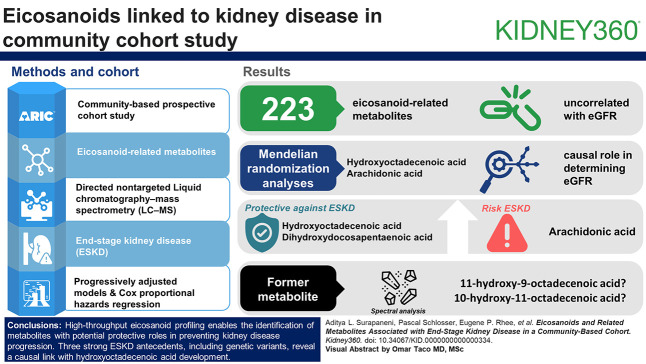
Visual Abstract
Keywords: CKD, clinical epidemiology, metabolism
Abstract
Key Points
High-throughput eicosanoid profiling can identify metabolites that may play a protective role in the development of kidney disease.
In contrast to many other nonlipid metabolites, eicosanoid levels are minimally related with kidney filtration cross-sectionally.
Background
Eicosanoids are derivatives of polyunsaturated fatty acids and participate in the inflammatory response and the maintenance of endothelial function. Specific eicosanoids have been linked to various diseases, including hypertension and asthma, and may also reduce renal blood flow. A systematic investigation of eicosanoid-related metabolites and adverse kidney outcomes could identify key mediators of kidney disease and inform ongoing work in drug development.
Methods
Profiling of eicosanoid-related metabolites was performed in 9650 participants in the Atherosclerosis Risk in Communities Study (visit 2; mean age, 57 years). The associations between metabolite levels and the development of ESKD was investigated using Cox proportional hazards regression (n=256 events; median follow-up, 25.5 years). Metabolites with statistically significant associations with ESKD were evaluated for a potential causal role using bidirectional Mendelian randomization techniques, linking genetic instruments for eicosanoid levels to genomewide association study summary statistics of eGFR.
Results
The 223 eicosanoid-related metabolites that were profiled and passed quality control (QC) were generally uncorrelated with eGFR in cross-sectional analyses (median Spearman correlation, −0.03; IQR, −0.05 to 0.002). In models adjusted for multiple covariates, including baseline eGFR, three metabolites had statistically significant associations with ESKD (P value < 0.05/223). These included a hydroxyoctadecenoic acid, a dihydroxydocosapentaenoic acid, and arachidonic acid, with higher levels of the former two protective against ESKD and higher levels of arachidonic acid having a positive association with risk of ESKD. Mendelian randomization analyses suggested a causal role for the hydroxyoctadecenoic and arachidonic acid in determining eGFR. Spectral analysis identified the former metabolite as either 11-hydroxy-9-octadecenoic acid or 10-hydroxy-11-octadecenoic acid.
Conclusions
High-throughput eicosanoid profiling can identify metabolites that may play a protective role in the development of kidney disease.
Introduction
ESKD affects more than 700,000 American individuals and confers dramatic decrements to quality of life and high risk of premature mortality.1 Discovering targetable pathways and molecules to forestall or prevent the development of ESKD is of critical importance. One class of plausibly targetable molecules are the eicosanoids. Eicosanoids are metabolites derived from polyunsaturated fatty acids (PUFAs) that, in health, participate in maintenance of the extracellular environment and regulation of BP homeostasis. For example, hydroxyeicosatetraenoic acids help regulate ion transport in the kidney via the Na-K ATPase and the Na-K-2Cl cotransporter while epoxyeicosatrienoic acids and CYP 450 epoxygenase metabolites are thought to affect the epithelial sodium channel activity. Animal and genetic models suggest that perturbations of eicosanoid levels decrease renal blood flow, leading to hypertension and CKD.2–5 In addition, eicosanoids are key mediators of the inflammatory response, and metabolites of arachidonic acid, including thromboxane and leukotrienes, have been linked to inflammatory damage in the kidney.6,7 Eicosanoids represent attractive molecules for intervention since they interact with several known drug targets.8,9 To our knowledge, however, there has been no systematic investigation with respect to the association between eicosanoids and development of adverse kidney outcomes.
Our overarching hypothesis is that identification of key metabolic mediators of kidney disease could inform ongoing work in the development of drugs and elucidate pathophysiology. To do this, we studied eicosanoid and PUFA precursors of ESKD in a community-based cohort. For the eicosanoids that demonstrated significant associations with outcomes, we used Mendelian randomization (MR) to evaluate evidence of a causal relationship.
Methods
Study Population
The study population was drawn from the Atherosclerosis Risk in Communities (ARIC) Study, a community-based prospective cohort study. Participants were enrolled from Forsyth County, North Carolina, Jackson, Mississippi, suburbs of Minneapolis, Minnesota, and Washington County, Maryland from 1987 to 1989. Eicosanoid-related metabolites were measured at a subsequent visit that occurred from 1990 to 1992 (visit 2). Participants who attended this visit, had eicosanoid measures, and were free from ESKD were included in this study (N=9650).
