Abstract
Epidemic outbreaks of group B meningococcal disease exhibit a clonal nature consisting of a common serotype-subtype. Subtype-specific monoclonal antibodies (MAbs) directed toward two variable regions (VR1 and VR2) of the class 1 protein of Neisseria meningitidis are used in this classification scheme. A new MAb was developed to classify a nonsubtypeable (NST) strain of N. meningitidis, 7967. This MAb bound to both the NST strain and the prototype subtype P1.14 strain, S3446, by dot blot analysis. However, a MAb produced to the prototype P1.14 strain did not bind to strain 7967. Sixteen additional strains were further identified as P1.14 with the prototype MAb; of these, 15 strains bound both MAbs. Differences in the characteristics of binding of both antibodies to the three apparently diverse P1.14 strains were studied further by using outer membrane complex proteins, immobilized peptides, and soluble peptides. Deduced amino acid analysis suggested that both MAbs bind to VR2 and that single amino acid changes within VR2 (KM, NM, or KK) might explain the differences in binding characteristics. These results demonstrated that minor variations which exist within subtype variable regions may be clearly identified only by a combination of molecular and immunologic testing. The impact of subtype variation will become more evident as subtype-specific vaccines are developed and tested for efficacy.
Neisseria meningitidis causes bacterial meningitis and septicemia worldwide. Epidemiological studies of endemic or epidemic outbreaks of meningococcal disease rely on polyvalent and monoclonal antibodies (MAbs) to capsular polysaccharides for monitoring serogroup specificity (27). This organism can be further classified into serotypes and subtypes with MAbs directed toward two major outer membrane proteins (OMPs) (6). Serotyping is based on MAbs which bind to the class 2 or class 3 OMP (PorB). Subtyping MAbs bind one of two immunodominant variable regions (VR1 and VR2) of the class 1 OMP (PorA) (1, 9, 10, 11, 13, 22). Complete subtyping requires identification of epitopes found in both variable regions (5).
MAbs to about 15 subtype epitopes have been developed and characterized; however, nonsubtypeable (NST) strains are still found. NST strains represent one of three categories: (i) strains possessing class 1 epitopes to which MAbs have not been developed and characterized, (ii) strains which do not express PorA, and (iii) strains in which the PorA subtype epitopes differ only slightly from known subtype epitopes due to genetic modifications, such as point mutations or duplication or deletion events, which eliminate binding of subtyping MAbs.
A significant part of recent group B meningococcal vaccine development efforts has been focused on OMPs as principal components of a subtype-serotype-specific vaccine (26). This vaccination approach is based on the observations that PorA elicits a human bactericidal antibody response (24) and that subtype-specific MAbs passively protect infant rats against challenge with N. meningitidis (17, 18). An effective subtype-specific vaccine should include the most prevalent subtype epitopes associated with the strains causing disease in the population in which the vaccine will be used. Human bactericidal antibodies induced by vaccination with a vaccine of one subtype are not equally effective in killing other subtype strains. Even single amino acid changes in VR1 and VR2 and deletions in regions flanking the epitopes may result in loss of reactivity with subtype-specific MAbs (12, 23), as observed in several recent outbreaks. One such variant also showed increased resistance to bactericidal activity (16), suggesting a possible influence of such subtype variants on the level of protection induced by a subtype-specific vaccine.
We have defined three different point mutations in the subtype-specific epitope P1.14 of N. meningitidis, using a combination of molecular and immunological techniques. The variability within this epitope highlights the importance of defining subtypes molecularly as well as immunologically.
MATERIALS AND METHODS
Growth and maintenance of bacterial strains.
Strains of N. meningitidis (strains 7967, 8659, 8778, 8779, and 9304) were obtained from the culture collection at the Walter Reed Army Institute of Research (WRAIR), and one strain (S3446) was kindly provided by C. E. Frasch, Food and Drug Administration, Rockville, Md. Strains were maintained at −70°C in skim milk or were lyophilized and stored at 4°C. Cultures were grown on supplemented GC agar (19) for 16 to 18 h at 37°C in a candle extinction jar.
