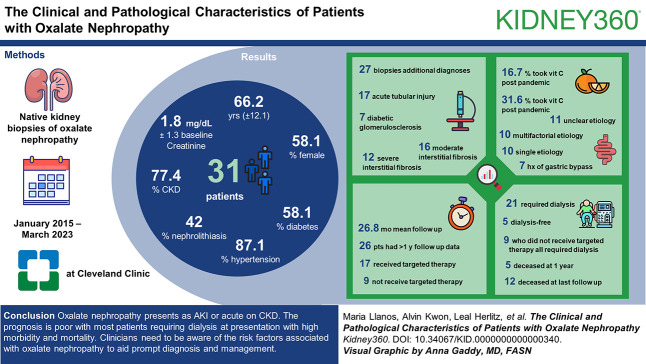
Visual Abstract
Keywords: oxalate nephropathy, enteric hyperoxaluria, calcium oxalate, AKI, CKD, COVID-19, drug nephrotoxicity, ESKD, gastrointestinal complications, vitamin C
Abstract
Key Points
Oxalate nephropathy is an underrecognized cause of CKD and ESKD
We present one of the largest native oxalate nephropathy cohorts to date from a tertiary care institution in the United States
Oxalate nephropathy has multiple etiologies and given its clinical course and poor prognosis, attention must be paid to screening for risk factors to guide prompt diagnosis and management
Background
Oxalate nephropathy (ON) is characterized by deposition of calcium oxalate crystals in the kidney and is commonly under-recognized. Causes of ON include primary hyperoxaluria, enteric hyperoxaluria, and ingestion of excess oxalate or its precursors.
Methods
We report the clinical and pathological characteristics of one of the largest series of native kidney ON to date, from January 2015 to March 2023 at the Cleveland Clinic.
Results
We identified 60 native biopsies with oxalate deposits and excluded patients with clinically insignificant biopsies (n=12) or lack of data (n=17). Thirty-one patients with native ON were described. The mean age at diagnosis was 66.2 years (±12.1), and 58.1% were female. 87.1% had hypertension, 58.1% had diabetes, 42% had nephrolithiasis, and 77.4% had underlying CKD, with a mean baseline creatinine of 1.8 mg/dl ±1.3.
The mean creatinine at biopsy was 5.2 mg/dl ±1.7. Kidney biopsies showed abundant calcium oxalate crystal deposits, and 27 of 31 biopsies had additional diagnoses, the most common of which were acute tubular injury n=17 (54.8%) and diabetic glomerulosclerosis n=7 (22.6%). Severe and moderate interstitial fibrosis was present in 38.7% (n=12) and 51.6% (n=16) of biopsies, respectively. Ten had a single etiology of ON, ten had a multifactorial etiology (both enteric hyperoxaluria and high precursor intake), and 11 had an unclear etiology. Notably, only seven patients had a history of gastric bypass.
The mean duration of follow-up was 26.8 months, and 26 patients had follow-up data >1 year. Of these, 21 required dialysis, and five were dialysis-free at presentation. Five of the 26 were deceased at 1 year, with 12 patients (38.7%) deceased at last follow-up. Seventeen patients received targeted management, while nine patients did not receive targeted treatment, and all nine required hemodialysis. More patients (31.6%) had vitamin C intake after the coronavirus disease 2019 pandemic (2020–2023) versus 16.7% before 2020.
Conclusions
ON presents as AKI or acute on CKD. The prognosis is poor with most patients requiring dialysis at presentation with high morbidity and mortality. Clinicians need to be aware of the risk factors associated with ON to aid prompt diagnosis and management.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/K360/2024_01_26_KID0000000000000340.mp3
Introduction
Oxalate nephropathy (ON) is a rare cause of kidney disease with varying etiologies, presentations, and outcomes. ON is diagnosed by kidney biopsy, with characteristic findings including the accumulation of calcium oxalate crystals in the kidney tubules, resulting in varying degrees of kidney dysfunction and often ESKD. The exact prevalence of ON is unknown and varies depending on methodology because it may be as low as 1% without the inclusion of concomitant diagnoses1 or as high at 4% with the inclusion of concomitant diagnoses and any degree of oxalate deposition.2
