Abstract
IMPORTANCE:
Sepsis and septic shock are major healthcare problems that need early and appropriate management.
OBJECTIVES:
To evaluate the association of daily cefepime pharmacokinetic/pharmacodynamic (PK/PD) parameters with change in Sequential Organ Failure Assessment (SOFA) score and vasopressors requirement.
DESIGN, SETTING, AND PARTICIPANTS:
This is a retrospective study. Adult ICU patients who received cefepime for Gram-negative pneumonia or bloodstream infection (BSI) and had cefepime concentrations measured were included. Daily cefepime exposure was generated and PK/PD parameters calculated for patients. Repeated-measures mixed-effect modeling was used to evaluate the impact of PK/PD on the outcomes.
MAIN OUTCOMES AND MEASURES:
Change in daily SOFA score and vasopressors requirement.
RESULTS:
A total of 394 and 207 patients were included in the SOFA and vasopressors analyses, respectively. The mean (±sd) age was 55 years (19) and weight 81 kg (29). For the change in SOFA score, daily SOFA score, mechanical ventilation, renal replacement therapy, and number of vasopressors were included. In the vasopressors analysis, daily SOFA score, day of therapy, and hydrocortisone dose were significant covariates in the final model. Achieving cefepime concentrations above the minimum inhibitory concentration (MIC) (T>MIC) for 100% of the dosing interval was associated with 0.006 µg/kg/min decrease in norepinephrine-equivalent dose. Cefepime PK/PD did not have an impact on the daily change in SOFA score.
CONCLUSIONS AND RELEVANCE:
Achieving 100% T>MIC was associated with negligible decrease in vasopressors requirement in ICU patients with Gram-negative pneumonia and BSI. There was no impact on the change in SOFA score.
Keywords: cefepime, critical care, organ dysfunction scores, pharmacokinetics, vasoconstrictor agents
KEY POINTS:
Question: To evaluate the association of daily cefepime pharmacokinetic/pharmacodynamic (PK/PD) parameters with change in Sequential Organ Failure Assessment (SOFA) score and vasopressors requirement.
Findings: Achieving cefepime concentrations above the minimum inhibitory concentration (MIC) (T>MIC) for 100% of the dosing interval was associated with 0.006 µg/kg/min decrease in daily vasopressors requirement. There was no impact of cefepime PK/PD target attainment on the change in daily SOFA score.
Meaning: Optimizing cefepime exposure may have negligible-to-no impact on vasopressors requirement and daily SOFA score in critical care.
Sepsis affects about 1.7 million adults in the United States each year and contributes to 30–50% of hospitalizations (1). Globally, 48.9 million sepsis cases and 11 million sepsis-related deaths were reported in 2017. Studies that reported the microbiologic profiles in hospital-acquired sepsis found that 34–64% of cases were caused by Gram-negative bacteria, and up to one third of cases were caused by drug-resistant bacteria (2).
One of the factors that impacts sepsis outcomes is the site and type of infection (3). Severe pneumonias, including both hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP), are the most frequently encountered bacterial infection in critical care settings (4). Severe pneumonia is associated with increased duration of mechanical ventilation, length of stay, and mortality rate (5, 6). Mortality due to VAP has been estimated at around 10% (7). In addition to pneumonias, bloodstream infections (BSIs) are associated with significant morbidity and mortality (4, 8). Gram-negative organisms are associated with serious therapeutic problems because of the increased incidence of antimicrobials resistance (6). Patients infected with Gram-negative BSIs may have higher early mortality rates compared to patients with BSIs caused by Gram-positive bacteria (5). Early initiation of appropriate antibacterial therapy may improve patients’ outcomes (7).
Beta-lactams, including cefepime, are considered essential for treating infections caused by Gram-negative bacteria. Because beta-lactams are time-dependent agents, the bacterial killing depends on the time the beta-lactam concentration exceeds the bacterial minimum inhibitory concentrations (T>MIC) (9). Standard doses of beta-lactams may be inadequate to treat infections in critically ill patients and achieve the pharmacokinetic/pharmacodynamic (PK/PD) targets reported in the literature (10–12). Additionally, critically ill patients may have regular changes in PK parameters which can contribute to unpredictable beta-lactam exposure at standard doses (11). As a result, it is essential to provide adequate exposure to beta-lactam antibiotics, both early and for the whole duration of therapy, to improve patient outcomes using therapeutic drug monitoring (TDM).
