This review summarizes how early life stress not only increases risks for chronic pain but also influences emotional responses to burn pain in adults.
Keywords: Expectancies, Early life, Stress, Symptom management, Coping
Abstract
Adverse childhood experiences (ACEs) affect over half of the adults in the United States and are known to contribute to the development of a wide variety of negative health and behavioral outcomes. The consequences of ACE exposure have been studied in patient populations that include individuals with gynecologic, orthopedic, metabolic, autoimmune, cardiovascular, and gastrointestinal conditions among others. Findings indicate that ACEs not only increase risks for chronic pain but also influence emotional responses to pain in many of these individuals. A growing body of research suggests that these effects may be the result of long-lasting changes induced by ACEs in neurobiological systems during early development. However, one area that is still largely unexplored concerns the effects of ACEs on burn patients, who account for almost 450,000 hospitalizations in the United States annually. Patients with severe burns frequently suffer from persistent pain that affects their well-being long after the acute injury, but considerable variability has been observed in the experience of pain across individuals. A literature search was conducted in CINAHL and PubMed to evaluate the possibility that previously documented ACE-induced changes in biological, psychological, and social processes might contribute to these differences. Findings suggest that better understanding of the role that ACEs play in burn outcomes could lead to improved treatment strategies, but further empirical research is needed to identify the predictors and mechanisms that dictate individual differences in pain outcomes in patients with ACE exposure and to clarify the role that ACE-related alterations play in early healing and recovery from burn injuries.
1. Introduction
Adverse childhood experiences (ACEs) affect over half of the adults in the United States and are known to contribute to the development of a wide variety of negative health and behavioral outcomes.43 Originally defined by Felitti et al.,23 these experiences include traumatic events such as physical, mental, and sexual abuse as well as neglect, divorce or separation, family incarceration, and violence, mental illness, or substance abuse in home. There is emerging evidence that one of the most intractable negative health outcomes associated with ACEs is chronic pain. Adverse childhood experiences have been shown to contribute to risks for chronic pain in both children and adults72,87 and are associated with increased severity of pain in medical conditions such as arthritis, back pain, and headaches.90 In recent years, there has been growing interest in studying the biological mechanisms that may underlie these associations. Findings of several lines of research have provided evidence that individual differences in pain responses and risks for chronic pain may be the result of ACE-induced alterations in the developing brain and in key neurobiological substates, such as the hypothalamic–pituitary–adrenal (HPA) axis, and autonomic and immune systems.7,31,86,110
Burn patients account for almost 450,000 hospitalizations in the United States annually.2 Anecdotal evidence suggests that up to 40% of the adult burn population has had ACE exposure.22 Yet, despite evidence that ACE exposure worsens pain outcomes in patients with other kinds of pain, only one published study has specifically explored the relationship between ACEs and burn injuries.22 Findings showed that a history of ACEs predicted more depressive symptoms, less resilience, greater probability of posttraumatic stress disorder (PTSD), and less social participation 3 months after burn injury. These findings provided preliminary evidence that ACE exposure may affect healing and coping after burn injuries. Although minimal differences in pain levels were observed between patients who reported >4 ACE events as compared with those who reported <4 events, several limitations of the study, such as the small sample size (N = 34) and lack of power analysis, suggest that further research is needed to definitively establish the impact of ACE exposure on burn pain, healing, and long-term morbidity and quality of life in burn patients.
Although the pathophysiology of acute burn pain is not yet fully understood, available data indicate that the mechanisms involve both peripheral and central processes and activation of nociceptive, neuropathic, and inflammatory pain pathways.34,50,51,69,81 In serious burn injuries, multiple surgical excisions occur during initial hospitalizations, often with only days between procedures. Acute pain is, therefore, repeatedly experienced by the patient. Repeated wound care and dressing change procedures during periods of nonsurgical intervention produce procedural pain, compounding the inflammatory process pain experienced during the initial cytokine storm.51 In burn patients with large total body surface area injuries, surgical pain is complicated by additional procedural insults, causing prolonged cascading effects. Psychogenic pain has also been shown in burn patients, particularly among patients with traumatic burn injuries from self-immolation, assault, or accident.84
Advances in the treatment of patients in the acute phase of their burn injuries has led to marked improvements in survival rates of burn patients over the past few decades. However, research on the tremendous impact that prolonged pain and disfigurement have on long-term quality of life and functional status of these patients is only beginning to emerge.17 Although mechanisms are poorly understood, it is estimated that approximately 60% of burn survivors develop chronic pain following the acute phase of the injury.64 This complication is most often believed to be neuropathic in origin, involving damage or dysfunction in nerve fibers,68 which may affect the entire nervous system.17 However, although it is generally assumed that the degree of pain that an individual experiences after a burn injury is determined by the severity and other characteristics of the burn itself, growing evidence suggests that considerable variability in both acute pain responses and long-term morbidity may be due to individual factors that are unrelated to these factors.20,51,93,103 In fact, findings of one study showed that a more negative experience of pain, operationalized as pain intensity and pain interference, was associated with decreased physical functioning 6 months after burn injury in pediatric burn patients irrespective of specific injury characteristics such as the burn or graft surface area.73 Pain interference at 6 months, which refers to an individual's perception of how pain is affecting their life, predicted both physical outcomes and mood or peer relationship impairment at 12 months above and beyond burn characteristics.73 Better understanding of factors that contribute to individual differences in cognitive appraisal or other aspects of the pain experience could lead to improved trauma-related care and long-term physical and psychological outcomes of burn patients.
For the purposes of this narrative review, a conceptual model was developed to highlight selected biopsychosocial mechanisms that could hypothetically influence responses to burn pain in persons with a history of ACEs. Although other mechanisms are possible, we limited our discussion to several key neurobiological processes (ie, brain, HPA axis, and immune function and epigenetic changes in gene expression) and psychosocial variables (ie, pain expectations, pain catastrophizing [PC], and peer groups) that have been previously shown to be significantly altered by ACE exposure or play an important role in pain outcomes. A literature search was conducted in CINAHL and PubMed for articles published between 2000 and 2021 using the biopsychosocial model to limit the search terms to Ace and “burn,” “burn pain,” “pain,” “peer group,” “pain expectations,” “pain and catastrophizing,” “central sensitization,” “epigenetics,” “neurobiology,” “hypothalamic-pituitary-adrenal axis,” and “childhood adversity and HPA axis.”
