Abstract
To adapt to different environmental conditions Sinorhizobium meliloti relies on finely tuned regulatory networks, most of them unexplored to date. We recently demonstrated that deletion of the two-component system ActJK renders an acid-vulnerable phenotype in S. meliloti and negatively impacts on bacteroid development and nodule occupancy, as well. To fully understand the role of ActJ in acid tolerance, S. meliloti wild-type and S. meliloti ΔactJ proteomes were compared in the presence or absence of acid stress by nanoflow ultrahigh-performance liquid chromatography coupled to mass spectrometry. The analysis demonstrated that proteins involved in the synthesis of exopolysaccharides (EPS) were notably enriched in ΔactJ cells in acid pH. Total EPS quantification further revealed that although EPS production was augmented at pH 5.6 in both the ΔactJ and the parental strain, the lack of ActJ significantly enhanced this difference. Moreover, several efflux pumps were found downregulated in the ΔactJ strain. Promoter fusion assays suggested that ActJ positively modulated its own expression in an acid medium but not at neutrality. The results presented here identifies several ActJ-regulated genes in S. meliloti, highlighting key components associated with ActJK regulation that will contribute to a better understanding of rhizobia adaptation to acid stress.
Keywords: ActJK, Sinorhizobium meliloti, acid stress, proteomics
Graphical Abstract

1. INTRODUCTION
Future generations will need at least a 20% increase in crop yields to meet the amounts necessary for worldwide food and nutritional security 1. Within the context of increasing agricultural productivity in a sustainable manner, symbiotic rhizobium-legume interactions play a crucial role in agricultural production systems through a reduction in industrial fertilizers use2,3. This natural approach provides the soil with nitrogen that enhances crop yields, thereby decreasing the use of industrial fertilizers and avoiding the contamination of cropping systems. Medicago sativa (alfalfa) is the most widely cultivated forage legume in many countries and is the highest-yielding perennial forage. To reach high yields and crop persistence, alfalfa requires well-drained soil, adequate fertilization, proper harvest management, and a soil pH above 6.1 4. Medicago – Sinorhizobium symbiosis and the survival of these symbiotic partners have been known for years to be extremely sensitive to acid stress 5–8. Therefore, the identification of acid tolerance genetic markers has been considered a key prerequisite for a better understanding of the mechanisms used by S. meliloti to survive and accomplish symbiosis under moderate acid stress.
Early studies have indicated that the rhizobial acid response displays a multigenic and pleiotropic phenotype in which the genes involved are associated with diverse cellular functions 9–16. Despite all these excellent studies, knowledge about the acid response network and its regulation in rhizobacteria remains incomplete.
Two-component systems (TCSs) are considered one of the main signal transduction mechanisms that enable bacteria to modify their gene expression in order to adapt to changing environments. A typical TCS comprises a sensor histidine kinase that detects certain changes in the environment and a response regulator, which generally acts as a transcriptional modulator. Upon activation, the histidine kinase becomes phosphorylated on a conserved histidine residue and modifies cognate response regulator activity by phosphorylation on a conserved aspartate 17.
In a recent study, we demonstrated that the TCS ActJK of S. meliloti played an essential role in survival at low pH and in symbiosis with alfalfa18. In particular, we observed that response regulator ActJ and histidine kinase ActK mutants had a reduced growth rate at pH 5.6 compared to wild-type strain. Regarding symbiotic performance, we found that both ActJ and ActK are necessary for full nodule occupancy and bacteroid development, since less bacteroids were observed in the plant nodule. Furthermore, we detected that plants inoculated with ΔactK showed a reduced shoot dry biomass production than plants inoculated with wild-type S. meliloti; while plants inoculated with ΔactJ did not. In the work reported here, an omics approach was performed to identify proteins under ActJ control when S. meliloti was exposed to acid stress. The proteomic responses of the S. meliloti 2011 wild type (wt) and the ΔactJ isogenic mutant (ΔactJ) to acid stress were compared through nanoflow ultrahigh-performance liquid chromatography coupled with a quadrupole Orbitrap mass spectrometer (LC-MS/MS). In order to corroborate the expression of some of the genes coding the differentially expressed proteins (DEPs) identified through a non-massive technique, the expression levels of some DEPs were analyzed by using a set of selected promoter fusions to the eGFP gene. In the end, this study provided new insights into S. meliloti acid stress response pathways that could contribute to the rational design of rhizobial inoculants with enhanced survival and symbiotic performance in moderately acid soils.