Etiologies of ON can be divided into three main groups, primary hyperoxaluria (PH), enteric hyperoxaluria, and ingestion of excess oxalate or its precursors, the latter two comprising the term secondary oxalosis.3 PH is an autosomal recessive condition characterized by hepatic enzyme defects resulting in excess production of the oxalate precursor glyoxylate. There are three known types which are diagnosed primarily with genetic testing. Type 1 PH is associated with a deficiency in alanine glyoxylate aminotransferase (80%),4 type 2 PH is with a deficiency in glycolate reductase/OH-pyruvate reductase, and type 3 PH with a deficiency in 4-hydroxyl-2-oxoglutarate aldolase. Clinically, patients tend to have higher plasma and urinary oxalate levels than patients with secondary oxalosis.5
Enteric hyperoxaluria occurs in the setting of fat malabsorption leading to steatorrhea, resulting in calcium being bound to free fatty acids rather than to oxalate. The result is excess oxalate absorption with a concomitant increase in urinary oxalate, leading to increased rates of kidney stones, and renal dysfunction. Enteric oxaluria is often iatrogenic in that, it occurs because of surgical manipulation of the bowel.6 Common causes of enteric hyperoxaluria include bariatric surgery such as Roux-en-Y gastric bypass7 (with the exception of sleeve gastrectomy), jejunoileal bypass, chronic pancreatitis,8 inflammatory bowel disease, and bowel resections in the setting of colonic diseases.9 Orlistat, which causes fat malabsorption, may also contribute.10 Enteric hyperoxaluria is often worsened by the presence of concomitant diarrhea, resulting in volume depletion, metabolic acidosis, and hypocitraturia. Ingestion of excess oxalate or its precursors is diet-driven.11 Some common high-oxalate foods are spinach, rhubarb, star fruit, and nuts. Oxalate precursors such as excess vitamin C in supplements,12,13 fruits, and toxic ingestions with ethylene glycol poisoning can also lead to excess oxalate production.5
To date, there have been a few case series, of which one of the largest native ON series has 30 patients and notably shows a trend toward supplements being an increasing cause of ON during the coronavirus disease 2019 (COVID) pandemic.14 Overall, these case series have helped to establish the clinical risk factors for secondary oxalosis, clinical presentations which are often severe, and described various attempts at management. However, there have not been a sufficient number of epidemiological studies to clarify further risk factors and associations. In this study, we present one of the largest native kidney biopsy cohorts of ON to date from a tertiary center in the United States, including demographic and kidney biopsy characteristics, clinical course outcomes, and associated factors.
Methods
Study Design
This study was approved by the Cleveland Clinic Institutional Review Board. We identified native kidney biopsies with calcium oxalate deposition from the Cleveland Clinic Kidney Biopsy Epidemiology Project15 from January 2015 to March 2023. ON was defined as the deposition of oxalate crystals in the renal tubules/parenchyma associated with acute renal dysfunction. Once we identified cases with calcium crystal deposition, we then excluded those cases with insufficient clinical data available, followed by those where the oxalate deposition was not considered to be significantly contributing to kidney dysfunction (Figure 1).
Figure 1.
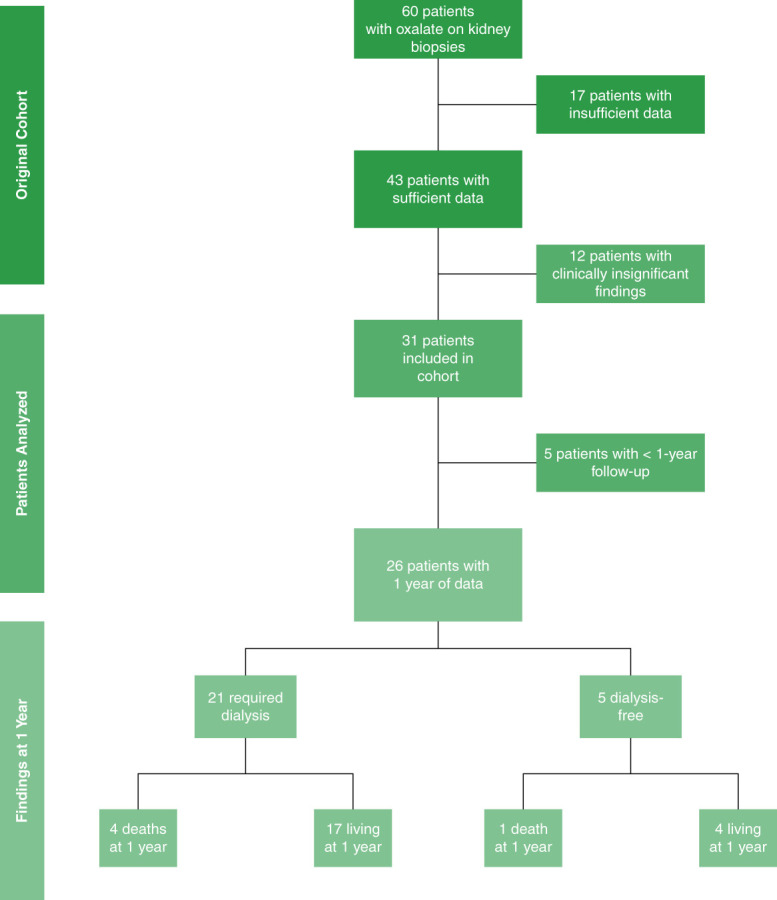
Study design.