MAbs.
MN21G3.17, the prototype P1.14 MAb, was kindly provided by J. T. Poolman, Rijsinstituut voor Volksgezondheit en Milieuhygiene, Bilthoven, The Netherlands (20), and is referred to as 1.14R in this paper. MAb BZ-1-P1.14 was produced in our laboratory and is referred to as 1.14W in this paper.
Briefly, BALB/c mice were immunized with a saline suspension of N. meningitidis 7967 (Z:4:NST) containing approximately 108 live bacteria per ml. The mice were injected intraperitoneally with 0.1 ml of the suspension at weeks 0, 3, and 7. Spleens were harvested 3 days after the final immunization, and lymphocytes were fused with P3X63-Ag 8.653 mouse myeloma cells at a ratio of 4:1, as previously described (14). Positive clones were selected by enzyme-linked immunosorbent assay (ELISA) using plates coated with 7967 outer membrane complex (OMC). Western blot analysis was used to confirm the binding of the MAb to the class 1 OMP (40.4 kDa) of strain 7967. Ascites fluid was produced by injection of 5 × 106 hybridoma cells into pristane-primed BALB/c mice. Ascites fluid was pooled, the titers were determined, and aliquots were stored at −20°C.
Dot blot analysis.
Cell suspension (2 to 3 μl of fresh live bacteria in 0.9% NaCl, with the cell density adjusted to between a no. 3 and no. 5 McFarland standard) was dotted onto nitrocellulose membranes, and the membranes were dried for 10 min at room temperature (RT). The membranes were used immediately or stored at 4°C until needed. Membranes were blocked for 30 min with 1% casein buffer and then washed once with phosphate-buffered saline (PBS). Casein buffer contained 10 g of casein in 400 ml of 0.1 N NaOH added to 400 ml of water containing 1.2 g of Tris, 8.8 g of NaCl, 1 g of MgCl2 · 6H2O, 1 g of sodium azide (pH 7.5), and water for a total volume of 1 liter. MAb diluted 1:10,000 in blocking buffer was added. Membranes were incubated overnight at RT on a rotator and then washed three times with PBS. The membranes were placed in a solution containing 1 μg of phosphatase-labeled goat anti-mouse immunoglobulin G (IgG) plus IgM (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) per ml diluted in blocking buffer and allowed to react for 2 h at RT on a rotator. The membranes were washed three times with PBS and one time with 50 mM Tris (pH 8.0) and developed with 1 mg of Naphthol AS-MX phosphate per ml–2 mg of Fast Red TR salt per ml in 50 mM Tris (pH 8.0) (Sigma Chemical Co., St. Louis, Mo.).
ELISA.
OMC antigen was prepared from strains 7967, S3446 (B:14:P1.14 prototype strain), and 8659 as previously described (25).
Immulon 4 plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with 20 μg of OMC per ml or 5 μg of peptide per ml diluted in PBS (pH 7.5) (Biowhittaker Inc., Walkersville, Md.) and incubated for 2 h at 37°C. The plates were washed four times with Dulbecco’s PBS (Life Technologies Inc., Gaithersburg, Md.) and blocked with 0.5% bovine serum albumin (BSA)–0.5% casein–PBS buffer (BSA-C) (3) for 1 h at 37°C. Plates were again washed four times with PBS. Triplicate ascites samples, diluted in BSA-C, were incubated overnight at RT.
Plates were again washed four times with PBS, and 1 μg of alkaline phosphatase-labeled goat anti-mouse IgG plus IgM (Kirkegaard & Perry Laboratories, Inc.) per ml in BSA-C was added to the plates. The plates were incubated overnight at RT. The plates were again washed, and color was developed by using Sigma 104 phosphatase substrate in 1 M Tris–0.3 mM MgCl2 buffer (pH 9.8). The reaction was stopped at 30 min by the addition of 3 N NaOH, and absorbance was read at a dual wavelength of 405 and 504 nm.
SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in 10% acrylamide gels measuring 16 by 16 by 0.15 cm. OMC at a concentration of 2.5 mg of protein per ml or low-molecular-weight protein standards (Bio-Rad Laboratories, Hercules, Calif.) were mixed 1:1 with treatment buffer (0.125 M Tris-Cl [pH 6.8], 4% SDS, 20% glycerol, 10% 2-mercaptoethanol) and boiled for 10 min before being loaded onto the gels. Gels were run at a constant current of 30 mA per gel at 10°C for approximately 4 h. The gels were stained with Coomassie brilliant blue R-250 or blotted onto nitrocellulose filters (0.45-μm pore size) at 30 V overnight at 10°C. Immunoblotting was performed as described for dot blotting, except the primary antibody was diluted 1:500.
PCR.
A fresh overnight culture of each strain was grown on supplemented GC agar. A single colony of each was suspended in 25 μl of 0.5 N NaOH for 15 min to lyse the bacteria. Twenty-five microliters of 1 N Tris (pH 8.0) was added for neutralization, followed by dilution with 450 μl of water. Ten microliters of this suspension was added to the PCR mix by hot start at 95°C. The PCR mix contained 10 μl of 10× reaction buffer, 200 μM of each deoxynucleoside triphosphate, 20 pmol each of primers (forward [GCGGCCGTTGCCGATGTCAGCC] and reverse [GCGGCATTAATTTGAGTGTAGTTGCC]), 3.0 U of Hot Tub Polymerase (Amersham Corp, Arlington Heights, Ill.), and water to 90 μl. Cycling was performed in two steps: 95°C for 1 min and 70°C for 2 min for 20 cycles in a thermocycler (Perkin-Elmer Cetus Corp., Norwalk, Conn.).
Partial sequence analysis.
Direct sequencing of PCR products was performed manually as previously described (15). Alternately, DNA from four PCRs was concentrated in Ultrafee-MC filter units (Millipore Corp., Bedford, Mass.). Concentrated DNA was separated in 1.2% SeaPlaque agarose (FMC BioProducts, Rockland, Me.) and cut from the gel under UV visualization. The amplified DNA was eluted and processed with Wizard PCR Preps (Promega Corp., Madison, Wis.). Sequencing primers included the forward amplification primer upstream of VR1 and AACAGCCTTCAGCCCTTCG or CACTCCCCTTAAAGCCGAT downstream of VR1 and CGGTTTCAGCGGCAGCGTC upstream of VR2 and CCCACATTGGCGTGTCTCGC or GTTCCCGGCAAAACCGCC downstream of VR2. Automated sequencing reactions were performed by using the DyeDeoxy Terminator Cycle Sequencing Kit on a model 373 DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.).
Solid-phase peptide synthesis (modified Geysen pin method).
Synthetic octapeptides covalently linked to derivatized polyethylene pins were prepared according to the modified technique of Geysen et al. (7). Synthesis of the overlapping peptides was directed from computer software and hardware designed at WRAIR (4). 9-Fluorenylmethyoxycarbonyl (Fmoc)-amino acid pentafluorophenyl esters were purchased from Peninsula Laboratories (Belmont, Calif.), and reagent grade chemicals were obtained from Aldrich Chemical Co. (Milwaukee, Wis.) and Fisher Scientific Co. (Springfield, N.J.). The synthesis was performed in duplicate, and the peptide composition was confirmed by amino acid analysis of one control pin per plate by complete hydrolysis with 6 N HCl at 110°C followed by analysis in a Beckman 6300 analyzer. Two control pins encompassing a previously mapped epitope were also included.
The pins were prepared for ELISA by sonication in 0.1 M sodium phosphate buffer (pH 7.2) with 1% SDS and 0.2% 2-mercaptoethanol at 70°C for 30 min followed by a hot water rinse and a boiling methanol bath for 5 min. After being air dried under a fume hood for 1 h, pins were stored at −70°C with silica desiccant.
Peptide synthesis.
