Abstract
A cysteine-containing peptide motif, EWSPCSVTCG, is found highly conserved in the circumsporozoite protein (CSP) and the thrombospondin-related anonymous protein (TRAP) of all the Plasmodium species analyzed so far and has been shown to be crucially involved in the sporozoite invasion of hepatocytes. We have recently shown that peptide sequences containing this motif, and also the antibodies raised against the motif, inhibit the merozoite invasion of erythrocytes. However, during natural infection, and upon immunization with recombinant CSP, this motif represents a cryptic epitope. Here we present the results of immunization studies with two linear multiepitopic constructs, a 60-residue (P60) and a 32-residue (P32) peptide, containing the conserved motif sequence. Both the peptides per se generated high levels of specific antibodies in BALB/c mice. P32 was found to be genetically restricted to H-2d and H-2b haplotypes of mice, whereas P60 was found to be immunogenic in five different strains of mice. The antibody response was predominantly targeted to the otherwise cryptic, conserved motif sequence in P60. Anti-P60 antibodies specifically stained the asexual blood stages of Plasmodium falciparum and Plasmodium yoelii in an immunofluorescence assay, recognized a 60- to 65-kDa parasite protein in an immunoblot assay, and blocked P. falciparum merozoite invasion of erythrocytes in a dose-dependent manner. Immunization with P60 also induced significant levels of the cytokines interleukin-2 (IL-2), IL-4, and gamma interferon in BALB/c mice. Moreover, >60% of mice immunized with P60 survived a heterologous challenge infection with a lethal strain of P. yoelii. These results indicate that appropriate medium-sized synthetic peptides might prove useful in generating specific immune responses to an otherwise cryptic but critical and putatively protective epitope in an antigen and could form part of a multicomponent malaria vaccine.
Several antigens from different stages of the life cycle of the malaria parasite Plasmodium falciparum have been characterized, and some of these, produced by recombinant DNA techniques or by chemical synthesis, are being tested as vaccine candidates (21, 22, 35). The circumsporozoite protein of P. falciparum (PfCSP) is the best-characterized antigen of the parasite because of its role in protective immunity against preerythrocytic stages of malaria (29, 30). This protein contains a stretch of highly conserved, immunodominant tetrapeptide repeats in the middle of its structure (13). However, clinical trials with PfCSP peptides or recombinant CSP and its fragments, aimed at developing specific antibody (Ab) responses to the repeats, have proved disappointing (2, 20). This has led to the suggestion that there might be other antigenic sites on the CSP; in fact, several B and T epitopes from the nonrepeat region of CSP have already been characterized (17, 37). The CSPs of all Plasmodium species contain a nonrepeat conserved portion termed region II. Further, a nonapeptide motif (W-S-P-C-S-V-T-C-G) within region II has been found highly conserved in all CSP sequences analyzed so far (32). This conserved motif is also found in a variety of biologically important proteins, such as thrombospondin, properdin, and components of the complement pathways (19, 32). Interestingly, this nonapeptide motif is also found in the thrombospondin-related anonymous protein (TRAP), first described from erythrocytic stages of P. falciparum. Subsequently, TRAP has also been shown on the surface of P. falciparum sporozoites, and a homolog of TRAP, termed sporozoite surface protein 2 (SSP-2), was found on the surface of sporozoites of Plasmodium yoelii (33, 34). Both CSP and TRAP (SSP-2) are thought to have crucial roles in recognition and entry of sporozoites into the liver cell, and in both, the conserved-motif sequence acts as a specific sporozoite ligand for putative hepatocyte receptors (7, 9, 10, 28). Recently a recombinant construct, RTS, S, containing a truncated version of CSP, inclusive of the region II sequence, attached to hepatitis core protein, has been synthesized. The construct has been found to be protective against sporozoite challenge in humans, raising hopes of a single-antigen-based malaria vaccine (41).
The role of TRAP and, indeed, its expression and location at the blood stages, is not yet known, although TRAP-specific mRNA has been detected in infected erythrocytes (32). We have recently shown that synthetic peptides representing the conserved motif sequences and the antisera raised against these peptides inhibited the merozoite invasion of erythrocytes (38). Further, the anti-peptide Abs recognized a TRAP-like molecule in the blood stage lysate of P. falciparum (38). A better understanding of the structure of the region II peptide sequences and immune responses against them may provide the basis for their inclusion in a subunit malaria vaccine.
Vaccine constructs based on generating only Ab response against well-characterized B epitopes from malaria antigens have not met with the expected success, for several reasons (2, 14, 20). There is now evidence to show that both Ab-mediated and Ab-independent T-cell-mediated protection mechanisms are operative at different stages of the parasite life cycle (4, 11, 45). Also, a successful malaria vaccine will be partly dependent on natural boosting to maintain high levels of protective Abs because of the impracticality of repeatedly administering a vaccine, particularly in the third-world countries where such a vaccine is most needed. To facilitate natural boosting, a vaccine would require T epitopes of parasite origin, and preferably the T and B epitopes should come from the same antigen in the parasite. With this perspective, we have investigated the immunogenicity of synthetic peptides containing B and T cell determinants, based on CSP and TRAP sequences of P. falciparum, in mice (13, 17). We have found that a linear 60-residue-long peptide (P60) containing the conserved region II sequence (amino acids [aa] 331 to 390 of PfCSP) is highly immunogenic in mice without the use of a carrier protein. We describe here in detail the immunological characteristics of P60. We also describe the synthesis and immunological properties of a chimeric peptide (P32) containing a T-cell epitope overlapping with the conserved motif sequence and show evidence that mice immunized with these two P. falciparum-based peptides are partially protected against lethal challenge with heterologous murine malaria blood stage parasites.
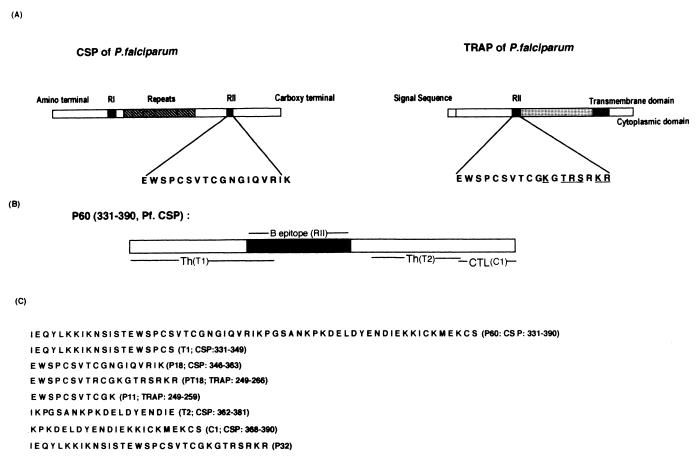
MATERIALS AND METHODS
Peptides.
