Abstract
We previously showed that acapsular transposon Tn916 mutants of Erysipelothrix rhusiopathiae are avirulent for mice. In this study, we constructed nonreverting acapsular mutants and examined the vaccine potential of the mutants in mice. A representative acapsular transposon mutant, 33H6, was plated on selective agar containing autoclaved chlortetracycline and quinaldic acid, and two tetracycline-sensitive mutants were obtained. Sequence analysis of chromosomal regions of the mutants in which Tn916 had flanked revealed that Tn916 had spontaneously excised from the region and that the six new nucleotides, which were presumably inserted with Tn916 into 33H6 chromosome, substituted for those present at the insertion site. The mutants were confirmed to be devoid of capsular antigen by Western immunoblotting and were nonvirulent for mice (subcutaneous 50% lethal dose [LD50], >109 CFU). The safety and efficacy of acapsular mutants for live vaccines was further studied by using one mutant strain, named YS-1. The YS-1 bacteria were cleared from the skin sites of inoculation, livers, and spleens of the inoculated mice by 7 days after subcutaneous (s.c.) inoculation. Mice immunized s.c. with doses ranging from 2 × 104 to 2 × 108 CFU of strain YS-1 were completely protected against challenge with 100 LD50 of the homologous, highly virulent strain Fujisawa-SmR 21 days postimmunization, and protective immunity conferred by immunization with 2 × 108 CFU of the strain lasted for as long as the 3 months of the observation period. In passive immunization experiments, sera collected from mice immunized with strain YS-1 at days 14 and 21 postimmunization provided protection against challenge with Fujisawa-SmR, whereas sera collected at days 4 and 7 did not. Furthermore, specific spleen cell responses to E. rhusiopathiae antigens were observed in mice immunized with strain YS-1, and cross-protection against the antigenically heterologous bacterium Listeria monocytogenes was observed at 7 days after immunization in the mice, suggesting that cell-mediated immunity had been induced. These results suggest that E. rhusiopathiae YS-1 may be a suitable choice for further studies of vaccine efficacy in swine.
Erysipelothrix rhusiopathiae is a gram-positive bacterium which causes a wide spectrum of disease in animals, birds, and humans (35). It is a cause of economic losses in swine and turkey industries (35). Vaccination against erysipelas infection in swine has been carried out by use of either live attenuated vaccines or bacterins (34, 35). Attenuation of current live vaccine strains was accomplished by air drying or by growth in media containing acridine dyes (35). However, the mechanisms of attenuation remain unknown, and it is possible that the attenuated vaccine strains can regain their virulence, casting doubt on their potential safety. In Japan, an acriflavin-fast attenuated live vaccine (24) has been used. However, this vaccine has the disadvantage of disease-causing potential in specific-pathogen-free pigs, indicating a clear need for urgent development of safer vaccines.
The development of highly effective vaccines requires an understanding of the pathogenesis of the organism at the molecular level so that genetically defined mutations may be introduced into the virulence genes to produce live vaccine strains. So far, studies on virulence mechanisms of the organism show an association between either hyaluronidase (19) or neuraminidase (13) production and virulence. In addition, virulent strains adhere better to porcine kidney cells in vitro than do avirulent strains (29). However, the roles of these factors in pathogenesis of the disease have not been well investigated.
Previously, using transposon mutagenesis with self-conjugative transposon Tn916, which confers resistance to tetracycline, we constructed avirulent transposon mutants by filter mating from the highly virulent strain Fujisawa-SmR (27). In contrast to the parent strain, these mutants lack a capsule on the cell surface, fail to resist phagocytosis by murine polymorphonuclear leukocytes (27), and cannot survive within murine macrophages (28), suggesting that the capsule is an important virulence factor of the organism.
To construct a mutant carrying a mutation in the gene involved in production of capsular material, we have exploited a property of Tn916. Transposon Tn916 can precisely excise from target DNA, and this restores the function of an insertionally inactivated gene (2, 9, 10, 27). However, excision of Tn916 from the target DNA can sometimes substitute six new nucleotides (coupling sequences), which are introduced with the transposon, for those present in the target sequences (2, 4), resulting in inactivation of the gene (2). In this study, using this property of Tn916, we constructed nonreverting acapsular mutants from a representative acapsular mutant 33H6 and examined the safety and protective capability of the mutants in mice.