Solid-phase peptide synthesis was performed with a 430A peptide synthesizer (Applied Biosystems, Inc.) with Fmoc-protected amino acids and side chain-protecting groups supported by p-hydroxymethylphenoxymethylpolystyrene resin. All solvents and side chain-protected amino acids were also obtained from Applied Biosystems, Inc. Following synthesis, protecting groups were removed by acid hydrolysis with trifluoroacetic acid in the presence of thioanisole, 1,2-ethanedithiol, and water as scavengers. Amino acid analysis and reverse-phase high-pressure liquid chromatography (HPLC) were used to determine the amino acid content and peptide purity, respectively.
Cyclization of peptides was achieved by dimethyl sulfoxide oxidation (21). Briefly, P1.14 peptides were synthesized with CC residues added at the N terminus and with GC residues added at the C terminus, resulting in disulfide bond formation upon oxidation. Following deprotection, the peptides, at a concentration of 0.5 mg/ml, were resuspended in PBS buffer (pH 6.0) with 20% dimethyl sulfoxide by volume. The progress of the oxidation was monitored by reverse-phase HPLC until completion (approximately 4 h). The crude peptide was then loaded onto a preparative C18 reverse-phase HPLC column, and the desired product was eluted.
RESULTS
Determination of the specificity of MAb 1.14W.
The specificity of MAb 1.14W was determined by testing a panel of prototype N. meningitidis strains, including strain 7967, by dot blot assay. The only strains binding MAb 1.14W were 7967 and S3446, the subtype P1.14 prototype strain. Strain 7967 was negative by dot blot assay with MAb 1.14R, the prototype P1.14 MAb (Table 1). To further test the specificity of 1.14W, a panel of 100 strains from the WRAIR culture collection (19 nonsubtypeable strains and 81 subtypeable strains) were tested by dot blot. Five of the six strains that were previously positive with 1.14R were positive with 1.14W; only strain 8659 was positive with 1.14R and not with 1.14W. Eleven additional strains were identified by dot blot with 1.14W. A total of 18 strains were identified as P1.14; 16 of these strains were positive with both antibodies.
TABLE 1.
Reactivity of subtype MAbs against three meningococcal strains
| Strain | Dot blot
|
Western blot
|
||
|---|---|---|---|---|
| 1.14W | 1.14R | 1.14W | 1.14R | |
| 7967 | + | − | + | − |
| S3446 | + | + | − | + |
| 8659 | − | + | − | + |
Western blot analysis.
The two MAbs 1.14W and 1.14R showed different patterns of binding to strains S3446, 7967, and 8659 analyzed by Western blotting. MAb 1.14W bound well to its homologous strain 7967 but not to the others, whereas 1.14R bound only to S3446 and 8659 and not to strain 7967 (Table 1).
Fourteen strains that were positive with both antibodies in dot blot analysis were further analyzed by Western blotting (data not shown). All of the strains reacted with 1.14R, and none reacted with 1.14W. The only strain found positive with 1.14W by immunoblotting was the parent strain, 7967.
It appeared that the reactivity of 1.14W was dependent on an epitope which was unavailable after denaturation of the class 1 protein in all strains except 7967. These data were insufficient to assess whether the epitopes of the two MAbs were both found on the same VR (P1.14 specific) or if they were actually directed toward different VRs and identified different subtypes. Alternatively, the epitopes of the two MAbs may have differed by only minor variations within one VR, as has been reported for other subtype-specific epitopes (12, 16, 23). Therefore, DNA sequence analysis of the class 1 gene porA was performed.
PCR-directed sequencing.
Six strains that were dot blot positive with 1.14W or 1.14R were selected for sequencing, including four strains which bound to both MAbs and two which showed different binding patterns. The relevant regions (VR1 and VR2) of porA were sequenced, and the data were analyzed (Table 2). The deduced amino acid sequences in VR1 differed significantly for several of the isolates and were not considered the probable binding site for either MAb. The previously described VR1 subtype epitope P1.7 (ASGQ) was present in two of the strains but not the others. The deduced amino acid sequences for VR2 were all similar, and the sequence for strain S3446 was consistent with published data (VDEKKM) (10). Strains 7967 and 8659 varied from strain S3446 by single base changes which would result in differences of one amino acid (M→K and K→N, respectively). It was predicted that the sequences of VR2 could represent the binding sites for 1.14W and 1.14R.