Eicosanoid Profiling and QC
Eicosanoids and related metabolites (hereafter referred to as eicosanoids) were measured from plasma that was collected at visit 2 and immediately stored at −80°C. Levels were ascertained using a directed nontargeted liquid chromatography-mass spectrometry (LC–MS) approach.10,11 Samples were run in 96-count sample batches, with each batch containing 93 samples and three internal quality control samples. All data were normalized using batch median normalization, where the median for each batch for each individual signal was normalized to its own global median across all batches. Quality control was evaluated by assessing for technical variation in internal standards and in 392 blind duplicate pairs. The median coefficient of variation across eicosanoids was 21%. Before analysis, missing eicosanoid levels were imputed with half of the minimum value for each individual eicosanoid. Eicosanoids missing in more than 50% of the samples were dropped, leaving 223 eicosanoids. Levels were log2 transformed because of skewed distributions, and values outside of five SDs from the mean were winsorized. The median number of outliers was three per eicosanoid, ranging from 0 to 132. For annotation of metabolites of interest, matching was performed on the basis of mass-to-charge ratio (m/z), retention time/index, and chromatographic data using theoretic and authentic standards where possible.
Outcomes
The main study outcome, ESKD, was assessed continuously through linkage to the United States Renal Data System.1 Time at risk began at visit 2 and continued until the first occurrence of study outcome, death, or December 31, 2019.
Covariate Definitions
Covariates were ascertained at study visit 2, concomitant with eicosanoid measurement. These included age, sex, self-reported race, study center, body mass index, systolic BP, use of antihypertensive medications within the prior 2 weeks, eGFR, smoking status (current/former/never), HDL, total cholesterol, diabetes, and history of heart disease. Systolic BP was estimated using three measurements with a random-zero sphygmomanometer, averaging the second and third measurements. Smoking was self-reported; history of heart disease was self-reported at visit 1 and additionally included adjudicated events from visit 1 to visit 2. HDL was assessed using the enzymatic method after precipitation with dextran sulfate–magnesium. eGFR was estimated using the CKD Epidemiology Collaboration 2021 equation that incorporated both serum creatinine and cystatin C. Creatinine was measured using the modified kinetic Jaffe method. Cystatin C was measured using the Roche Cobas 6000 chemistry analyzer.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics (mean, SD, or median, interquartile range, as indicated). Binary variables were summarized using percentages. We examined cross sectional associations of eicosanoids with eGFR using Spearman correlations. Associations of log2-transformed eicosanoids with ESKD were examined using Cox proportional hazards models adjusted for age, sex, race, study center, baseline cholesterol, high-density lipoprotein cholesterol diabetes, systolic BP, antihypertension medication, body mass index, history of heart disease, and eGFR, and hazard ratios (HRs) calculated. We used Bonferroni correction to account for multiple testing, dividing 0.05 by the number of eicosanoids investigated. In sensitivity analyses, we also assessed the effect of adjusting for batch. We tested the proportional hazards assumption using Schoenfeld residuals.
Genetic Association Studies
For the eicosanoids associated with ESKD, we performed genomewide association studies (GWAS) to identify associated genetic variants. Genotyping procedures in ARIC have previously been described12; in brief, genotyping was performed on the Affymetrix 6.0 DNA microarray with exclusion of single-nucleotide polymorphisms (SNPs) with call rates <95%, Hardy–Weinberg equilibrium P < 0.001, or minor allele frequencies <1%. Data were then imputed to a common set of SNPs using the Trans-Omics for Precision Medicine reference (Freeze 5b).13–15 After QC, 6496 White participants and 1910 Black participants had eicosanoid and genotype data. GWAS were performed using the inverse-rank normalized residuals of the log-transformed eicosanoid regressed on age, sex, and the first ten genetic and eicosanoid principal components separately by race. GWAS were then meta-analyzed using fixed effects across the White and Black populations.