MATERIALS AND METHODS
Mice.
Seven- to nine-week-old female BALB/c mice were used throughout this study. They were purchased from Japan SLC, Inc., Hamamatsu, Japan.
Bacterial strains and growth media.
E. rhusiopathiae Fujisawa-SmR, a streptomycin-resistant spontaneous mutant of the highly virulent strain Fujisawa, and its transposon mutant derivative strain 33H6 (27), which is deficient in capsule production, were used. Bacterial strains were usually grown in brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) containing 0.1% Tween 80 (pH 7.6) (BHI-T80). Listeria monocytogenes EGD was used for cross-protection experiments. L. monocytogenes was grown in BHI (Difco).
Isolation of nonreverting acapsular mutants.
Nonreverting acapsular mutants were selected from tetracycline-sensitive (Tcs) clones, which were generated when Tn916 was excised from the chromosome, from a transposon mutant strain 33H6. Positive selection of Tcs clones from 33H6 was performed by using autoclaved chlortetracycline as previously described (1, 17), with modifications. Briefly, one solution containing 37 g of BHI (Difco), 15 g of Bacto Agar (Difco), 0.05 g of chlortetracycline hydrochloride (Sigma Co., St. Louis, Mo.), and 1 ml of Tween 80 (Tokyo Kasei, Tokyo, Japan) in 500 ml of distilled water and another solution containing 3 g of Tris in 500 ml of distilled water (pH 7.4) were separately autoclaved for 20 min, mixed, and cooled at pouring temperature (ca. 50°C); 5 ml of ZnCl2 (20 mM) and 10 ml of quinaldic acid (10 mg/ml) were added to the mixture, and the selective medium was dispensed into plates. Strain 33H6 was grown in BHI-T80 for 14 h at 37°C, diluted with BHI-T80, and then plated on the selective medium at a cell density of 106 per plate.
Virulence testing.
Bacterial strains were grown in BHI-T80 overnight at 37°C and diluted with BHI-T80. Groups of five mice were inoculated subcutaneously (s.c.) with 0.1 ml of serial dilutions of bacterial suspension and observed for death over a period of 4 weeks. The 50% lethal doses (LD50) of the mutants were determined as previously described (21).
PCR and DNA sequencing.
Chromosomal DNA from Erysipelothrix strains was prepared by the method of Gálan and Timoney (8). With primers HM1-1 (5′-TATCTTTGTAGCGGTAGTTGG-3′) and HM1-2 (5′-CAATAAAAGGAAATACCAGTGC-3′), which were designed from sequences adjacent to the inserted transposon in 33H6 (25), the target DNA region was amplified. For amplification of the left hand end of the Tn916-chromosomal junction region in 33H6 chromosome, primers TNLO-2 (5′-GTGAAGTATCTTCCTAC-3′) (4) and Tn-Insert1 (5′-TCCATACGAATTTTACG-3′) were used. PCR was performed on a model 2400 thermal cycler (Perkin-Elmer). Amplifications were performed for 30 cycles, with each cycle consisting of 60 s of melting at 94°C, 30 s of annealing at 50°C, and 60 s of extension at 72°C. All DNA products of PCR were cloned into pCRII (Original TA Cloning kit; Invitrogen), and both strands of DNA were sequenced by the dideoxy-sequencing technique of Sanger et al. (23), using a model 377 DNA sequencer (Applied Biosystems).
Detection of capsular antigens.