TABLE 2.
Deduced amino acid sequences of VR1 and VR2 of six strains of N. meningitidis which bound to one or both MAbs 1.14W and 1.14R
| Straina | Group: serotype | Sequence for:
|
|
|---|---|---|---|
| VR1 | VR2 | ||
| S3446 | B:14 | TEQPSRTQGQTSNQVKVT---KAKS | PAYVDEKKMVHAA |
| 7967 | Z:4 | TEQPSKAQGQTNNQVKVT---KAKS | PAYVDEKKKVHAA |
| 8659 | B:NT | TEQPSKAQGQTNNQVKVT---KAKS | PAYVDEKNMVHAA |
| 8778 | B:8 | TEAQAANGGASGQVKVTKVTKAKS | PAYVDEKKMVHAA |
| 8779 | B:8 | TEAQAANGGASGQVKVTKVTKAKS | PAYVDEKKMVHAA |
| 9304 | B:NT | TEQPSRTQGQTSNQVKVT---KAKS | PAYVDEKKMVHAA |
Strains are in WRAIR collection and were obtained from the following individuals or locations: Carl Frasch (S3446), Harry Feldman (7967), Chile (8659), Dominican Republic (8778 and 8779), and Brazil (9304).
Epitope mapping.
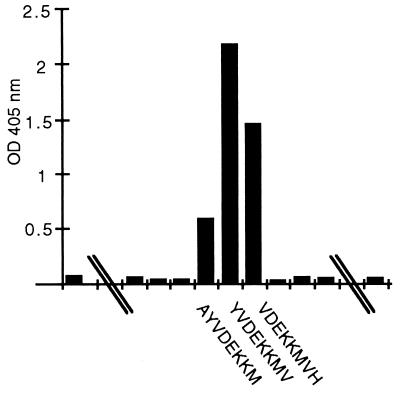
The deduced sequences of 39 amino acids encompassing VR2 of strains 7967, S3446, and 8659 were selected in order to identify the epitopes of 1.14W and 1.14R. Overlapping octamers representing these sequences were synthesized on Geysen pins. MAb 1.14R bound to three pins of S3446, representing the sequence VDEKKM (Fig. 1). MAb 1.14W did not bind to solid-phase peptides synthesized from the VR2 sequence of 7967, S3446, or 8659. The possibility existed that the epitope for 1.14W was within this region, but determination of that epitope required either a longer peptide or a unique conformational structure of the epitope. Therefore, longer peptides which could be used for quantitative analysis of MAb binding were prepared.
FIG. 1.
Reactivity of MAb 1.14R by ELISA with overlapping octapeptides representing region VR2 of meningococcal strain S3446.
Peptide analysis.
Three peptides consisting of 20 amino acids deduced from the sequences of VR2 of strains S3446, 7967, and 8659 were synthesized for quantitative analysis by ELISA with both MAbs. The additional C residues at the termini of the peptide were synthesized to allow the formation of a disulfide bond, thus mimicking the predicted exposed loop formation of VR2.
The patterns of binding of both MAbs to peptides and the OMC of each strain were compared (Table 3). MAb 1.14W bound well to the native form of the antigen in the OMC of both S3446 and 7967, but it bound well only to the peptide unique to the parent strain (VDEKKK) and weakly to the sequence of S3446 (VDEKKM). The binding affinity of 1.14W was 200-fold less with 8659 OMC, the native antigen which possessed two amino acid changes within the epitope (KK→NM). Binding to the soluble peptide with this sequence was totally abrogated.
TABLE 3.