MR
MR is a method that uses genetic variants related to the exposure of interest as instruments to minimize confounding and study causal effects. We used a two sample MR method to combine our GWAS summary statistics with previously published GWAS results of eGFR without the need for individual-level data.16 We integrated the eicosanoid GWAS results derived in ARIC with summary statistics for eGFR done by the CKDGen Consortium.17 Because most data from the CKDGen consortium is derived from participants of European ancestry and MR analyses are ancestry specific, we compared summary statistics from the ARIC eicosanoid GWAS in White patients with eGFR GWAS of European ancestry. We used the R package TwoSampleMR to examine the causal effect of the eicosanoids on eGFR and vice versa, as previously published.18 For the eicosanoid to eGFR direction, we used P < 5×10−7 as a filter for selection of significant SNPs. Correlated SNPs were removed using linkage disequilibrium clumping (ld_clump function) with the reference European population in a window of 500 kb and an R2 threshold of 0.01. We removed SNPs that were palindromic or had possible strand mismatch (harmonise_data function) and applied Steiger filtering. For the eGFR to eicosanoid direction, we used a stricter threshold for the genetic instruments (P < 5×10−8), leveraging the greater power available from the CKDGen data. An inverse variance–weighted method was our primary analysis, and the associations were also evaluated for robustness using weighted median, MR-Egger, and MR-mixed.19–21
Transcriptomewide Association Studies
To provide additional support for the genes implicated in the GWAS of the relevant eicosanoids and potential tissue-specific sites of action, we performed transcriptomewide association studies (TWASs) using models from the GTEx project v8 (http://gusevlab.org/projects/fusion/).22 For each of the eicosanoids related to the development of ESKD, we evaluated TWAS models for genes within 500 kb of the GWAS significant index SNPs. We used Bonferroni correction to determine statistical significance, accounting for the number of investigated genes and tissues for each eicosanoid. We allowed the model to determine the best fit for each gene before application to the eicosanoid GWAS summary statistics.
Results
The 9650 participants from ARIC visit 2 included in this study had a mean age of 57 years; 56% were women, and 24% were Black. The cohort had a mean eGFR of 90 ml/min per 1.73 m2. Over a follow-up of 25 years, there were 256 ESKD events (Table 1).
Table 1.
Characteristics of study participants in the Atherosclerosis Risk in Communities Cohort, by the development of ESKD during 25 years of follow-up
| Characteristic | Overall | No ESKD | ESKD |
|---|---|---|---|
| N | 9650 | 9394 | 256 |
| Age, yr | 56.8 (5.7) | 56.8 (5.7) | 57.0 (5.5) |
| Black race, n (%) | 2330 (24.1) | 2193 (23.3) | 137 (53.5) |
| Female, n (%) | 5451 (56.5) | 5312 (56.5) | 139 (54.3) |
| Systolic BP, mm Hg | 121.0 (18.7) | 120.7 (18.3) | 133.3 (25.1) |
| Hypertension, n (%) | 3311 (34.5) | 3144 (33.6) | 167 (65.2) |
| Diabetes, n (%) | 1375 (14.3) | 1245 (13.3) | 130 (51.2) |
| NSAID use, n (%) | 4730 (49.1) | 4599 (49.1) | 131 (51.6) |
| Current smoker, n (%) | 2080 (21.6) | 2024 (21.6) | 56 (22.0) |
| Former smoker, n (%) | 3611 (37.5) | 3519 (37.5) | 92 (36.1) |
| eGFRcrcys (ml/min per 1.73 m2), mean (SD) | 98.3 (16.6) | 98.8 (15.9) | 82.3 (27.6) |
NSAID, nonsteroidal anti-inflammatory drug.
In cross-sectional analysis, eicosanoids were minimally correlated with eGFR (median Spearman r, −0.05; 25th–75th percentile, −0.03 to 0.002) (Supplemental Figure 1 and Supplemental Table 1). Of the 223 eicosanoids tested, three were associated with ESKD in the fully adjusted model (Bonferroni P value < 2.2e-04 [0.05/223]) (Figure 1 and Supplemental Table 2). These included a hydroxyoctadecenoic acid (HR, 0.71; 95% confidence interval [CI], 0.61 to 0.83), a dihydroxydocosapentaenoic acid (HR, 0.78; 95% CI, 0.71 to 0.85), and arachidonic acid (HR, 1.67; 95% CI, 1.37 to 2.05). The proportional hazards assumption was violated at the nominal level (P value < 0.05) for dihydroxydocosapentaenoic acid. Introducing a time interaction demonstrated that the protective association was stronger in the first 10 years of follow-up compared with after 10 years (HR, 0.67; 95% CI, 0.58 to 0.79 versus HR, 0.95; 95% CI, 0.83 to 1.08). These associations were similar when the models were further adjusted for batch. The associations were not significantly different by diabetes, hypertension status, or the use of nonsteroidal anti-inflammatory drugs, except for that of arachidonic acid, which was stronger in the presence of nonsteroidal anti-inflammatory drug use (Table 2). Cross-sectional analysis of each metabolite with diabetes, hypertension, and cardiovascular use revealed a strong negative association between the hydroxyoctadecenoic acid and diabetes (P < 0.001), a negative association between the dihydroxydocosapentaenoic acid and cardiovascular disease (P = 0.003), and positive associations between arachidonic acid and all three conditions (diabetes, P < 0.001; hypertension, P < 0.001; cardiovascular disease, P = 0.046) (Supplemental Table 3). Detailed LC-MS/MS analyses performed to confirm analyte annotations are shown in Supplemental Figure 2. On the basis of these analyses, the hydroxyoctadecenoic acid was determined to be either 11-hydroxy-9-octadecenoic acid (M+Na+Acetate-H)- or 10-hydroxy-11-octadecenoic acid (M+Na+Acetate-H)-, and the dihydroxydocosapentaenoic acid to have the formula C22H34O4 of unknown structure (Supplemental Figure 2). Arachidonic acid was confirmed by spike-in of an authentic standard. The coefficient of variation for each metabolite across 392 blind duplicates was 16.2% for the hydroxyoctadecenoic acid, 24% for the dihydroxydocosapentaenoic acid, and 18.5% for arachidonic acid.