1.1. Potential biological mechanisms
1.1.1. Central sensitization
Central sensitization is characterized by a heightened responsiveness of the central nervous system which leads to hypersensitivity to both painful and innocuous stimuli.91 This condition is observed in many chronic pain disorders and has been used to explain both increased intensity of pain in patients with a history of ACE exposure and chronic pain62 and heightened affective responses to pain.42,57 Importantly, functions of brain regions, such as the prefrontal cortex, amygdala, and nucleus accumbens, which are involved in both central sensitization and affective components of pain, are significantly altered by ACEs.39 Amygdala–prefrontal circuits have been shown to be particularly sensitive to the effects of environmental influences early in life. Changes in these brain networks, which are involved in stress responses, cognition, and emotion, may help to shape the development of neural and behavioral phenotypes that persist well into adulthood.28,101 Although very few studies have directly examined the involvement and consequences of such changes about pain, there is evidence that early life adversity leads to altered functional activation of nodes within central pain pathways in rats, which may be related to central amplification of sensory input.40 Changes induced by early life adversity in these regions (eg, thalamo–cortico–amygdala pathway) have also been shown to lead to exacerbated pain sensitivity in animal models.86 In patients with chronic abdominal pain, ACE-related alterations in intrinsic connectivity have been observed within the salience or executive control network, which is implicated in the pathophysiology of central pain amplification.31 A dose–risk relationship has also been observed between ACE history and central sensitivity in several chronic pain syndromes.44
It is notable that in burn patients, there is evidence that central sensitization within the spinal cord, which seems to be part of the long-lasting changes in neuronal and nonneuronal tissue damage from burns, contributes to excessive pain after burn injuries.76 Although links with burn pain are speculative, Chung et al.10 showed that maternal separation upregulates the activity of ascending pathways at the spinal level as well as the thalamo–cortico–amygdala pathway in rats, which contributed to sensitization. Thus, it is possible that the effects of burn injuries could be intensified in pathways that have already been sensitized by ACEs. Postburn inflammation in combination with increased ACE-related neuroinflammation85 could also potentially contribute to central sensitization and its accompanying hyperalgesia through upregulation of IL-8 and centrally mediated hyperalgesia.69
1.1.2. Hypothalamic–pituitary–adrenal axis function
The HPA axis is a complex system that mediates the body's endocrine response to stress through a series of hormonal responses that ultimately lead to the release of glucocorticoids from the adrenal cortex. Animal models simulating psychological ACEs through maternal deprivation or maternal separation have demonstrated that early life trauma is associated with profound and enduring changes in HPA axis function.58 Both hypercortisolemia and hypocortisolemia have been reported in humans with a history of ACEs, which have been shown to contribute to increased stress reactivity and vulnerability for a host of psychopathological, metabolic, and chronic inflammatory conditions.5,80,107
Importantly, one consequence of such HPA axis dysregulation is increased vulnerability for the development of chronic pain.99 It has been suggested that enhanced salivary cortisol secretion is associated with greater pain intensity in response to acute pain; however, hypocortisolemia may be a marker for pain chronicization.71 Increased demand on the HPA axis during the acute recovery from a burn injury alters the stress response.75 In fact, Akita and colleagues showed that HPA axis activity is a good marker of disease severity and prognosis in patients with extensive burns.1 Although little is known about the influence of HPA axis function on burn pain, there is evidence that 8 weeks of cortisol administration by drinking water is coupled with thermal hyperalgesia in rodents.33 Taken together, these findings suggest that preexisting alterations in HPA axis function resulting from ACE exposure could be mechanistically linked to disadvantageous outcomes after a burn injury in adults with this history. This would be consistent with notions that prolonged activation of stress systems contributes to central sensitization and exacerbated responses to pain and noxae.
1.1.3. Immune system function
Findings of a growing body of research have shown that ACEs are associated with chronic activation of the immune system. Although in the short term, a stronger immune response could be expected to lead to more effective healing after a burn injury, chronic inflammation, which has been implicated in a range of conditions from type 2 diabetes to cancer and is a key biological mechanism linking ACEs to psychopathology and cardiometabolic disease,15,52 is associated with increased susceptibility to inflammatory disease and exaggerated responses to injury in later life.7 Ganguly and Brenhouse25 suggested that early life adversity precipitates deleterious suppression of inflammatory markers during early development but causes a shift toward a proinflammatory state later in life. There is evidence of alterations in peripheral markers, such as IL-6, TNFα, C-reactive protein, and leukocytes, in ACE-exposed individuals.6 Alterations in neuroinflammatory gene expression in brain regions associated with pain and mood have also been observed.7
Zouikr and Karshikoff110 suggested that the immune system has a profound effect on pain systems throughout life by neuroimmune and neuroendocrine interactions. Findings from several lines of preclinical studies indicate that dysregulation of immune function early in life may lead to long-lasting hyperalgesia, enhanced nociceptive behavior to inflammatory stimuli, and altered nociceptive processing, which together suggest sensitization of nociceptive circuits.7 Findings from both preclinical and clinical studies have shown that burns cause an acute inflammatory response that can be either restricted to the site of injury or involve systemic inflammatory processes.69 The cytokines and inflammatory molecules that are released mediate wound healing contribute to burn-related allodynia and hyperalgesia. Thus, although the research has yet to be conducted, it is reasonable to speculate that an immune system that has been dysregulated by ACE exposure could increase risks for significant morbidity and pain after a burn injury.
