Abstract
Background:
Glomus tumors are rare benign tumors that were first described in 1812 by Wood. They arise from normal glomus apparatus, usually located in the reticular dermis of the body. Although glomus tumors are universal in occurrence, the sub-Saharan Africa experience has not been well documented.
Methods:
The authors performed a systematic literature review of eligible studies between 1960 and August 2023, using the terms “glomus,” “tumor,” “glomangioma,” “glomangiomyoma,” and “Africa.” We also performed a search of the AIC Kijabe Hospital pathology department database of about 140,000 records, covering 30 years, for the terms “glomus tumor,” “glomangioma” and “glomangiomyoma.”
Results:
The systematic literature search and institutional database search produced a total of 74 patients who had glomus tumors. These patients had a lag of between 3 months and 20 years from symptom development to definitive treatment.
Conclusions:
There are very few reports of glomus tumors from sub-Saharan Africa in the current literature: the authors’ histopathology database of 140,000 specimens had 46 glomus tumors (0.03%), and only 28 additional patients were found in literature from sub-Saharan Africa. The low numbers of African patients may indicate racial differences in the occurrence of glomus tumors, although this may also be due to failure of clinicians to recognize glomus tumors. The prolonged lag period between symptom development and definitive treatment for glomus tumors indicates the need for diligence in the diagnosis and treatment of a simple problem that is otherwise the cause of incapacitating pain and misery.
Takeaways
Question: What is the experience of surgeons in sub-Saharan Africa with patients who have glomus tumors?
Findings: A 30-year institutional pathology database with 140,000 records had 46 glomus tumors (0.03%), while literature from the rest of sub-Saharan Africa reported 28 cases between 1969 and August 2023. The lag period between symptom development and definitive treatment for glomus tumors was between 3 months and 20 years.
Meaning: The low number of patients with glomus tumors reported from sub-Saharan Africa may be due to failure of clinicians to recognize these lesions, or the result of racial differences in occurrence. Surgical excision is curative for this debilitating pathology.
INTRODUCTION
Glomus tumors are rare benign tumors first described by Wood in 1812. These tumors arise from normal glomus apparatus,1 a contractile neuromyoarterial receptor complex that controls blood pressure and temperature by regulating flow in the cutaneous microvasculature.2 Glomus tumors may be divided into three broad categories, on the basis of their biologic potential: glomus tumors, glomus tumors of uncertain malignant potential, and malignant glomus tumors.3 Histopathologically, glomus tumors may be subdivided into solid glomus tumors, glomangioma, and glomangiomyoma, based on the relative predominance of the three major constituents: round glomus cells in solid glomus tumor, blood vessels in glomangioma, and spindle cells in glomangiomyoma. The solid glomus tumor is the most common, followed by glomangioma, with glomangiomyoma making up about 8% of all glomus tumors.3,4
Normal glomus bodies are located in the reticular dermis and subcutis, and the tumors can therefore occur in any part of the body; because of the high concentration of glomus bodies in the tips of digits, especially under the nail, glomus tumors are most commonly subungal. Extra-cutaneous glomus tumors have also been reported in such anatomic sites as intravascular, bone, respiratory, intraneural, genitourinary, and gastrointestinal tracts.5–10
Solitary glomus tumors are more frequent in adults than in children. Multiple glomus tumors, on the other hand, develop 10–15 years earlier than solitary lesions, and are more common in children.11 Congenital glomus tumors are rare, plaque-like in appearance and are considered a variant of multiple glomus tumors.11
The etiology of glomus tumors is yet to be elucidated. Although many issues remain unknown, a number of associations and theories of glomus tumor origin have been proposed. The association between glomus tumors and neurofibromatosis type 1 (Nf1) is now well established.12
While glomus tumors in 30% of Nf1 patients are multifocal, solitary glomus tumors have a similar distribution and clinical presentation as in the general population.12,13 Multifocality has been associated with children, familial inheritance, and pregnancy. Multifocal tumors constitute about 10% of all glomus tumors. “Multiple hereditary glomus tumors” is an entity that is distinct from solitary glomus tumors; they develop at a much earlier age than solitary tumors, and are inherited in an autosomal dominant fashion through a glomulin gene mutation.12–15
Glomus tumors are generally benign neoplasms; however, malignant glomus tumors should be considered if the tumors are in a deep location and are larger than 2 cm, or have histologic features of malignancy, such as nuclear atypia, necrosis, or mitotic activity.3 Also known as glomangiosarcomas, malignant glomus tumors are exceedingly rare and account for less than 1% of all reported glomus tumor cases.3,16,17 Patients with glomus tumors generally present with the triad of tenderness, paroxysmal pain and hypersensitivity to cold4; delay in diagnosis and treatment may result in untold suffering from intractable pain,18 whilst the treatment may be only a 30-minute procedure under local anesthesia. Familiarity with this rare diagnosis is therefore important both for primary care physicians and surgeons.
