or a grower diet. Furthermore, the capacity of B. licheniformis to improve starch degradation has been demonstrated. This unique ability becomes particularly significant when considering its potential interaction with a diet containing high-starch concentration, which can lead to an expedited fermentation process (Ferrari et al., 1993; Deng et al., 2018; Pan et al., 2022). Thus, the inclusion of a bacterial mixture that contains Bacillus species in the diet of animals has shown promising results in enhancing fiber digestion and potentially improving the digestibility of complex carbohydrates.
Feeding behavior assessment allows the evaluation of potential nutritional disturbances, such as ruminal acidosis, in cattle. This can be determined by observing simple behaviors such as animals with head/ears down at the end of the pen, changes in intake levels, as well as analyzing the number of meals and duration of meals. By monitoring feeding behavior, it is possible to identify if an animal is experiencing such issues and gain insights into the severity of the problem (Gonzales et al., 2012). In the current project, only subtle effects were observed for feeding behavior variables. Major feeding behavior variables currently observed, such as rumination time and chewing activity were similar to those observed by (Ovinge et al., 2018; De Melo et al., 2019) which offered steam-flaked corn-based diets to beef cattle, as in the current experiment. The current experiment detailed assessment of feeding behavior and the consequent absence of responses for the variables measured suggests that the DFM offered were not connected with any potential signaling of satiety triggered by the host, and that the positive effects observed for nutrient digestion and ruminal degradation were perhaps directly related to chemical digestion.
Ruminal fermentation characteristics
The energy supplementation from grain sources was expected to depress ruminal pH (Caton and Dhuyvetter, 1997). Regardless of treatment, it was evident in the current experiment that animals experienced the lowest pH average between 8 and 16 h after feeding, which is in agreement with several other experiments that also continuously measured ruminal pH from beef steers offered steam-flaked corn-based finishing diets (Barajas and Zinn, 1998; Sindt et al., 2002; May et al., 2009). Steers offered Treat-A showed an increased ruminal pH average compared to Control, while also experiencing 300 min less time under a ruminal pH of 5.6, which is known as the threshold for ruminal subacute acidosis (Firkins et al., 1985; Larson et al., 1993; Owens et al., 1998). In addition, steers offered Treat-A also had a slightly lower ruminal temperature compared to Control. Paradoxically at first instance, such a dietary treatment (Treat-A) contained a greater concentration of B. licheniformis, which was expected to increase ruminal digestion of highly fermentable carbohydrates (such as starch) and perhaps consequently decrease ruminal pH and increase ruminal temperature. The precise mechanism of action of the bacteria used in the current research has not yet been fully elucidated. However, some of the unique factors described below, in the DFM mixture used for Treat-A may offer partial explanations for the present findings. First, Mingmongkolchai and Panbangred (2018) reported that the inclusion of B. Licheniformis improved fiber digestion, which was also observed in the current experiment. Greater ruminal fiber degradation can improve the synthesis of short-chain fatty acids that contain a greater pKa than lactic acid, and so increase ruminal pH. Ruminal fluid collections occurred at 0, 2, 4, 8, 16, and 23 h after feeding, and it was hypothesized that the inclusion of the DFM would modify the ruminal VFA profile, although such finding was not evident in the current experiment. Similarly, Dias et al. (2022) offered a combination of B. subtilis and B. licheniformis to Nellore bulls in a finishing phase and did not observe any difference in the VFA profile. Additionally, Kenney et al. (2015) offered high-concentrate diets supplemented with lactic-acid-producing and utilizing bacteria, containing L. acidophilus and P. freudenreichii (1 × 109 CFU/animal-daily) and did not observe differences in the ruminal VFA profile. Similar evidence has been also reported by Lawrence et al. (2020), which conducted a study involving L. animalis and P. freudenreichii as a top-dressed supplement to lactating dairy cows (offered twice daily at 1 × 109 and 2 × 109 CFU/animal-daily, respectively). Other than the fact that these studies were conducted with ruminants, they were also combining a lactic-acid-producing bacteria (L. acidophilus or L. animalis) with a lactic-acid-utilizing bacteria (P. freudenreichii). The rationale for such a combination is based on the fact that greater nutrient assimilation can be achieved, initially at the ruminal level, while minimizing adverse effects on the ruminal environment (Yoon and Stern, 1995; Nocek and Kautz, 2006; Elghandour et al., 2015). Thus, an increase in ruminal pH for a treatment that brings such combination of DFM may be partially justified. Secondly, a greater concentration of ruminal NH3–N was observed for steers offered Treat-A, with this concentration being more than twice as much as the Control group. Such finding is likely to be related to a potential greater ruminal degradation of protein, which can consequently induce the deamination of amino acids and the generation of NH3-N (Eschenlauer et al., 2002), affecting the ruminal buffering capacity (Kertz, 2010). Therefore, independent effects, although more likely to be the consequence of a combination, both factors could have contributed to the greater ruminal pH observed for steers offered Treat-A.