Clinical Parameters
The medical chart was reviewed for demographics; medical comorbidities such as diabetes (and duration), hypertension, kidney stones, alcohol and tobacco use, high oxalate food intake; and medications such as diuretics, renin–angiotensin aldosterone system blockade, antibiotic use, and vitamin C/oxalate precursor intake. Laboratory parameters such as baseline kidney function, to determine CKD stage, creatinine at presentation and biopsy, to determine presence of AKI and serum and/or urine oxalate levels were recorded when available. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2021 equation. In addition, charts were reviewed for the presence of genetic testing for PH, gastric bypass/bowl surgery, pancreatitis, diarrhea, high oxalate precursor intake (on the basis of treating clinician judgment), and the treating nephrologists notes to help determine the specific cause of ON and whether more than one cause was present.
Follow-up data were collected from diagnosis until last available follow-up for each patient, during the time frame of 2015–2023. Regarding outcomes, treatments attempted (low oxalate diet, administration of pyridoxine or K citrate, calcium citrate, kidney transplant, and renal replacement therapy), and clinical outcomes such as kidney failure needing renal replacement therapy in the form of dialysis or kidney transplant, and mortality data were analyzed.
Kidney Biopsy
All biopsy reports were reviewed by a nephropathologist at the Cleveland Clinic and processed according to the standardized protocol for light microscopy (hematoxylin and eosin, periodic acid–Schiff, trichrome, and Jones stains) and immunofluorescence (IgG, IgA, IgM, C3, C1q, kappa, lambda, and fibrinogen). The presence of calcium oxalate crystals was determined by observing their positive birefringence under polarized light. To establish a diagnosis of ON, patients had to have multiple calcium oxalate crystals per 1 cm biopsy core associated with progressive/acute kidney dysfunction. Those with only focal calcium oxalate crystals were not included. Additional diagnoses such as acute tubular necrosis, acute interstitial nephritis, and diabetic kidney disease were noted. The presence of interstitial fibrosis and tubular atrophy were graded by the amount of renal cortex as absent/insignificant (<10%), mild (10%–25%), moderate (26%–50%), or severe (>50%).1
Statistical Analysis
Patient characteristics were presented using descriptive statistics, including mean±SD for continuous variables and percentages for categorical variables. Student's t test and Fisher's exact test were used for analyzing continuous and categorical variables, respectively. All statistical analyses were conducted using Stata version 16.1 (StataCorp, College Station, TX).
Results
We identified 60 native biopsies with oxalate deposits and excluded patients with clinically insignificant biopsies (n=12) or lack of data (n=17) (Figure 1). Thirty-one patients with native ON were described. Table 1 summarizes the characteristics of patients with ON. For our cohort, the mean age at diagnosis was 66.2 years (±12.1), and 58.1% were female. 87.1% had hypertension, 58.1% had diabetes, 42% had nephrolithiasis, and 77.4% had underlying CKD with a mean baseline creatinine of 1.8 mg/dl ±1.3 and mean serum eGFR of 48.2 ml/min per 1.73 m2 ±29.3. The mean serum creatinine at presentation was 7.6 mg/dl ±3.4. The mean creatinine at biopsy was 5.2 mg/dl ±1.7 with a mean body mass index in our cohort of 30.0±9.2.
Table 1.
Patient characteristics
| Characteristic | Value (n=31) |
|---|---|
| Age, yr | 66.2±12.1 |
| Sex, female | 18 (58.1) |
| Diabetes | 18 (58.1) |
| Hemoglobin A1c, % (n=17) | 7.2±1.4 |
| Duration of diabetes, mo (n=16) | 17.0±10.8 |
| Hypertension | 27 (87.1) |
| History of nephrolithiasis | 13 (42.0) |
| Alcohol use | 10 (32.3) |
| Smoking/tobacco use | 10 (32.3) |
| CKD, yes (n=30) a | 24 (77.4) |
| Stage; 2/3/4/5 (n=23) | 1/16/4/2 |
| Serum creatinine at baseline (mg/dl) (n=30) | 1.8±1.3 |
| Serum creatinine at presentation (mg/dl) (n=29) | 7.6±3.4 |
| Serum creatinine at biopsy (mg/dl) (n=30) | 5.2±1.7 |
| Baseline eGFR (ml/min per 1.73 m2) (n=30) | 48.0±29.3 |
| Serum oxalate (mcmol/L) (n=9) | 19.0±15.4 |
| 24-h urine oxalate (mg) (n=7) | 47.1±8.9 |
| Vitamin C, yes | 8 (25.8) |
| Daily vitamin C amount; 500/1000 mg/other (iv or multivitamin form) (n=8) | 1/5/2 |
| Diuretic use (n=30)a | 13 (43.3) |
| RAAS inhibitor use | 15 (48.4) |
| Antibiotic use (n=28)a | 10 (32.3) |
| Polyethylene glycol-3350 use (within 1 yr) | 19 (61.3) |
| BMI (kg/m2) | 30.0±9.2 |
| Follow-up length, mo (n=26) | 26.8±21.3 (13–40) |
| Time until death event, mo (n=12)b | 19.8±18.9 (6–29) |
Mean±SD, n (%). BMI, body mass index; RAAS, renin–angiotensin aldosterone system.
Percentage was calculated from numerator of n=31.
Time until death event among 12 patients who were deceased at entire follow-up.