Endpoint titers of MAb binding to OMC and VR2 peptides by ELISA
| Strain | Peptide | Titer
|
|||
|---|---|---|---|---|---|
| 1.14W
|
1.14R
|
||||
| OMC | Peptide | OMC | Peptide | ||
| S3446 (P1.14a) | PAYVDEKKMVHAA | 1:32,000 | 1:80 | 1:128,000 | ≥1:10,240 |
| 7967 (P1.14b) | PAYVDEKKKVHAA | 1:64,000 | 1:2,500 | 1:1,600 | <1:10 |
| 8659 (P1.14c) | PAYVDEKNMVHAA | 1:320 | <1:10 | 1:64,000 | 1:10,240 |
MAb 1.14R bound strongly to OMC and peptides of S3446 and 8659 (Table 3). The K→N substitution within the epitope had little effect on the binding of the MAb. The MAb was much less reactive (80-fold reduction) with the epitope as exposed in OMC of 7967, where the M→K substitution occurred. No binding of 1.14R was detected in testing with the peptide representing the sequence of strain 7967.
DISCUSSION
Typing of N. meningitidis includes determination of group, serotype, and subtype by either whole-cell ELISA or dot blot assays using MAbs. The subtype specificity of a MAb is defined by its reactivity to the approximately 40-kDa OMP (PorA) of the immunizing strain by Western blot analysis. Subtyping MAbs usually bind to one of two VRs of PorA, and many of these epitopes are known. In recent years, several mutants of subtypeable strains carrying point mutations and deletions in VR1 and VR2 that fail to bind subtype-specific MAbs have been isolated (12, 16, 23). Subtype variants of this kind which have been identified are designated as such by the addition of a lowercase letter to the subtype. Suggested designations for the variants of subtype P1.14 are presented in Table 3.
We produced a new subtype-specific MAb with NST strain Z:4:NST as the immunogen. MAb 1.14W bound to the prototype P1.14 strain as well as to the Z:4:NST strain. Although the MAb generated by strain Z:4:NST reacted with subtype P1.14 N. meningitidis, the P1.14 subtyping MAb did not bind to the Z:4:NST strain. However, both MAbs did bind to 16 of 18 strains tested. DNA sequence analysis of six of these strains suggested that the VR1 sequences differed among these strains, while the VR2 sequences were very similar. We concluded that neither antibody bound to the different VR1 epitopes present in the six strains. Therefore, we directed our attention to the VR2 epitope.
Four forms of the subtype-specific antigen were compared to determine the functional epitopes of the two antibodies: (i) native antigen (whole organism or OMC) as it is exposed on the surface of the microbe by dot blot or ELISA, (ii) denatured antigen in Western blot analysis, (iii) soluble 20-amino-acid peptides circularized to mimic the predicted exposed VR2 loop of PorA, and (iv) solid-phase peptides designed to measure continuous epitopes.
MAb 1.14R bound native and denatured antigen in 17 of 18 strains. MAb 1.14R bound to the VR2 epitope VDEKKM or VDEKNM. However, substitution of the nonpolar amino acid (M) with a charged amino acid (K) eliminated binding of the MAb to the native antigen by dot blotting or ELISA or to denatured antigen.
MAb 1.14W appeared to bind to an epitope in a conformation-dependent manner. This antibody bound to native antigen which contained either the sequence VDEKKM or the sequence VDEKKK. After denaturation in SDS-PAGE, MAb 1.14W bound only the antigen with the VDEKKK epitope, the sequence of the immunizing antigen. It did not bind any solid-phase octamer peptides, a conformationally constrained antigen, but it did bind to the longer peptide analogous to the sequence of the immunogen and very weakly to that which differed by one amino acid. As expected, the antibody did not bind a conformationally intact antigen which varied from the immunizing antigen by two amino acids (VDEKNM).
Any protein molecule displays multiple determinants or epitopes which vary in immunological availability. A denatured molecule may renature to a conformation different from the original, thus exposing a modified array of epitopes. This is particularly true of B-cell epitopes, which when characterized at the level of X-ray crystallography are usually discontinuous or conformational (2, 8). Because most of the binding energy for antigen-antibody interactions occurs within a few of the critical residues to which the MAb binds, the epitope often can be mapped to the region where those few residues are located. Linearization or unfolding-refolding of the core region of the epitope will modify the interactions of the amino acids and hence modify the binding site of the MAb. This result has been demonstrated by comparisons of the two MAbs described in this study.