The absence of capsular antigen on the cell surface of the mutants was confirmed by Western immunoblotting with monoclonal antibody (MAb) ER21, which is specific for the capsular antigen (26). Cell surface antigens of bacterial strains were prepared by extraction with Triton X-100 as described by others (8, 14). Briefly, the bacterial strains were grown in 10 ml of BHI-T80 for 14 h at 37°C. Cells were harvested by centrifugation, washed once with 20 mM Tris-HCl (pH 7.6), and suspended in 0.5 ml of 20 mM Tris (pH 7.6) containing 0.5% Triton X-100. Cells were incubated at 37°C for 1 h with rotation. Cells were removed by centrifugation, and the supernatants were used for capsular antigen detection by immunoblotting analysis described below. Cell surface antigens were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by the method of Laemmli (15), with a 12.5% separating gel and a 4% stacking gel. Immunoblotting analysis was performed as described by Towbin et al. (32). Antigens transferred to a polyvinylidene difluoride membrane were probed with MAb ER21. After incubation with ER21, the membrane was treated first with biotin-labeled rabbit anti-mouse immunoglobulin A (IgA), IgG, and IgM (Zymed Laboratories, Inc., San Francisco, Calif.) and next with peroxidase-labeled streptavidin (Zymed). Staining of immunoreactive bands was performed with 0.03% 3,3′-diaminobenzidine tetrahydrochloride dihydrate and 0.003% hydrogen peroxide in phosphate-buffered saline.
Growth of strain YS-1 in vivo.
Mice were inoculated s.c. with 0.1 ml of bacterial suspension (2 × 109/ml). At 12 h and on days 1, 2, 4, 7, 14, and 21 postinoculation, groups of three mice were killed with ether, and skin sites of inoculation, spleens, and livers were removed and weighed. The tissues were homogenized in BHI-T80, diluted, and plated onto BHI-T80 agar supplemented with crystal violet (10 μg/ml; Merck, Darmstadt, Germany). After incubation for 48 h at 37°C, bacterial colonies were counted.
Protection experiments.
For protection experiments, groups of mice were immunized s.c. with 0.1 ml of appropriate dilutions of the bacterial suspension of strain YS-1. After immunization, mice were challenged s.c. with 100 LD50 of Fujisawa-SmR and were observed for clinical symptoms and death over a period of 4 weeks.
Passive protection experiments.
Passive protection experiments were performed as previously described (33). Briefly, serum was collected from a group of nonimmunized mice and pooled for use as a control. Mice were immunized s.c. with 2 × 108 cells of YS-1, and groups of these mice were bled 4, 7, 14, and 21 days later. The sera from the mice within a group were pooled for test samples. For opsonization, 0.5 ml of bacterial suspension of Fujisawa-SmR was incubated at 37°C for 30 min with 0.5 ml of the pooled sera and diluted with BHI-T80. Mice were challenged s.c. with 0.1 ml of the dilutions containing 100 LD50 of Fujisawa-SmR and observed for clinical symptoms and death over a period of 4 weeks.
Spleen cell proliferation assay.
Groups of three mice were immunized s.c. with 2 × 108 CFU of strain YS-1. Spleen cells were obtained from nonimmunized mice and mice immunized with YS-1 at 7, 14, and 21 days postimmunization. Spleen cells were suspended in RPMI (Sigma) supplemented 10% fetal bovine serum, 2 mM l-glutamine, 1 mM pyruvate, 50 U of penicillin per ml, 50 μg of streptomycin per ml and 5 × 10−5 M 2-mercaptoethanol (complete medium) at a cell density of 2 × 106 per ml. One hundred microliters of cell suspension was incubated at 37°C in 5% CO2 with 100 μl of complete medium containing 10 μg of formalin-killed whole Fujisawa-SmR cells per ml in wells of 96-well microtiter plates. After the 4-day incubation, cell cultures were pulsed for 12 h with 1.0 μCi of [3H]thymidine per well. Cells were then harvested, and radioactivity was measured as counts per minute in a liquid scintillation counter. Cell proliferation results were expressed as mean of the stimulation index ± standard error of the mean (SEM). The stimulation index is derived as follows: mean counts per minute of treated cells/mean counts per minute of controls (three replicates of each).
Cross-protection experiments.
Mice were challenged intravenously at 7 and 14 days after YS-1 immunization with 4 × 103 CFU of L. monocytogenes EGD suspended in phosphate-buffered saline. Three days after challenge, spleens and livers of the mice were removed, homogenized, diluted, and plated onto BHI (Difco) agar. After incubation for 48 h at 37°C, bacterial colonies were counted.
Statistical methods.
Statistical analyses were performed with Student’s unpaired t test.
RESULTS
Isolation of nonreverting acapsular mutants.