The amino acid sequences of the peptides P60 and P32 and their constituent peptide fragments representing T1, T2, C1, P18, and PT18 are shown in Fig. 1. The sequence of the 60-residue-long peptide P60 is from CSP of the 7G8 clone of P. falciparum, corresponding to residues 331 to 390 (13). The other peptide, P32, is a chimeric peptide containing the T-helper sequence (T1; aa 331 to 347) from PfCSP as in P60, followed by the region II motif sequence (PT18; aa 249 to 266) from TRAP of P. falciparum (PfTRAP) (13, 17). The differences between region II of PfTRAP and that of PfCSP are shown in Fig. 1. The peptide P32 is therefore highly homologous to the N-terminal half of P60. All the peptides were synthesized on a 0.1-mmol scale with an automated peptide synthesizer (model 430A; Applied Biosystems). The peptides were cleaved and deprotected by treatment with either anhydrous hydrogen fluoride or trifluoromethanesulfonic acid, with thioanisole and ethanedithiol as scavengers. Deprotected peptides were placed in reducing conditions, subjected to gel filtration on Sephadex G-25 or G-10 columns, and purified by high-pressure liquid chromatography on a C18 reverse-phase column with a gradient of acetonitrile in 0.1% trifluoroacetic acid as the eluant. Peptides P60 and P32 were characterized by protein sequencing (model 477A sequencer; Applied Biosystems) and amino acid analysis. All other peptides were checked by amino acid analysis.
FIG. 1.
(A) Schematic representation of CSP and TRAP of P. falciparum indicating the location and sequence of region II (RII) in these proteins. Differences between the two sequences of RII are underlined. (B) Schematic representation of P60 indicating the positions of T-helper (Th) T1 and T2, B RII, and CTL C1 epitopes. (C) Amino acid sequences of P60, P32, P18, and related peptides used in this study. The sequence of P60 is from the 7G8 clone of P. falciparum, whereas P32 is a chimeric peptide containing a T-helper epitope; T1 is from CSP of P. falciparum (7G8 clone), and PT18 is from TRAP of P. falciparum.
Immunization of animals.
Four- to 6-week-old mice of different inbred strains, namely, BALB/c (H-2d), C3H/He (H-2k), C57BL/6 (H-2b), FVB (H-2b), DBA/2 (H-2d), and SJL (H-2S), were purchased from the Small Animal Facility of the National Institute of Immunology, New Delhi, India. Two rabbits (New Zealand White) were also procured from the same facility.
To find out whether P60, P32, and P18 were immunogenic on their own, three groups of inbred mice (BALB/c; six per group) were immunized with 50 μg of the given peptide dissolved in phosphate-buffered saline (PBS; pH 7.4) and emulsified in complete Freund’s adjuvant (CFA) via the intraperitoneal route. The animals were boosted on days 28 and 35 with the same inoculum emulsified in incomplete Freund’s adjuvant. The animals were bled on days 0 (preimmune), 14, 28, 35, 49, 64, 70, 100, and 120, and the sera were separated for immunoassays. Similarly, to analyze the pattern of genetic restriction of the immune response to P60, mice belonging to different haplotypes were immunized as described above and their sera were collected. All sera were heat inactivated and stored at −20°C until used.
The rabbits were prebled and immunized subcutaneously at multiple sites with 250 μg of P60 dissolved in PBS (pH 7.4) per injection in an emulsion with CFA. The first boost with the same amount of P60 in incomplete Freund’s adjuvant was given 4 weeks later, followed by a second boost another 4 weeks later. Blood was taken on days 0, 14, 28, 49, and 63. The sera were separated out in each case and inactivated at 56°C for 30 min. The inactivated sera were diluted in 0.5% casein in PBS (pH 7.4) prior to immunological analysis.
Antibody assays. (i) Affinity purification of immunoglobulins.
Peptide-specific Abs were purified from the rabbit anti-P60 sera by immunoaffinity chromatography essentially as described in our earlier work (38). Briefly, gamma globulin fraction from rabbit serum was obtained by ammonium sulfate precipitation followed by ion-exchange chromatography on an Econo-Pac immunoglobulin G (IgG) purification column (Bio-Rad Laboratories, Richmond, Calif.) according to the manufacturer’s instructions. The purified IgG was then applied to the immunoadsorbent column prepared by the coupling of P60 to a cyanogen bromide-activated Sepharose 4B column. The peptide-specific IgG Abs were eluted with glycine-HCl buffer (0.1 M; pH 2.5), and the fractions were neutralized by the addition of Tris base (2.0 M). The fractions were pooled and characterized as described previously (38).
(ii) ELISA.
Antibody levels in the sera from the mice immunized with the peptides were assayed by enzyme-linked immunosorbent assay (ELISA), using appropriate synthetic peptides as capture antigens. Briefly, the wells of flat-bottom 96-well microtiter plates (Greiner, Nurtingen, Germany) were coated with the relevant antigen. Uncoated reactive sites in the wells were blocked by incubation with a 5% solution of a nonfat dried milk powder in PBS, pH 7.2, for 1 h. The plates were washed three times with washing buffer (0.15 M NaCl solution containing 0.05% Tween 20). All serum samples were serially diluted in PBS, pH 7.2, containing 0.5% milk powder and incubated in antigen-coated wells for 90 min at room temperature in a humid chamber. The wells were washed thoroughly with the washing buffer, and the plates were incubated with 50 μl of optimally diluted horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-human IgG (Sigma) for 90 min in the respective assays. The enzyme reaction was developed with 100 μl of substrate solution (o-phenylenediamine dihydrochloride [2 mg/ml] and H2O2 in citrate buffer, pH 5.0). The reaction was stopped with 8 N H2SO4 (50 μl/well), and the optical density (OD) of the reaction product was obtained with a microplate reader (Molecular Devices) at 490 nm. The last dilution of a test serum giving an OD value greater than twice the OD value obtained with the respective preimmune serum diluted 1/100 was taken as the endpoint titer. Sera obtained from the control mice receiving only CFA were also screened for Abs to the relevant peptides.
(iii) Inhibition ELISA.
Different concentrations of the relevant peptides were preincubated with optimally diluted polyclonal anti-P60 Abs for 1 h at 4°C. The plates, coated with P60 (1 μg/well) and blocked as described above, were then incubated with 50 μl of the Ab-peptide solution for 30 min at room temperature along with the polyclonal anti-P60 Abs diluted in 0.5% casein in PBS (pH 7.2) without peptide. The plates were washed extensively with the washing buffer and incubated with 50 μl of optimally diluted goat anti-mouse IgG for 90 min. Following washings with washing buffer, the reaction was developed with 100 μl of substrate solution (o-phenylenediamine dihydrochloride [2 mg/ml] and H2O2 in citrate buffer, pH 5.0). The reaction was stopped with 8 N H2SO4 (50 μl/well), and the OD of the reaction product was obtained with a microplate reader (Molecular Devices) at 490 nm. An unrelated linear 23-residue-long peptide, VH-1, based on a plant protein (FLTTYAQAANTHLFLLKDAQIYG) was used as a negative control in this assay.
(iv) Subtyping of IgG.
The ELISA plate was coated with P60 and washed three times with the washing buffer, followed by incubation of 50 μl of serially diluted mouse anti-P60 serum samples in duplicate for 90 min. The plate was washed and incubated with different goat anti-mouse IgG subtypes, namely, IgG, IgG1, IgG2a, IgG2b, and IgG3 (diluted 1/1,000 in PBS [pH 7.4] containing 0.5% nonfat dried milk) for 90 min. The plate was washed another three times with the washing buffer and incubated with horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin (1/500) for 90 min, and the assay was completed as described above.
IFA.