1.1.4. Epigenetic changes
Broadly defined, epigenetic mechanisms are processes, such as DNA methylation, chromatin remodeling, or histone posttranslational modification, that regulate gene expression without changing the genetic code.56 Accumulating evidence suggests that epigenetic mechanisms mediate the long-term effects of ACEs on the function of the HPA axis and stress-sensitive brain regions,5,65,70,98 which may play a pivotal role in the etiology of several neuropsychiatric, neurodegenerative, and autoimmune illnesses.18
Interestingly, epigenetic mechanisms involving stress-related genes have also been shown to be involved in the development and maintenance of persistent pain states.30 One gene whose function has been shown to be altered by early trauma is the stress regulator FDBP prolyl isomerase 5 (FKBP5). FKBP5 is an important modulator of the stress response, helping to regulate not only the glucocorticoid receptor activity but also a multitude of other cellular process in the brain and periphery. Interactions between FKBP5 and environmental stressors have been shown to contribute to a number of aberrant phenotypes in both rodents and humans.108 Importantly, this gene also seems to play an important role in nociceptive processing, regulating chronic pain states through modulation of glucocorticoid signaling.61 Collectively, these findings suggest that the epigenetic changes induced by ACEs could underlie the impact of early adversity on both the emotional responses to pain and susceptibility to developing chronic pain later in life.7 Although little is known about these relationships in burn patients, one could postulate that the dysregulation of metabolic processes and cortisol regulation that occurs after severe burns might interact with alterations induced in the FKBP5 gene to increase susceptibility for chronic pain.
1.2. Potential psychological mechanisms
1.2.1. Expectancies or expectations
Expectancies are psychophysical predictions of future events that can occur without full awareness (ie, implicit expectancies). On the contrary, expectations refer to conscious cognitive dynamic constructs referring to predictions of future events and outcome anticipations that can be measured through validated scales and verbal self-reports.11,74 Expectations as predictors of future outcomes can be positive and negative and can affect neural pain processes.11–13 In orthopedic patients, for example, particularly knee and hip replacement patients, belief in treatment efficacy (positive expectations) has been associated with lower pain scores and better patient outcomes.49 Positive and negative expectancies influence outcomes by driving behavioral and neurobiological pain systems and processes.13 Expectations of positive or negative outcomes are often not met in daily clinical practice. We demonstrated that expectation violation expressed as a misalignment between what it is expected and what is actually experienced alerts responses to pain. Such discrepancies resulted in the activation of the left inferior parietal cortex that redirects attention load in the presence of misalignment between sensorial stimulations and cognitive events in healthy participants.13
Expectations are dynamic in nature and, therefore, can be shaped to optimize outcomes. Rief et al.83 conducted a trial preoperatively to optimize patient expectations to improve long-term outcome in heart surgery patients. The study was designed as a 3-arm randomized clinical trial including a follow-up of 6 months in 124 patients undergoing coronary artery bypass graft surgery. The 3 arms included a short-lasting psychological presurgery session with the scope to optimize outcome expectations (EXPECT), a psychological control session which focused on emotional support and general advice alone (SUPPORT), and a standard medical care session. Both presurgery sessions were comparable in duration. The authors demonstrated that a brief session tailored explicitly to optimize expectations improved disability up to 6 months after surgery. This study along with many others from this team pave the way to psychological approaches that align expectations to optimize long-term outcomes even in the presence of invasive surgical interventions and other procedures.26,37,53
Although the role of changing expectations has not yet been validated in patients experiencing burn injuries, the idea of aligning expectations with healing prognosis is important in injuries that require prolonged or repeated hospitalizations. Blalock et al.3 found that patients who had low expectations about rehabilitative outcomes but who attached high importance to the need for improvement exhibited the most psychological distress after major or moderate thermal burn injuries. Positive and negative expectations have also been shown to contribute to conditioned pain modulation24 and to engage descending pain modulatory circuits during exposure to thermal pain,14 suggesting that the development of strategies to optimize expectations may be useful for the management of both acute and chronic burn pain.
In one of the few studies that have examined the impact of ACE history on pain expectations, Walton et al.104 found that high ACE scores together with threat appraisal were associated with increased expectations that events would be painful or traumatic in patients with acute, musculoskeletal trauma. Although causation could not be inferred from the cross-sectional data, the findings were consistent with those of animal studies showing effects of early life trauma on threat processing. Exposure to maltreatment is thought to bias attentional and emotional processes towards greater threat generalization and reduced flexibility in appraising challenges, potentially leading to higher risks for psychopathology later in life.46,54,77 In one human laboratory study with healthy adults, individuals with attachment insecurity, which could be a consequence of ACE exposure,19,55,60 had significantly lower expectations of perceived control over experimentally induced pain and reported less ability to decrease pain than secure individuals.67 Taken together, these findings extend notions that subjective expectations related to pain can be shaped by trauma, highlighting the role that preexisting vulnerabilities or resilience may play in the process. Further research into the role expectations play in the experience of acute burn pain in adult survivors of ACEs could potentially help to substantiate the proposed pathways.
1.2.2. Pain catastrophizing
Pain catastrophizing has been defined as a maladaptive orientation toward actual or anticipated pain27 that has 3 distinct characteristics: rumination (inability to inhibit pain-related thoughts), magnification (exaggerated threat value of the pain stimulus), and helplessness.96 Catastrophizing is a strong predictor of disability in patients with chronic pain95,102 and has been associated with several dimensions of pain experience, including heightened affective responses,102 increased severity,45,47 and longer duration.4 Not surprisingly, given its links with affective responses to pain, PC is also associated with depression and anxiety 16,94 and, in fact, may mediate relationships between psychological distress and pain experiences.9,79 Findings of one recent brain imaging study showed that several brain regions involved in PC are also involved in mood regulation and pain processing,41,89 which, at least in theory, could help to explain these relationships.
Strong associations have also been observed between child maltreatment and PC. In one study, the relationship was shown to be independent of other risk factors, including anxiety and depression.59 Reported relationships between ACEs and PC include all 3 dimensions of PC and several different types of ACEs, although emotional abuse seems to show the strongest association.78,88,109 Although there has been only limited study of PC in burn patients, evidence of a relationship with pain sensitization and duration has been reported.35 Van Loey et al.100 further found that PC 6 months after a burn injury predicted both chronic pain and symptoms of PTSD at 12 months postburn. This suggests that screening for a history of child maltreatment and tendency to catastrophize could lead to early identification of individuals who are at greater risk of developing PTSD and chronic pain after a burn injury. However, to date, PC effects on acute burn pain have not been explored in burn patients with ACEs.