Regardless of treatment, a time effect on the VFA profile was observed. Ruminal fermentation is a continuous process occurring over the period of 24 h and considering that even though animals were offered diets once daily, multiple meals within the day were observed, thus the time of collection effects observed for ruminal VFA profile are in line with physiological expectations. Current findings showed branched-chain, valerate, and acetate as part of the initial stages of acid production around feeding time, similar to the findings observed from previous studies (Soto-Navarro Et al., 2000; May et al., 2009) that offered diets with approximately 65% of steam-flaked corn.
Ruminal in situ degradability
The ruminal in situ disappearance of distinct substrates used for incubation provides useful insights into the effects of ruminal environment conditions, substrate quality, and fractions potentially degradable, while also the combination of factors. It is important to highlight that ruminal degradability assessments cannot be used to make inferences regarding the apparent total tract digestibility, as they do not account for the effects of other digestive tract compartments on nutrient digestion, but rather indicate the kinetic uniqueness of one sole compartment. The current experiment utilized a finisher diet and low-quality forage as substrates for in situ incubations. Although comparing both was not part of the objectives, an anticipated and observed outcome was the likelihood that the finisher diet would demonstrate higher degradation rates, increased effective degradable fractions, and reduced undegradable fractions compared to the low-quality forage substrates. This expectation stems from the influential role of the finisher diet that the steers were consuming. The diet significantly shapes the ruminal environment by affecting microbial populations, enzyme availability, rumen microbe numbers, their activity, and directly impacting nutrient degradation efficiency (Hungate, 1966). It is also important to highlight that the NDF fraction of the diet-substrate ruminally incubated contained less fiber content but was composed of a greater proportion of fermentable NDF, mainly represented by fiber from corn bran, wet corn gluten feed, and alfalfa hay. On the other hand, the forage-substrate had a greater quantity of fiber content, from low-quality forage, nevertheless, this fiber content was characterized by a decreased amount of fermentable NDF. As reported by Owens et al. (1998), the greater inclusion of dietary roughage is expected to reduce the incidence of acidosis due to the relationship between fiber digestion and ruminal pH. As the dietary inclusion of roughage increases, it will lead to a greater ruminal pH, which creates an optimal environment for the activity of enzymes such as cellulase and hemicellulose that will be ultimately responsible for the degradation of fiber fractions inside the rumen.
Current ruminal degradation kinetics data were collected from steers adapted and offered a steam-flaked corn-based diet, which elicits a greater microbiota of amylolytic bacteria rather than fibrolytic ones. Thus, the inclusion of current bacterial DFM mixtures were physiologically challenged in respect of fiber degradation, given that the current ruminal environment did not offer ideal conditions for fiber degradation. The bacterial DFM mixtures used in the current experiment contained B. subtilis and B. licheniformis. These bacteria were expected to enhance fiber utilization (Pan et al., 2022). Such hypothesis aligned with the current findings, as the animals offered the Treat-A exhibited tendencies for a numerical improvement in the effective degradable fraction of NDF on diet-substrates. This effect on fiber degradation was likely to be induced by the more degradable fraction of the NDF incubated, given that hemicellulose was the most positively affected fraction when Treat-A was offered. Interestingly, hemicellulose was also the only fraction with improved degradation when the forage-substrate was incubated. It is suggestive that the combination of bacteria in Treat-A enhanced the ruminal digestion of high-quality fiber components, as such results occurred within the more degradable fractions from ingredients used in the diet-substrate and the forage-substrate.