Figure 1.
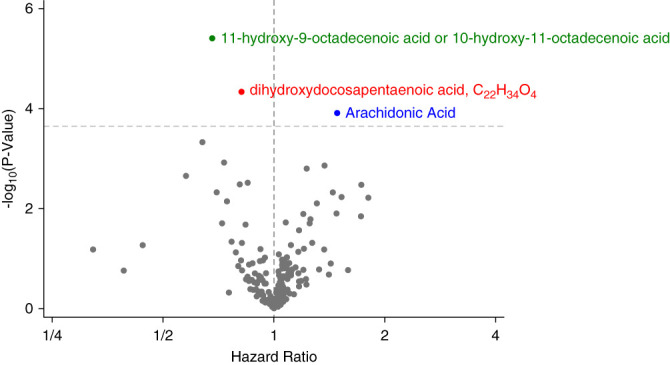
Hazard ratios for ESKD per doubling of eicosanoid levels adjusted hazard ratio of ESKD per doubling of eicosanoid levels. Models are adjusted age, sex, race, study center, eGFR, systolic BP, use of hypertensive medications, diabetes, smoking, BMI, prevalent heart disease, total cholesterol, and HDL cholesterol. BMI, body mass index.
Table 2.
Eicosanoids associated with ESKD overall and by subgroups
| Metabolite Class | Hydroxyoctadecenoic Acid | Dihydroxydocosapentaenoic Acid | Arachidonic Acid |
|---|---|---|---|
| Eicosanoid ID | Eic_14_15_EET_b | Eic_EPA_a | Eic_Arachidonic_Acid_c |
| Annotation | 11-hydroxy-9-octadecenoic acid (M+Na+Acetate-H)-, or 10-hydroxy-11-octadecenoic acid (M+Na+Acetate-H)- | C22H34O4 (M-H)− | Arachidonic acid (M-H)− |
| m/z | 379.2494 | 361.2378 | 303.2336 |
| RT, min | 5.17 | 6.22 | 6.53 |
| Overall HR (95% CI) | 0.68 (0.58 to 0.80) | 0.82 (0.74 to 0.90) | 1.48 (1.21 to 1.82) |
| Subgroup Analysis | HR (95% CI) | Interaction P Value | HR (95% CI) | Interaction P Value | HR (95% CI) | Interaction P Value |
|---|---|---|---|---|---|---|
| No diabetes | 0.75 (0.60 to 0.94) | 0.81 (0.70 to 0.94) | 1.45 (1.09 to 1.93) | |||
| Diabetes | 0.62 (0.50 to 0.77) | 0.17 | 0.82 (0.72 to 0.93) | 0.90 | 1.52 (1.14 to 2.02) | 0.84 |
| No hypertension | 0.65 (0.51 to 0.83) | 0.88 (0.74 to 1.05) | 1.56 (1.09 to 2.22) | |||
| Hypertension | 0.57 (0.48 to 0.69) | 0.38 | 0.76 (0.68 to 0.86) | 0.18 | 1.57 (1.23 to 2.01) | 0.96 |
| No NSAID use | 0.61 (0.49 to 0.76) | 0.79 (0.68 to 0.92) | 1.29 (0.99 to 1.70) | |||
| NSAID use | 0.58 (0.48 to 0.71) | 0.73 | 0.82 (0.72 to 0.93) | 0.74 | 1.94 (1.45 to 2.59) | 0.04 |
Interaction P value for the difference in effect between participants with and without diabetes etc. CI, confidence interval; RT, retention time; m/z, mass-to-charge ratio; NSAID, nonsteroidal anti-inflammatory drug.
The GWAS of the hydroxyoctadecenoic acid revealed a significant locus (rs603424) on chromosome 10 (P = 1.94×10−113) (Figure 2). There were also significant genetic loci for the dihydroxydocosapentaenoic acid near the LECT2/TGFBI, ACOT4/6, and UGT2B7 regions (chromosome 5, 14, and 4, respectively) and for arachidonic acid in the FADS1/2 region on chromosome 11 (rs28456).
Figure 2.