At the appropriate concentrations, each MAb described herein effectively defines most strains with the P1.14 subtype in screening procedures but fails to detect at least one variant strain. It would be impractical to include multiple antibodies for each subtype variant which has been identified, since epidemiological screens already require about 15 MAbs. In the case of P1.14, MAb 1.14R is probably the more appropriate MAb to use, because its immunogen, strain S3446, is the prototype P1.14 strain and VDEKKM appears to be the dominant subtype epitope. The investigator must be aware that some subtype variants may not be detected.
Determination of the most appropriate screening methods and subtype analysis depends on the immediate requirements of an investigation. A panel of well-characterized subtype-specific MAbs proposed for use in screening a large number of isolates during an outbreak should be selected to identify the majority of strains. This method of epidemiological screening has proven effective in assessing outbreaks during the last 10 years.
Data collected in vaccine trials during this 10-year period have focused attention on the importance of the subtype antigen in stimulating a protective immune response. More-rigorous studies of this protein, in particular VR1 and VR2, have been performed, yielding detailed analyses of single base changes, deletions, and duplications that modify subtype specificity. This molecular approach to subtype identification highlights the variability which is more difficult to detect immunologically and offers a molecular basis for description of subtle differences within the immunogenic class 1 epitopes. Rapid advances in PCR and automated sequencing technology have made this molecular approach to subtype identification a viable alternative for future investigations focusing on the protective immune response directed toward this antigen. Data concerning clonal variation within epidemic populations as well as identification of immunologically nonsubtypeable variants can be assessed. The effect that this subtype variation may have on the efficacy of a subtype-specific vaccine is yet to be determined.
REFERENCES
- 1.Barlow A K, Heckels J E, Clarke I N. The class 1 outer membrane protein of Neisseria meningitidis: gene sequence and structural and immunological similarities to gonococcal porins. Mol Microbiol. 1989;3:131–139. doi: 10.1111/j.1365-2958.1989.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin D C. B-cell epitopes: fact and fiction. In: Aledort L M, Hoyer L W, Lusher J M, Reisner H M, White II G C, editors. Inhibitors to coagulation factors. New York: Plenum Press; 1995. pp. 95–108. [Google Scholar]
- 3.Burke D S, Brandt B L, Redfield R R, Lee T, Thorn R M, Beltz G A, Hung C. Diagnosis of human immunodeficiency virus infection by immunoassay using a molecularly cloned and expressed virus envelope polypeptide. Ann Int Med. 1987;106:671–676. doi: 10.7326/0003-4819-106-5-671. [DOI] [PubMed] [Google Scholar]
- 4.Carter J M, Van Albert S, Lee J, Lyon J, Deal C D. Shedding light on peptide synthesis. Bio/Technology. 1992;10:509–513. doi: 10.1038/nbt0592-509. [DOI] [PubMed] [Google Scholar]
- 5.Feavers I M, Heath A B, Bygraves J A, Maiden M C. Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol. 1992;6:489–495. doi: 10.1111/j.1365-2958.1992.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 6.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 7.Geysen H M, Meloen R H, Barteling S J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laver W G, Air G M, Webster R G, Smith-Gill S J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990;61:553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- 9.Maiden M C, Suker J, McKenna A J, Bygraves J A, Feavers I M. Comparison of the class 1 outer membrane proteins of eight serological reference strains of Neisseria meningitidis. Mol Microbiol. 1991;5:727–736. doi: 10.1111/j.1365-2958.1991.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 10.Maiden M C, Bygraves J A, McCarvil J, Feavers I M. Identification of meningococcal serosubtypes by polymerase chain reaction. J Clin Microbiol. 