As the ATTND of ADF was increased by ~26%, reaching levels of 52% to 54% when Treat-A was offered, and NDF and ADF ruminal effective degradation (regardless of the kp used) accounted for ~37% of such overall degradation only, it is suggestive that post-ruminal fermentation potentially triggered by Bacilli spores activity at the hindgut (potentially cecum). It could potentially establish the hindgut as an important site of digestion when such a combination of bacterial DFM is offered to beef cattle fed finishing diets.
Conclusions
The DFM mixtures offered did not affect intake and major feeding behavior variables. The DFM combination containing a greater proportion of B. licheniformis (Treat-A) seemed to elicit an improved total tract core-fiber digestibility and a healthier ruminal pH pattern, in which the ruminal environment showed to be more prone to induce the effective degradability of fiber fractions, while also releasing more NH3-N.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ATTND
apparent total tract nutrient digestibility;
- BW
body weight;
- CTL
control;
- DM
dry matter
- DMI
dry matter intake
- ED
effective degradability
- HEM
hemicellulose
- iNDF
indigestible neutral detergent fiber
- NDF
neutral detergent fiber
- OM
organic matter
- OMI
organic matter intake
- pH
potential of hydrogen
- Treat
treatment
- VFA
volatile fatty acid
Contributor Information
Kaliu G Scaranto Silva, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Jhones O Sarturi, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Bradley J Johnson, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Dale R Woerner, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Alejandra M Lopez, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Barbara M Rodrigues, Center for Natural Resource Technology Information, Texas A&M AgriLife Research, College Station, TX 77840, USA.
Kaue T Nardi, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Camron J Rush, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, 79409, USA.
Conflict of interest statement
The authors report no conflicts of interest.
Literature Cited
- AOAC. 1990. Official Method of Analysis. 15th ed. Arlington, VA: AOAC Int. Association of Official Agricultural Chemists. [Google Scholar]
- AOAC. 2005. Official Methods of Analysis. 18th ed. 3rd rev. Gathersburg, MD: AOAC Int. Association of Official Agricultural Chemists. [Google Scholar]
- Ban, Y., and Guan L. L... 2021. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 12:109. doi: 10.1186/s40104-021-00630-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas, R., and Zinn R. A... 1998. The feeding value of dry-rolled and steam-flaked corn in finishing diets for feedlot cattle: influence of protein supplementation. J. Anim. Sci. 76:1744–1752. doi: 10.2527/1998.7671744x [DOI] [PubMed] [Google Scholar]
- Broderick, G. A., and Kang J. H... 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8 [DOI] [PubMed] [Google Scholar]
- Buntyn, J. O., Schmidt T. B., Nisbet D. J., and Callaway T. R... 2016. The role of direct-fed microbials in conventional livestock production. Annu. Rev. Anim. Biosci. 4:335–355. doi: 10.1146/annurev-animal-022114-111123 [DOI] [PubMed] [Google Scholar]
- Caton, J., and Dhuyvetter D... 1997. Influence of energy supplementation on grazing ruminants: requirements and responses. J. Anim. Sci. 75:533–542. doi: 10.2527/1997.752533x [DOI] [PubMed] [Google Scholar]
- Caton, J. S., Freeman A. S., and Galyean M. L... 1988. Influence of protein supplementation on forage intake, in situ forage disappearance, ruminal fermentation and digesta passage rates in steers grazing dormant blue grama rangeland. J. Anim. Sci. 66:2262–2271. doi: 10.2527/jas1988.6692262x [DOI] [Google Scholar]