1.3. Potential influences of social learning
1.3.1. Peer group
Peer groups are often discussed in ACE literature as both moderating and mediating the impact of ACE exposure, particularly on normal development in adolescence.106 Explorations of the relationship between ACEs and deviant peer groups have demonstrated that exposure to ACEs predisposes adolescents to seek out and develop such peer groups.97
Involvement in deviant peer groups has been shown to lead to further trauma and has been linked to poor health outcomes. The converse, that supportive peer groups for survivors of ACEs are associated with better outcomes, has also been demonstrated.48 Supportive peer groups need not always take the form of same-age peers but can be fulfilled with supportive older adults or communities.105 In adult burn patients, who may have significant barriers to inclusion in adult peer groups due to scarring from catastrophic injuries, the consequences of exclusions from adolescent peer groups or inclusion in deviant peer groups may be compounded. Further research into how deviant or nonexistent peer groups for adult survivors of ACEs affects their affiliation with adult peer groups and the experience of pain is warranted.
1.3.2. Conceptual framework
The biopsychosocial model of pain, which guided us in writing this review, hypothesizes that pain is dependent on interconnected biological, psychological, and social variables specific to the individual experiencing pain.35,66 As such, the model allows for exploration of the complex mechanisms by which ACE exposure colors the experience of acute and chronic burn pain in survivors of childhood adversity (Fig. 1). In the biological domain, these mechanisms may include central sensitization, alterations in HPA axis and immune functions, and changes in epigenetic processes. Although examination of the effects of ACEs on the development of central sensitization and epigenetic processes are relatively new areas of inquiry, there is a growing body of literature supporting the important role that both play in the experience of acute pain and the development of chronic pain. In the psychological domain, a small body of evidence has demonstrated that ACE exposure may influence expectations related to painful experiences, as well as tendencies for PC, which may in turn heighten risks for adverse outcomes related to pain. Finally, although not yet studied about pain, in the social domain, there is evidence that ACE exposure may be associated with greater tendencies to seek out deviant peer groups, which may also be linked to poor health outcomes.
Figure 1.
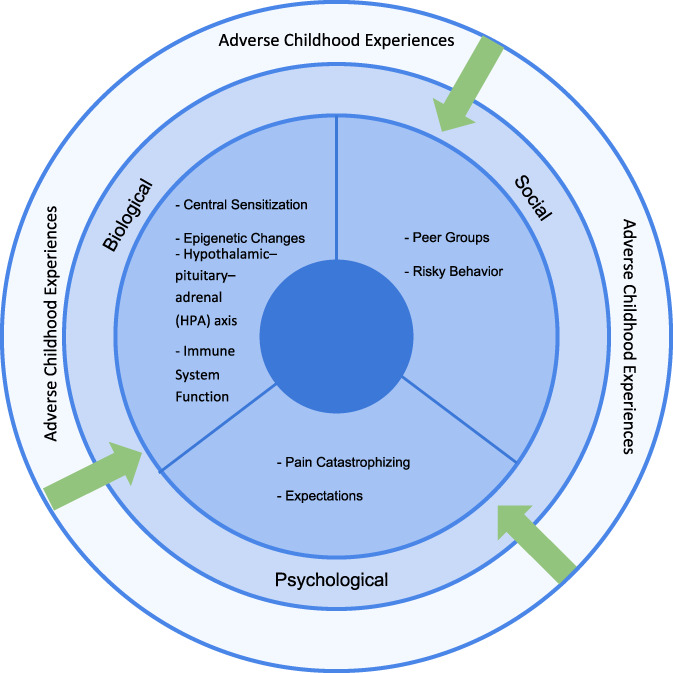
Biopsychosocial model allowing an exploration of the complex mechanisms by which adverse childhood experience exposure colors the experience of acute and chronic burn pain in survivors of childhood adversity.
2. Discussion
Taken together, the findings of this review highlight several etiological pathways that may help to explain relationships between a history of ACEs and adverse pain-related outcomes in adulthood. What is remarkable, however, is the paucity of research that has explored these relationships in patients with burn injuries. Not only has there been minimal research exploring the consequences of ACE exposure in burn patients, but also there has been little investigation of the biological, psychological, or social mechanisms that may underlie individual differences in pain experiences and response to treatment in these individuals, regardless of cause. The evidence from human laboratory and clinical studies showing that ACEs alter perceptual and affective responses to other kinds of pain support theories that the pathways and assumptions described in this article may hold true for burn patients. Moreover, what we do know about the unique physiology and psychological ramifications of burns suggests that the consequences of ACE exposure may be compounded in these individuals. Postburn inflammation and the high demand on HPA axis and brain stress systems during acute recovery from burns, for example, may interact with preexisting deficits inflicted in these systems by ACE exposure to significantly affect recovery. Given that ACE exposure has also been associated with greater catastrophizing and more negative expectations related to pain, it might also be expected that ACE exposure would be associated with greater psychological morbidities in burn patients. Burn injuries are among the most painful experiences that individuals can encounter, treatments often contribute to ongoing occurrences of acute pain, the duration of pain and disability from these injuries can last from months to years, and the pain is often accompanied by psychological trauma and alienation from significant others.