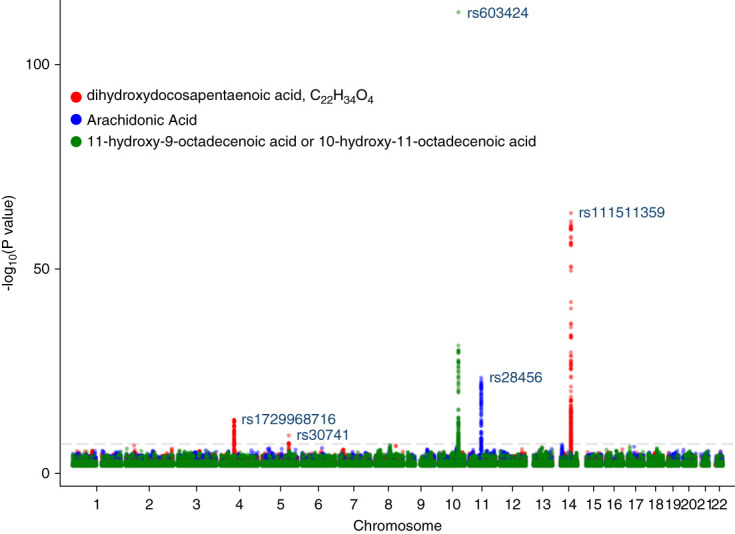
Genomewide association study of eicosanoids associated with ESKD in White and Black participants.
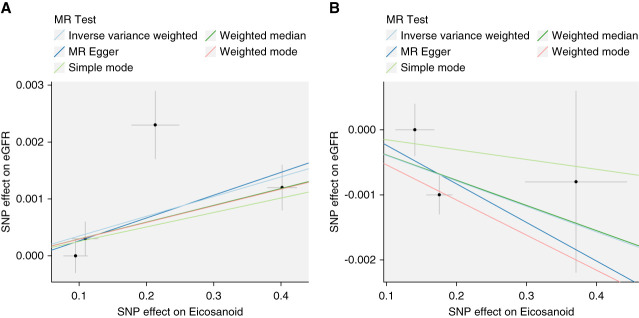
There was a small but significant association for higher hydroxyoctadecenoic acid causing higher eGFR that was robust to different methods of MR (Figure 3). Arachidonic acid was also associated with a small lowering of eGFR. By contrast, although the inverse variance–weighted method of MR suggested a causal association between the dihydroxydocosapentaenoic acid and lower levels of eGFR, the results were not robust to all sensitivity analyses. None of the three eicosanoids were implicated in the opposite direction, that is, as being influenced by eGFR (Supplemental Table 4, A and B).
Figure 3.
MR to estimate the causal effect of hydroxyoctadecenoic acid and arachidonic acid on kidney function. MR, Mendelian randomization; SNP, single-nucleotide polymorphism.
For the hydroxyoctadecenoic acid, the strongest associations between TWAS models and the genetic determinants of the eicosanoid were for stearoyl-CoA desaturase expression in adipose tissue in the omentum and subcutaneous space and polycystin 2 like 1 in the brain, testis, and lung (Supplemental Table 5A). For the dihydroxydocosapentaenoic acid, acyl-CoA thioesterase 2 expression in whole blood was among the strongest associations for the chromosome 14 region, and UDP glucuronosyltransferase family 2 member B11 expression in the liver was among the strongest associations for the chromosome 4 region, whereas associations in the chromosome 5 region were relatively weak (Supplemental Table 5, B–D). For arachidonic acid, expression of fatty acid desaturase 1 in a variety of tissues was highly associated with genetic determinants of arachidonic acid (Supplemental Table 5E).
Discussion
In this large, community-based cohort, we evaluated >200 eicosanoids and related metabolites and their associations with ESKD over a median follow-up of 25 years. We found that, in contrast to many other nonlipid metabolites, eicosanoid levels were only minimally related with baseline kidney filtration. We identified three metabolites with significant associations with the development of ESKD in fully adjusted models. To distill whether the metabolites might be causal or merely prognostic biomarkers, we performed GWAS to identify genetic instruments and integrated these results with that of a large GWAS of eGFR, finding evidence for a causal role for one of the eicosanoids, hydroxyoctadecenoic acid, as a protective factor against the development of ESKD and another, arachidonic acid, as having a positive association with risk of ESKD. The results suggest that additional study of eicosanoids and related metabolites may help identify and refine drug targets to prevent or forestall the development of ESKD.