- Cole, N. A., McCuistion K., Greene L. W., and McCollum F. T... 2011. Effects of concentration and source of wet distillers grains on digestibility of steam-flaked corn-based diets fed to finishing steers. Prof. Anim. Sci. 27:302–311. doi: 10.15232/s1080-7446(15)30493-9 [DOI] [Google Scholar]
- De Melo, A. H. F., Marques R. S., V. N.Gouvêa,, De Souza J., Batalha C. D. A., Basto D. C., Millen D. D., Drouillard J. S., and, Santos F. A. P... 2019. Effects of dietary roughage neutral detergent fiber levels and flint corn processing method on growth performance, carcass characteristics, feeding behavior, and rumen morphometrics of Bos indicus cattle. J. Anim. Sci. 97:3562–3577. doi: 10.1093/jas/skz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, K., Xiao D., Ma Y., Tu T., Diao Y., Chen Q., and Jiang Y. H... 2018. Ruminal fermentation, nutrient metabolism, and methane emissions of sheep in response to dietary supplementation with Bacillus licheniformis. Anim. F. Sci. Tech. 241:38–44. doi: 10.1016/j.anifeedsci.2018.04.014 [DOI] [Google Scholar]
- Dias, B. G. C., Santos F. A. P., Meschiatti M., Brixner B. M., Almeida A. A., Queiroz O., and Cappellozza B. I... 2022. Effects of feeding different probiotic types on metabolic, performance, and carcass responses of Bos indicus feedlot cattle offered a high-concentrate diet. J. Anim. Sci. 100:1–10. doi: 10.1093/jas/skac289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghandour, M. M. Y., Salem A. Z. M., Castañeda J. S. M., Camacho L. M., Kholif A. E., and Chagoyán J. C. V... 2015. Direct-fed microbes: a tool for improving the utilization of low-quality roughages in ruminants. J. Integr. Agric.. 14:526–533. doi: 10.1016/s2095-3119(14)60834-0. [DOI] [Google Scholar]
- Eschenlauer, S. C. P., McKain N., Walker N. D., McEwan N. R., Newbold C. J., and Wallace R. J... 2002. Ammonia production by ruminal microorganisms and enumeration, isolation, and characterization of bacteria capable of growth on peptides and amino acids from the sheep rumen. Appl. Environ. Microbiol. 68:4925–4931. doi: 10.1128/AEM.68.10.4925-4931.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, E., Jarnagin A. S., and Schmidt B. F... 1993. Commercial production of extracellular enzymes. In Bacillus subtilis and other gram-positive bacteria. doi: 10.1128/9781555818388.ch62 [DOI] [Google Scholar]
- Firkins, J. L., Berger L. L., and G. C.Fahey, Jr. 1985. Evaluation of wet and dry distillers grains and wet and dry corn gluten feeds for ruminants. J. Anim. Sci. 60:847–860. doi: 10.2527/jas1985.603847x [DOI] [Google Scholar]
- Fuerniss, L. K., Kreikemeier K. K., Reed L. D., Cravey M. D., and Johnson B. J... 2022. Cecal microbiota of feedlot cattle fed a four-species Bacillus supplement. J. Anim. Sci. 100:10. doi: 10.1093/jas/skac258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, L. M., Calsamiglia X., Schwartzkopf-Genswein S., and Alfred K. F... 2012. Ruminal acidosis in feedlot cattle: interplay between feed ingredients, rumen function and feeding behavior (a review). Anim. F. Sci. Tech. 172:66–79. doi: 10.1016/j.anifeedsci.2011.12.009 [DOI] [Google Scholar]
- Green, D. H., Wakeley P. R., Page A., Barnes A., Baccigalupi L., Ricca E., and Cutting S. M... 1999. Characterization of two Bacillus probiotics. Appl. Environ. Microbiol. 65:4288–4291. doi: 10.1128/AEM.65.9.4288-4291.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorini, P., Gunter S. A., and Beck P. A... 2008. Matching plant and animal processes to alter nutrient supply in strip-grazed cattle: timing of herbage and fasting allocation. J. Anim. Sci. 86:1006–1020. doi: 10.2527/jas.2007-0432 [DOI] [PubMed] [Google Scholar]