Sequential Organ Failure Assessment (SOFA) score may be used to determine the level of organ dysfunction and mortality risk (13), whereas vasopressor requirements may reflect the degree of shock and hypoperfusion in critically ill patients (14). Both can be used as outcome variables in septic patients. Previous studies reported these outcome variables as a snapshot at a certain time point during therapy and/or at the end of therapy. Therefore, the purpose of this study was to evaluate the impact of daily cefepime target attainment on the daily change of SOFA scores and vasopressors requirement in critically ill patients with Gram-negative HAP/VAP or BSI.
MATERIALS AND METHODS
This was an analysis of two previously published datasets which included patients who were admitted to the ICU at University of Florida (UF) Health Shands Hospital between January 2016 and May 2021 (15, 16). Patients were included if they were older than 18 years, had Gram-negative HAP/VAP or BSI confirmed by culture, received cefepime for the treatment of their infection for at least 2 days, and had cefepime plasma concentration measured as part of the usual TDM service. Pregnant women, prisoners, and patients allergic to cefepime were excluded. Data collected included patients’ age, sex, weight, calculated creatinine clearance (CrCl) using the Cockcroft-Gault equation, renal replacement therapy (RRT), cefepime dosing information and plasma concentrations, cultures and susceptibility including MICs when available, daily SOFA scores, number of vasopressors administered each day, mean daily vasopressors (epinephrine, norepinephrine, dopamine, phenylephrine, and vasopressin) requirement in µg/kg/min or units/min, total daily dose of systemic steroids in mg/day, and daily mechanical ventilation status (on/off). The mean daily norepinephrine-equivalent dose calculation was based on the following equation (17):
As per standard of care, patients had their cefepime plasma concentration measured typically in the first 48 hours of therapy, and concentrations were repeated as needed. Clinicians order peak (1 hr after the end of the infusion) and trough (before starting the next dose) samples for patients on intermittent or extended infusion beta-lactams. Patients on continuous infusion may have one to two random samples collected. The quantification of cefepime plasma concentration was done at the Infectious Disease Pharmacokinetics Laboratory at UF using validated liquid chromatography with tandem mass spectrometry assays. The calibration range was 2–100 mg/L and the inter- and intraday precision and accuracy were less than 10% (18). The cefepime doses, concentrations, actual timing, and covariates (baseline weight and daily CrCl) were used to calculate the median posterior predictions for each patient using published nonparametric population PK model (Pmetrics v1.9; Laboratory of Applied Pharmacokinetics and Bioinformatics, Los Angeles, CA) (19, 20). The individual predictions were then imported to Phoenix WinNonlin v8.1 (Certara, St. Louis, MO) and the time the concentration remained above the MIC (T>MIC) and above four multiples of the MIC (T>4×MIC) were calculated for each patient on every day of therapy.
Bacteria were identified by standard microbiologic methods using VITEK Mass Spectrometry and VITEK II (bioMérieux, Durham, NC) and MIC quantified. MICs were quantified by E-test only for Burkholderia cepacia complex, and previously isolated Acinetobacter species and Gram-negative nonfermenters. In the case of polymicrobial infection, the highest MIC was used for the PK/PD calculations. If no MIC reported, the European Committee on Antimicrobial Susceptibility (EUCAST) breakpoint for the bacteria identified was used.
This study was reviewed by the institutional review board at UF and approved as exempt (institutional review board number: 201902910, January 2020, “Clinical experience with a TDM program”). Informed consent was waived.
Statistical Analysis
Continuous data were presented as median and IQR or mean and sd, whereas the categorical data as counts and percentages. A random-intercept, linear, mixed-effects model was applied to determine the impact of PK/PD parameters on the mean daily vasopressors requirement and change in SOFA score. For mean vasopressor requirement analysis, parameters of interest included the day of therapy, type of infection (HAP/VAP or BSI), SOFA score, dose of systemic steroids, and RRT (yes/no) on every day of therapy. For change in SOFA scores, in addition to the parameters included in the vasopressors analysis, the following parameters were added: mechanical ventilation status (on/off) and number of and mean vasopressors requirement on every day of therapy.
A full model was specified using the variables above, excluding the PK/PD parameters, as fixed effects and the subject-specific identifier as a random effect in order to control for subject-specific variation. The reduced model was chosen based on minimizing Akaike’s Information Criterion (AIC). The likelihood ratio test was used to detect significant differences in model performance. PK/PD parameters were added to the reduced model as a last step. Multicollinearity was considered acceptable if the variable inflation factor value was less than 10. A p value less than 0.05 was considered statistically significant.