Little is known about hydroxyoctadecenoic acids. To our knowledge, no human studies have been published about either 11-hydroxy-9-octadecenoic acid or 10-hydroxy-11-octadecenoic acid, the annotation of our molecule of interest. As shown in Supplemental Figure 2, the molecule comprises an 18-carbon monounsaturated fatty acid with an added hydroxyl group. We found a strong negative association between the molecule and diabetes and a protective association between the molecule and the development of ESKD. In GWAS, we found an association with rs603424. This SNP is intronic in the PKD2L1 gene, which encodes an integral membrane protein of the polycystin family with no obvious lipid-related function. However, a recent study showed that rs603424 is an expression quantitative trait locus for the gene SCD in adipocytes, a finding corroborated by our TWAS. SCD encodes stearoyl-CoA desaturase, which converts saturated fatty acids to monounsaturated fatty acids with the introduction of a single double bond, for example stearic acid to oleic acid (9-octadecenoic acid). Addition of a hydroxyl group to oleic acid would yield 11-hydroxy-9-octadecenoic acid, providing substantial biological plausibility to the genetic association and the annotation: Although this reaction has not been documented in humans, it has been observed in bacteria.23 Finally, the association between the genetic determinants of the hydroxyoctadecenoic acid and SCD expression in adipocytes provides face validity to the strong correlation of the metabolite and diabetes.24
More work is required to determine how 11-hydroxy-9-octadecenoic acid or 10-hydroxy-11-octadecenoic acid confer kidney protection. Given the range of biologic processes affected by eicosanoids, experiments that assess the effect of these metabolites in vivo should consider their potential impact on BP, renal blood flow, electrolyte and glucose homeostasis, and inflammation, among others. Indeed, each of the identified eicosanoids had distinct patterns of associations, with lower levels of the hydroxyoctadecenoic acid associated with diabetes, lower levels of the dihydroxydocosapentaenoic acid associated with cardiovascular disease, and higher levels of arachidonic acid in diabetes, hypertension, and cardiovascular disease. Direct protective effects could also be considered on kidney cells in vitro. Because SCD plays such a fundamental role in lipid metabolism, its deficiency or overexpression in model systems would not provide a clean window into hydroxyoctadecenoic acid biology. Many eicosanoids act through cognate receptors; thus, identification of a specific receptor for 11-hydroxy-9-octadecenoic acid or 10-hydroxy-11-octadecenoic acid would greatly accelerate elucidation of their biologic actions.
Eicosanoids and related metabolites have been increasingly implicated in kidney injury and hypertension and as both activators and suppressors of systemic inflammation. Arachidonic acid, a polyunsaturated omega-6 fatty acid, can be found in meat, fish, and eggs and is a key component of cell structure, although free levels must be kept low to avoid cytotoxicity.25 Although our analyses identify a statistically significant, deleterious association for arachidonic acid, we acknowledge that this does not provide definitive evidence for a causal role in disease. As an alternative, it is possible that a downstream arachidonic acid metabolite, not measured by our platform, is the biologic mediator of the observed association. Thus, the associations between genetic determinants of arachidonic acid and those of eGFR should be viewed as strictly hypothesis generating, particularly because FADS1/2 may also affect the levels of other eicosanoids, including arachidonic acid metabolites.16,26 Interestingly, supplementation of other PUFAs has been explored as a possible therapy in kidney disease. For example, eicosapentaenoic acids are low in ESKD, particularly when indexed to arachidonic acid levels.27 Three open-label randomized studies tested supplementation with eicosapentaenoic acid in patients receiving maintenance hemodialysis, finding large reductions in all-cause or cardiovascular mortality.28–30 By contrast, supplements containing eicosapentaenoic acid and docosahexaenoic acid did not help slow eGFR decline or prevent the increase in albuminuria among patients with diabetes.31 Eicosapentaenoic acid has also been studied as an antidote to cancer cachexia with limited success.32
Although this study is one of the largest of eicosanoids with a focus on kidney outcomes, there are some inherent limitations. Blood for eicosanoid profiling were collected in a standardized manner at a research study visit; however, only a single measure was used to evaluate risk for ESKD up to 25 years, and we did not adjust for the interval development of established risk factors, such as diabetes and hypertension. We view this as both a strength and limitation: Early targets are needed to prevent the development of kidney disease and may be less likely to represent glomerular filtration itself, yet additional measures over time might yield enhanced biological insight. Our MR analyses focused on participants of European descent because we had less power to identify genetic instruments for both eicosanoids and eGFR among participants with other ancestries. International research efforts to broaden genetic studies to include participants of diverse ancestries are critical. Although we do not suspect that biology differs by ancestry, a diverse representation in genetic studies enhances our ability to develop genetic prediction models for use in causal inference analyses. Many of the eicosanoids described are novel, precluding classification into known pathways, and several related metabolites have been demonstrated to have distinct genetic determinants, suggesting the complexity of interrelated classes. Finally, we focused on eicosanoid levels in blood only. There are likely tissue-specific mechanisms of action that require additional investigation.
In summary, our study demonstrates the potential utility of eicosanoid profiling as a method for understanding disease pathogenesis and identifying new treatment targets. We identify three robust antecedents of ESKD, a hydroxyoctadecenoic acid, a dihydroxydocosapentaenoic acid, and arachidonic acid. We identify specific genetic variants associated with levels of these eicosanoids which, when combined with genetic determinants of eGFR, support a causal role of the hydroxyoctadecenoic acid with the development of ESKD.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions. The opinions presented do not necessarily represent those of the NIDDK, the NIH, the Department of Health and Human Services, or the US Government. Some of the data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
See related editorial, “Prognostic Value of Oxylipins for the Development of ESKD,” on pages 1–2.