To isolate nonreverting acapsular mutants, we modified the medium used in the studies reported by Bochner et al. (1) and Maloy and Nunn (17) for application to E. rhusiopathiae. In those studies, autoclaved chlortetracycline and quinaldic acid or fusaric acid were used for positive selection of Tcs cells from a population of tetracycline-resistant (Tcr) Escherichia coli cells carrying tetracycline resistance transposons, such as Tn10. Interestingly, autoclaved chlortetracycline and quinaldic acid in BHI-T80 were found to be toxic to Tcr cells (33H6) carrying Tn916 but not to isogenic Tcs cells (Fujisawa-SmR) (data not shown). These results suggested that the selective medium could be used for positive selection of Tcs cells with Tn916 excision from a predominantly Tcr population. The concentrations of the reagents added to selective medium and pH were optimized as described in Materials and Methods, and 106 cells of 33H6 were plated on the selective medium.
Approximately 300 to 500 colonies per plate arose on the selective media. However, most of the colonies were found to be Tcr. A total of 10,000 colonies were screened for tetracycline susceptibility by replica plating, and two independent Tcs clones were obtained from different experiments. The mutants, designated YS-1 and YS-2, were further analyzed.
DNA sequencing.
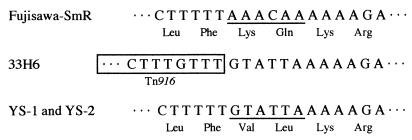
To determine whether sequence differences occurred after Tn916 excision from the chromosome compared to native sequences, the corresponding regions of Fujisawa-SmR, 33H6, and the mutants were sequenced and compared. The results revealed that Tn916 presumably inserted into the chromosome with six nucleotides (GTATTA) and that when Tn916 was excised from the chromosome of 33H6, a substitution of the six nucleotides for those (AAACAA) present in the target gene occurred, resulting in change of two amino acids in the gene product (Fig. 1).
FIG. 1.
Nucleotide sequence of the target site of Fujisawa-SmR and nonreverting acapsular mutants and of the corresponding regions of 33H6. The deduced amino acid sequence is shown under the nucleotide sequence. The mutated sequences are underlined. The sequences of left end of Tn916 are boxed. Only one strand of DNA is presented.
Virulence testing.
Virulence of the mutants was evaluated by determining their LD50 after subcutaneous inoculation in mice. The LD50 of Fujisawa-SmR was estimated to be about 101.2 bacteria per mouse, whereas the mutants were nonvirulent at the dose of 109. The mutants, which were recovered from the livers of the infected mice 2 days after inoculation, were found to be avirulent. They did not revert to virulence after several passages through mice, suggesting that the mutants are genetically stable.
Detection of capsular antigen.
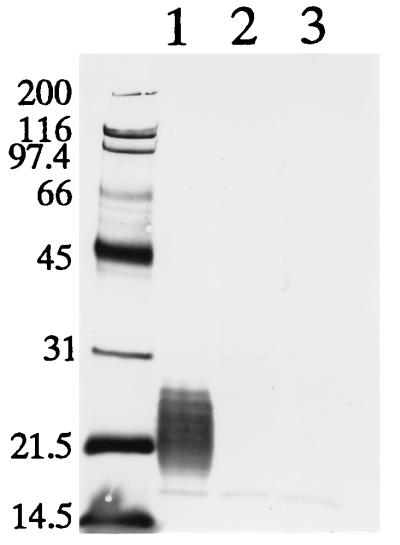
To examine whether the mutants were devoid of capsular antigen, we performed Western immunoblotting with MAb ER21 against capsular antigen (26). Immunoblotting analysis showed that low-molecular-weight capsular antigen which was abundant in a sample from strain Fujisawa-SmR was absent in the samples from the mutant strains (Fig. 2), showing that substitutions of two amino acids in the gene product resulted in the loss of capsule expression. This result, taken together with virulence testing, suggests that a major virulence determinant of E. rhusiopathiae in mice is the capsule.
FIG. 2.
Immunoblotting analysis of capsular antigen of E. rhusiopathiae strains with MAb ER21. The bacterial surface antigens prepared by extraction with Triton X-100 were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was treated with MAb ER21 specific for capsular antigen. Lane 1, Fujisawa-SmR; lane 2, YS-1, lane 3, YS-2. Molecular size markers (in kilodaltons) are shown on the left.