All statistical analyses were conducted with R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) using the lme4 package (21) with models fit using maximum likelihood.
RESULTS
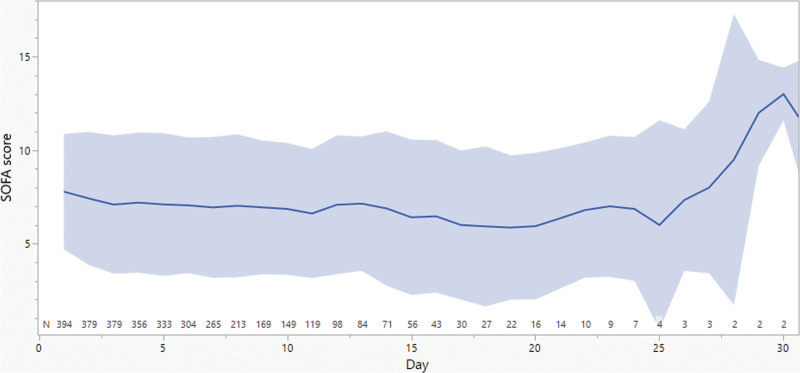
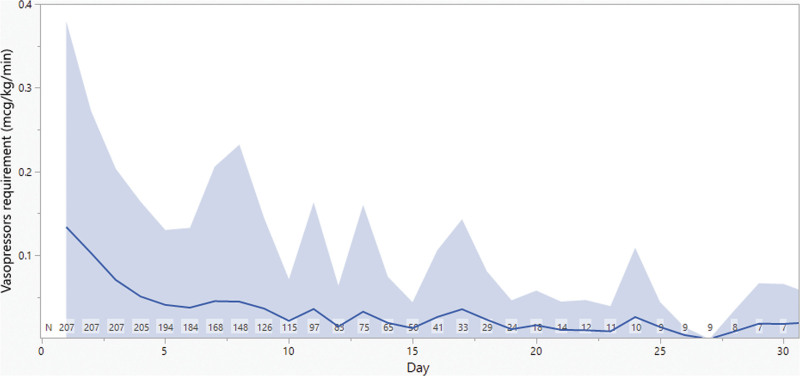
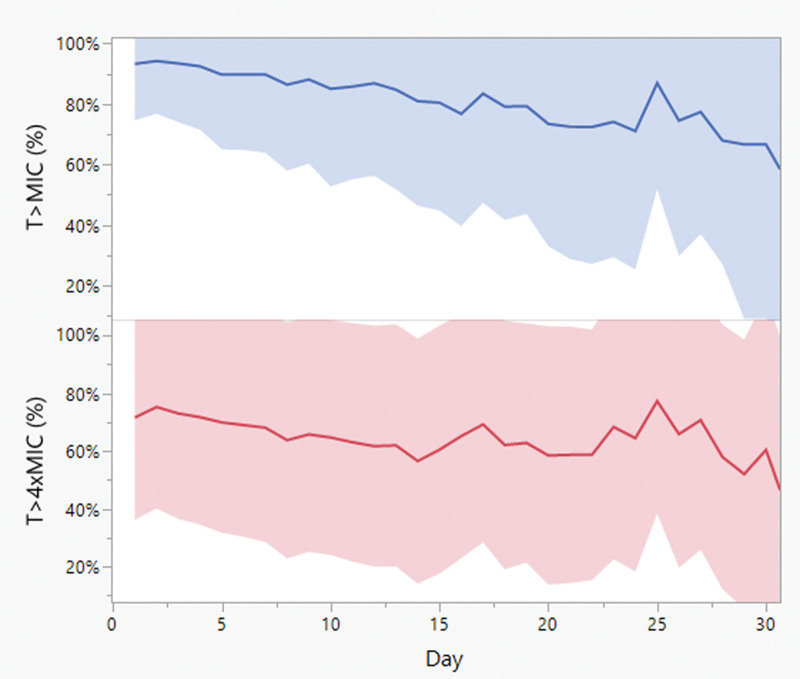
A total of 394 and 207 patients were included in the SOFA and vasopressors analyses, respectively. Table 1 summarizes patients’ demographics. The mean (±sd) age was 55 years (±19) and weight 81 kg (±29). Sixty-one percent of patients were male and 16% received RRT. The mean (±sd) follow-up duration was 11 days (±7). Eighty-five percent of patients had MIC reported for the isolated bacteria; 43% of them had MIC less than or equal to 1 mg/L. Only 15% of patients had no MIC reported for the isolated bacteria, half of them had Pseudomonas aeruginosa, and hence, EUCAST breakpoints were used. Daily SOFA score and vasopressor requirement are summarized in Figures 1 and 2. Mean (±sd) SOFA score was 7 to 8 (±3 to 4) in the first 25 days of therapy. The mean (±sd) daily vasopressor dose started at 0.13 µg/kg/min (±0.25) and showed more variability over days of therapy than SOFA score, but it dropped slowly over the course of therapy. Individual daily cefepime exposure was generated for the entire duration of therapy for all patients. Figure 3 summarizes the mean and sd PK/PD target attainment daily during therapy.