Disclosures
M. Alotaibi reports the following: Research Funding: American Lung Association and Bayer Pulmonary Hypertension Accelerated Awards (PHAB). S. Cheng reports the following: Research Funding: NIH and Sapient LLC; and Honoraria: Viz.AI and Zogenix. J. Coresh reports the following: Consultancy: Scientific Advisory Board—Healthy.io and SomaLogic; Ownership Interest: Healthy.io; and Research Funding: National Institute of Health and NKF. M.E. Grams reports the following: Advisory or Leadership Role: American Journal of Kidney Diseases, ASN Publication Committee, CJASN, JASN Editorial Fellowship Committee, KDIGO Executive Committee (co-chair elect), NKF Scientific Advisory Board, and USRDS Scientific Advisory Board; and Other Interests or Relationships: Grant funding from NKF—which receives funding from multiple pharmaceutical companies; grant funding from NIH; payment from academic institutions for grand rounds; payment from NephSAP. M. Jain reports the following: Sapient Bioanalytics, LLC; Ownership Interest: Sapient Bioanalytics, LLC; Research Funding: Sapient Bioanalytics, LLC; Patents or Royalties: Sapient Bioanalytics, LLC; and Advisory or Leadership Role: Sapient Bioanalytics, LLC. All remaining authors have nothing to disclose.
Funding
National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services (HHSN268 201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). M.E. Grams: National Institute of Diabetes and Digestive and Kidney Diseases (R01DK108803, R01DK124399) and National Heart, Lung, and Blood Institute (K24HL155861).
Author Contributions
Conceptualization: Morgan E. Grams, Eugene P. Rhee.
Data curation: Mohit Jain.
Formal analysis: Aditya L. Surapaneni.
Funding acquisition: Josef Coresh, Morgan E. Grams, Eugene P. Rhee.
Methodology: Morgan E. Grams, Mohit Jain, Pascal Schlosser.
Resources: Susan Cheng.
Supervision: Josef Coresh, Morgan E. Grams.
Writing – original draft: Aditya L. Surapaneni.
Writing – review & editing: Mona Alotaiabi, Susan Cheng, Josef Coresh, Morgan E. Grams, Mohit Jain, Eugene P. Rhee, Pascal Schlosser.
Data Sharing Statement
Partial restrictions to the data and/or materials apply. Preexisting data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee, and these requests will be promptly reviewed for confidentiality or intellectual property restrictions and will not be refused unreasonably. Please refer to the data sharing policies of these studies; the ARIC Study follows the NIH data sharing guidelines. Individual-level patient or protein data may further be restricted by consent, confidentiality, or privacy. In addition, the ARIC Study Coordinating Center will release newly generated data 1 year after quality control procedures are complete via BioLINCC and/or dbGAP.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A417.
Supplemental Figure 1. Histogram of Spearman correlations of eicosanoids and kidney function.
Supplemental Figure 2. LC-MS/MS analyses to confirm analyte annotations.
Supplemental Table 1. Correlation of eicosanoids with eGFR estimated using the 2021 CKD-EPI equation that uses serum creatinine and cystatin.
Supplemental Table 2. Hazard ratios for log2-transformed eicosanoids for ESKD.
Supplemental Table 3. Demographic adjusted, cross-sectional associations between log2-transformed eicosanoids and diabetes, hypertension, and cardiovascular disease.
Supplemental Table 4A. Mendelian randomization estimating the causal effect of GFR on eicosanoids.
Supplemental Table 4B. Mendelian randomization estimating the causal effect of eicosanoids on GFR.
Supplemental Table 5A. TWAS analysis of 11-hydroxy-9-octadecenoic acid or 10-hydroxy-11-octadecenoic acid using GWAS summary statistics from ARIC and expression data from GTEx v8, statistically significant results (Bonferroni correction).
Supplemental Table 5B. TWAS analysis of dihydroxydocosapentaenoic acid, C22H34O4 using GWAS summary statistics from ARIC and expression data from GTEx v8, statistically significant results (Bonferroni correction).
Supplemental Table 5C. TWAS analysis of dihydroxydocosapentaenoic acid, C22H34O4 using GWAS summary statistics from ARIC and expression data from GTEx v8, statistically significant results (Bonferroni correction).
Supplemental Table 5D. TWAS analysis of dihydroxydocosapentaenoic acid, C22H34O4 using GWAS summary statistics from ARIC and expression data from GTEx v8, statistically significant results (Bonferroni correction).
Supplemental Table 5E. TWAS analysis of arachidonic Acid using GWAS summary statistics from ARIC and expression data from GTEx v8, statistically significant results (Bonferroni correction).