Etiologies of ON
Of the 31 patients, 20 patients were determined to have a clear etiology of ON (Table 2). Of these, ten had a single etiology of ON, and the remaining ten had a multifactorial etiology (both enteric hyperoxaluria and high precursor intake). Eleven patients had an unclear etiology. Seventeen patients were found to have enteric oxaluria, of which, only seven patients had a history of gastric bypass (three out of the seven had concomitant vitamin C use). Three patients had a history of short gut syndrome, five patients had a history of chronic diarrhea (one of which had a history of carcinoid syndrome), one had a history of chronic pancreatitis, and one had a history of octreotide use. Among the 15 patients with high precursor intake (vitamin C, tea, high-oxalate diet [Table 2]), eight patients had high vitamin C intake, five had high tea intake, and two patients had a high-oxalate diet. One patient had PH determined by genetic testing. Interestingly, more patients (31.6%) had vitamin C intake after the COVID pandemic (2020–2023) versus 16.7% before 2020 (Supplemental Table 1). In addition, 13 patients (43.3%) were taking a diuretic, and 15 patients (48.4%) were taking a renin–angiotensin system inhibitor (Table 1). Of note, in our cohort of 31 patients, 23 of them had taken PEG-3350, and 19 of these 23 were using it within 1 year of biopsy (Table 1). Additionally, five patients had a colonoscopy performed within 1 year of biopsy diagnosis of ON, of which four of five had a known preparation, and all four used PEG 3350. The duration from PEG-3350 colonoscopy preparation to biopsy diagnosis was 5 days, 6 days, 5 months, and 1 year in these four patients. Patients diagnosed within 1 week of PEG-3350 preparation had their risk factors as enteric hyperoxaluria (1 Roux en Y, one colectomy).
Table 2.
Clinical symptoms and laboratory data
| Characteristic | Value (n=31) |
|---|---|
| Known/clear etiology of oxalate nephropathy | 20 (64.5) |
| Single etiology | 10 |
| Multifactoriala | 10 |
| Unclear etiology | 11 |
| Etiology | Value (n=20) |
| Primary hyperoxaluria type 1 | 1 |
| Enteric hyperoxaluria | 17 |
| Gastric bypassb | 7 |
| Diarrhea | 5 |
| Short gut syndrome | 3 |
| Chronic pancreatitis | 1 |
| Octreotide | 1 |
| High precursor intake (oxalate, vitamin C, tea) | 15 |
| Vitamin C intake | 8 |
| Tea intake | 5 |
| High oxalate diet | 2 |
n (%).
Enteric hyperoxaluria and high precursor intake (oxalate, vitamin C, tea).
Three of seven had concomitant vitamin C use.
Among our patient cohort, 11 patients had ON of unclear etiology. Chart review and/or nephrologist notes did not reveal clear contributing risk factors for the development of ON in these patients.
Pathology Findings
Kidney biopsies showed abundant calcium oxalate crystal deposits (Figure 2), with 27 of 31 biopsies having superimposed findings, the most common of which were acute tubular injury, n=17 (54.8%), and concomitant diabetic glomerulosclerosis, n=7 (22.6%). Additional diagnoses included acute interstitial nephritis, podocytopathy, and chronic lithium nephropathy in one patient each. Severe and moderate interstitial fibrosis was present in 38.7% (n=12) and 51.6% (n=16) of biopsies, respectively, Table 3. Therefore, 90.3% of patients had a moderate-to-severe level of fibrosis at the time of biopsy with 9.7% with mild fibrosis.
Figure 2.
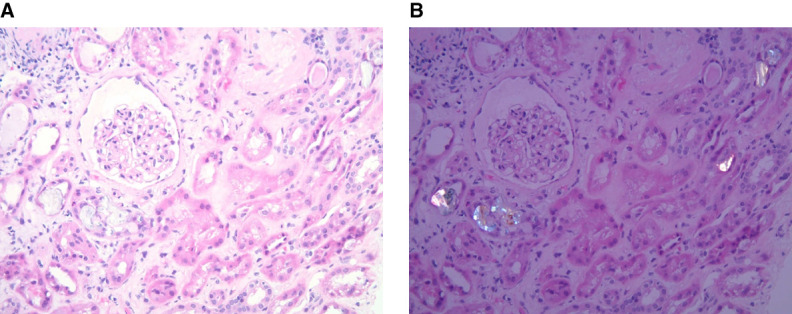
Oxalate nephropathy. (A) Light microscopy and (B) polarized light, showing oxalate deposits in tubules.
Table 3.
Kidney biopsy dataa
| Characteristic | |
|---|---|
| Additional diagnosis | (n=27) |
| Acute interstitial nephritis | 1 (3.2) |
| Acute tubular injury | 17 (54.8) |
| Diabetic glomerulosclerosis | 7 (22.6) |
| Podocytopathy | 1 (3.2) |
| Chronic lithium nephropathy | 1 (3.2) |
| Degree of fibrosis | (n=31) |
| Mild | 3 (9.7) |
| Moderate | 16 (51.6) |
| Severe | 12 (38.7) |
n (%).