TABLE 1.
Patients Baseline Demographicsa
| Characteristics | Vasopressors Analysis (n = 207) | SOFA Analysis (n = 394) |
|---|---|---|
| Age, yr | 59 (16) | 56 (17) |
| Sex, male | 122 (59) | 253 (64) |
| Weight, kg | 85 (30) | 83 (29) |
| Renal replacement therapy | 64 (31) | 77 (20) |
| Mechanical ventilation | 186 (90) | 326 (83) |
| Baseline SOFA score | 9 (3) | 8 (3) |
| Vasopressors starting doseb | 0.13 (0.25) | 0.07 (0.19) |
| Dataset Hospital-acquired pneumonia/ventilator-associated pneumonia Bloodstream infection |
151 (73) 56 (27) |
302 (77) 92 (23) |
| Days of follow-up | 12 (8) | 11 (6) |
| Common isolated bacteriac Pseudomonas aeruginosa Escherichia coli Serratia marcescens Klebsiella pneumoniae |
83 (4) 22 (1) 21 (1) 19 (1) |
169 (4) 35 (2) 37 (1) 36 (1) |
| No. of cefepime samples | 791 | 459 |
SOFA = Sequential Organ Failure Assessment.
Data presented as mean (sd) or n (%) unless specified.
Reported as norepinephrine-equivalent dose in µg/kg/min.
n (median minimum inhibitory concentration).
Figure 1.
Daily Sequential Organ Failure Assessment (SOFA) scores and vasopressors requirement. The solid line represents the mean and the band represents the sd.
Figure 2.
Daily vasopressors requirement. The solid line represents the mean and the band represents the sd.
Figure 3.
Daily pharmacokinetic/pharmacodynamic target attainment. The solid line represents the mean and the band represents the sd. T>MIC and T>4×MIC = time the beta-lactam concentration was above the minimum inhibitory concentration or four multiples of the minimum inhibitory concentration, respectively.
Table 2 shows the initial and final mixed-effect model for the daily change in SOFA score. The final model included daily SOFA score, mechanical ventilation, RRT, and number of vasopressors. There was no impact of PK/PD target attainment on the change of SOFA score.
TABLE 2.
Mixed-Effect Model for Change in Sequential Organ Failure Assessment Scorea
| Initial Model | Final Model | |||
|---|---|---|---|---|
| Predictors | Coefficient | p | Coefficient | p |
| Day of therapy | 0.012 | 0.0159 | — | — |
| Current day Sequential Organ Failure Assessment | −0.091 | < 0.0001 | −0.298 | < 0.0001 |
| No. of vasopressors | 0.078 | 0.41 | 0.313 | < 0.0001 |
| Fludrocortisoneb | -0.625 | 0.34 | — | — |
| Hydrocortisoneb | 0.001 | 0.44 | — | — |
| Renal replacement therapyc | 0.167 | < 0.0001 | 0.822 | < 0.0001 |
| Type of infectiond | −0.020 | 0.62 | — | — |
| Mechanical ventilationc | −0.150 | < 0.0001 | 0.185 | 0.0007 |
| Mean daily vasopressors requiremente | 0.897 | 0.14 | — | — |
| 100% T>MICc | — | — | 0.009 | 0.88 |
| 100% T>4×MICc | — | — | 0.087 | 0.055 |
T>MIC and T>4×MIC = time the free beta-lactam concentration was above the minimum inhibitory concentration or four multiples of the minimum inhibitory concentration, respectively.