References
- 1.United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [Google Scholar]
- 2.Sun D Cuevas AJ Gotlinger K, et al. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol. 2014;306(8):H1146–H1153. doi: 10.1152/ajpheart.00920.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward NC Tsai I-J Barden A, et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51(5):1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463 [DOI] [PubMed] [Google Scholar]
- 4.Kujal P Čertíková Chábová V Škaroupková P, et al. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clin Exp Pharmacol Physiol. 2014;41(3):227–237. doi: 10.1111/1440-1681.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65(3):476–482. doi: 10.1161/HYPERTENSIONAHA.114.03585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rand AA Barnych B Morisseau C, et al. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci U S A. 2017;114(17):4370–4375. doi: 10.1073/pnas.1616893114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T Fu X Chen Q, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. 2019;20(15):3683. doi: 10.3390/ijms20153683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niphakis MJ Lum KM Cognetta AB 3rd, et al. A global map of lipid-binding proteins and their ligandability in cells. Cell. 2015;161(7):1668–1680. doi: 10.1016/j.cell.2015.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell. 2021;81(18):3708–3730. doi: 10.1016/j.molcel.2021.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagerborg KA, Watrous JD, Cheng S, Jain M. High-throughput measure of bioactive lipids using non-targeted mass spectrometry. Methods Mol Biol. 2019;1862:17–35. doi: 10.1007/978-1-4939-8769-6_2 [DOI] [PubMed] [Google Scholar]
- 11.Watrous JD Niiranen TJ Lagerborg KA, et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem Biol. 2019;26(3):433–442.e4. doi: 10.1016/j.chembiol.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellinor PT Lunetta KL Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44(6):670–675. doi: 10.1038/ng.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S Forer L Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–1287. doi: 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31(5):782–784. doi: 10.1093/bioinformatics/btu704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taliun D Harris DN Kessler MD, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590(7845):290–299. doi: 10.1038/s41586-021-03205-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee EP Surapaneni AL Schlosser P, et al. A genome-wide association study identifies 41 loci associated with eicosanoid levels. Commun Biol. 2023;6(1):792. doi: 10.1038/s42003-023-05159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanzick KJ Li Y Schlosser P, et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat Commun. 2021;12(1):4350. doi: 10.1038/s41467-021-24491-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Z Coresh J Qi G, et al. A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int. 2020;98(3):708–716. doi: 10.1016/j.kint.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gusev A Ko A Shi H, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48(3):245–252. doi: 10.1038/ng.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez E, Espuny M, Manresa A, Guerrero A. Identification of (E)-11-hydroxy-9-octadecenoic acid and (E)-9-hydroxy-10-octadecenoic acid by biotransformation of Oleic acid by pseudomonas sp. 32T3. J Am Oil Chemists' Soc. 2001;78(6):593–597. doi: 10.1007/s11746-001-0310-3 [DOI] [Google Scholar]
- 24.Perrin HJ Currin KW Vadlamudi S, et al. Chromatin accessibility and gene expression during adipocyte differentiation identify context-dependent effects at cardiometabolic GWAS loci. PLoS Genet. 2021;17(10):e1009865. doi: 10.1371/journal.pgen.1009865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taber L, Chiu CH, Whelan J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids. 1998;33(12):1151–1157. doi: 10.1007/s11745-998-0317-4 [DOI] [PubMed] [Google Scholar]
- 26.Rhee EP Surapaneni A Zheng Z, et al. Trans-ethnic genome-wide association study of blood metabolites in the Chronic Renal Insufficiency Cohort (CRIC) study. Kidney Int. 2022;101(4):814–823. doi: 10.1016/j.kint.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoji T Kakiya R Hayashi T, et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am J Kidney Dis. 2013;62(3):568–576. doi: 10.1053/j.ajkd.2013.02.362 [DOI] [PubMed] [Google Scholar]
- 28.Inoue T Okano K Tsuruta Y, et al. Eicosapentaenoic acid (EPA) decreases the all-cause mortality in hemodialysis patients. Intern Med. 2015;54(24):3133–3137. doi: 10.2169/internalmedicine.54.4931 [DOI] [PubMed] [Google Scholar]
- 29.Nasu M Seino K Tamura Y, et al. Eicosapentaenoic acid restrains the development of the cardiovascular events independent of triglyceride and C-reactive protein reduction in Japanese hemodialysis patients. Eur Heart J. 2013;34(suppl 1):P1428. doi: 10.1093/eurheartj/eht308.p1428 [DOI] [Google Scholar]
- 30.Umemoto N Ishii H Sakakibara T, et al. Administration of eicosapentaenoic acid reduces cardiovascular and all-cause mortality in chronic hemodialysis patients. Eur Heart J. 2015;36:662. [Google Scholar]
- 31.de Boer IH Zelnick LR Ruzinski J, et al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2019;322(19):1899–1909. doi: 10.1001/jama.2019.17380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy RA, Yeung E, Mazurak VC, Mourtzakis M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer. 2011;105(10):1469–1473. doi: 10.1038/bjc.2011.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial restrictions to the data and/or materials apply. Preexisting data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee, and these requests will be promptly reviewed for confidentiality or intellectual property restrictions and will not be refused unreasonably. Please refer to the data sharing policies of these studies; the ARIC Study follows the NIH data sharing guidelines. Individual-level patient or protein data may further be restricted by consent, confidentiality, or privacy. In addition, the ARIC Study Coordinating Center will release newly generated data 1 year after quality control procedures are complete via BioLINCC and/or dbGAP.