Percentage was calculated from numerator of n=31.
Treatment and Outcome
The mean duration of follow-up was 26.8 months, and 26 patients had follow-up data more than 1 year. Of these, 21 required dialysis, and five were dialysis-free at presentation. Five of the 26 were deceased at 1 year (Figure 1), with 12 patients (38.7%) deceased at last follow-up (median time to death 19.8 months) (Table 4). Among the 21 who required dialysis, 14 remained dialysis-dependent, two received a kidney transplant, and one recovered with residual CKD. The remaining four patients were dialysis-dependent until death. Among the five patients who were dialysis-free at presentation, one received a kidney transplant, two recovered with residual CKD, and one recovered without residual CKD. The remaining patient passed away after recovering with residual CKD within 1 year (Table 4).
Table 4.
Clinical outcomes at 1 year
| Characteristic | Value (n=26) |
|---|---|
| Required dialysis | 21 (80.8) |
| Living at 1 year (n=17) | |
| Continue to be dialysis-dependent | 14 |
| Kidney transplant | 2 |
| Recovered with residual CKD | 1 |
| Death at 1 year (n=4) | |
| Continue to be dialysis-dependent | 4 |
| Dialysis-free | 5 (19.2) |
| Living at 1 yr (n=4) | |
| Kidney transplant | 1 |
| Recovered with residual CKD | 1 |
| Residual CKD | 1 |
| Recovered without residual CKD | 1 |
| Death at 1 yr (n=1) | |
| Recovered with residual CKD until died | 1 |
n (%).
The three alive patients who recovered kidney function with residual CKD had a serum creatinine of 1.3, 3.0, and 3.3 mg/dl at follow-up.
Management of ON
Among 26 patients with 1 year data available, a total of 17 patients received targeted management, while nine patients did not receive targeted treatment (Table 5). Of the nine without specific treatment, 100% of patients required hemodialysis initiation. At 1 year, 7 (77.8%) remained dialysis-dependent on hemodialysis (n=5) and peritoneal dialysis (n=2). While the 2 (22.2%) remaining patients died within 1 year from cardiogenic shock and unclear cause. Of the 17 patients who did receive specific treatment, 70.6% (n=12) required dialysis initiation, and 17.4% (n=3) died within 1 year (Table 5). Causes of death among these individuals were attributed to conditions such as chronic myelogenous leukemia progression, septic shock, and dialysis discontinuation.
Table 5.
Clinical outcomes at 1 year and management
| Characteristic | Value (n=26) |
|---|---|
| Treatment, yes | 17 |
| Kidney transplant (n=2) | |
| Alive | 1 |
| Alive, dialysis-dependent | 1 |
| Kidney transplant, pyridoxine, potassium citrate, and calcium citrate (n=1) | |
| Alive, dialysis-dependent | 1 |
| Low oxalate diet (n=2) | |
| Alive, dialysis-dependent | 1 |
| Deceased, recovered with residual CKD | 1 |
| Low oxalate diet and pyridoxine (n=1) | |
| Alive, dialysis-dependent | 1 |
| Pyridoxine (n=2) | |
| Deceased, dialysis-dependent | 2 |
| Pyridoxine and calcium citrate (n=1) | |
| Alive, dialysis-dependent | 1 |
| Pyridoxine and potassium citrate (n=1) | |
| Alive, recovered without residual CKD | 1 |
| Potassium citrate and hydrochlorothiazide (n=1) | |
| Alive | 1 |
| Calcium citrate (n=3) | |
| Alive, dialysis-dependent | 3 |
| Calcium citrate and stop vitamin C (n=1) | |
| Alive, recovered without residual CKD | 1 |
| Stop vitamin C (n=2) | |
| Alive, dialysis-dependent | 1 |
| Alive, started on dialysis and recovered with residual CKD | 1 |
| Treatment, no | 9 |
| Alive (n=7) | |
| Dialysis-dependent | 7 |
| Deceased (n=2) | |
| Dialysis-dependent | 2 |
n (%).
Target therapies consisted of stopping vitamin C supplementation (n=3), low oxalate diet (n=3), pyridoxine (n=6), potassium citrate initiation (n=3), and calcium citrate initiation (n=6). Various combinations of these therapies were attempted (Table 5). Of the three patients who underwent kidney transplantation, one of them underwent combined liver, kidney transplantation for type 1 PH, and another had biopsy-proven recurrence of ON in the transplanted kidney.
Discussion
We report one of the largest cohorts of native ON to date from a multicenter, tertiary institution in the Unites States. Key findings include an older population, high rates of concomitant diabetes, kidney stones, multifactorial risk factors, and severe presentations. Over 67% required renal replacement therapy, and over 90% had moderate-to-severe fibrosis on biopsy with a large burden of resulting CKD and ESKD.