Indirect immunofluorescence assays (IFAs) were performed with sera obtained from BALB/c mice and rabbits immunized with P60 or P32. Briefly, the wells of slides were coated with P. yoelii- or P. falciparum-infected erythrocytes. The cells were fixed on slides by immersing them in cold acetone at −20°C for 2 h. The slides were incubated with different dilutions of sera in individual wells for 1 h. After extensive washing with PBS, the slides were incubated with a 1:40 dilution of goat anti-mouse IgG conjugated to fluorescein isothiocyanate for 1 h in the dark in a humid chamber. Following washings, the slides were observed under a fluorescence microscope (Nikon) by visible and UV light alternately to see specific binding of the antibody to the infected erythrocytes. Serum samples obtained from rabbits immunized with adjuvant alone were also tested in this assay and served as a negative control.
Western blot analysis.
P. falciparum proteins were fractionated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels under reducing conditions. Recombinant PfTRAP (a truncated version lacking the signal and transmembrane sequences; residues 26 to 503) expressed in the pQE vector and recombinant PfCSP (a kind gift from P. Sinnis) were also included in the gel. The fractionated proteins were then electroblotted onto nitrocellulose paper. The parasite proteins were probed with the polyclonal Ab raised against P60 in rabbit serum (preadsorbed on human erythrocytes), followed by incubation with horseradish peroxidase labelled anti-rabbit IgG. The reaction was developed with 3,3′-diaminobenzidine as a substrate. In each case rabbit anti-parasite antibodies and preimmune rabbit IgG or serum were used as a positive and negative control, respectively. Monoclonal Ab (MAb) 2A10 (a kind gift from P. Sinnis) directed against PfCSP and polyclonal serum raised against recombinant PfTRAP in inbred female BALB/c mice served as positive controls for the respective recombinant proteins. The serum obtained from the adjuvant-immunized rabbit was also screened for generation of the parasite-specific Abs.
Merozoite invasion inhibition and parasite growth inhibition assays.
The FID-3 isolate of P. falciparum was used for the merozoite invasion inhibition assays. The parasite was cultured following methods described by Trager and Jenson (42). For the merozoite invasion inhibition assay, cultures of the FID-3 isolate of P. falciparum were synchronized by two treatments with 5% sorbitol (25) and incubated for about 30 h so that at the time of setting up the assay, more than 95% of the parasites were late trophozoites. For the merozoite invasion inhibition assay, the cultures were incubated for about 20 h with various concentrations of the immunoglobulin IgG, obtained from the rabbits immunized with P60, as well as with the sera obtained from P32-immunized BALB/c mice. Only the ring-stage-infected erythrocytes were counted as parasitized cells for calculating percent parasitemia. In each case the sera obtained from rabbits immunized with the synthetic peptide, P8 (LDNIKGNVGKMEDYIKKNNKC), from merozoite surface protein 1 (MSP1) of P. falciparum, was used as the negative control (39). Each serum or immunoglobulin concentration was tested in triplicate. Percent invasion inhibition was calculated as follows: 100 − (percent parasitemia in test serum [immune immunoglobulin]/percent parasitemia in preimmune serum [or immunoglobulin] × 100).
Cellular immune responses. (i) Lymphocyte proliferation assays.
Two groups of four mice each were primed with 50 μg of P60 or P32 in PBS emulsified with equal volumes of CFA via tail base inoculations, while the group of control mice received emulsified PBS alone. Twelve days later, the inguinal lymph nodes (LN) were extracted and crushed to release the cells. The cells were washed twice with RPMI 1640 medium (Sigma) and plated at 4 × 105/well in 96-well tissue culture plates (Costar) in RPMI 1640 medium supplemented with 15 mM HEPES, 0.2% sodium bicarbonate, 50 μM β-mercaptoethanol (Bio-Rad), 2 mM glutamine, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 10% fetal calf serum (Sigma). Appropriate peptides were incubated with the seeded lymphocytes at different concentrations. All cultures were set up in quadruplicate. The plates were incubated at 37°C in 5% CO2 (Forma Scientific). Tritiated thymidine (0.5 μCi; Amersham, Buckinghamshire, United Kingdom) was added to each well in the last 15 h of the 5 days of culture. Cells were harvested, and the tritiated thymidine incorporation was determined with a liquid scintillation counter (Betaplate; Pharmacia, Uppsala, Sweden). Counts were derived from the averages of four separate experiments and expressed as the stimulation index (SI) (SI = counts per minute of stimulated culture/counts per minute of control culture). The T-cell mitogen concanavalin A (Sigma) was used as a positive control.
(ii) Cytokine analysis.
Supernatants were collected from in vitro lymphoproliferative cultures, growing in the presence or absence of the peptide, after 72 h. Cytokine levels were estimated with the appropriate commercially available murine ELISA kits (Endogen) according to the manufacturer’s instructions. The cytokines interleukin-2 (IL-2), IL-4, and gamma interferon (IFN-γ) were measured with 50 μl of supernatant diluted four times. The plates were read at a wavelength of 450 nm. The concentration of each cytokine was calculated from standard curves obtained with known concentrations of the positive control provided with the respective kits.
Protection in mice.
A group of 15 inbred mice (BALB/c) were immunized intraperitoneally with 50 μg of P60 emulsified in CFA. Control mice received only the adjuvant in PBS. All the mice received boosts on days 28 and 42. On day 49, the mice were bled and sera were collected. A week later, the immunized and control mice were challenged with an inoculum of 104 Plasmodium yoelii nigeriensis (lethal strain)-infected erythrocytes. From the third day after the challenge, thin blood smears obtained from each mouse were stained with Giemsa stain and percent parasitemia was determined by microscopy. To assess the protective potential of P32, a separate group of 10 BALB/c mice were immunized with the peptide and challenged with P. yoelii parasites as described above. The protection experiments were repeated twice to confirm the observations.
RESULTS
Immunoassays.
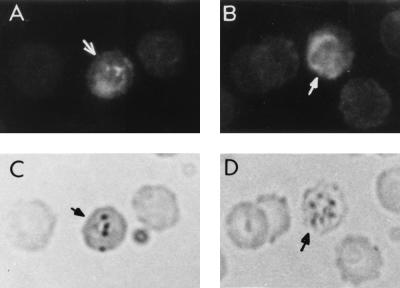
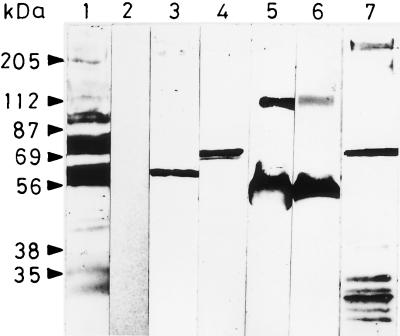
We had found earlier that anti-region II peptide Abs immunoreacted with a TRAP-like molecule in the P. falciparum blood lysate (38). Since both P60 and P32 contained the conserved region II sequences which are present in both CSP and TRAP and which are also found conserved in different plasmodium species (32), we wondered if anti-P60 Abs would also recognize antigens from the blood stages of P. falciparum and P. yoelii. To investigate this, an indirect immunofluorescence Ab test was carried out with sera from mice immunized with P60 (Fig. 2). In an immunoblot the crude P. falciparum lysate was probed with the rabbit anti-P60 serum preadsorbed on human erythrocytes. Anti-peptide Abs recognized a protein with a molecular mass in the range of 60 to 65 kDa (Fig. 3) in the lysate, as determined by prestained molecular mass markers (SDS-7B; Sigma). The position of this protein band is somewhat lower than that we had observed in our earlier work (38). In order to compare this protein band with the other two well-known sporozoite stage proteins (CSP and TRAP), which contain the conserved thrombospondin binding motif, recombinant PfCSP and PfTRAP were also included in this assay. Anti-peptide Abs recognized recombinant PfCSP as well as the truncated version of PfTRAP (37a) expressed in our laboratory (Fig. 3). No further attempt was made to characterize the 60- to 65-kDa protein at this stage. Anti-P. falciparum hyperimmune serum generated in a rabbit was used as a positive control, whereas preimmune serum or purified immunoglobulins served as a negative control in this Western blot analysis. Sera raised against the recombinant PfTRAP and MAb 2A10 directed against the NANP repeats in PfCSP were used to identify these proteins in this assay.