Although glomus tumors are universal in occurrence, the African experience has not been well documented. The authors reviewed reports from sub-Saharan Africa on glomus tumors in the English language, and herein describe their findings. The authors hope that this review will inform healthcare workers in sub-Saharan Africa and stimulate interest and early diagnosis and management of patients with this benign, but potentially disabling pathology.
MATERIALS AND METHODS
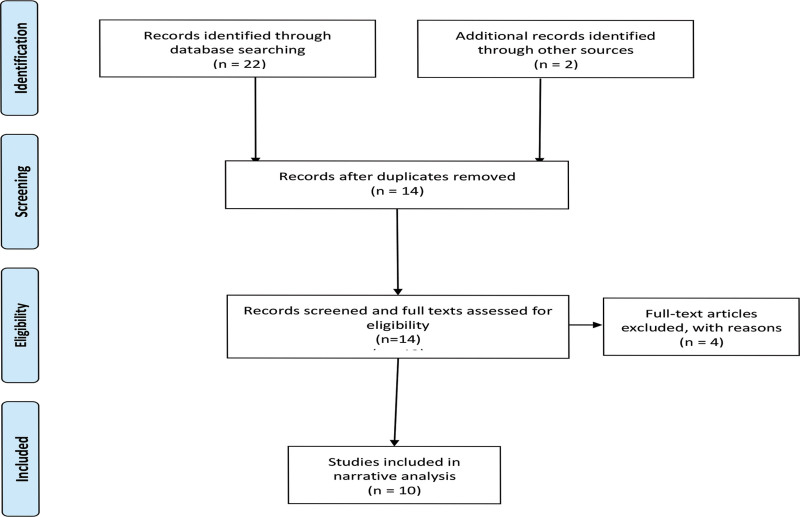
The authors conducted a systematic review of included studies, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines19 We performed a systematic literature review and narrative synthesis of eligible studies between 1960 and August 2023 from the following databases: MEDLINE, Embase, Bioline International, AJOL, Web of Science, Scopus, and grey literature, using the tearms “glomus,” “tumor,” “glomangioma,” “glomangiomyoma,” and “Africa” (Fig. 1). (See appendix, Supplemental Digital Content 1, which displays the search terms used in the Embase database. http://links.lww.com/PRSGO/D42.) The search yielded 14 papers.18,20–32 Four papers (two each from Tunisia and Morocco, respectively) were excluded from the review.29–32
Fig. 1.
PRISMA flow diagram for systematic literature search.
The authors also made a search of the hospital pathology department database of 140,000 entries between 1992 and August 2023, for “glomus tumor” and found 43 cases, whereas the terms “glomangioma,” and “glomangiomyoma” yielded two, and one patient, respectively, a total of 46 patients (Table 1).
Table 1.