Although the data presented in this review provide convincing evidence of the plausibility of mechanistic relationships between ACE history and burn outcomes, it should be noted that most of the proposed relationships are speculative at this point. In addition, most of the studies that have examined the effects of ACEs on health outcomes are correlational, which precludes causal conclusions, and they rely on retrospective self-reports of ACEs, which could be biased by memory distortions or misreporting. Nevertheless, the evidence of correspondence between the animal and human literature in many of the areas studied7,32,36,63 supports the validity of the findings. Moderate agreement has also been demonstrated between retrospective and prospective measures of adversity, especially when outcomes were objectively assessed.82
Finally, although the preponderance of human and animal studies conducted to date have linked trauma-induced alterations in HPA axis, brain, and immune system function to unfavorable mental and physical health outcomes, there is some evidence that ACE exposure may lead to accelerated maturation of neural circuits and greater capacity for stress and emotion regulation.28 For example, findings of a few studies have shown that children29 and youths38 with ACE exposure had a more mature pattern of medial PFC–amygdala connectivity, which was associated with lower separation anxiety and lower internalizing symptoms in the respective groups. These findings are consistent with notions that, rather than impairing development, exposure to stress biases development towards strategies that are adaptive under stressful conditions.8,21 From this perspective, growing up in a traumatic environment might foster the development of skills and abilities that enhance adaptation within stressful contexts, making an individual more equipped to cope with challenging situations that occur later in life, such as burn injuries.
3. Conclusion
Pain may remain an ongoing source of disability long after the acute recovery period is over in burn patients. Further research is needed to provide critical insights into the mechanisms, conditions, and specific elements of ACE exposure that help to determine individual differences in phenotypic outcomes and to identify strategies for leveraging this knowledge to target areas of both vulnerability and strength in the care of individual burn patients. It is our hope that better understanding of the relationship between ACEs and burn pain will help to improve the prognostic, diagnostic, and therapeutic approaches to burn patients. New knowledge will support the development of novel approaches (eg, nonpharmacological virtual reality interventions among others92) to target the as yet unspecified, underlying pathways in ACE-exposed individuals who experience burn injuries.
Funding
This research was supported by the MPower the State Grant (PI: Colloca) and National Center for Complementary and Integrative Health (NCCIH, R01 AT011347–01A1; PI: Colloca). The views expressed here are the authors own and do not reflect the position or policy of the National Institutes of Health or any other part of the federal government.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Emily H. Werthman, Email: ewerthman@umaryland.edu.
Lynn M. Oswald, Email: oswald@umaryland.edu.
References
- [1].Akita S, Akino K, Imaizumi T, Tanaka K, Anraku K, Yano H, Hirano A. The quality of pediatric burn scars is improved by early administration of basic fibroblast growth factor. J Burn Care Res 2006;27:333–8. [DOI] [PubMed] [Google Scholar]
- [2].American Burn Association. Vol. 2021, 2021. Available at: https://ameriburn.org/. Accessed May 6, 2022. [Google Scholar]
- [3].Blalock SJ, Bunker BJ, DeVellis RF. Psychological distress among survivors of burn injury: the role of outcome expectations and perceptions of importance. J Burn Care Rehabil 1994;15:421–7. [DOI] [PubMed] [Google Scholar]
- [4].Brecht DM, Gatchel RJ. An overview of a biopsychosocial model of epigenetics and pain catastrophizing. J Appl Biobehav Res 2019;24:e12171. [Google Scholar]
- [5].Brydges CR, Fox AM, Reid CL, Anderson M. The differentiation of executive functions in middle and late childhood: a longitudinal latent-variable analysis. Intelligence 2014;47:34–43. [Google Scholar]
- [6].Brydges NM, Reddaway J. Neuroimmunological effects of early life experiences. Brain Neurosci Adv 2020;4:2398212820953706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res 2017;95:1257–70. [DOI] [PubMed] [Google Scholar]
- [8].Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci 2016;7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cassar GE, Knowles S, Youssef GJ, Moulding R, Uiterwijk D, Waters L, Austin DW. Examining the mediational role of psychological flexibility, pain catastrophizing, and visceral sensitivity in the relationship between psychological distress, irritable bowel symptom frequency, and quality of life. Psychol Health Med 2018;23:1168–81. [DOI] [PubMed] [Google Scholar]
- [10].Chung EK, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience 2007;149:685–95. [DOI] [PubMed] [Google Scholar]
- [11].Colloca L, Akintola T, Haycock NR, Blasini M, Thomas S, Phillips J, Corsi N, Schenk LA, Wang Y. Prior therapeutic experiences, not expectation ratings, predict placebo effects: an experimental study in chronic pain and healthy participants. Psychother Psychosom 2020;89:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Colloca L, Miller FG. Role of expectations in health. Curr Opin Psychiatry 2011;24:149–55. [DOI] [PubMed] [Google Scholar]
- [13].Colloca L, Schenk LA, Nathan DE, Robinson OJ, Grillon C. When expectancies are violated: a functional magnetic resonance imaging study. Clin Pharmacol Ther 2019;106:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crawford LS, Mills EP, Hanson T, Macey PM, Glarin R, Macefield VG, Keay KA, Henderson LA. Brainstem mechanisms of pain modulation: a within-subjects 7T fMRI study of placebo analgesic and nocebo hyperalgesic responses. J Neurosci 2021;41:9794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Danese A, Baldwin JR. Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol 2017;68:517–44. [DOI] [PubMed] [Google Scholar]
- [16].Darnall BD, Colloca L. Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: a review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol 2018;139:129–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dauber A, Osgood PF, Breslau AJ, Vernon HL, Carr DB. Chronic persistent pain after severe burns: a survey of 358 burn survivors. Pain Med 2002;3:6–17. [DOI] [PubMed] [Google Scholar]