- Hungate, R. E. 1966. The Rumen and its microbes. New York: Academic Press. [Google Scholar]
- Kenney, N. M., Vanzant, E. S., Harmon, D. L., & McLeod, K. R. (2015). Direct-fed microbials containing lactate-producing bacteria influence ruminal. J. Anim. Sci. 93:2336–2348. [DOI] [PubMed] [Google Scholar]
- Kertz, A. F. 2010. Urea feeding to dairy cattle: a historical perspective and review. Prof. Anim. Scientist 26:257–272. doi: 10.15232/S1080-7446(15)30593-3 [DOI] [Google Scholar]
- Krehbiel, C. R., Rust S. R., Zhang G., and Gilliland S. E... 2003. Bacterial direct-fed microbials in ruminant diets: performance response and mode of action. J. Anim. Sci. 81:120–132. doi: 10.2527/2003.8114_suppl_2E120x [DOI] [Google Scholar]
- Krizsan, S. J., and Huhtanen P... 2013. Effect of diet composition and incubation time on feed indigestible neutral detergent fiber concentration in dairy cows. J. Dairy Sci. 96:1715–1726. doi: 10.3168/jds.2012-5752 [DOI] [PubMed] [Google Scholar]
- Larson, E. M., Stock R. A., Klopfenstein T. J., Sindt M. H., and Huffman R. P... 1993. Feeding value of wet distillers by products for finishing ruminants. J. Anim. Sci. 71:2228–2236. doi: 10.2527/1993.7182228x [DOI] [PubMed] [Google Scholar]
- Lawrence, M., Polukis S., Barnard A. M., Miller M. A., L.Kung, Jr, and Gressley T. F... 2020. Evaluating the effects of Lactobacillus animalis and Propionibacterium freudenreichii on performance and rumen and fecal measures in lactating dairy cows. J. Dairy Sci. 104:4119–4133. doi: 10.3168/jds.2020-19291 [DOI] [PubMed] [Google Scholar]
- May, M. L., Quinn M. J., Reinhardt C. D., Murray L., Gibson M. L., Karges K. K., and Drouillard J. S... 2009. Effects of dry-rolled or steam-flaked corn finishing diets with or without twenty-five percent dried distillers grains on ruminal fermentation and apparent total tract digestion. J. Anim. Sci. 87:3630–3638. doi: 10.2527/jas.2008-0857 [DOI] [PubMed] [Google Scholar]
- McAllister, T. A., Beauchemin K. A., Alazzeh A. Y., Baah J., Teather R. M., and Stanford K... 2011. Review: the use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 91:193–211. doi: 10.4141/cjas10047 [DOI] [Google Scholar]
- Mccollum, F. T., and Galyean M. L... 1985. Influence of cottonseed meal supplementation on voluntary intake, rumen fermentation and rate of passage of prairie hay in beef steers. J. Anim. Sci. 60:570–577. doi: 10.2527/jas1985.602570x [DOI] [Google Scholar]
- Mingmongkolchai, S., and Panbangred W... 2018. Bacillus probiotics: an alternative to antibiotics for livestock production. J. Appl. Microbiol. 124:1334–1346. doi: 10.1111/jam.13690 [DOI] [PubMed] [Google Scholar]
- Nardi, K. T., Sarturi J. O., Huerta-Leidenz N., Henry D. D., Woerner D. R., Ciriaco F. M., Sánchez-Escalante A., Torrescano-Urrutia G. R., Silva K. G. S., and Favero I. G... 2023. The effects of a Nutritional Packet (live yeast, vitamins C and B1, and electrolytes) offered during the final phase of feedlot steers on growth performance, nutrient digestion, and feeding behavior. J. Anim. Sci. 101:skac416. doi: 10.1093/jas/skac416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle. 8th revised ed.. Washington, DC: The National Academies Press. doi: 10.17226/19014 [DOI] [Google Scholar]
- Nocek, J. E., and Kautz W. P... 2006. Direct-fed microbial supplementation on ruminal digestion, health, and performance of pre- and postpartum dairy cattle. J. Dairy Sci. 89:260–266. doi: 10.3168/jds.S0022-0302(06)72090-2 [DOI] [PubMed] [Google Scholar]