FIG. 2.
Immunofluorescence staining of the trophozoites P. falciparum with anti-peptide serum. (A and B) Parasites showing bright fluorescent staining under UV light. (C and D) The same fields under visible light, showing the parasite-infected erythrocytes; some uninfected erythrocytes can also be seen in these panels.
FIG. 3.
Immunoblot showing the blood stage parasite protein recognized by rabbit anti-P60 serum. A whole-cell lysate of the asexual blood stages of P. falciparum and recombinant PfTRAP and PfCSP (without the signal sequence and transmembrane domain) (lanes 3, 4, and 5, respectively) were fractionated by SDS–10% polyacrylamide gel electrophoresis and probed with rabbit anti-P60 serum preadsorbed on human erythrocytes. Hyperimmune serum raised against P. falciparum blood stages served as a positive control for the P. falciparum blood stage lysate (lane 1), while MAb 2A10 served as a positive control for the recombinant PfCSP (lane 6). Polyclonal sera raised in BALB/c mice served as positive controls for the recombinant TRAP in this assay (lane 7) and the preimmune serum served as a negative control for the P. falciparum blood stage lysate (lane 2). Prestained molecular mass markers (SDS-7B; Sigma) are shown on the left.
Merozoite invasion inhibition assay.
The results of an experiment to ascertain the effect of anti-peptide Abs on the merozoite invasion of erythrocytes are summarized in Table 1. We found that the addition of up to 10% normal rabbit serum to the culture growing with 10% human serum did not affect the rate of growth of the parasites. Incorporation of up to 10% rabbit anti-P60 serum caused nearly 70% inhibition of merozoite invasion. To further establish that the inhibition was due to the presence of anti-peptide Abs, we tested affinity-purified IgG fraction, obtained from rabbit anti-P60 serum, at different concentrations in the above assay. We found (Table 1) that while the rabbit immune IgG could inhibit merozoite invasion in a dose-dependent manner (2 mg of purified IgG/ml caused 70% inhibition), the normal (preimmune) IgG showed a negligible effect on the invasion. Abs raised against a 21-residue peptide, P8, representing a sequence from PfMSP1 (38), showed no inhibition and served as a negative control in these assays.
TABLE 1.
In vitro inhibition of P. falciparum FID-3 merozoite invasion by purified anti-P60 IgG
| IgG | Concn (μg/ml) | % Parasitemiaa | % Inhibition |
|---|---|---|---|
| Preimmune | 2,000 | 4.03 ± 0.03 | 0 |
| Purified anti-P60 | 125 | 2.90 ± 0.2 | 28 |
| 250 | 2.76 ± 0.1 | 32 | |
| 500 | 2.5 ± 0.16 | 38 | |
| 1,000 | 1.70 ± 0.04 | 60 | |
| 2,000 | 1.25 ± 0.1 | 70 |
Each value represents the mean (± standard deviation) of the percent parasitemia obtained in triplicate wells. The parasitemia at zero hour was 0.5%.
Protection in mice immunized with synthetic peptides.
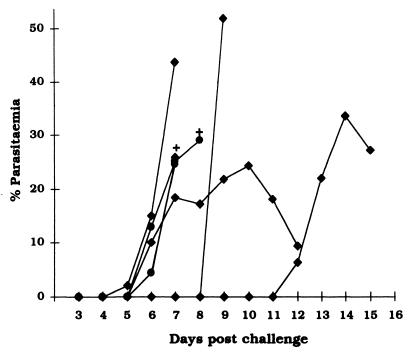
Since the anti-P60 Abs showed reactivity with asexual blood stages of both P. falciparum and P. yoelii in an IFA, we wondered if the peptide immunization would also provide protection in mice against murine malaria infection. To investigate this, a group of 15 BALB/c mice immunized with P60 were challenged with a lethal dose of asexual blood stages of P. yoelii nigeriensis. Ten of the 15 immunized mice remained slide negative for the parasite and survived the challenge. In two immunized mice, parasitemia developed and reached a peak on days 6 and 12, respectively, but decreased quite significantly thereafter. However, these mice died by day 14, perhaps due to other reasons, such as anemia. In our analysis these mice were considered unprotected. The three remaining mice showed a delayed onset of parasitemia compared to that in the control mice, but they all died by day 9 after challenge. In a repeat experiment essentially similar results were obtained. Control mice which received only the adjuvant died within 10 days after the challenge inoculation (Fig. 4).
FIG. 4.
Time course of P. yoelii nigeriensis infection in 15 BALB/c mice immunized with P60 and challenged 10 days after the last boost. Parasitemia is expressed as the percentage of infected erythrocytes. Ten immunized mice which did not develop parasitemia and remained slide negative are not included in the figure. •, profiles of parasitemia in two control mice which received adjuvant only; ⧫, course of parasitemia in five immunized mice which developed parasitemia; +, death of the animal.
Mice (BALB/c) immunized with P32 and challenged as described above also showed a similar pattern of protection; a total of 7 of the 10 immunized mice survived the challenge. In fact, six mice remained slide negative for the parasite, and in the one in which parasitemia developed, the parasitemia decreased by day 14. In the three remaining mice there was a delayed onset of parasitemia compared to that in the control mice, but these mice died by day 14 (data not shown).
Humoral responses.
Sera from mice immunized with P60, P32, P18, and PT18 were tested by ELISA for the presence of peptide-specific Abs. Anti-P60 Ab titers reached a peak at day 49, 7 days after the second boost (Fig. 5). The Ab levels remained essentially the same for up to 150 days. Immunization with P32 showed a similar pattern of Ab response (data not shown). However, BALB/c mice immunized with carrier-free P18 or PT18 did not produce detectable levels of peptide-specific Abs. Control mice receiving adjuvant alone also did not show any peptide-specific Abs.
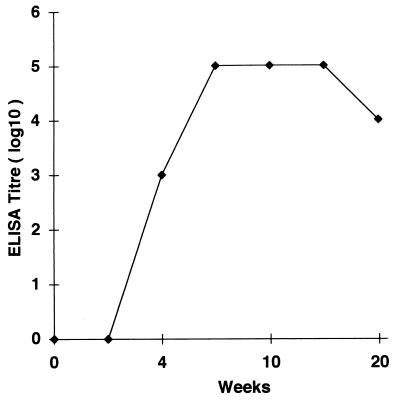
FIG. 5.
Kinetics of peptide-specific IgG response in BALB/c mice immunized with P60 as monitored by ELISA. Immunization of control mice with CFA in PBS did not induce detectable level of peptide-specific Abs.