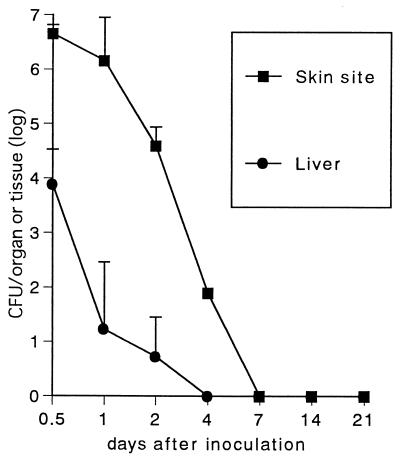
Growth of YS-1 strain in vivo.
The safety and live vaccine potential of acapsular mutants was further studied in mice by using strain YS-1. To examine whether nonreverting acapsular mutants can colonize in mouse tissues after s.c. inoculation, growth curves of YS-1 in skin inoculation sites, spleens, and livers were monitored. As seen in Fig. 3, organisms were recovered from livers until day 2 and cleared between 2 and 4 days postinoculation. From the skin lesions, large numbers of the organisms were recovered until day 2 postinoculation; however, bacterial counts in the tissue sharply dropped thereafter, and organisms could not be detected at 7 days postinoculation. YS-1 cells could not be recovered from spleens of infected mice throughout the experiments.
FIG. 3.
Growth curve of strain YS-1 in mouse tissues. Mice were inoculated s.c. with 2 × 108 CFU of the bacteria and killed at various time points over 21 days. Skin sites of the inoculation, spleens, and livers of mice were removed, weighed, and homogenized, and then bacterial cells were counted. No bacteria were recovered from the spleens throughout the experiments. Results are expressed as the mean ± SEM for three mice.
Protection experiments.
Mice immunized s.c. with 0.1 ml of serial dilutions of the YS-1 suspension were challenged s.c. with 100 LD50 of Fujisawa-SmR. Following vaccination, all mice remained healthy with no symptoms before challenge, and vaccinated mice challenged with the virulent strain 21 days postimmunization were completely protected without any clinical symptoms. Furthermore, the protective immunity conferred by immunization with 2 × 108 CFU of the strain lasted for up to 3 months (Table 1), showing that immunization of strain YS-1 could induce long-lasting immunity. All control mice died within 4 days after challenge.
TABLE 1.
Protection of mice against virulent E. rhusiopathiae strain by graded doses of YS-1
| Immunizationa | Time of challengeb | Immunizing dose (CFU) | No. of survivors/totalc |
|---|---|---|---|
| YS-1 | 21 days | 2 × 108 | 10/10 |
| 2 × 107 | 10/10 | ||
| 2 × 106 | 10/10 | ||
| 2 × 105 | 10/10 | ||
| 2 × 104 | 10/10 | ||
| BHI-T80 | 0/10 | ||
| YS-1 | 2 mo | 2 × 108 | 7/7 |
| BHI-T80 | 0/7 | ||
| YS-1 | 3 mo | 2 × 108 | 7/7 |
| BHI-T80 | 0/8 |
Mice were immunized s.c. with 0.1 ml of bacterial suspension. Controls were injected with 0.1 ml of broth medium.
Time after immunization.
Mice were challenged s.c. with 100 LD50 of virulent E. rhusiopathiae Fujisawa-SmR.
Passive protection experiments.
In erysipelas infection, antibodies are known to play an important role in protection as opsonins (34–36). To determine whether vaccination with acapsular mutants could induce protective antibodies against infection, passive protection experiments were performed with sera from mice immunized with strain YS-1. Mice challenged with the virulent strain opsonized with serum from nonimmunized mice or with sera collected at 4 and 7 days postimmunization died within 4 days after challenge, whereas mice challenged with the strain opsonized with sera collected at 14 and 21 days postimmunization survived without any clinical symptoms (Table 2).
TABLE 2.
Passive protection of mice against virulent E. rhusiopathiae strain by the serum from mice immunized with strain YS-1
Mice were immunized s.c. with 2 × 108 of bacteria in 0.1 ml of broth medium. Controls were injected with 0.1 ml of broth medium.
Time of collection of the mouse serum after immunization with strain YS-1.