Currently there are a few case series of ON. One of the early series of 11 patients highlighted the role of gastric bypass in the pathophysiology of enteric hyperoxaluria.7 Most recently, a case series of 30 patients with native ON demonstrated a higher percentage of ingestion-related ON in the context of the COVID-19 pandemic, especially due to vitamin C and supplement intake, demonstrating its role in ON.14 When looking at both studies, compared with ours, we noted only seven patients with gastric bypass, of which three also had concomitant vitamin C use. This highlights the importance to the nephrology community of awareness of nonkidney comorbidities/medications that may inadvertently contribute to CKD and shows that the nephrologist needs to be aware of comorbidities other than gastric bypass surgery.
The other three case series in order of publication have 12,8 21,1 and 252 patients, respectively. In our case series of 31 patients with native ON, we see similar age (60s), rates of hypertension, and diabetes; however, our study was more female-predominant, and we note higher rates of kidney stones (42% versus 25%,8 14%,1 and 4%2) and baseline CKD 77.4% versus 58.3%8 and 62%.1 The overall clinical presentation of severe renal dysfunction, as evidenced by a mean creatinine on presentation of 7.6 mg/dl, is consistent with the prior case series, demonstrating the severity of this under-recognized condition.
Of the nine patients with plasma oxalate levels, the mean level was 19.0 mcmol/L and mean urinary oxalate level 47.1 mg/24 hours. It is known that high plasma oxalate levels are associated with poor outcomes,16 and as eGFR declines, plasma oxalate levels rise making the ability to suspect/diagnose disorders of oxalate metabolism difficult.17 Given this finding, we highlight the need for increased awareness of screening for PH in the setting of high plasma oxalate levels, especially when the eGFR is not decreased, and recently, derived models have been developed to better interpret plasma oxalate levels when PH is suspected, even when eGFR is reduced.17
When looking at etiologies of ON, we report one case of PH among 10 with a single etiology, while 10 and 11 patients had multifactorial and unknown etiologies, respectively. Most single etiology ON was related to enteric hyperoxaluria, while the overarching theme when looking at multifactorial etiologies of ON included a combination of enteric hyperoxaluria with concomitant ingestion of oxalate and/or its precursors. While we report 11 patients classified as unknown etiology, many of these patients had potential risk factors such as antibiotic, PEG-3350, and diuretic use; however, thorough review of the chart and treating nephrologists' notes did not allow for a definitive cause to be established. Because ethylene glycol ingestion is a cause of ON, recently, the US Food and Drug Administration (FDA) has raised concerns about the contamination of PEG 3350, a common over-the-counter osmotic laxative, and bowel prep with ethylene glycol and diethylene glycol.18 In our study, 61.3% of patients used PEG-3350 within 1 year of biopsy, with two patients having PEG-3350 preparation for colonoscopy within 1 week of diagnosis. While these data are concerning, our study does not provide any conclusive evidence of a direct causal link but rather raises awareness and supports the current plan implemented by the FDA to investigate this further. Moving forward, strong consideration for multiple risk factors contributing to the development of ON must be a part of the clinical assessment of these patients. In addition, care must be given to lower the threshold for genetic testing, especially in patients with elevated plasma oxalate levels and to have patients with risk factors consider using food diaries to help with identifying sources of high precursor intake.
In our cohort, similar to previous works, we see a significant amount of Acute Tubular Necrosis (54.8%) and diabetic kidney disease (22.6%); however, the standout feature was the presence of a significant amount of interstitial fibrosis and tubular atrophy with over 90% of patients having at least moderate chronicity on kidney biopsy. Apart from ON being underrecognized, one possible explanation for this is that hyperoxaluria with resultant calcium oxalate crystal deposition contributes to interstitial fibrosis. The exact mechanism is not known; however, TGF-β1, a well-known, profibrotic cytokine, is produced by macrophages when exposed to calcium oxalate and leads to epithelial to mesenchymal transition which contributes to fibrosis.19 Because the proximal tubule is the area that is most exposed to calcium oxalate crystals, they can take up calcium oxalate crystals through endocytosis and have demonstrated the production of TGF-β1 in this setting, thus a possible contributor to peritubular epithelial to mesenchymal transition/fibrosis.19 Given this finding, focusing on reducing hyperoxaluria and in turn, calcium oxalate deposition may help alleviate the fibrosis in ON because fibrosis is often a major driver of the development of underlying CKD and progression to ESKD. Given our findings, future ideas for consideration could focus on differences in pathology findings/degree of fibrosis among those with and without comorbidities such as Acute Tubular Necrosis and diabetes, as well as noninvasive biomarkers/risk factors contributing to worse outcomes but will require a larger number of patients to effectively do so.
This leads us to focus on treatment strategies noted in this cohort. While lumasiran is an FDA-approved treatment for PH,20,21 there are currently no FDA-approved therapies for enteric hyperoxaluria22; various strategies were attempted such as pyridoxine, potassium citrate, calcium citrate (alone or in combination), and along with the elimination of oxalate precursors in the diet and from vitamin C supplementation. While several patients had enteric hyperoxaluria as an etiology, we did not see any attempts at surgical restoration, e.g., reversal of gastric bypass which has been shown to be beneficial,23 and further study needs to conducted in this setting. Attention must be paid to the fact that many patients were dialysis-dependent at the time of presentation with most having moderate-to-severe fibrosis, contributing largely to the no targeted therapy group, (other than receiving renal replacement therapy), likely because of the clinical lack of reversibility.