Body Regions of Patients in the Kenyan Study from which Glomus Tumors Were Removed
| Body region | Site | Number |
|---|---|---|
| Upper limb | ||
| Site not indicated | 2 | |
| Shoulder | 3 | |
| Arm | 4 | |
| Forearm | 6 | |
| Palm | 2 | |
| Thumb | 2 | |
| Fingers | 11 | |
| Total | 30 | |
| Lower limb | ||
| Pelvis | 3 | |
| Thigh | 2 | |
| Leg | 1 | |
| Knee | 1 | |
| Foot | 2 | |
| Total | 9 | |
| Head and Neck | ||
| Neck | 2 | |
| Ear | 4 | |
| Trunk | Anterior chest | 1 |
| Total | 7 | |
| TOTAL | 46 |
RESULTS
The systematic review yielded 29 cases of glomus tumors (of which one was a publication from the current authors’ institution),18 whereas the authors’ institutional database gave a total of 46 patients, including the published case report18 (Table 2). The mean age of Kenyan patients with glomus tumors was 43.5 (range 11–90) years. Of the 74 patients reported in this review, only one, an 18-year-old woman, had two glomus tumors; one other patient had five recurrences.25 None of the patients included in this review were reported as having neurofibromatosis type 1 (Nf1), or any other notable associations. A diagnosis of Nf1 in most of sub-Saharan Africa is based on the clinical criteria proposed by the 1987 National Institute of Health Consensus statement.33
Table 2.
Demographics of African Patients with Glomus Tumors
| Country | Patients | Age (Mean) | M:F | Subungal | Pulp | Other Areas | Time to Diagnosis (y) | Digit Side R/L |
|---|---|---|---|---|---|---|---|---|
| Zimbabwe | 1 | 55 | N/A | 1 | — | — | 10 | |
| SA | 15 | 40 | 2:9 | 11 | 0 | 0 | 0.5–16 | |
| Nigeria | 12 | 41 | 2:9 | 2 | 2 | 7 (fingers) | 0.3–4 | 6:5 |
| Kenya | 45 + 1 | 45 | 1:2 | 7 | 7 | Many other regions | 0.25–20 |
The male-to-female ratio of the patients accumulated from the rest of sub-Saharan Africa was 1:2, with mean ages of 49.4 for men and 41 years for women (this difference was not significant, P = 0.1); their mean age was 43.9 years. Twenty seven of the 29 patients had an average presentation-to-surgery waiting period of 5.6 years.
DISCUSSION
There are very few reports of glomus tumors from Africa in the current literature. Indeed, from the institutional pathology database of 140,000 specimen in the Kenyan study, there were only 46 glomus tumor cases (0.03%). A further 28 African patients were found in the literature (excluding one case report from the authors’ institution).18 Mofikoya also reported the rarity of glomus tumors in reports from West Africa.22
All the included reports in this review had a preponderance of female patients. While 37% of the specimen from Kenya were from outside the upper limb regions, only two (7%) were extra-upper limb in the rest of the African data (Tables 1 and 2). In the Kenyan study, although the mean age of 43 years was consistent with the findings of other African reports, men with glomus tumors were on average 13 years older than the women.
The rarity of reports of glomus tumors in sub-Saharan African literature may be a reflection of a rarity of the lesion amongst populations from the region. However, other plausible explanations exist, amongst them the possibility that glomus tumors may not be well known by healthcare workers, and may therefore be misdiagnosed18 or unreported. Another possible explanation is that a large number of excised tumors from the region are not histologically examined, and cannot therefore be reported as glomus tumors.