Five mice were challenged s.c. with 100 LD50 of virulent E. rhusiopathiae Fujisawa-SmR opsonized with the serum for 30 min at 37°C.
Spleen cell proliferation assay.
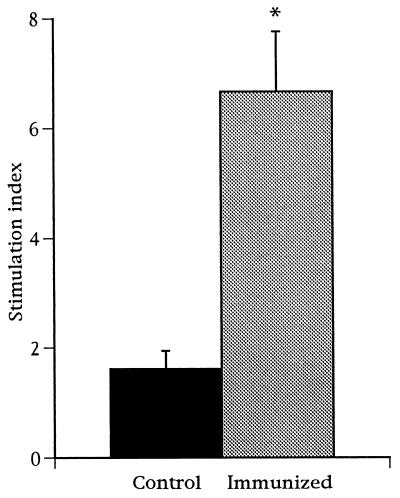
Although protective immunity against erysipelas infection is mediated by antibodies, the involvement of cell-mediated responses as well has been suggested (28, 30, 31). To test whether immunization with strain YS-1 can induce cell-mediated immunity, spleen cells were obtained from mice at days 7, 14, and 21 postimmunization and cellular proliferation in response to E. rhusiopathiae antigens was assayed in vitro. As shown in Fig. 4, spleen cells from YS-1-immunized mice proliferated significantly in response to E. rhusiopathiae antigens.
FIG. 4.
Spleen cell proliferation in response to E. rhusiopathiae antigens in mice immunized with strain YS-1. Mice were immunized with 2 × 108 CFU of the bacteria 1 to 3 weeks before preparation of spleen cells. Spleen cell cultures (2 × 105/well) were incubated with 10 μg of formalin-killed E. rhusiopathiae per ml for 4 days. Results are expressed as the mean of stimulation index ± SEM. Asterisk indicates difference (P < 0.05) between immunized group (n = 9) and control group (n = 3), using Student’s unpaired t test.
Cross-protection experiments.
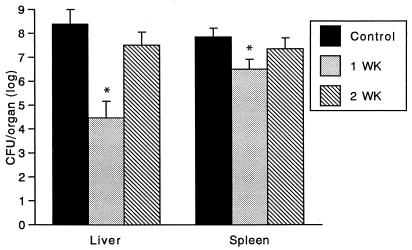
Nonspecific resistance to heterologous bacteria is an indicator of cell-mediated immunity in intracellular bacterial infection (6, 7, 16, 37). To examine this, mice were challenged 7 and 14 days after YS-1 immunization with the antigenically unrelated intracellular parasite L. monocytogenes, and growth of the bacteria in the spleens and livers of the mice was monitored. As shown in Fig. 5, inhibition of growth of L. monocytogenes in liver and spleen from mice immunized with YS-1 was observed at 7 days after immunization, indicating that cross-protection against L. monocytogenes had occurred. These results suggest that immunization with YS-1 induced specific and nonspecific cell-mediated resistance and that the cell-mediated immune response contributed to protection against E. rhusiopathiae.
FIG. 5.
Effect of immunization of mice with strain YS-1 on clearance of L. monocytogenes from the liver and spleen. Mice were immunized s.c. with strain YS-1 (2 × 108 CFU) 1 or 2 weeks prior to intravenous challenge with 4 × 103 CFU of L. monocytogenes EGD. L. monocytogenes bacteria in the organs were enumerated 3 days after challenge infection. Results are expressed as the mean ± SEM for four mice. Asterisks indicate differences (P < 0.05) between immunized group (n = 4) and control group (n = 4), using Student’s unpaired t test.