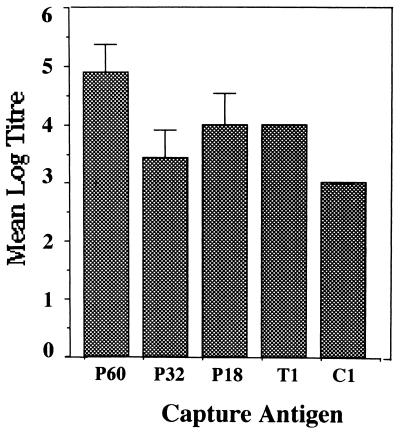
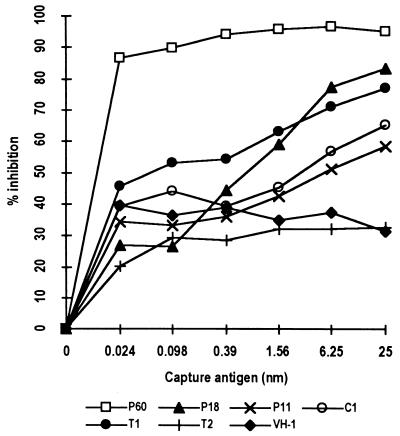
In order to investigate the fine specificity of the humoral response to P60 in BALB/c mice, ELISA was performed with peptides representing different epitope sequences and peptide fragments spanning more than one epitope (Fig. 1). The highest Ab response was seen against the peptides represented by P18 and T1: the endpoint ELISA titers were as high as 1/10,000 for these peptides (Fig. 6). Peptide PT18 showed a reactivity similar to that of P18 in this assay. In the case of P32 also the response was focused on the sequences represented by PT18 or P18 (data not shown). We also performed ELISA in which specific Abs could bind competitively to a given peptide in solution or to P60 coating the wells. Results of the competitive ELISA experiments further supported the above observations, and a dose-dependent inhibition was observed with the constituent peptides, except the unrelated control peptide (Fig. 7). Of all the constituent peptides of P60, P18 was the most effective, causing an inhibition of 77.1% at a concentration of 6.25 nmol.
FIG. 6.
Fine specificities of humoral responses generated in BALB/c mice immunized with P60. The animals were primed on day 0 and boosted on day 28 with P60. Sera collected on day 35 were tested in an ELISA with different peptide constructs as capture antigens.
FIG. 7.
Inhibition of binding of anti-P60 mouse Ab to P60 in an ELISA. Mouse serum diluted to 1/2,000 was preincubated with the indicated amounts (final concentrations) of different peptide fragments before being added to the ELISA plate coated with P60. VH-1 is an unrelated peptide used as a negative control in this assay.
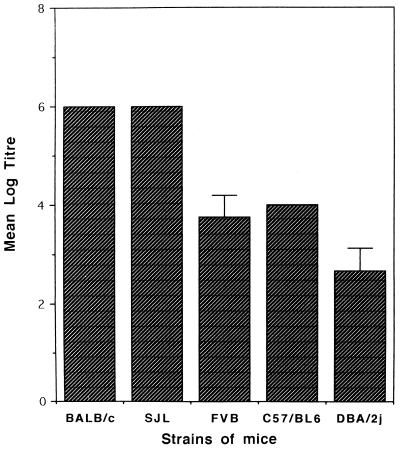
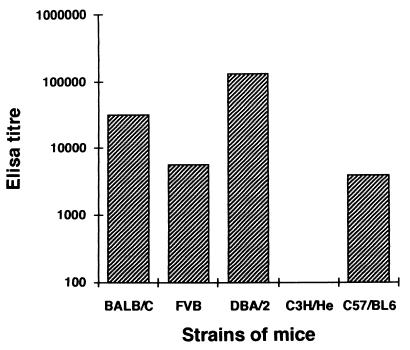
To determine whether humoral response to the peptides P60 and P32 was genetically restricted, mice of different inbred strains, namely, FVB, BALB/c, SJL, DBA/2j, and C3H/He were primed and boosted twice with both peptides. All the immunized mice generated an Ab response to P60, although the response varied in different haplotypes (Fig. 8). Both peptides generated the highest response in H-2d mice, where Ab endpoint titers of 1/100,000 were obtained. Immunization with P32 showed genetic restriction of the immune response: high levels of anti-peptide Abs were generated in BALB/c (H-2d) and C57BL/6 (H-2b) mice, whereas it failed to induce Abs in C3H/He (H-2k) mice (Fig. 9).
FIG. 8.
Ab response to P60 as measured by ELISA in sera from different strains of mice immunized with carrier-free P60. Sera obtained from mice immunized with the adjuvant alone from each of these strains did not show the presence of Abs against P60. The ELISA titers shown are the geometric means (± standard deviations) of the endpoint titers obtained from eight immunized mice.
FIG. 9.
Genetic restriction profile of P32 in different strains of inbred mice. Mice receiving CFA alone did not show detectable levels of peptide-specific Abs in either of the strains used in this study. The titers shown are the geometric means of the individual titers obtained from six to eight immunized mice.
Subtyping of IgG.
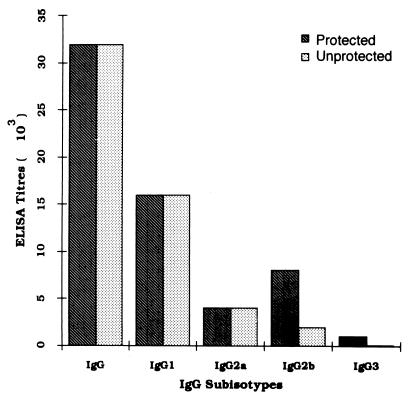
Since the isotype of an Ab is considered important in determining the protective nature of the immune response to malaria infection (5, 45), we also carried out subtyping of IgG response in BALB/c mice, induced upon P60 immunization. Endpoint analysis of IgG subtypes obtained from protected and unprotected mice indicated that IgG1, IgG2a, IgG2b, and IgG3 were generated (Fig. 10). While IgG3 could be detected at up to 1/1,000 dilution in protected mice, the levels of IgG3 were somewhat lower (1/100) in unprotected mice. We observed a minor difference between the levels of IgG2b in the protected and unprotected mice: endpoint titers of 1/8,000 and 1/2,000 were obtained for the protected and unprotected mice, respectively. It is noteworthy that no difference between the levels of the subisotype IgG2a, which has been implicated in altering the course of parasitemia in P. yoelii infection, in the protected and unprotected mice were observed in the present analysis (5).
FIG. 10.
Profile of peptide-specific IgG subisotype responses in protected and unprotected BALB/c mice as determined by ELISA.
Cellular immune response to peptide immunization.
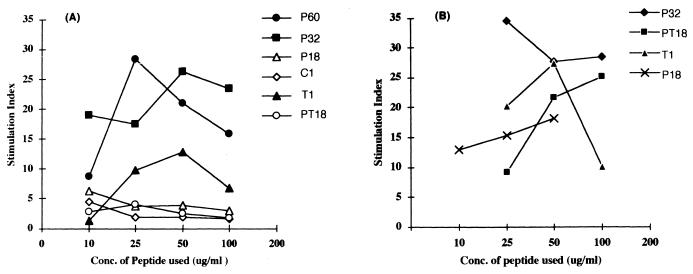
To determine whether the T-epitope sequences incorporated in P60 induced T-helper cell functions, in vitro experiments involving peptide-driven cell proliferation were carried out. A group of inbred BALB/c mice (four mice) were immunized with P60, and 12 days later, cells from the draining LN were cocultured in vitro with the homologous peptide as well as with the constituent peptides at varying concentrations. The maximum proliferation in each case was observed with the immunizing peptide (P60) (100 μg/ml; SI = 28.3). In P60-immunized mice, the proliferation of LN cells was also observed in response to (in decreasing order) P32 (SI at 100 μg/ml, 23.4), T1 (SI at 50 μg/ml, 12.8), and PT18 (SI at 25 μg/ml, 4.0) (Fig. 11A). However, we did not observe any significant lymphocyte proliferation in response to the peptide T2 (aa 362 to 381), which has been previously described as a T-helper epitope (17). The results of cellular proliferation experiments with P32 are summarized in Fig. 11B.