Focusing on outcomes, given the severity of initial presentation of ON in our series with many patients needing dialysis, we looked at 1-year outcomes. At 1 year, data were available for 26 patients, of which five were deceased at 1 year (19%). Among all 31 patients at last follow-up, 12 of 31 (38.7) were deceased, compared with a recent systematic review by Lymlertgul et al.,6 where the overall mortality rate for ON was 33%. Of these 26 patients with 1-year data, 21 (80.8%) needed dialysis, and four of these 21 were deceased at 1 year, compared with only one in the dialysis-free group (n=5). In addition, 18 of the 21 patients who needed dialysis, remained dialysis-dependent until last follow-up or death, only one recovered with residual CKD, and two patients received a kidney transplant. In the dialysis-free cohort, one patient recovered without residual CKD. Finally, the patient with PH who underwent combined liver–kidney transplant was liberalized from dialysis with 1.3 mg/dl creatinine at follow-up, consistent with previous published outcomes in this population.24 These outcomes show an overall poor prognosis, but more importantly, demonstrate the need for early suspicion/screening for PH given its better prognosis and defined treatment strategy, thorough review of medications and supplements, as well as surgical history given the multifactorial nature of this condition.
Our study has several strengths including a review of all cases of native ON within an 8-year period with clinical data available, a multicenter cohort, with a consistent pathology review process and large numbers compared with previous works. Limitations include the lack of genetic testing in all patients and lack of clinical data, e.g., serum and urine oxalate levels, given the retrospective nature of this cohort.
ON presents as AKI or acute on CKD. The prognosis is poor with most patients requiring dialysis at presentation with high morbidity and mortality. Clinicians need to be aware of the risk factors associated with ON to aid prompt diagnosis and management.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Kurt Spindler and Elizabeth Sosic for their administrative and technical support.
Disclosures
S.A. Bobart reports the following: Ownership Interest: RNLX, prior; Honoraria: Travere Therapeutics; and Other Interests or Relationships: Faculty, GlomCon fellowship. S. Cohen reports the following: Consultancy: Dane Street LLC; Sidley Austin LLC. L. Herlitz reports the following: Consultancy: ChemoCentryx, Novartis; Honoraria: Novartis; and Other Interests or Relationships: Kidney360 editorial board member. T. Shafi reports the following: Consultancy: Allucent (DSMB Member); Research Funding: Active: Clinical site investigator (Numares); Inactive: Clinical site investigator (Baxter, Bayer, CVS, Goldfinch, Natera, Vertex); and Advisory or Leadership Role: Clinical Journal of the American Society of Nephrology; Kidney360; American Journal of Medicine. All remaining authors have nothing to disclose.
Funding
This work is supported by Cleveland Clinic Florida Regional Grant.
Author Contributions
Conceptualization: Shane A. Bobart, Hanny Sawaf.
Data curation: Shane A. Bobart, Leal Herlitz, Alvin Kwon, Maria Llanos, Hanny Sawaf.
Formal analysis: Alvin Kwon.
Funding acquisition: Shane A. Bobart, Surafel K. Gebreselassie.
Investigation: Shane A. Bobart, Maria Llanos, Hanny Sawaf.
Methodology: Shane A. Bobart, Leal Herlitz, Alvin Kwon, Maria Llanos, Hanny Sawaf.
Project administration: Shane A. Bobart, Surafel K. Gebreselassie.
Resources: Shane A. Bobart, Surafel K. Gebreselassie.
Software: Alvin Kwon, Hanny Sawaf.
Supervision: Shane A. Bobart, Surafel K. Gebreselassie.
Validation: Alvin Kwon, Maria Llanos.
Visualization: Hanny Sawaf.
Writing – original draft: Shane A. Bobart, Alvin Kwon, Maria Llanos.
Writing – review & editing: Shane A. Bobart, Scott Cohen, Surafel K. Gebreselassie, Leal Herlitz, Tariq Shafi.
Data Sharing Statement
Partial restrictions to the data and/or materials apply. Data use agreement required per CCF IRB.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A420.
Supplemental Table 1. Vitamin C intake before and after COVID-19 pandemic.