The change in Sequential Organ Failure Assessment was evaluated as the difference between the current and next day’s score.
Per 1 mg/d.
Yes compared with no.
Blood vs. pneumonia.
Per 1 µg/kg/min.Values in boldface font are p < 0.05.
Table 3 shows the initial and final mixed-effect model for the daily vasopressor requirement. In addition to the day of therapy, daily SOFA score, and hydrocortisone dose, achieving 100% T>MIC was associated with a statistically, but not clinically, significant decline in the mean daily vasopressors requirement of 0.006 µg/kg/min. Achieving 100% T>4×MIC was not a significant predictor in this analysis.
TABLE 3.
Mixed-Effect Model for Vasopressor Requirementa
| Initial Model | Final Model | |||
|---|---|---|---|---|
| Predictors | Coefficient | p | Coefficient | p |
| Day of therapy | −0.001 | < 0.0001 | −0.002 | 0.0002 |
| Current day Sequential Organ Failure Assessment | 0.008 | < 0.0001 | −0.007 | < 0.0001 |
| Fludrocortisoneb | 0.159 | 0.0055 | — | — |
| Hydrocortisoneb | 0.0004 | < 0.0001 | 0.0003 | < 0.0001 |
| Renal replacement therapyc | 0.020 | < 0.0001 | — | — |
| Type of infectiond | −0.004 | 0.24 | — | — |
| 100% T>MICc | — | — | −0.006 | 0.0318 |
| 100% T>4×MICc | — | — | 0.002 | 0.38 |
T>MIC and T>4×MIC = time the free beta-lactam concentration was above the minimum inhibitory concentration or four multiples of the minimum inhibitory concentration, respectively.
Vasopressors requirement was evaluated as norepinephrine-equivalent dose based on µg/kg/min.
Per 1 mg/d.
Yes compared with no.
Blood vs. pneumonia.Values in boldface font are p < 0.05.
DISCUSSION
We presented the impact of cefepime PK/PD on the daily change in SOFA score and the requirement of vasopressors in critically ill patients with Gram-negative bacterial pneumonia and BSI. We used repeated-measures, mixed-effect modeling to investigate this relationship daily (22). Given the longitudinal nature of our data, with daily measurements of both clinical outcomes (i.e., vasopressors requirement and SOFA score) and predictors (i.e., PK/PD target attainment), we employed repeated-measures, mixed-effect modeling. This analytical approach was chosen for its ability to account for intrasubject correlation due to repeated measurements, handle unbalanced datasets (i.e., different numbers of measurements across subjects), and model individual patient trajectories. Specifically, the mixed-effect model was deemed most appropriate to account for the variability in outcomes and predictors for each patient, capturing individual baseline variations and unobserved heterogeneity among patients. Our choice of a random intercept was driven by the need to account for individual differences that are not captured by the fixed effects in the model. T>MIC was a statistically, but not clinically, significant predictor of the decline in daily vasopressors requirement, but not change in SOFA score. T>4×MIC was not a significant predictor in any of the analyses.
In a systematic review and meta-regression which included 87 randomized clinical trials, change in SOFA score was found reliably and consistently associated with mortality (slope = 0.70, p = 0.004) and explained 32% of the overall mortality. Although fixed-day SOFA was the most frequently reported outcome in the included trials, it was not significantly associated with mortality (slope = 0.35, p = 0.08) and explained 3% of the overall mortality (23). TARGET trial was a multicenter study randomizing septic patients receiving piperacillin/tazobactam continuous infusions to either a TDM-guided arm or a fixed-dose arm. The primary outcome was the mean daily total SOFA score up to day 10. Two hundred forty-nine patients were randomized; 62% had pneumonia. The piperacillin exposure was similar between the study arms; hence no difference in mean SOFA scores, 28-day mortality, and clinical and microbiologic cure was detected (24, 25). A few other studies looked at the impact of beta-lactam target attainment on the clinical outcomes based on the Acute Physiology and Chronic Health Evaluation (APACHE) score with mixed results regarding the impact of alternative beta-lactam dosing strategies on APACHE scores and potentially lower mortality in patients with higher APACHE scores that achieved appropriate PK/PD targets (26–28). In our study, we evaluated cefepime PK/PD target attainment and the change in the daily SOFA score, rather than comparing the baseline to end-of-therapy SOFA scores. However, our results showed no significant impact of achieving 100% T>MIC on the daily change in SOFA score.