- Ørskov, E. R., and Mcdonald I... 1970. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92:499–503. doi: 10.1017/s0021859600063048 [DOI] [Google Scholar]
- Ovinge, L. A., Sarturi J. O., Galyean M. L., Ballou M. A., Trojan S. J., Campanili P. R. B., Alrumaih A. A., and Pellarin L. A... 2018. Effects of a live yeast in natural-program finishing feedlot diets on growth performance, digestibility, carcass characteristics, and feeding behavior. J. Anim. Sci. 96:684–693. doi: 10.1093/jas/sky011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, F. N., Secrist D. S., Hill W. J., and Gill D. R... 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275–286. doi: 10.2527/1998.761275x [DOI] [PubMed] [Google Scholar]
- Pan, L., Harper K., Queiroz O., Copani G., and Cappellozza B. I... 2022. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Transl. Anim. Sci. 17:6. doi: 10.1093/tas/txac067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner, G. B., Beauchemin K. A., and Mutsvangwa T... 2006. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 89:2132–2140. doi: 10.3168/jds.S0022-0302(06)72284-6 [DOI] [PubMed] [Google Scholar]
- Qiao, G. H., Shan A. S., Ma N., Ma Q. Q., and Sun Z. W... 2010. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Nutr. 1:429–436. doi: 10.1111/j.1439-0396.2009.00926.x [DOI] [PubMed] [Google Scholar]
- Retta, K. 2016. Role of probiotics in rumen fermentation and animal performance: a review. Int. J. Livest. Prod. 7:24–32. doi: 10.5897/IJLP2016.0285 [DOI] [Google Scholar]
- Sindt, J. J., Drouillard J. S., Thippareddi H., Phebus R. K., Lambert D. L., Montgomery S. P., Farran T. B., Labrune H. J., Higgins J. J., & Ethington R. T.. 2002. Evaluation of finishing performance, carcass characteristics, acid-resistant E. coli and total coliforms from steers fed combinations of wet corn gluten feed and steam-flaked corn. J. Anim. Sci. 80:3328–3335. [DOI] [PubMed] [Google Scholar]
- Soto-Navarro, S. A., Krehbiel C. R., Duff G. C., Galyean M. L., Brown M. S., and Steiner R. L... 2000. Influence of feed intake fluctuation and frequency of feeding on nutrient digestion, digesta kinetics, and ruminal fermentation profiles in limit-fed steers. J. Anim. Sci. 78:2215–2222. doi: 10.2527/2000.7882215x [DOI] [PubMed] [Google Scholar]
- Springer-Verlag, A., De Boer S., Priest F., and Diderichsen B... 1994. Applied microbiology biotechnology mini review on the industrial use of Bacillus licheniformis: a review. Appl. Microbiol. Biotechnol. 40:595–598. doi: 10.1007/BF00173313 [DOI] [Google Scholar]
- Sun, P., Wang J. Q., and Deng L. F... 2013. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal. 7:216–222. doi: 10.1017/S1751731112001188 [DOI] [PubMed] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A... 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Vanzant, E. S., Cochran R. C., Titgemeyer E. C., Stafford S. D., Olson K. C., Johnson D. E., and St Jean G... 1996. In vivo and in situ measurements of forage protein degradation in beef cattle. J. Anim. Sci. 74:2773–2784. doi: 10.2527/1996.74112773x [DOI] [PubMed] [Google Scholar]
- Wilson, B. K., and Krehbiel C. R... 2012. Current and future status of practical applications: beef cattle. In: Direct-fed microbials and prebiotics for animals: science and mechanisms of action 9:137–152. doi: 10.1007/978-1-4614-1311-0_9 [DOI] [Google Scholar]
- Yoon, I., and Stern M... 1995. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants - a review. Anim. Biosci. 8:533–555. doi: 10.5713/ajas.1995.553 [DOI] [Google Scholar]