- [18].Deyo RA, Katrina R, Buckley DI, Michaels L, Kobus A, Eckstrom E, Forro V, Morris C. Performance of a Patient Reported Outcomes Measurement Information System (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med 2016;17:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Donadio M, Valera P, Sinangil N. Understanding attachment styles, adverse childhood events, alcohol use, and trauma in Black and Latino Men with criminal justice histories. J Community Psychol 2021:1–13. [DOI] [PubMed] [Google Scholar]
- [20].Edwards RR, Magyar-Russell G, Thombs B, Smith MT, Holavanahalli RK, Patterson DR, Blakeney P, Lezotte DC, Haythornthwaite JA, Fauerbach JA. Acute pain at discharge from hospitalization is a prospective predictor of long-term suicidal ideation after burn injury. Arch Phys Med Rehabil 2007;88:S36–42. [DOI] [PubMed] [Google Scholar]
- [21].Ellis BJ, Bianchi J, Griskevicius V, Frankenhuis WE. Beyond risk and protective factors: an adaptation-based approach to resilience. Perspect Psychol Sci 2017;12:561–87. [DOI] [PubMed] [Google Scholar]
- [22].Fassel M, Grieve B, Hosseini S, Oral R, Galet C, Ryan C, Kazis L, Pengsheng N, Wibbenmeyer LA. The impact of adverse childhood experiences on burn outcomes in adult burn patients. J Burn Care Res 2019;40:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- [24].France CR, Burns JW, Gupta RK, Buvanendran A, Chont M, Schuster E, Orlowska D, Bruehl S. Expectancy effects on conditioned pain modulation are not influenced by naloxone or morphine. Ann Behav Med 2016;50:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ganguly P, Brenhouse HC. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cogn Neurosci 2015;11:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gasteiger C, Sherriff R, Fraser A, Shedden-Mora MC, Petrie KJ, Serlachius AS. Predicting patient reassurance after colonoscopy: the role of illness beliefs. J Psychosom Res 2018;114:58–61. [DOI] [PubMed] [Google Scholar]
- [27].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- [28].Gee DG. Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am J Psychiatry 2021;178:998–1013. [DOI] [PubMed] [Google Scholar]
- [29].Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 2013;110:15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geranton SM. Does epigenetic 'memory' of early-life stress predispose to chronic pain in later life? A potential role for the stress regulator FKBP5. Philos Trans R Soc Lond B Biol Sci 2019;374:20190283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gupta A, Puri S, Puri V. Bioinformatics unmasks the maneuverers of pain pathways in acute kidney injury. Sci Rep 2019;9:11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 2003;79:471–8. [DOI] [PubMed] [Google Scholar]
- [33].Hache G, Guiard BP, Le Dantec Y, Orvoen S, David DJ, Gardier AM, Coudore F. Antinociceptive effects of fluoxetine in a mouse model of anxiety/depression. Neuroreport 2012;23:525–9. [DOI] [PubMed] [Google Scholar]
- [34].Haddock NT, Weichman KE, Reformat DD, Kligman BE, Levine JP, Saadeh PB. Lower extremity arterial injury patterns and reconstructive outcomes in patients with severe lower extremity trauma: a 26-year review. J Am Coll Surg 2010;210:66–72. [DOI] [PubMed] [Google Scholar]
- [35].Haythronthwaite JA, Lawrence JW, Fauerbach JA. Brief cognitive interventions for burn pain. Ann Behav Med 2001;23:42–9. [DOI] [PubMed] [Google Scholar]
- [36].Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001;49:1023–39. [DOI] [PubMed] [Google Scholar]
- [37].Heisig SR, Shedden-Mora MC, Hidalgo P, Nestoriuc Y. Framing and personalizing informed consent to prevent negative expectations: an experimental pilot study. Health Psychol 2015;34:1033–7. [DOI] [PubMed] [Google Scholar]
- [38].Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging 2016;1:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Herzberg MP, Gunnar MR. Early life stress and brain function: activity and connectivity associated with processing emotion and reward. Neuroimage 2020;209:116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holschneider DP, Guo Y, Mayer EA, Wang Z. Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiol Stress 2016;3:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014;1:e20 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Johnson BN, Lumley MA, Cheavens JS, McKernan LC. Exploring the links among borderline personality disorder symptoms, trauma, and pain in patients with chronic pain disorders. J Psychosom Res 2020;135:110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jones CM, Merrick MT, Houry DE. Identifying and preventing adverse childhood experiences: implications for clinical practice. JAMA 2020;323:25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jones GT. Psychosocial vulnerability and early life adversity as risk factors for central sensitivity syndromes. Curr Rheumatol Rev 2016;12:140–53. [DOI] [PubMed] [Google Scholar]
- [45].Kadimpati S, Zale EL, Hooten WM, Ditre JW, Warner DO. Correction: associations between neuroticism and depression in relation to catastrophizing and pain-related anxiety in chronic pain patients. PLoS One 2015;10:e0129871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kalia V, Knauft K, Hayatbini N. Cognitive flexibility and perceived threat from COVID-19 mediate the relationship between childhood maltreatment and state anxiety. PLoS One 2020;15:e0243881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kanzawa-Lee GA, Harte SE, Bridges CM, Brummett C, Clauw DJ, Williams DA, Knoerl R, Lavoie Smith EM. Pressure pain phenotypes in women before breast cancer treatment. Oncol Nurs Forum 2018;45:483–95. [DOI] [PubMed] [Google Scholar]
- [48].Karatekin C, Ahluwalia R. Effects of adverse childhood experiences, stress, and social support on the health of college students. J Interpers Violence 2020;35:150–72. [DOI] [PubMed] [Google Scholar]
- [49].Kastner A, Ng Kuet Leong VSC, Petzke F, Budde S, Przemeck M, Muller M, Erlenwein J. The virtue of optimistic realism—expectation fulfillment predicts patient-rated global effectiveness of total hip arthroplasty. BMC Musculoskelet Disord 2021;22:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim DE, Pruskowski KA, Ainsworth CR, Linsenbardt HR, Rizzo JA, Cancio LC. A review of adjunctive therapies for burn injury pain during the opioid crisis. J Burn Care Res 2019;40:983–95. [DOI] [PubMed] [Google Scholar]