Compared with SOFA score outcome, there were fewer reports published comparing the vasopressors requirement between patients receiving beta-lactam therapy. Richter et al reported a before (intermittent infusion, n = 114) and after (prolonged infusion, n = 290) retrospective study investigating the outcomes associated with beta-lactam infusion strategy. Although mortality was lower in the prolonged infusion arm, there was no difference between the groups in terms of vasopressor dependence at days 0, 2, 4, 7, and 14 (28). Another retrospective study reported the outcomes in patients receiving meropenem either as extended (n = 52) or intermittent (n = 96) infusion. The ICU mortality was lower, clinical response was higher, and median total vasopressor days were shorter in the extended infusion group (2 vs. 3 days, p = 0.032) (29). In our analysis, achieving 100% T>MIC was associated with a marginal decline in daily vasopressors requirement which may not be clinically relevant. Based on these results and previously published work, beta-lactam PK/PD target attainment might be more important for the final therapy outcomes compared with daily changes in vasopressors requirement and SOFA score.
Different cefepime PK/PD targets were reported in the literature. Early preclinical work suggested that bacterial stasis was associated with 30% to 40% T>MIC, whereas bactericidal activity was associated with 60% to 70% T>MIC (30–32). Similar targets, 68% and 74% T>MIC, were suggested for survival in 180 ICU patients using exposures generated using population PK models (33). Clinically, patients who achieved 100% T>MIC had higher chances of clinical cure and microbiologic eradication and had higher ventilator-free days (34, 35). In our study, we evaluated the daily outcomes associated with achieving 100% T>MIC and T>4×MIC. Only T>MIC was associated with lower daily vasopressors requirement; however, the difference was not clinically significant and unlikely to impact the overall patient outcomes.
Our study has some limitations. First, it was a single-center, retrospective study including mainly patients with Gram-negative bacterial pneumonia treated with cefepime only. Second, we had to use breakpoints for some of the patients to evaluate the PK/PD target attainment which might not have been reflective of the true MICs. Third, cefepime exposure was calculated using population PK which fixes the PK parameters per patient for the entire therapy duration while these parameters may change in an ICU setting. Finally, that PK model was used to generate cefepime exposure in patients receiving RRT although it was not built including such patients. These limitations may be addressed in future studies by robust daily plasma samples collection using prospective study design combined with advanced statistical methods.
CONCLUSIONS
In conclusion, achievement of 100% T>MIC was associated with negligible decline in daily vasopressor requirements in ICU patients receiving cefepime for the treatment of Gram-negative pneumonia or BSI. There was no impact on the daily SOFA score.
ACKNOWLEDGMENT
We acknowledge the University of Florida Integrated Data Repository and the UF Health Office of the Chief Data Officer for providing the analytic data set for this project.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
The research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards UL1 TR000064 and UL1TR001427.
REFERENCES
- 1.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program: Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA network open 2019; 2:e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization: 2020 Global report on the epidemiology and burden of sepsis: Current evidence, identifying gaps and future directions. https://www.who.int/publications/i/item/9789240010789. Accessed March 3, 2023.