FIG. 11.
In vitro cellular proliferative responses with various peptides. BALB/c mice were immunized with either P60 (A) or P32 (B), and the LN cells were stimulated with the indicated peptides. Concanavalin A (1 mg/ml) was used as a positive test antigen. Each data point represents an average of values obtained in quadruplicate wells. The maximum background incorporations in the absence of antigen were 2,229 cpm (A) and 1,475 cpm (B).
Cytokine profile.
In order to determine the type of T-helper cells involved in lymphocyte proliferation, cytokine analysis was carried out. The supernatants were collected from lymphoproliferative cultures after 72 h of incubation following in vitro peptide stimulation. Production of TH-1-derived IFN-γ and IL-2 and TH-2-derived IL-4 was estimated by using ELISA kits at varying concentrations of P60. At the antigen concentration of 25 μg/ml (the maximum SI was observed at this concentration), the levels of IFN-γ, IL-2 and IL-4 secreted from the stimulated lymphocytes were significantly higher than those in the unstimulated lymphocytes, indicating stimulation of both the TH-1 and TH-2 subsets of cells (e.g., the amount of IFN-γ released on stimulation with 10 μg of P60/ml was 980 pg/ml) (Table 2).
TABLE 2.
In vitro production of cytokines from LN cells from immunized mice following stimulation with P60
| Concn of P60 (μg/ml) | Level of cytokine released (pg/ml)a
|
||
|---|---|---|---|
| IL-4 | IL-2 | IFN-γ | |
| 0 | 12 | 68 | 380 |
| 10 | 80 | 200 | 960 |
| 25 | 200 | 240 | 1,700 |
| 50 | 240 | 220 | 2,200 |
| 100 | 260 | 320 | 1,260 |
Supernatants obtained after 72 h of in vitro lymphoproliferative culture were used to assay the generation of cytokines.
DISCUSSION
The main aim of the present study was to design peptide sequences containing malaria-related T epitopes which could generate a boostable region II-specific Ab response in addition to stimulating effector T cells. With this in view, peptides P60 and P32, both containing T-cell determinants and the region II sequence (Fig. 1), were synthesized and characterized. We found that immunization with the synthetic peptides produced an amanestic Ab response in mice and that significant levels of Abs are maintained for an extended period of time. The high-titer anti-peptide Ab response in mice and rabbits was elicited without conjugating the peptides to any carrier protein. We also found that BALB/c mice immunized with these two P. falciparum-based peptides developed partial protection against a heterologous challenge with P. yoelii blood stage parasites. It thus appears that both P32 and P60 contain determinants capable of producing protective immune responses in mice. We showed earlier that anti-region II peptide Abs inhibited merozoite invasion of erythrocytes and recognized a TRAP-like protein in the P. falciparum blood stage lysate (38). The results of immunoassays (Western blot and IFA) with anti-P60 Abs in the present study show that these Abs can recognize some protein(s) of the blood stage of P. falciparum and can also cross-react with P. yoelii blood stage parasites, and they may be at least partially involved in protective immunity in mice. However, the protein that is recognized by the anti-P60 Abs seems to have lower mobility than the protein identified by anti-region II Abs earlier (38). From the Western blot assays it appears that the protein recognized by anti-P60 Abs is different from the well-characterized sporozoite stage P. falciparum antigens CSP and TRAP (SSP-2), at least as far as mobility in SDS-polyacrylamide gel electrophoresis is concerned. Since we repeatedly failed to observe the faster-moving band beyond the 70-kDa mark (38), it is difficult to say from these results alone if there is more than one blood stage protein that cross-reacts with anti-region II peptides or if the observed difference in protein mobility is due to experimental conditions with respect to the parasite lysate preparation or a discrepancy in the marker proteins. However, it may be emphasized that with anti-peptide Abs only one major band was observed in both the previous (38) and the present study. No further attempts to characterize this protein were undertaken at this time.
Immune responses to the synthetic peptides were further analyzed to investigate their fine specificities. Results of different immunoassays have indicated that in mice a significant amount of the polyclonal Ab response to immunization with P60 was focused on the region II sequence in the peptide. The ELISA results also showed that P18 and PT18 reacted equally well with the peptide antisera. This indicates that despite a few differences in the amino acid sequences (Fig. 1) these two otherwise homologous peptides share common B-cell epitopes. We found that the P60-primed LN cells proliferated on stimulation with the peptide itself and the constituent peptides P32, T1, and P18. Stimulation with the homologous peptide PT18 also produced significant proliferation, indicating the presence of cross-reactive T-cell determinants in P18 and PT18. Similarly, the LN cells from P32-primed mice also proliferated upon stimulation with P32 itself and its constituent peptides, T1 and PT18 (P18), indicating that both T1 and P18 contain T-helper determinants in their sequences. While T1 represents a well-known T-helper epitope (18) and a cytotoxic-T-lymphocyte epitope within its sequence (24, 37), we were somewhat surprised to find that the region II peptide sequences, P18 and PT18, also contain T-helper epitopes. The fact that we also observed a strong Ab response focused on this epitope upon P32 and P60 immunization indicates that these peptide sequences contain overlapping B- and T-cell epitopes. Clearly, P32 and P60, lying downstream of the immunodominant repeats, represent high-epitopic-density regions of CSP. The occurrence of overlapping B- and T-cell epitopes in the above-mentioned peptides may not be surprising; in several antigenic proteins the B- and T-cell epitopes are often located close to each other (15, 27). On the other hand, immunization with either P18 or PT18, which apparently contain both B- and T-cell determinants, did not induce any detectable Ab response in rabbits or in BALB/c mice. Keeping in mind that short peptides containing B- and T-cell epitopes can produce high levels of specific Ab responses, the reasons for this observation are not clear. But it has been shown that in short peptides, T cells may not always induce an Ab response in B cells when their determinants overlap (36). It is also suggested that pathogens causing the dominant T-cell determinants to overlap with the critical B-cell determinant may interfere with Ab responses detrimental to the pathogen. This may well be one of the several possible reasons why the B-cell determinants in region II sequences remain largely cryptic during the course of natural infection (3).
The fine specificities of the Ab humoral immune responses in P. falciparum-infected individuals are generally dominated by the repeat peptide structures, and the region II conserved sequence seems to be a cryptic B epitope, at least during the course of natural infection (3). One of the reasons for this may be that the CSP repeats dominate the structural features of the protein in such a manner that the region II sequences, which lie downstream of the repeats, are not easily accessible to the immune system in the intact protein. On the other hand, it has also been reported that B cells may respond best against rigid and highly repetitive surface antigens of pathogens and may not even require T-helper cells for this (1). The repeats are most likely to represent highly structured B epitopes (6), the immune response to which could easily dominate the responses to other regions of CSP even if they are exposed. However, these explanations will remain speculative until structural details of malaria proteins containing repeats, such as CSP, become available. In another study, immunization with a repeatless CSP construct of P. falciparum in mice showed that the region II sequences still remained poorly immunogenic when Ab specificity was determined by using overlapping octapeptides (44).