References
- 1.Buysschaert B, Aydin S, Morelle J, Gillion V, Jadoul M, Demoulin N. Etiologies, clinical features, and outcome of oxalate nephropathy. Kidney Int Rep. 2020;5(9):1503–1509. doi: 10.1016/j.ekir.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y Sharma PD Nair V, et al. Kidney oxalate crystal deposition in adult patients: a relatively common finding. Clin Nephrol. 2020;93(5):243–250. doi: 10.5414/CN109980 [DOI] [PubMed] [Google Scholar]
- 3.Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3(6):1363–1372. doi: 10.1016/j.ekir.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milliner DS, Harris PC, Sas DJ, Cogal AG, Lieske JC. Primary hyperoxaluria type 1. In: Adam MP Mirzaa GM Pagon RA, et al. GeneReviews((R)); 1993. [PubMed] [Google Scholar]
- 5.Rosenstock JL, Joab TMJ, DeVita MV, Yang Y, Sharma PD, Bijol V. Oxalate nephropathy: a review. Clin Kidney J. 2022;15(2):194–204. doi: 10.1093/ckj/sfab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witting C Langman CB Assimos D, et al. Pathophysiology and treatment of enteric hyperoxaluria. Clin J Am Soc Nephrol. 2021;16(3):487–495. doi: 10.2215/CJN.08000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasr SH D'Agati VD Said SM, et al. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3(6):1676–1683. doi: 10.2215/CJN.02940608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartery C Faguer S Karras A, et al. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol. 2011;6(8):1895–1902. doi: 10.2215/CJN.00010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hylander E, Jarnum S, Jensen HJ, Thale M. Enteric hyperoxaluria: dependence on small intestinal resection, colectomy, and steatorrhoea in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13(5):577–588. doi: 10.3109/00365527809181767 [DOI] [PubMed] [Google Scholar]
- 10.Buysschaert B, Aydin S, Morelle J, Hermans MP, Jadoul M, Demoulin N. Weight loss at a high cost: Orlistat-induced late-onset severe kidney disease. Diabetes Metab. 2016;42(1):62–64. doi: 10.1016/j.diabet.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Crivelli JJ Mitchell T Knight J, et al. Contribution of dietary oxalate and oxalate precursors to urinary oxalate excretion. Nutrients. 2020;13(1):62. doi: 10.3390/nu13010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana F Cazzato S Giovanella S, et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. 2020;5(10):1815–1822. doi: 10.1016/j.ekir.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colliou E, Mari A, Delas A, Delarche A, Faguer S. Oxalate nephropathy following vitamin C intake within intensive care unit. Clin Nephrol. 2017;88(12):354–358. doi: 10.5414/CN109118 [DOI] [PubMed] [Google Scholar]
- 14.Fong P Wusirika R Rueda J, et al. Increased rates of supplement-associated oxalate nephropathy during COVID-19 pandemic. Kidney Int Rep. 2022;7(12):2608–2616. doi: 10.1016/j.ekir.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobart SA Portalatin G Sawaf H, et al. The Cleveland Clinic kidney biopsy epidemiological project. Kidney360. 2022;3(12):2077–2085. doi: 10.34067/KID.0005882022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah RJ, Vaughan LE, Enders FT, Milliner DS, Lieske JC. Plasma oxalate as a predictor of kidney function decline in a primary hyperoxaluria cohort. Int J Mol Sci. 2020;21(10):3608. doi: 10.3390/ijms21103608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perinpam M Enders FT Mara KC, et al. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. 2017;50(18):1014–1019. doi: 10.1016/j.clinbiochem.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. From our perspective: FDA actions to continue to ensure the safety of the nation’s drug supply. Accessed November 15, 2023. https://www.fda.gov/drugs/our-perspective/our-perspective-fda-actions-continue-ensure-safety-nations-drug-supply
- 19.Convento MB, Pessoa EA, Cruz E, da Glória MA, Schor N, Borges FT. Calcium oxalate crystals and oxalate induce an epithelial-to-mesenchymal transition in the proximal tubular epithelial cells: contribution to oxalate kidney injury. Sci Rep. 2017;7:45740. doi: 10.1038/srep45740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacchetta J, Lieske JC. Primary hyperoxaluria type 1: novel therapies at a glance. Clin Kidney J. 2022;15(suppl 1):i17–i22. doi: 10.1093/ckj/sfab245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrelfs SF Frishberg Y Hulton SA, et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384(13):1216–1226. doi: 10.1056/NEJMoa2021712 [DOI] [PubMed] [Google Scholar]
- 22.Langman CB Assimos D Blank M, et al. On behalf of the rare kidney stone consortium kidney health initiative O, hyperoxaluria foundation enteric hyperoxaluria W: end point considerations for clinical trials in enteric hyperoxaluria. Clin J Am Soc Nephrol. 2023;18(12):1637–1644. doi: 10.2215/CJN.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal V, Wilfong JB, Rich CE, Gibson PC. Reversal of gastric bypass resolves hyperoxaluria and improves oxalate nephropathy secondary to Roux-en-Y gastric bypass. Case Rep Nephrol Dial. 2016;6(3):114–119. doi: 10.1159/000449128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhondup T, Lorenz EC, Milliner DS, Lieske JC. Combined liver-kidney transplantation for primary hyperoxaluria type 2: a case report. Am J Transplant. 2018;18(1):253–257. doi: 10.1111/ajt.14418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial restrictions to the data and/or materials apply. Data use agreement required per CCF IRB.