2. MATERIALS AND METHODS
2.1. Bacterial strains and growth conditions
Table S1 and S2 list the respective bacterial strains and plasmids used in this work. Rhizobia were grown at 28°C in tryptone-yeast medium (TY) 19 or in a defined glutamate-sucrose medium (GSM modified) containing 27.45 mM sucrose, 2.67 mM Na-glutamate, 0.15 mM K2HPO4·3H2O, 0.15 mM KH2PO4, 0.7 mM Na2SO4, 1.0 mM MgSO4·7H2O, 1.0 mM CaCl2·2H2O, 2.95 μM thiamine-HCl, 4.2 μM Ca-pantothenate, 0.08 μM biotin, 48.0 μM H3BO3, 10.0 μM MnSO4, 1.0 μM ZnSO4, 0.5 μM CuSO4, 0.5 μM CoCl2, 1.0 μM Na2MoO4·2H2O, and 1.0 μM FeCl3·6H2O 20. GSM was supplemented with 20 mM MES (2-[N-morpholino] ethanesulfonic acid) or 20 mM PIPES (piperazine N,N’-bis[2-ethanesulfonic acid]) buffer to maintain the pH at 5.6 or 7.0, respectively. When required, tryptone-yeast (TY) media were supplemented with 400 μg mL−1 streptomycin (Sm) or 5 μg mL−1 tetracycline (Tc). Escherichia coli strains were grown at 37 °C in Lysogeny Broth medium (LB) 21. When required, LB media were supplemented with 10 μg mL−1 Tc.
2.2. Total protein extraction and subcellular fractionation
To analyze the differential proteomes between S. meliloti 2011 wt and S. meliloti ΔactJ, GSM batch cultures at pH 7.0 (neutral condition) or pH 5.6 (stress condition) were grown to the exponential phase at OD600nm 0.50 ± 0.05. In this regard, an OD600nm of 0.5 in spectrophotometer cuvettes with an optical path of 1 cm is equivalent to an OD600nm of 0.25 in the microplate reader (Figure 1, optical path length of 0.516 cm). The samples were obtained from three independent cultures per condition and per strain.
Figure 1.

Growth curves in GSM at (A) pH = 7.0 (neutral condition) and (B) pH = 5.6 (acid stress). In the figure, the optical density OD600nm is plotted on the ordinate as a function of time on the abscissa. The black points represent S. meliloti 2011 wild-type cells (wt) and the light blue points represent the ΔactJ mutant. The error bars in each point represent the standard deviation of the mean among three replicates.
For protein extraction, three independent 250 mL bacterial cultures per condition and per strain were centrifuged at 10,000 g for 30 min at 4 °C. The cells were then washed twice in ice-cold Low Salt buffer (3 mM KCl, 9mM NaH2PO4, 1.5 mM KH2PO4, 6 mM NaCl). The pellets were resuspended in 6.5 mL of ice-cold 10 mM TRIS solution containing the protease inhibitor phenylmethylsulfonyl fluoride (0.25 M final concentration).
The samples were placed in vials containing 500 mg of glass beads (0.1 to 0.3 mm diameter) and lysed by 4 20-sec pulses at 5,500 rpm in a high-power homogenizer (Precellys, Bertin Instruments). The beads and cell debris were next separated by centrifugation at 10,000 g for 20 min at 4 °C and the resulting supernatants recovered and treated with DNase and RNase (0.2 U mL−1 and 8 ug mL−1, respectively) before storage at 4 °C.