Whatever the reason may be, it is quite clear that region II is not as immunodominant as some other malaria epitopes, and it is likely that the immune response to such cryptic epitopes is raised only very slowly. It is well known that natural immunity to malaria in individuals living in areas where it is endemic is not fully acquired before adolescence, even following repeated infections (11, 12). Masking of the crucial protective epitopes in an antigen during the course of natural infection has also been reported in the case of tryptomastigote surface antigen 1 (TSA-1) of Trypanosoma cruzi (46). When mice were immunized with the intact recombinant protein, the Ab response was found to be mainly focused on the carboxy-terminal region of the protein, which did not provide any protection against a challenge infection. On the other hand, immunization with a recombinant N-terminal fragment provided protection, leading to the conclusion that in TSA-1, the protective epitopes of the N-terminal region remain cryptic in the intact protein and that the removal of the immunodominant carboxy-terminal region from the protein allows the immune response to be focused on these cryptic, but crucial, epitopes (46). The results of the present study also suggest that through the use of synthetic peptides it may be possible to focus Ab response on the epitopes which tend to remain cryptic during immunization with the intact protein. But it should also be emphasized that it may not always be possible to predict the nature of immune responses from multiepitopic peptides. We and others have shown that such immunogens may be polar, and there are no rules yet to design these molecules for specific immune responses (10, 16, 39).
In general, immune response to short synthetic peptides is genetically restricted. Inclusion of appropriate T-cell determinants may help to overcome this problem. In fact, in the case of the shorter peptide, P32, the immune response was restricted to H-2d and H-2b haplotypes of mice. On the other hand, immunization with P60, which contains several T-cell determinants, produced significant response in all the haplotypes tested. These results suggest that in a synthetic peptide immunogen, inclusion of more than one T epitope may at times be a reasonable way to circumvent the problem of genetic restriction of the immune response. In a multiple-antigen peptide vaccine construct designed to produce high levels of Abs against P. yoelii CSP repeats, two T-helper epitopes were used (43).
Differential activation of T-cell subsets, TH-1 and TH-2, seems to play a crucial role in parasitic diseases (26, 30, 31). In malaria also, TH subsets have been implicated in modulating the course of infection during different stages of the parasite life cycle (31). The results of our cytokine analyses of the supernatants obtained from in vitro cellular proliferation experiments with P60 indicated that both the TH-1 and TH-2 subsets of T cells were activated upon peptide immunization. Since mature erythrocytes do not bear or express major histocompatibility complex class I or II antigens, it is difficult to envisage a direct role for T cells in protective immunity against blood stages of the parasite. But at the same time, activated lymphocytes release a battery of cytokines, which could mediate the functions of phagocytic cells and possibly promote phagocytosis of the intraerythrocytic parasite. For example, IFN-γ, which is known to play an important role in modulating infection (40), is released by both TH-1 and CD8+ cells. Specific activation of T cells could have a role in inducing protective immunity against malaria (29, 31).
The relative roles of different immunoglobulin subtypes have also been assessed, and there is evidence that in both human and rodent malaria, the distribution of Ab subisotypes can modulate the course of infection (5, 45). We found noticeably higher levels of IgG3 and IgG2b in protected mice compared to those in unprotected mice. However, no differences were seen between the levels of IgG2a in protected and unprotected mice. In an earlier study, the IgG2a subisotype alone was found to alter the course of parasitemia in mice infected with P. yoelii (45). The qualitative and quantitative roles of Abs in malaria are not well understood and need to be evaluated for the development of malaria vaccine (4).
Can a functional conserved malaria protein sequence which is also a part of self molecules like thrombospondin and properdin be considered for inclusion in a peptide malaria vaccine construct? It can be validly argued that the induction of an immune response to the conserved motif could give rise to autoimmune responses, as shown for the heat shock protein 70 (hsp-70) cognate parasite protein. At the same time, several P. falciparum proteins, viz., Pf25, PfMSP-1, and PfP41, contain sequences homologous to those of host proteins, such as epidermal growth factor (23), the intermediate filament protein, and the human aldolase enzyme, respectively (8); and, significantly, none of these sequences have been shown to induce or be a target of any autoimmune response. In conclusion, the present study indicates that (i) through appropriate synthetic peptides it may be possible to focus immune response on the epitopes that remain cryptic when the whole antigen is presented to the immune system, (ii) that linear, nonpolymeric peptide can be a potent immunogen, (iii) that inclusion of more than one T epitope may be necessary to circumvent the problem of genetic restriction in peptide immunization, and (iv) that highly conserved motifs in malaria surface antigens may be useful targets for inclusion in synthetic peptide malaria vaccine constructs.
ACKNOWLEDGMENTS
We thank V. N. Sailaja, J. Ananya, Sachhidanand, and Mridul Mukherjee for their help in peptide synthesis and immunological assays. We also thank V. S. Dattu, P. Sejwali, and Photini Sinnis for providing us samples of recombinant P. falciparum TRAP and CSP. We are grateful to Photini Sinnis for also providing us a sample of MAb 2A10 specific to CSP.
This work was partly supported by EC grant TS CT 9302.
REFERENCES
- 1.Bachmann M F, Hengartner H, Zinkernegel R M. T helper cell independent neutralising B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur J Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- 2.Ballou W R, Hoffman S L, Sherwood J A, Hollingdale M R, Neva F A, Hockmeyer W T, Gordon D M, Schneider I, Wirtz R A, Young J F, Waserman J F, Reeve P, Diggs C L, Chulay J D. Safety and efficacy of recombinant Plasmodium falciparum sporozoite DNA vaccine. Lancet. 1987;i:1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 3.Ballou W R, Rothbard J, Wirtz R A, Gordon D M, Williams J S, Gore R W, Schneider I, Hollingdale M R, Beaudoin R L, Maloy W L, Miller L H, Hockmeyer W T. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985;228:996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Altanals P, Sabchareon A, Changsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H D, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks B R, Pastor R W, Carson F W. Theoretically determined three dimensional structure for the repeating tetrapeptide unit of the circumsporozoite coat protein of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1987;84:4470–4474. doi: 10.1073/pnas.84.13.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerami C, Frevert U, Sinnis P, Tackacs B, Clavejo P, Santos M J, Nussenzweig V. The basolateral domain of hepatocyte plasma membrane bears the receptor for CSP of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1023. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 8.Certa U, Ghersa P, Dobeli H, Matile H, Kochar H P, Srivastava I K, Shaw A R, Perrin L H. Aldolase activity of Plasmodium falciparum protein with protective properties. Science. 1988;240:1036–1038. doi: 10.1126/science.3285469. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Wery M, Sharma P, Chauhan V S. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect Immun. 