- [51].Klifto KM, Dellon AL, Hultman CS. Prevalence and associated predictors for patients developing chronic neuropathic pain following burns. Burns Trauma 2020;8:tkaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lacey RE, Pinto Pereira SM, Li L, Danese A. Adverse childhood experiences and adult inflammation: single adversity, cumulative risk and latent class approaches. Brain Behav Immun 2020;87:820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Laferton JA, Kube T, Salzmann S, Auer CJ, Shedden-Mora MC. Patients' expectations regarding medical treatment: a critical review of concepts and their assessment. Front Psychol 2017;8:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lange I, Goossens L, Bakker J, Michielse S, van Winkel R, Lissek S, Leibold N, Marcelis M, Wichers M, van Os J, van Amelsvoort T, Schruers K. Neurobehavioural mechanisms of threat generalization moderate the link between childhood maltreatment and psychopathology in emerging adulthood. J Psychiatry Neurosci 2019;44:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Le TL, Geist R, Bearss E, Maunder RG. Childhood adversity and attachment anxiety predict adult symptom severity and health anxiety. Child Abuse Negl 2021;120:105216. [DOI] [PubMed] [Google Scholar]
- [56].Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 2005;6:108–18. [DOI] [PubMed] [Google Scholar]
- [57].Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67:942–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol 2014;26:707–23. [DOI] [PubMed] [Google Scholar]
- [59].MacDonald TM, Fisk JD, Bernstein CN, El-Gabalawy R, Hitchon CA, Kornelsen J, Patten SB, Tisseverasinghe A, Marrie RA. The association between childhood maltreatment and pain catastrophizing in individuals with immune-mediated inflammatory diseases. J Psychosom Res 2021;145:110479. [DOI] [PubMed] [Google Scholar]
- [60].Madsen EB, Smith-Nielsen J, Egmose I, Lange T, Vaever MS. The impact of childhood adversity on parenting stress is mediated by adult attachment and depressive symptoms. Scand J Psychol 2022;63:47–54. [DOI] [PubMed] [Google Scholar]
- [61].Maiaru M, Tochiki KK, Cox MB, Annan LV, Bell CG, Feng X, Hausch F, Geranton SM. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci Transl Med 2016;8:325ra319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mansiz-Kaplan B, Ayhan FF, Cagli M, Atik F, Ece I. A preliminary study of the child abuse and central sensitization in adolescent patients with chronic non-organic chest pain and an overlooked condition: juvenile fibromyalgia syndrome. Pediatr Rheumatol Online J 2020;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matosin N, Cruceanu C, Binder EB. Preclinical and clinical evidence of DNA methylation changes in response to trauma and chronic stress. Chronic Stress (Thousand Oaks) 2017;1:2470547017710764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mauck MC, Barton CE, Tungate A, Shupp JW, Karlnoski R, Smith DJ, Williams FN, Jones SW, McGrath KV, Cairns BA, McLean SA. Peritraumatic vitamin D levels predict chronic pain severity and contribute to racial differences in pain outcomes following major thermal burn injury. J Burn Care Res 2021;42:1186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009;12:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 2018;87:168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Meredith PJ, Strong J, Feeney JA. The relationship of adult attachment to emotion, catastrophizing, control, threshold and tolerance, in experimentally-induced pain. PAIN 2006;120:44–52. [DOI] [PubMed] [Google Scholar]
- [68].Mewa Kinoo S, Singh B. Complex regional pain syndrome in burn pathological scarring: a case report and review of the literature. Burns 2017;43:e47–52. [DOI] [PubMed] [Google Scholar]
- [69].Morgan M, Deuis JR, Frosig-Jorgensen M, Lewis RJ, Cabot PJ, Gray PD, Vetter I. Burn pain: a systematic and critical review of epidemiology, pathophysiology, and treatment. Pain Med 2018;19:708–34. [DOI] [PubMed] [Google Scholar]
- [70].Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct 2014;219:1287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nees TA, Rosshirt N, Zhang JA, Reiner T, Sorbi R, Tripel E, Walker T, Schiltenwolf M, Hagmann S, Moradi B. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J Clin Med 2019;8:1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nelson S, Beveridge JK, Mychasiuk R, Noel M. Adverse childhood experiences (ACEs) and internalizing mental health, pain, and quality of life in youth with chronic pain: a longitudinal examination. J Pain 2021;22:1210–20. [DOI] [PubMed] [Google Scholar]
- [73].Nelson S, Uhl K, Wright LA, Logan D. Pain is associated with increased physical and psychosocial impairment in youth with a history of burn injuries. J Pain 2020;21:355–63. [DOI] [PubMed] [Google Scholar]
- [74].Okusogu C, Colloca L. Placebo hypoalgesia: above and beyond expectancy and conditioning. Curr Opin Behav Sci 2019;26:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Palmieri TL, Levine S, Schonfeld-Warden N, O'Mara MS, Greenhalgh DG. Hypothalamic-pituitary-adrenal axis response to sustained stress after major burn injury in children. J Burn Care Res 2006;27:742–8. [DOI] [PubMed] [Google Scholar]
- [76].Patwa S, Benson CA, Dyer L, Olson KL, Bangalore L, Hill M, Waxman SG, Tan AM. Spinal cord motor neuron plasticity accompanies second-degree burn injury and chronic pain. Physiol Rep 2019;7:e14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Peverill M, Sheridan MA, Busso DS, McLaughlin KA. Atypical prefrontal-amygdala circuitry following childhood exposure to abuse: links with adolescent psychopathology. Child Maltreat 2019;24:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pieritz K, Rief W, Euteneuer F. Childhood adversities and laboratory pain perception. Neuropsychiatr Dis Treat 2015;11:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pinto PR, McIntyre T, Almeida A, Araujo-Soares V. The mediating role of pain catastrophizing in the relationship between presurgical anxiety and acute postsurgical pain after hysterectomy. PAIN 2012;153:218–26. [DOI] [PubMed] [Google Scholar]
- [80].Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003;160:1554–65. [DOI] [PubMed] [Google Scholar]
- [81].Retrouvey H, Adibfar A, Shahrokhi S. Extremity mobilization after split-thickness skin graft application: a survey of current burn surgeon practices. Ann Plast Surg 2020;84:30–4. [DOI] [PubMed] [Google Scholar]