To separate cytoplasmic from membrane-associated proteins, an ultracentrifugation at 100,000 g for 2 h at 4 °C was performed (Beckman Coulter, swinging rotor model SW 41 Ti). The membrane-enriched pellets were thereafter kept at 4 °C. The supernatants containing soluble proteins were incubated overnight in four volumes of cold acetone and afterwards precipitated by centrifugation (10,000 g, 30 min, 4 °C). Both cytoplasmic and membrane fractions were resuspended in a rehydration solution (7 M urea, 2 M thiourea, 10% [v/v] isopropanol, 2% [v/v] Triton X-100) and finally stored at −80 °C.
Proteins were quantified through the use of the Protein Assay Kit (Bio-Rad) and the sample quality was monitored by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on a 12% (w/v) polyacrylamide separating gel followed by Coomassie blue staining.
2.3. Protein reduction, alkylation, and precipitation
Samples containing 50 μg of total protein were reduced with 10 mM dithiothreitol dissolved in 50 mM of ammonium bicarbonate (56 °C, 45 min) and alkylated with 20 mM iodoacetamide dissolved in 50 mM of ammonium bicarbonate (25 °C, 40 min in the dark). The samples were then incubated in trichloroacetic acid, 1:5 [v/v], at −20 °C for 2 h and precipitated by centrifugation (10 min, 12,000 g, 4 °C). The pellets were washed twice with cool acetone and dried at room temperature.
2.4. Protein identification by liquid chromatography plus tandem mass spectrometry (LC/MS-MS)
For protein identification by LC/MS-MS, proteins were resuspended in 50 mM ammonium bicarbonate pH 8.0 and digested with trypsin (Promega V5111). The samples were then desalted with ZipTip C18 columns (Millipore) and the digests analyzed by nano LC-MS/MS in a QExactive Mass Spectrometer coupled to a nanoHPLC EASY-nLC 1000 (Thermo Scientific). For LC-MS/MS analysis, approximately 1 μg of peptides was loaded onto the column and eluted for 120 min from a reverse phase column [C18, 2 μm x 10 nm particle size, 100 A, 50 μm x 150 mm, serial number: 10487094, temperature 35 °C, Thermo Scientific, Easy-Spray Column PepMap RSLC (P/N ES801)] suitable for separating protein complexes with a high degree of resolution. The injection volume was 4 μl, and the flow rate used for the nanocolumn was 300 nL min−1 along with a two-solution gradient was used. Solvent A was 0.1% (v/v) formic acid in water and solvent B was 0.1% (v/v) formic acid in acetonitrile. The series of sequential solvent combinations were: 5% B for the first 5 min, 35% B for the next 100 min, followed by 100% B for 5 min and, finally, 100% B for a further 10 min. The MS equipment featured a high collision dissociation cell (HCD) for fragmentation and an Orbitrap analyzer (Thermo Scientific, model: Q-Exactive). A voltage of 3.5 kV was used for Electro Spray Ionization (Thermo Scientific, EASY-SPRAY).
XCalibur 3.0.63 software (Thermo Scientific) was used for data acquisition with an equipment configuration allowing peptide identification at the moment of their chromatographic separation. Full-scan mass spectra were acquired in the Orbitrap analyzer. The scanned mass range was 400-1800 m/z, at a resolution of full MS 70,000 and tandem MS-MS of 17,500 at 400 m/z. The 15 most intense ions in each cycle were sequentially isolated, fragmented by high-collision dissociation, and measured in the Orbitrap analyzer. The threshold for precursor ion selection was 10,000. The mass window for precursor ion selection was 1.6 m/z and the normalized collision energy 27. The charge state screening settings were all charges (excepting +1 and unassigned), while the dynamic exclusion parameters were 20.0 sec. This analysis was performed at the National University of Buenos Aires by the Center for Chemical and Biological Studies by Mass Spectrometry [Centro de Estudios Químicos y Biológicos por Espectrometria de Masa (CEQUIBIEM)].