One of the authors’ patients received such relief after surgery, following eight years of excruciating pain with multiple diagnoses, that he decided to make his experience public.34 Six other patients with glomus tumors were treated by the author immediately after the newspaper article was published. The average time from initial presentation with a complaint to diagnosis amongst 27 patients was 5.6 years, with a range of 3 months to 20 years (Table 2); this prolonged delay in treatment is universal, and not peculiar to this sub-Saharan series.35
Clinical Examination
Clinical examination includes the well-known Love test (pin head) and the cold test, both of which elicit pain on application, as well as Hildreth test, which renders the site of the glomus tumor painless upon the application of a proximal tourniquet. The Joseph Posner test involves the application of methyl alcohol on the affected site, which produces pain as the alcohol evaporates and causes a cooling effect.36
Imaging
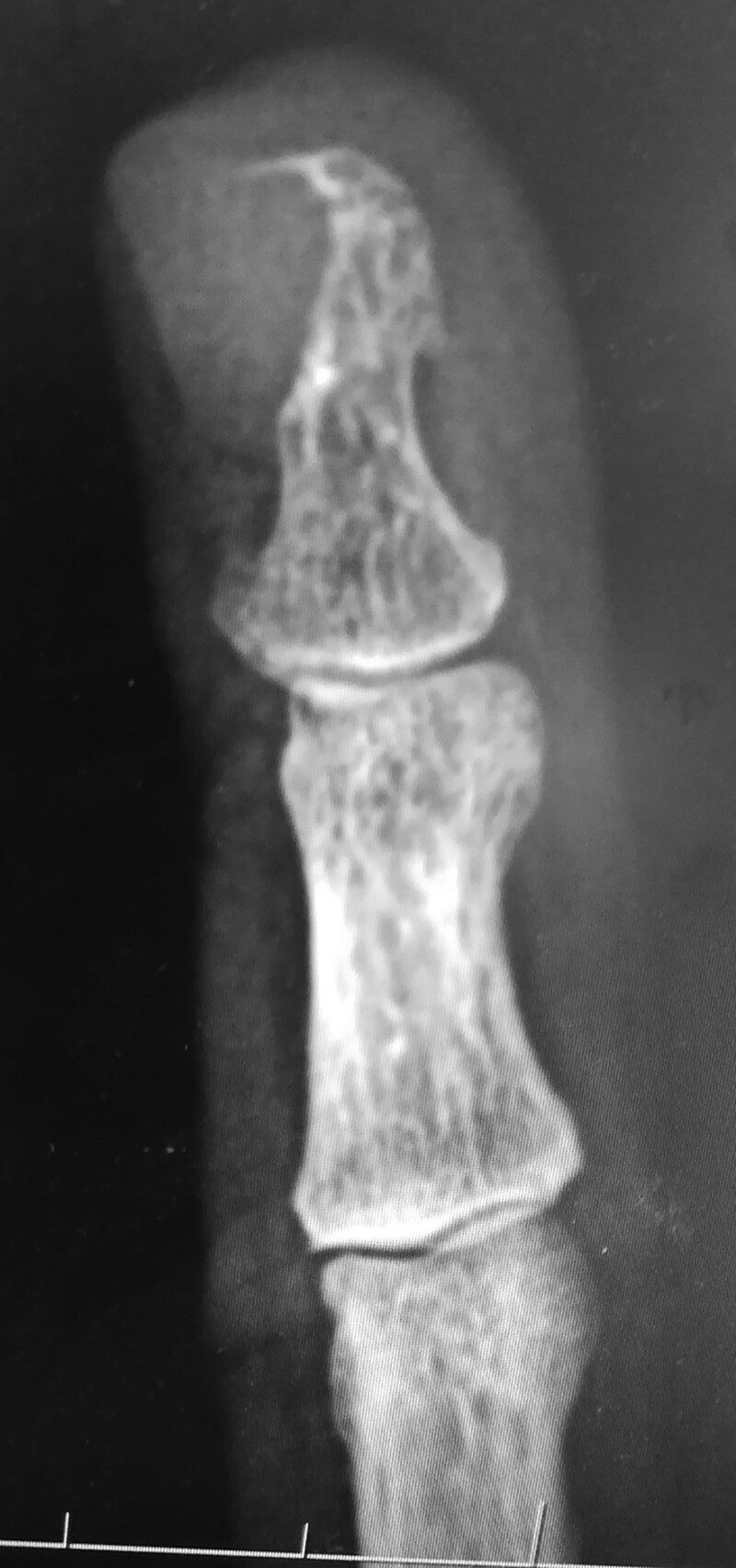
Plain radiography, ultrasound, computerized tomography scan, positron emission tomography scan, and magnetic resonance imaging (MRI) have all been used to aid clinical diagnosis of glomus tumors. These become important especially when the history and/or clinical examination is inconclusive.4 Plain radiography is of limited use, as it depends on erosive bone changes caused by the glomus tumor; changes present in only 22% of patients with glomus tumors.37,38 (Fig. 2).