- 3.Knaus WA, Sun X, Nystrom P-O, et al. : Evaluation of definitions for sepsis. Chest 1992; 101:1656–1662 [DOI] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team: Emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollef MH, Hamilton CW, Ernst FR: Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012; 33:250–256 [DOI] [PubMed] [Google Scholar]
- 6.Muscedere JG, Day A, Heyland DK: Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 2010; 51:S120–S125 [DOI] [PubMed] [Google Scholar]
- 7.Papazian L, Klompas M, Luyt CE: Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med 2020; 46:888–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388. [DOI] [PubMed] [Google Scholar]
- 9.Melsen WG, Rovers MM, Groenwold RH, et al. : Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from harmacome prevention studies. Lancet Infect Dis 2013; 13:665–671 [DOI] [PubMed] [Google Scholar]
- 10.Crandon JL, Bulik CC, Kuti JL, et al. : Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 2010; 54:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JA, Paul SK, Akova M, et al. ; DALI Study: DALI: Defining antibiotic levels in intensive care unit patients: Are current b-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58:1072–1083 [DOI] [PubMed] [Google Scholar]
- 12.Heffernan AJ, Sime FB, Lipman J, et al. : Individualising therapy to minimize bacterial multidrug resistance. Drugs 2018; 78:621–641 [DOI] [PubMed] [Google Scholar]
- 13.Vincent J-L, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 14.Einav S, Helviz Y, Ippolito M, et al. : Vasopressor and inotrope treatment for septic shock: An umbrella review of reviews. J Crit Care 2021; 65:65–71 [DOI] [PubMed] [Google Scholar]
- 15.Alshaer MH, Maranchick N, Bai C, et al. : Using machine learning to define the impact of beta-lactam early and cumulative target attainment on outcomes in intensive care unit patients with hospital-acquired and ventilator-associated pneumonia. Antimicrob Agents Chemother 2022; 66:e0056322–e0056322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alshaer MH, Maranchick N, Alexander KM, et al. : Beta-lactam target attainment and associated outcomes in patients with bloodstream infections. Int J Antimicrob Agents 2023; 61:106727. [DOI] [PubMed] [Google Scholar]
- 17.Goradia S, Sardaneh AA, Narayan SW, et al. : Vasopressor dose equivalence: A scoping review and suggested formula. J Crit Care 2021; 61:233–240 [DOI] [PubMed] [Google Scholar]
- 18.Al-Shaer MH, Alghamdi WA, Graham E, et al. : Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit 2020; 42:129–132 [DOI] [PubMed] [Google Scholar]
- 19.Neely M, van Guilder M, Yamada W, et al. : Accurate detection of outliers and subpopulations with Pmetrics, a non-parametric and parametric harmacometrics modeling and simulation package for R. Ther Drug Monit 2012; 34:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alshaer MH, Goutelle S, Santevecchi B, et al. : Cefepime precision dosing tool: From standard to precise dose using nonparametric population pharmacokinetics. Antimicrob Agents Chemother 2021; 66:AAC-02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates D, Mächler M, Bolker B, et al. : Fitting linear mixed-effects models Usinglme4. J Stat Soft 2015; 67:1–48 [Google Scholar]
- 22.Plate JDJ, van de Leur RR, Leenen LPH, et al. : Incorporating repeated measurements into prediction models in the critical care setting: A framework, systematic review and meta-analysis. BMC Med Res Methodol 2019; 19:1–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Grooth H-J, Geenen IL, Girbes AR, et al. : SOFA and mortality endpoints in randomized controlled trials: A systematic review and meta-regression analysis. Crit Care 2017; 21:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagel S, Bach F, Brenner T, et al. ; TARGET Trial Investigators: Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med 2022; 48:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshaer MH, Peloquin CA: Another trial for the TARGET trial. Intensive Care Med 2022; 48:774–775 [DOI] [PubMed] [Google Scholar]
- 26.Miglis C, Rhodes NJ, Kuti JL, et al. : Defining the impact of severity of illness on time above the MIC threshold for cefepime in Gram-negative bacteraemia: A “Goldilocks” window. Int J Antimicrob Agents 2017; 50:487–490 [DOI] [PubMed] [Google Scholar]
- 27.Lodise TP, Lomaestro B, Drusano GL: Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007; 44:357–363 [DOI] [PubMed] [Google Scholar]
- 28.Richter DC, Dietrich M, Lalev LD, et al. : Prolonged infusion of β-lactams decreases mortality in patients with septic shock: A retrospective before-and-after study. Antibiotics (Basel) 2021; 10:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed N, Jen SP, Altshuler D, et al. : Evaluation of meropenem extended versus intermittent infusion dosing protocol in critically ill patients. J Intensive Care Med 2020; 35:763–771 [DOI] [PubMed] [Google Scholar]
- 30.Craig WA: Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26:1–10; quiz 11 [DOI] [PubMed] [Google Scholar]
- 31.Craig WA: Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003; 17:479–501 [DOI] [PubMed] [Google Scholar]
- 32.Tam VH, Chang KT, Zhou J, et al. : Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J Antimicrob Chemother 2017; 72:1421–1428 [DOI] [PubMed] [Google Scholar]
- 33.Rhodes NJ, Kuti JL, Nicolau DP, et al. : Defining clinical exposures of cefepime for Gram-negative bloodstream infections that are associated with improved survival. Antimicrob Agents Chemother 2016; 60:1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. : Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016; 42:1535–1545 [DOI] [PubMed] [Google Scholar]
- 35.McKinnon PS, Paladino JA, Schentag JJ: Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T> MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008; 31:345–351 [DOI] [PubMed] [Google Scholar]