2.5. Analysis of MS data
Q Exactive raw data were processed by means of the Proteome Discoverer software (version 2.1.1.21 Thermo Scientific) in combination with the S. meliloti 2011 protein sequences database with trypsin specificity and a maximum of two missed cleavages permitted per peptide. Proteome Discoverer searches were performed with a precursor mass tolerance of 10 ppm and product ion tolerance of 0.05 Da. Carbamidomethylation of cysteine residues was set as a static modification, and oxidation of methionine was set as variable modification. Protein hits were filtered for high confidence peptide matches with a maximum protein and peptide false-discovery rate (FDR) of 1% calculated by a reverse database strategy.
Finally, the data were analyzed with Perseus software (Max Planck Institute of Biochemistry – version 1.5.8.5). The replicates of each sample were used to construct volcano plots for the comparison of samples by the logarithmic values of the ratio of the areas under the peaks along with the statistical significance of those values assessed by the Student t-test. Hence, for each condition (neutral or stressed) and each fraction (cytoplasmic or membrane enriched) we plotted - log10 of the Student t-test p-value on the y axis versus Student t-test difference between the strains S. meliloti ΔactJ and S. meliloti 2011 wt. The DEPs were considered those with fold-changes greater than 2 (less than −1 or more than 1 on the x axis of the graph) and with p-values lower than 0.05 (of values of ≥1.3 on the y axis of the graph). The protein functional assignment and protein-protein interaction analyses were performed manually with the repositories of the Kyoto Encyclopedia of Genes and Genomes (KEGG) and STRING (https://string-db.org) 22, respectively.
2.6. Total EPS extraction and quantification
Cultures of S. meliloti 2011 wt and S. meliloti ΔactJ grown in GSM at pH 7.0 or pH 5.6 were used to extract the total EPS. The procedure stated in brief: cultures were centrifuged for 45 min at 10,000 g and the supernatants were incubated with 3 volumes of ethanol overnight at −20 °C. The total EPS were then precipitated by centrifugation (10,000 g, 30 min, 4 °C) and the resulting pellets air-dried and resuspended in Milli-Q water. Finally, quantification was performed by the anthrone method 23.
2.7. Transcriptional fusions and fluorescence assays
To evaluate promoter activities, approximately 300 base pairs (bp) upstream emrA, acrA, macA and degP1 transcription start sites were PCR-amplified and cloned in the pPHU231 vector24 in XbaI/PstI sites through the use of the primers listed in Table S2. Plasmids were introduced in S. meliloti by conjugation as previously described 25.
Cells containing reporter plasmids were cultured in GSM in a microplate with continuous agitation at 28 °C. To control and register cell growth (OD600) at 24 h postincubation (exponential phase of growth) we used a microplate reader (BMG Labtech, Germany). At the appropriate density, the cells were centrifuged and resuspended in 0.9% (w/v) NaCl at 1:2 volume ratio in order to eliminate background fluorescence of the medium. Fluorescence was measured in a fluorometer with blue excitation at ~470 nm and the relative fluorescence units (RFU) at emission collected between 514 and 567 nm. To determine the background fluorescence of the cells, we used strains carrying the pPHU231 vector with a promoterless enhanced-green-fluorescence protein (eGFP).
3. RESULTS AND DISCUSSION
3.1. Identification of ActJ-regulated proteins under neutral and acid conditions through a comparative proteomic approach
TCSs play a key role in adapting bacteria to environmental stresses. In S. meliloti the deletion of the gene that encodes the response regulator protein ActJ of the TCS ActJK leads to a S. meliloti strain with impaired ability to grow under suboptimal acid conditions and results in negative impacts on rhizobia symbiotic life, such as a diminished nodule occupancy and impaired bacteroid differentiation 18. In that initial work, we screened a S. meliloti mutant library to search for response regulator mutants with impaired ability to grow in a suboptimal acid condition. Such stress condition was previously stablished as pH 5.6 since S. meliloti was not able to grow below pH 5.6, while in the range of pH between 5.8 to 7.0, only minor deviations from the growth curve at pH 7.0 occurred 20. At pH 5.6, S. meliloti showed a reduced growth rate but the cell titer counts documented that this pH was not yet lethal18.