1995;63:4375–4381. doi: 10.1128/iai.63.11.4375-4381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S, Sharma P, Kumar S, Chauhan V S. Fine specificity of immune responses to epitopic sequences in synthetic peptides containing B and T epitopes from conserved P. falciparum blood stage antigens. Vaccine. 1994;13:1474–1481. doi: 10.1016/0264-410x(94)00052-o. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Butcher G A, Mitchell G H, Deans J A, Langhorn J. Acquired immunity and vaccination in malaria. Am J Trop Med Hyg. 1977;26:223–227. doi: 10.4269/ajtmh.1977.26.223. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, McGregor I A, Carrington S C. Gamma globulin and acquired immunity to malaria. Nature (London) 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 13.Dame J B, Williams J L, McCutchan T F, Weber J L, Wirtz R A, Rockmeyer W T, Maloy W L, Haynes J D, Schneider I, Roberts D, Sanders G S, Reddy E P, Diggs C L, Miller L H. Structure of the gene encoding the immunodominant surface antigen in the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 14.Dolan S A, Miller L H, Wellems T E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis M J, Fry C M, Rowlands D J, Bittle J L, Houghton R A, Lerner R A, Brown F. Immune response to uncoupled peptides of foot and mouth disease virus. Immunology. 1987;61:1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Golvano J, Lasarte J L, Sarobe P, Gullan A, Prieto J, Cuesta F B. Polarity of immunogen: implications for vaccine design. Eur J Immunol. 1990;20:2363–2366. doi: 10.1002/eji.1830201031. [DOI] [PubMed] [Google Scholar]
- 17.Good M F, Pombo D, Quakyi I A, Riley E M, Houghton R A, Menon A, Alling D W, Berzfosky J A, Miller L H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci USA. 1988;85:1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good M F, Moloy W L, Lunde M N, Margalit H, Cornetto J L, Smith G L, Moss B, Muller L H, Berzofsky J A. Construction of a synthetic immunogen: use of a new T-helper epitope on malaria circumsporozoite protein. Science. 1987;235:1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- 19.Goundis D, Reid B M. Properdin, the terminal complement components, thrombospondin and CSP of malaria parasites contain similar sequence motifs. Nature (London) 1988;335:82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- 20.Herrington D A, Clyde D F, Losonsky G, Cortesia M, Murphy J R, Dais J, Baqar S, Felix A M, Heighmer E P, Gillesen G, Nardin E, Nussenzweig R S, Nussenzweig V, Hollingdale M R, Levine M M. Safety and immunogenicity in man of synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature (London) 1987;328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman S L, Jones T R. Malaria vaccine development. Clin Microbiol Rev. 1994;7:303–310. doi: 10.1128/cmr.7.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard R J, Paloske B L. Target antigen for asexual malaria vaccine development. Parasitol Today. 1993;9:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 23.Kaslow D C, Quakyi I A, Syin C, Raum M G, Keister D B, Coligan J E, McCutchan T F, Miller L H. A vaccine candidate from sexual stage of human malaria that contains EGF like domains. Nature (London) 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Miller L H, Quakyi I A, Keister D B, Houghten R A, Maloy W L, Moss B, Berzfosky J A, Good M F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature (London) 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 25.Lambrose C, Vanderberg J. Synchronization of P. falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 26.Locksley R M, Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991;12:58–60. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 27.Milich D R, McLachlan A, Thornton G B, Hughes J L. Antibody production to the nucleocapsid and envelope of hepatitis B virus primed by single synthetic T-cell site. Nature (London) 1987;329:547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- 28.Muller H M, Reckman I, Hollingdale M R, Bujard H, Robson K J H, Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glyco conjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardin E H, Nussenzweig R S. T-cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 30.Nussenzweig R S, Nussenzweig V. Development of sporozoite vaccines. Philos Trans R Soc Lond. 1981;307:117–128. doi: 10.1098/rstb.1984.0113. [DOI] [PubMed] [Google Scholar]
- 31.Robinson-Taylor A W. Regulation of immunity to malaria: valuable lessons learned from murine model. Parasitol Today. 1995;11:334–341. doi: 10.1016/0169-4758(95)80186-3. [DOI] [PubMed] [Google Scholar]
- 32.Robson K J H, Hall J R S, Jennings M W, Harris T J R, Marsh K, Newbold C I, Tate W E, Weatherall D J. A highly conserved amino acid sequence in thombospondin, properdin, and sequence from sporozoites and blood stages of human malaria parasites. Nature (London) 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 33.Rogers W O, Rogers M D, Hedstrom R C, Hoffman S L. Characterisation of the gene encoding sporozoite surface protein, a protective Plasmodium yoelii sporozoite antigen. Mol Biochem Parasitol. 1992;53:45–52. doi: 10.1016/0166-6851(92)90005-5. [DOI] [PubMed] [Google Scholar]
- 34.Rogers W O, Malik A, Mellouck S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein-2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero P. Malaria vaccines. Curr Opin Immunol. 1992;4:432–441. doi: 10.1016/s0952-7915(06)80035-x. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai T, Ametani A, Nakamura Y, Shimizu N, Idota T, Kaminogawa S. Cryptic B cell determinant: in a short peptide T-cells do not induce antibody response of B-cells when their determinants entirely overlap each other. Int Immunol. 1995;5:793–800. doi: 10.1093/intimm/5.7.793. [DOI] [PubMed] [Google Scholar]
- 37.Sedegah M, Sim B K L, Mason C, Nutman T, Malik A, Roberts C, Johnson A, Ochola J, Koech D, Were B, Hoffman S L. Naturally acquired CD8+ cytotoxic T-lymphocytes against the Plasmodium falciparum circumsporozoite protein. J Immunol. 1992;149:966–971. [PubMed] [Google Scholar]
- 37a.Sejwali, P., et al. Unpublished data.
- 38.Sharma P, Bharadwaj A, Bhasin V K, Sailaja V N, Chauhan V S. Antibodies to a conserved-motif peptide sequence of the Plasmodium falciparum thrombospondin-related anonymous protein and circumsporozoite protein recognize a 78-kilodalton protein in the asexual blood stages of the parasite and inhibit merozoite invasion in vitro. Infect Immun. 1996;64:2172–2179. doi: 10.1128/iai.64.6.2172-2179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma P, Kumar A, Batni S, Chauhan V S. Codominant and reciprocal T-helper cell activity of epitopic sequences and formation of junctional B-cell determinants in synthetic T:B chimeric immunogens. Vaccine. 1993;11:1321–1326. doi: 10.1016/0264-410x(93)90102-4. [DOI] [PubMed] [Google Scholar]
- 40.Shear L H, Srinivasan R, Nolan T, Ng C. Role of IFN-γ in lethal and non-lethal malaria in susceptible and resistant murine hosts. J Immunol. 1989;143:2038–2044. [PubMed] [Google Scholar]
- 41.Stoute J A, Saloui M, Happner D G, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 42.Trager W, Jensen J B. Human malaria parasite in continuous culture. Science. 1975;143:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Charoenvit Y, Corradin G, Porozzi R, Hunter R L, Glenn G, Alving C R, Church P, Hoffman S L. Induction of polyclonal antibodies by immunisation with Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J Immunol. 1995;154:2784–2793. [PubMed] [Google Scholar]
- 44.White K, Krzych U, Gordon D M, Porter T G, Richards R L, Alving C R, Deal C D, Hollingdale M, Silverman C, Sylvester D R, Ballou W R, Gross M. Induction of cytolytic and antibody response using Plasmodium falciparum repeatless circumsporozoite protein encapsulated in liposomes. Vaccine. 1993;11:1341–1346. doi: 10.1016/0264-410x(93)90105-7. [DOI] [PubMed] [Google Scholar]
- 45.White W I, Evans C B, Taylor D W. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991;59:3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrightsman R A, Dawson B D, Fouts D L, Manning J E. Identification of immunodominant epitopes in Trypanosoma cruzi trypomastigote surface antigen-1 protein that mask protective epitopes. J Immunol. 1994;153:3148–3154. [PubMed] [Google Scholar]