Fig. 2.
Plain radiograph of a subungal glomus tumor showing scalloped distal phalanx.
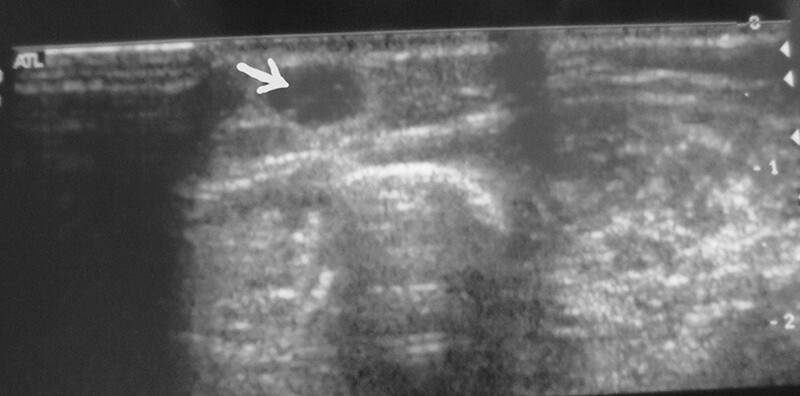
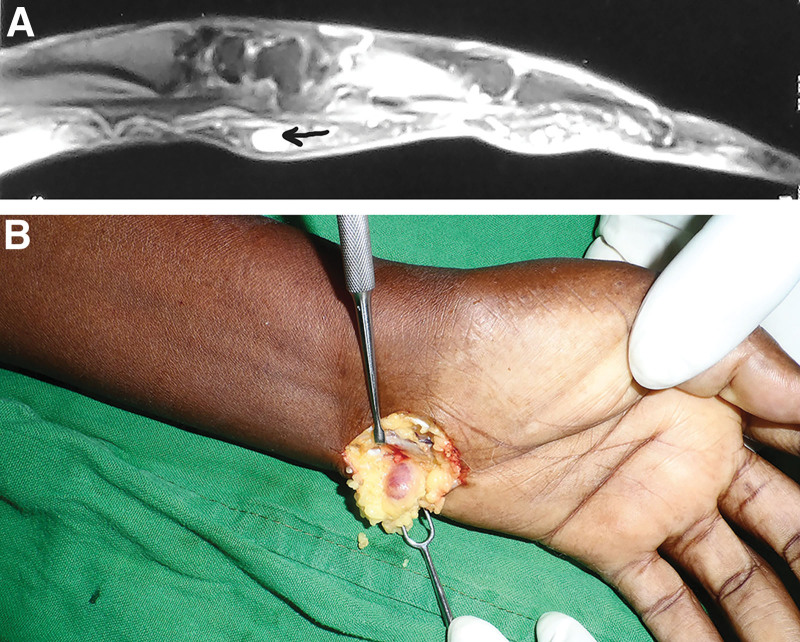
On ultrasonography, glomus tumors present as circumscribed solid hypoechoic masses, while on color Doppler, these lesions show marked blood flow.38 (Fig. 3). Workers have reported different experiences with the use of the MRI scan for identifying glomus tumors (Fig. 4A and B): although its reliability in detecting glomus tumors has been reported by some, others have found it unreliable.4,38 Positron emission tomography scan and the computerized tomography scan may be useful for lesions that are deep-seated and difficult to define or find.38,39
Fig. 3.
Ultrasound picture of a glomus tumor in the anterior aspect of the distal leg. White arrow is pointing at glomus tumor.