DISCUSSION
Using the conjugative transposon Tn916, we previously isolated acapsular mutants from the highly virulent strain Fujisawa-SmR and showed that these mutants are totally avirulent in mice (27). In subsequent experiments with transposon mutant 33H6, we found that this mutant caused no clinical symptoms of the disease in germ-free piglets at a dose of 108 but conferred on the animals almost complete protection against a challenge with 6 × 107 cells of the highly virulent, wild-type strain Fujisawa (data not shown). These results prompted us to construct nonreverting mutants with a mutation within the gene which Tn916 inserted in 33H6 for vaccine studies in swine erysipelas. We found that a single Tn916 insertion occurred within the 33H6 chromosome (27) and that it insertionally inactivated a gene which potentially encodes a protein showing significant homology with glycosyltransferases of other gram-positive and gram-negative encapsulated bacteria (unpublished data). To induce a stable mutation in this gene and construct nonreverting acapsular mutants, we exploited the property of conjugative transposon Tn916, in which the element sometimes generates new sequences at the insertion point after spontaneous excision (2, 4), resulting in a mutation within the gene (2). To our knowledge, this is the first approach using this property of Tn916 to isolate nonreverting mutants in gram-positive bacteria. We previously showed that a capsule-producing, Tcs revertant clone in which Tn916 precisely excised from the chromosome was obtained from 33H6 at a rate of 10−8 per bacterium (27). In the present study, we could select two nonreverting acapsular mutants from 104 colonies of 33H6. Thus, this method greatly facilitated isolation of the clones with excision of Tn916 from the chromosome. The conjugative transposon Tn916 has been successfully used for genetic studies in gram-positive bacteria (9–12, 18, 22). Therefore, the strategy used in this study may allow the immediate development of genetically defined attenuated strains for vaccine studies from other gram-positive bacterial species for which no electroporation system is yet available.
We evaluated E. rhusiopathiae mutant YS-1 as a live vaccine candidate in a mouse model. No growth of bacteria was observed in the tissues of inoculated mice, suggesting that the strain is highly safe for animals. Despite the low infectivity, the mutant was nevertheless highly immunogenic. A possible explanation for this may be the persistence of a large number of bacteria in the skin lesions at the initial stage of vaccination. This hypothesis may be strengthened by the findings (3, 6, 20) that the important event for the development of an immune response to an antigen is the initial amount of antigens that stimulate immune systems and not prolonged persistence of the antigens. However, to support the hypothesis, further studies are needed.
Recombinant live vaccines may offer an attractive approach to the control of various infectious diseases. Gram-negative bacteria have been extensively examined as live vectors to deliver foreign antigens and some recombinant bacterial species strains, especially Salmonella spp., have shown to trigger both humoral and cell-mediated immunity (5). However, studies with recombinant gram-positive bacteria which can trigger both humoral and cell-mediated immunity have been few. In this study, immunization with the acapsular E. rhusiopathiae strain YS-1 induced not only humoral immunity but also cell-mediated immunity. These properties may have important consequences for the abilities of live-carrier vaccines to present cloned antigens. Our results also suggest that E. rhusiopathiae YS-1 may be a good candidate for a recombinant vaccine vector.
ACKNOWLEDGMENTS
We thank K. Arai for excellent technical assistance. We also thank M. Osaki for helpful suggestions regarding the experiments.
This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Bochner B R, Hung H, Steven G L, Ames B N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143:926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caparon M G, Scott J R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989;59:1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas L, Clements J D. Stability, immunogenicity and expression of foreign antigens in bacterial vaccine vectors. Vaccine. 1993;11:126–135. doi: 10.1016/0264-410x(93)90007-k. [DOI] [PubMed] [Google Scholar]
- 4.Clewell D B, Flannagan S E, Ike Y, Jones J M, Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988;170:3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtiss R, III, Kelly S M, Hassan J O. Live oral avirulent Salmonella vaccines. Vet Microbiol. 1993;37:397–405. doi: 10.1016/0378-1135(93)90038-9. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein T K, Killar L M. Immunity to Salmonella typhimurium in C3H/HeJ and C3H/HeNCrIBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenstein T K, Killar L M, Stocker B A D, Sultzer B M. Cellular immunity by avirulent Salmonella in LPS-defective C3H/HeJ mice. J Immunol. 1984;133:958–961. [PubMed] [Google Scholar]