1992;30:2835–2841. doi: 10.1128/jcm.30.11.2835-2841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuinness B, Barlow A K, Clarke I N, Farley J E, Anilionis A, Poolman J T, Heckels J E. Deduced amino acid sequences of class 1 protein (PorA) from three strains of Neisseria meningitidis. J Exp Med. 1990;171:1871–1882. doi: 10.1084/jem.171.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuinness B T, Clarke I N, Lambden P R, Barlow A K, Poolman J T, Jones D M, Heckels J E. Point mutation in meningococcal por A gene associated with increased endemic disease. Lancet. 1991;337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness B T, Lambden P R, Heckels J E. Class 1 outer membrane protein of Neisseria meningitidis: epitope analysis of the antigenic diversity between strains, implications for subtype definition and molecular epidemiology. Mol Microbiol. 1993;7:505–514. doi: 10.1111/j.1365-2958.1993.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan R B, Drabick J J, Williams J C, Fortier A H, Meltzer M S, Sadoff J C, Bolt C R, Nacy C A. Immunotherapy of tularemia: characterization of monoclonal antibody reactive with Francisella tularensis. J Leukocyte Biol. 1993;53:112–116. doi: 10.1002/jlb.53.1.112. [DOI] [PubMed] [Google Scholar]
- 15.Rao V B, Saunders N B. A rapid polymerase-chain-reaction-directed sequencing strategy using a thermostable DNA polymerase from Thermus flavus. Gene. 1992;113:17–23. doi: 10.1016/0378-1119(92)90665-c. [DOI] [PubMed] [Google Scholar]
- 16.Rosenqvist E, Hoiby E A, Wedege E, Caugant D A, Froholm L O, McGuinness B T, Brooks J, Lambden P R, Heckels J E. A new variant of serosubtype P1.16 in Neisseria meningitidis from Norway, associated with increased resistance to bactericidal antibodies induced by a serogroup B outer membrane protein vaccine. Microb Pathog. 1993;15:197–205. doi: 10.1006/mpat.1993.1070. [DOI] [PubMed] [Google Scholar]
- 17.Saukkonen K, Abdillahi H, Poolman J T, Leinonen M. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb Pathog. 1987;3:261–267. doi: 10.1016/0882-4010(87)90059-3. [DOI] [PubMed] [Google Scholar]
- 18.Saukkonen K, Leinonen M, Abdillahi H, Poolman J T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989;7:325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 19.Schneider H, Cross A S, Kuschner R A, Taylor D V, Sadoff J C, Boslego J W, Deal C D. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11 mKC are infective. J Infect Dis. 1995;172:180–185. doi: 10.1093/infdis/172.1.180. [DOI] [PubMed] [Google Scholar]
- 20.Scholten R J P M, Bijlmer H A, Poolman J T, Kuipers B, Caugant D A, Van Alphen L, Dankert J, Valkenberg H A. Meningococcal disease in the Netherlands, 1958–1990: a steady increase in the incidence since 1982 partially caused by new serotypes and subtypes of Neisseria meningitidis. Clin Infect Dis. 1993;16:237–246. doi: 10.1093/clind/16.2.237. [DOI] [PubMed] [Google Scholar]
- 21.Tam J P, Wu C, Liu W, Zhang J. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J Am Chem Soc. 1991;113:6657–6662. [Google Scholar]
- 22.van der Ley P, Heckels J E, Virji M, Hoogerhout P, Poolman J T. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedege E, Dalseg R, Caugant D A, Poolman J T, Froholm L O. Expression of an inaccessible P1.7 subtype epitope on meningococcal class 1 proteins. J Med Microbiol. 1993;38:23–28. doi: 10.1099/00222615-38-1-23. [DOI] [PubMed] [Google Scholar]
- 24.Wedege E, Froholm L O. Human antibody response to a group B serotype 2a meningococcal vaccine determined by immunoblotting. Infect Immun. 1986;51:571–578. doi: 10.1128/iai.51.2.571-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zollinger W D, Mandrell R E, Griffiss J M, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zollinger W D. New and improved vaccines against meningococcal disease. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 469–488. [Google Scholar]
- 27.Zollinger W D, Boslego J. Immunologic methods for diagnosis of infections by gram-negative cocci. In: Rose N R, Folds J D, Lane H C, Nakamura R M, editors. Manual of clinical laboratory immunology. 5th ed. Washington, D.C: American Society of Microbiology; 1997. pp. 473–483. [Google Scholar]