Fig. 4.
Left wrist glomus tumor. A, MRI of a glomus tumor in the volar aspect of the wrist. Black arrow is pointing at glomus tumor. B, Clinical picture of the wrist glomus tumor.
Angiography was used in the past, but because of its invasiveness, attendant risks, and cost, the procedure has gradually fallen out of favor.13 There is currently no gold standard imaging modality. Although plain radiography and ultrasonography are relatively inexpensive, and almost ubiquitously available, ultrasonography is operator dependent, and plain radiography, unreliable. All the other modalities are expensive and may fail to visualize the lesion. However, even when imaging fails to reveal the lesion, clinical suspicion should necessitate surgical exploration.4
Treatment
Treatment of glomus tumors is by surgical excision, irrespective of the anatomical site of the lesion; a complete excision is curative. Before the operation, the point of maximal pain and tenderness is marked, and the appropriate incision is made under loupe magnification. The lesion is then gently dissected out.
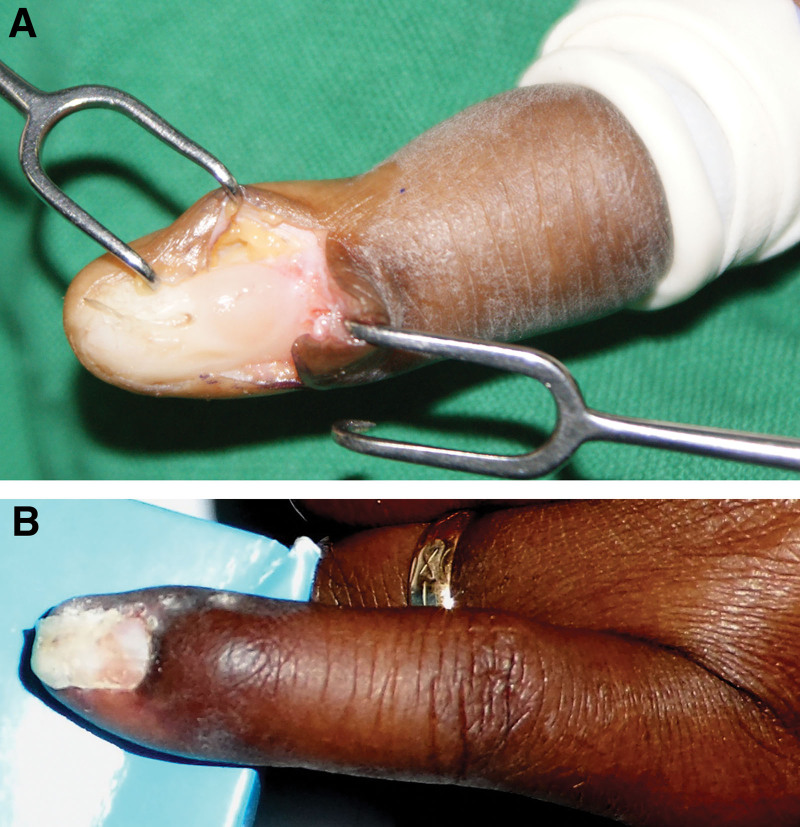
For subungal lesions, an incision is made along the lateral border of the nail of the involved digit, and the nail is lifted off gently using a Freer. An incision is made on the nail bed, and the tumor is exposed and removed (Fig. 5A). The incision is repaired, and the nail is placed back as a dressing and held in place with a few stitches40 (Fig. 5B). The lateral and volar approaches used in some centers for subungal lesions are primarily designed to minimize injury to the nail bed.40–43 Appropriate incisions are made as required for lesions in other anatomical areas.
Fig. 5.
Surgical management of a subungal glomus tumor. A, Subungal glomus tumor. Nail removed to expose glomus tumor growing beneath nail bed. B, Three weeks after excision of glomus tumor. The sutures have been removed, and the patient is now fully recovered and using hand for activities of daily living.