- 8.Gálan J E, Timoney J F. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. 1990;58:3116–3121. doi: 10.1128/iai.58.9.3116-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gawron-Burke C, Clewell D B. A transposon in Streptococcus faecalis with fertility properties. Nature. 1982;300:281–284. doi: 10.1038/300281a0. [DOI] [PubMed] [Google Scholar]
- 10.Gawron-Burke C, Clewell D B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984;159:214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivins B E, Welkos S L, Knudson G B, Leblanc D J. Transposon Tn916 mutagenesis in Bacillus anthracis. J Bacteriol. 1988;56:176–181. doi: 10.1128/iai.56.1.176-181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasemann C, Müller H E. The virulence of Erysipelothrix rhusiopathiae strains and their neuraminidase production. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig Reihe A. 1975;231:206–213. [PubMed] [Google Scholar]
- 14.Lachmann P G, Deicher H. Solubilization and characterization of surface antigenic components of Erysipelothrix rhusiopathiae T28. Infect Immun. 1986;52:818–822. doi: 10.1128/iai.52.3.818-822.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Mackaness G B. The immunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–120. [PubMed] [Google Scholar]
- 17.Maloy S R, Nunn W D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981;145:1110–1112. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nida K, Cleary P P. Insertional inactivation of streptolysin S expression in Streptococcus pyogenes. J Bacteriol. 1983;155:1156–1161. doi: 10.1128/jb.155.3.1156-1161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nørrung V. Studies on Erysipelothrix insidiosa s. rhusiopathiae. 1. Morphology, cultural features, biochemical reactions and virulence. Acta Vet Scand. 1970;11:577–585. doi: 10.1186/BF03547956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral responses in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:693–697. [Google Scholar]
- 22.Ruben C E, Wessels M R, Heggen L M, Kasper D L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci USA. 1987;84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;86:699–703. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seto K, Nishimura Y, Fujiki M, Azechi H, Suzuki K. Studies on acriflavin-fast attenuated Erysipelothrix insidiosa. Comparison on pathogenicity and immunogenicity between mice and pigs. Jpn J Vet Sci. 1971;33:161–171. doi: 10.1292/jvms1939.33.161. [DOI] [PubMed] [Google Scholar]
- 25.Shimoji Y, Mori Y, Hyakutake K, Sekizaki T, Yokomizo Y. Use of an enrichment broth cultivation-PCR combination assay for rapid diagnosis of swine erysipelas. J Clin Microbiol. 1998;36:86–89. doi: 10.1128/jcm.36.1.86-89.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoji, Y., Y. Mori, M. Kobayashi, and Y. Yokomizo. Unpublished data.
- 27.Shimoji Y, Yokomizo Y, Sekizaki T, Mori Y, Kubo M. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect Immun. 1994;62:2806–2810. doi: 10.1128/iai.62.7.2806-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoji Y, Yokomizo Y, Mori Y. Intracellular survival and replication of Erysipelothrix rhusiopathiae within murine macrophages: failure of induction of the oxidative burst of macrophages. Infect Immun. 1996;64:1789–1793. doi: 10.1128/iai.64.5.1789-1793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi T, Hirayama N, Sawada T, Tamura Y, Muramatsu M. Correlation between adherence of Erysipelothrix rhusiopathiae strains of serovar 1a to tissue culture cells originated from porcine kidney and their pathogenicity in mice and swine. Vet Microbiol. 1987;13:57–64. doi: 10.1016/0378-1135(87)90098-8. [DOI] [PubMed] [Google Scholar]
- 30.Timoney J. The inactivation of Erysipelothrix rhusiopathiae in macrophages from normal and immune mice. Res Vet Sci. 1969;10:301–302. [PubMed] [Google Scholar]
- 31.Timoney J. The inactivation of Erysipelothrix rhusiopathiae in pig buffy-coat leukocytes. Res Vet Sci. 1970;11:189–190. [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter A J, Duncan J R, Santisteban C G, Douglas J T, Adams L G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989;57:3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood R L. Swine erysipelas—a review of prevalence and research. J Am Vet Med Assoc. 1984;184:944–949. [PubMed] [Google Scholar]
- 35.Wood R L. Erysipelas. In: Leman A D, et al., editors. Diseases of swine. Ames, Iowa: Iowa State University Press; 1992. pp. 475–486. [Google Scholar]
- 36.Yokomizo Y, Isayama Y. Antibody activity of IgM and IgG fractions from rabbit anti-Erysipelothrix rhusiopathiae sera. Res Vet Sci. 1972;13:294–296. [PubMed] [Google Scholar]
- 37.Zinkernagel R M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976;13:1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]