To more completely understand the regulation mediated by the ActJ response regulator in S. meliloti, we compared the protein expression profiles of S. meliloti 2011 wt and ΔactJ under acid and neutral conditions by using LC-MS/MS. Although growth differences were noticed in both the exponential and the stationary phases (Figure 1), the cells from the batch cultures were harvested at the mid-exponential phase (OD600 0.5 ± 0.05, after monitoring growth with spectrophotometer cuvettes having an optical path length of 1 cm) to take advantage of the high protein synthesis at that stage.
To deepen the proteome analysis, we performed a subcellular protein fractionation by ultracentrifugation (resulting in cytoplasmic- and membrane-enriched proteins) (Figure S2). With respect to both the cytoplasmic and the membrane-associated proteins, we identified a total of 2,013 and 1,981 hits between the wt and ΔactJ cells cultivated at pH 7.0, respectively; and a total of 2,260 and 2,254 hits between the two cell strains at pH 5.6, respectively. These detections accounted for more than 30% of the total protein coding regions predicted in the S. meliloti 2011 genome 26. At a cut-off value of ≥2 or ≤−2 fold and at statistically significant p-value of ≤0.05, the analysis under acid stress pointed to 59 and 24 unique proteins as being respectively up- and downregulated in ΔactJ cells under acid stress (Figures 2A and 2B, Tables 1 and 2). At pH 7.0, the proteomic analysis identified 47 and 19 unique proteins as being up- and downregulated in cultured ΔactJ vs. wt cells, respectively (Figures 2C and 2D, Tables S3 and S4).
Figure 2.

Distribution of the abundance of cytoplasmic (A and C) and membrane enriched (B and D) proteins obtained from ΔactJ cells cultured in GSM at either pH 5.6 (panels A, B) or pH 7.0 (panels C, D) with respect to proteins obtained from wt cells. In each panel, the −log10(p value) is plotted on the ordinate as a function of the log2(fold change). In identifying differentially expressed proteins we included those which met the following two conditions: fold-change, FC ≥ 2 and FC ≤ −2 (corresponding to values greater than 1 or −1 on the abscissa) and p value ≤ 0.05 (corresponding to values greater than 1.3 in the ordinate). The purple circles represent overexpressed, and the pink squares represent underexpressed proteins present in ΔactJ cells relative to those in the wt at pH = 5.6. The blue circles represent overexpressed and the light-blue squares underexpressed proteins present in ΔactJ cells relative to those in the wt at pH = 7.0. The proteins located on the 0 line correspond to the on/off proteins since those proteins had no associated p-value.
Table 1.
Upregulated proteins of Sinorhizobium meliloti ΔactJ cells grown in GSM minimal medium at pH 5.6 in relation to S. meliloti wild type 2011 (wt). Three biological replicates were used to perform relative quantification (Fold Change) between strains. The univariate Student t test was conducted between samples. Only proteins detected in the membrane (M) or cytoplasmatic (C) fraction with a p-value<0.05 are indicated. On proteins are those detected in all ΔactJ biological replicates but not in any wt replicates. Proteins were classified according to the Cluster Orthologous Group (COG): energy production and conversion (C), amino acid metabolism and transport (E), carbohydrate metabolism and transport (G), translation (J), replication and repair (L), membrane biogenesis (M), inorganic ion transport and metabolism (P), intracellular trafficking and secretion (U), and unknown function (S).