The main problems after surgery are nail deformity and recurrence. Recurrence rates in the literature range between 1% and 33%.38,39,44 In this review, there was only one patient reported with recurrence (five recurrences).25
CONCLUSIONS
The low number of patients with glomus tumors reported from sub-Saharan Africa may be due to failure of clinicians to recognize these lesions, or the result of racial differences in occurrence. Surgical excision is curative. The lag period between symptom development and definitive treatment for glomus tumors of between 3 months and 20 years, though universal, is a reminder of the need for diligence in the diagnosis and treatment of a problem that is easily treated and cured, but untreated, is the cause of incapacitating pain and misery.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 1 February 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Wood W. On painful subcutaneous tubercle. Edinb Med Surg J. 1812;8:283–291. [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54:691–703. [PubMed] [Google Scholar]
- 3.Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1–12. [DOI] [PubMed] [Google Scholar]
- 4.McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397–1400. [DOI] [PubMed] [Google Scholar]
- 5.Gaertner EM, Steinberg DM, Huber M, et al. Pulmonary and mediastinal glomus tumors--report of five cases including a pulmonary glomangiosarcoma: a clinicopathologic study with literature review. Am J Surg Pathol. 2000;24:1105–1114. [DOI] [PubMed] [Google Scholar]
- 6.Acebo E, Fernando Val-Bernal J, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24:384–389. [DOI] [PubMed] [Google Scholar]
- 7.Lee SK, Song DG, Choy WS. Intravascular glomus tumor of the forearm causing chronic pain and focal tenderness. Case Reports in Orthopedics. 2014;2014:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett S, Lam M, Wasserman J, et al. A case series of two glomus tumors of the gastrointestinal tract. J Surg Case Rep. 2015;2015:rju144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park DS, Choe WJ, Chun YI, et al. Glomus Tumor in the femoral nerve. J Korean Neurosurgical Society. 2013;54:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong GN, Nandini CL, Teoh LC. Multiple intraneural glomus tumors on a digital nerve: case report. J Hand Surg Am. 2013;38:1972–1975. [DOI] [PubMed] [Google Scholar]
- 11.Lim SW, Lee J, Goh C-L. A case of congenital plaque-type glomuvenous malformations. J Paediatr Child Health. 2013;49:E443–E447. [DOI] [PubMed] [Google Scholar]
- 12.Harrison B, Sammer D. Glomus tumors and neurofibromatosis: a newly recognized association. Plast Reconstr Surg Global Open. 2014;2:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niechajev IA. Multiple glomus tumours: Special reference to radiological findings. Scand J Plast Reconstr Surg. 1982;16:183–190. [DOI] [PubMed] [Google Scholar]
- 14.Reed WB. Multiple glomus tumors with a family history. Arch Dermatol. 1969;100:496–497. [DOI] [PubMed] [Google Scholar]
- 15.Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Zaid A, Azzam A, Amin T, et al. Malignant glomus tumor (glomangiosarcoma) of intestinal ileum: a rare case report. Case Rep Pathol. 2013;2013:305321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097–1101. [DOI] [PubMed] [Google Scholar]
- 18.Macharia C, Nthumba PM. Glomus tumor presenting as complex regional pain syndrome of the left upper limb: a case report. J Med Case Rep. 2015;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grave GF. Glomus tumour in an African. Cent Afr J Med. 1969;15:56–57. [PubMed] [Google Scholar]
- 21.Nggada H, Gali B, Tahir C. A histopathological pattern of vascular tumours in north eastern Nigeria. Highland Med Res J. 2005;1:1. [Google Scholar]