- [82].Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry 2016;57:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rief W, Shedden-Mora MC, Laferton JA, Auer C, Petrie KJ, Salzmann S, Schedlowski M, Moosdorf R. Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: results of the randomized controlled PSY-HEART trial. BMC Med 2017;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rietschel CH, Reese JB, Hahn AP, Fauerbach JA. Clinical and psychiatric characteristics of self-inflicted burn patients in the United States: comparison with a nonintentional burn group. J Burn Care Res 2015;36:381–6. [DOI] [PubMed] [Google Scholar]
- [85].Salberg S, Sgro M, Brady RD, Noel M, Mychasiuk R. The development of adolescent chronic pain following traumatic brain injury and surgery: the role of diet and early life stress. Dev Neurosci 2020;42:2–11. [DOI] [PubMed] [Google Scholar]
- [86].Salberg S, Yamakawa GR, Griep Y, Bain J, Beveridge JK, Sun M, McDonald SJ, Shultz SR, Brady RD, Wright DK, Noel M, Mychasiuk R. Pain in the developing brain: early life factors alter nociception and neurobiological function in adolescent rats. Cereb Cortex Commun 2021;2:tgab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Salonsalmi A, Pietilainen O, Lahelma E, Rahkonen O, Lallukka T. Contributions of childhood adversities to chronic pain among mid-life employees. Scand J Public Health 2021;50:1403494820981509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sansone RA, Watts DA, Wiederman MW. The demographics of pain catastrophizing in a primary care sample. Innov Clin Neurosci 2013;10:12–14. [PMC free article] [PubMed] [Google Scholar]
- [89].Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. PAIN 2006;120:297–306. [DOI] [PubMed] [Google Scholar]
- [90].Sheinberg R, Campbell C, Kearson A, Burton E, Letzen J. (112) childhood adversity linked to heightened pain sensitivity in adults. J Pain 2019;20:S4–5. [Google Scholar]
- [91].Sherman AL, Morris MC, Bruehl S, Westbrook TD, Walker LS. Heightened temporal summation of pain in patients with functional gastrointestinal disorders and history of trauma. Ann Behav Med 2015;49:785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Smith KL, Wang Y, Colloca L. Impact of virtual reality technology on pain and anxiety in pediatric burn patients: a systematic review and meta-analysis. Front Virtual Reality 2022;2:751735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Spronk I, Polinder S, van Loey NEE, van der Vlies CH, Pijpe A, Haagsma JA, van Baar ME. Health related quality of life 5-7 years after minor and severe burn injuries: a multicentre cross-sectional study. Burns 2019;45:1291–9. [DOI] [PubMed] [Google Scholar]
- [94].Sturgeon JA, Ziadni MS, Trost Z, Darnall BD, Mackey SC. Pain catastrophizing, perceived injustice, and pain intensity impair life satisfaction through differential patterns of physical and psychological disruption. Scand J Pain 2017;17:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Subramanian M, Venkatesan P. The predictors for altered central pain modulation in individuals with nonspecific chronic low back pain: a systematic review. Pain Pract 2021;22:276–84. [DOI] [PubMed] [Google Scholar]
- [96].Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. PAIN 2005;113:310–15. [DOI] [PubMed] [Google Scholar]
- [97].Trinidad JE. Childhood adversity and deviant peers: considering behavioral selection and cultural socialization pathways. Child Youth Serv Rev 2021:121:105844. [Google Scholar]
- [98].Tyrka AR, Parade SH, Welch ES, Ridout KK, Price LH, Marsit C, Philip NS, Carpenter LL. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: associations with early adversity and depressive, anxiety and substance-use disorders. Transl Psychiatry 2016;6:e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain 2013;136:815–27. [DOI] [PubMed] [Google Scholar]
- [100].Van Loey NE, Klein-Konig I, de Jong AEE, Hofland HWC, Vandermeulen E, Engelhard IM. Catastrophizing, pain and traumatic stress symptoms following burns: a prospective study. Eur J Pain 2018;22:1151–9. [DOI] [PubMed] [Google Scholar]
- [101].VanTieghem MR, Tottenham N. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr Top Behav Neurosci 2018;38:117–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Varallo G, Scarpina F, Giusti EM, Suso-Ribera C, Cattivelli R, Guerrini Usubini A, Capodaglio P, Castelnuovo G. The role of pain catastrophizing and pain acceptance in performance-based and self-reported physical functioning in individuals with fibromyalgia and obesity. J Pers Med 2021;11:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Velmahos CS, Herrera-Escobar JP, Al Rafai SS, Chun Fat S, Kaafarani H, Nehra D, Kasotakis G, Salim A, Haider AH. It still hurts! Persistent pain and use of pain medication one year after injury. Am J Surg 2019;218:864–8. [DOI] [PubMed] [Google Scholar]
- [104].Walton DM, Tremblay P, Seo W, Elliott JM, Ghodrati M, May C, MacDermid JC. Effects of childhood trauma on pain-related distress in adults. Eur J Pain 2021;25:2166–76. [DOI] [PubMed] [Google Scholar]
- [105].Wang D, Choi JK, Shin J. Long-term neighborhood effects on adolescent outcomes: mediated through adverse childhood experiences and parenting stress. J Youth Adolesc 2020;49:2160–73. [DOI] [PubMed] [Google Scholar]
- [106].Wang X, Yang J, Wang P, Zhang Y, Li B, Xie X, Lei L. Deviant peer affiliation and bullying perpetration in adolescents: the mediating role of moral disengagement and the moderating role of moral identity. J Psychol 2020;154:199–213. [DOI] [PubMed] [Google Scholar]
- [107].Womersley JS, Martin L, van der Merwe L, Seedat S, Hemmings SMJ. Genetic variation in neuropeptide Y interacts with childhood trauma to influence anxiety sensitivity. Anxiety Stress Coping 2021;34:450–64. [DOI] [PubMed] [Google Scholar]
- [108].Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology 2016;41:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ziadni MS, You DS, Sturgeon JA, Mackey SC, Darnall BD. Perceived injustice mediates the relationship between perceived childhood neglect and current function in patients with chronic pain: a preliminary pilot study. J Clin Psychol Med Settings 2021;28:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zouikr I, Karshikoff B. Lifetime modulation of the pain system via neuroimmune and neuroendocrine interactions. Front Immunol 2017;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]