- 22.Mofikoya BO, Anunobi CC, Enweluzo GO. Glomus tumours of the hand in Lagos, Nigeria. East Central African J Surg. 2011;16:16123–16128. [Google Scholar]
- 23.Sefeane T, Aden A, Peters F. Subungual glomus tumours: Report on 11 cases. SA Orthopaedic J. 2013;12:1242–1245. [Google Scholar]
- 24.Onuigbo WI. Pattern of angiomas in the Igbos of Nigeria. Angiology. 1977;28:803–805. [DOI] [PubMed] [Google Scholar]
- 25.Nkosi C, Sefeane T. A subungual glomus tumour of the finger with five reappearances: a rare case report. SA Orthopaedic J. 2023;22:48–51. [Google Scholar]
- 26.Yakubu A, Mohammed A, Edino S, et al. Glomus tumour—the report of a case in an adult Nigerian. Niger J Med. 2006;14:97–99. [DOI] [PubMed] [Google Scholar]
- 27.Workman M, Saragas N, Ferrao P. Glomus tumors in the foot: two case reports. J Foot Ankle. 2020;14:183–186. [Google Scholar]
- 28.Anley C, Vrettos B, Roche S, et al. A glomus tumour of the elbow: a case report and review of the literature. Shoulder & Elbow. 2014;6:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdi MF. Glomus tumour of fingertip: report of eight cases and literature review. Musculoskeletal Surg. 2011;95:237–240. [DOI] [PubMed] [Google Scholar]
- 30.Sbai MA, Benzarti S, Gharbi W, et al. Glomus tumor of the leg: a case report. Pan Afr Med J. 2018;31:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bousbaa H, Amhajji L. Case study of glomus tumor of the index finger. Pan Afr Med J. 2017;26:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margad O, Bousselmame N. Glomus tumor of the thigh: a new case report and literature review. Pan Afr Med J. 2017;28:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stumpf DA. Neurofibromatosis. Conference statement, national institute of health development conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 34.Okeyo V. I just had to share my amazing story with you. Daily Nation. 2014. Available at https://nation.africa/kenya/life-and-style/living/i-just-had-to-share-my-amazing-story-with-you-1013990. Acessed December 20, 2023.
- 35.Guedes GVC, Jácome DT, Alves GF, et al. Epidemiological analysis of glomus tumors of the hand and association with recurrence rate. Revista Iberoamericana de Cirugía de la Mano. 2022;50:e27–e33. [Google Scholar]
- 36.Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8:918–920. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberghe L, De Smet L. Subungual glomus tumours: a technical tip towards diagnosis on plain radiographs. Acta Orthop Belg. 2010;76:396–397. [PubMed] [Google Scholar]
- 38.Glazebrook KN, Laundre BJ, Schiefer TK, et al. Imaging features of glomus tumors. Skeletal Radiol. 2011;40:855–862. [DOI] [PubMed] [Google Scholar]
- 39.Nadrous HF, Allen MS, Bartholmai BJ, et al. Glomus tumor of the trachea: value of multidetector computed tomographic virtual bronchoscopy. Mayo Clin Proc. 2004;79:237–240. [DOI] [PubMed] [Google Scholar]
- 40.Lee H-J, Kim P-T, Kyung H-S, et al. Nail-preserving excision for subungual glomus tumour of the hand. J Plast Surg Hand Surg. 2014;48:201–204. [DOI] [PubMed] [Google Scholar]
- 41.Lee IJ, Park DH, Park MC, et al. Subungual glomus tumours of the hand: diagnosis and outcome of the transungual approach. J Hand Surg Eur Vol. 2009;34:685–688. [DOI] [PubMed] [Google Scholar]
- 42.Madhar M, Bouslous J, Saidi H, et al. Which approach is best for subungual glomus tumors? Transungual with microsurgical dissection of the nail bed or periungual? Chir Main. 2015;34:39–43. [DOI] [PubMed] [Google Scholar]
- 43.Vasisht B, Watson HK, Joseph E, et al. Digital glomus tumors: a 29-year experience with a lateral subperiosteal approach. Plast Reconstr Surg. 2004;114:1486–1489. [DOI] [PubMed] [Google Scholar]
- 44.Nazerani S, Motamedi MH, Keramati MR. Diagnosis and management of glomus tumors of the hand. Tech Hand Up Extrem Surg. 2010;14:8–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.