| Acc. Number | Protein Name | Locus tag | Fold Change | p-value | Annotation/Possible function | Functional Classification (COG) | Fraction |
|---|---|---|---|---|---|---|---|
| Q92KJ4 | - | SMc00096 | On | - | Uncharacterized protein | S | M |
| Q92KL8 | - | SMc00924 | On | - | YkuD domain-containing protein | M | C |
| Q92T41 | CpaC1 | SMc04111 | On | - | Pilus assembly protein | U | C |
| Q92RX4 | PotF | SMc00770 | 8.6 | 0.0196 | Putrescine-binding periplasmic protein | P | M |
| Q92VA6 | - | SMb21298 | 8.0 | 0.0004 | Uncharacterized protein | S | M |
| Q92L25 | RpmF | SMc03881 | 7.5 | 0.0156 | 50S ribosomal protein L32 | J | C |
| Q92KC7 | - | SMc01173 | 7.4 | 0.0403 | Uncharacterized protein | S | C |
| Q92KG5 | LptD | SMc00582 | 6.3 | 0.0010 | LPS-assembly protein | M | M |
| P33691 | ExoA | SMb20957 | 6.1 | 0.0259 | Succinoglycan biosynthesis protein | G | M |
| P33696 | ExoN | SMb20690 | 4.7 | 0.0011 | Glycosyltranferase involved in EPSI synthesis | G | M |
| Q92WA5 | CuxR | SMb20457 | 4.6 | 0.0097 | Transcriptional activator involved in EPS production | K | M |
| Q92SF7 | - | SMc01723 | 4.5 | 0.0299 | Uncharacterized protein | S | C |
| Q92VZ1 | - | SMb20816 | 3.7 | 0.0049 | AsmA domain-containing protein | M | M |
| Q7ANQ7 | - | SMb21310 | 3.5 | 0.0166 | Uncharacterized protein | S | M |
| Q92N86 | CspA3 | SMc01585 | 3.5 | 0.0398 | Putative cold shock transcription regulator | K | C |
| Q92KL8 | - | SMc00924 | 3.5 | 0.0032 | Uncharacterized protein | S | M |
| Q92MU4 | - | SMc01981 | 3.5 | 0.0323 | Putative cytochrome C transmembrane protein | C | C |
| Q926E2 | - | SMb20605 | 3.5 | 0.0071 | ABC transporter, periplasmic solute-binding protein | E | C |
| Q9Z3Q5 | RhtA | SMa2414 | 3.2 | 0.0455 | Rhizobactin receptor | P | C |
| Q92K28 | - | SMc01549 | 3.2 | 0.0152 | Uncharacterized protein | S | C |
| Q9Z3R5 | AglE | SMc03061 | 3.0 | 0.0169 | Alpha-glucosides-binding periplasmic protein AglE | G | M |
| Q92R52 | RpoZ | SMc02408 | 2.9 | 0.0208 | DNA-directed RNA polymerase subunit omega | K | M |
| Q92TC5 | TrxA | SMc02761 | 2.9 | 0.0161 | Thioredoxin | O | C |
| Q92ZZ1 | NrtA | SMa0585 | 2.8 | 0.0031 | Nitrate transporter, periplasmic nitrate binding protein | P | C |
| Q92Q36 | FadL | SMc02079 | 2.8 | 0.0047 | Long-chain fatty acid transport protein | I | M |
| Q926F1 | - | SMb21432 | 2.8 | 0.0478 | Putative iron uptake ABC transporter periplasmic solute-binding protein | P | M |
| Q926C6 | FhuA2 | SMc01657 | 2.7 | 0.0164 | Putative ferrichrome-iron receptor protein | P | M |
| Q930U8 | CspA7 | SMa0181 | 2.6 | 0.0012 | CspA7 cold shock protein | K | C |
| Q92KE5 | - | SMc00996 | 2.6 | 0.0078 | Penicillin-binding protein | M | M |
| Q92T52 | Cdd | SMc04124 | 2.6 | 0.0205 | Cytidine deaminase | F | C |
| P58393 | GlgA1 | SMc03924 | 2.6 | 0.0042 | Glycogen synthase 1 | G | M |
| Q92VZ8 | KpsF1 | SMb20809 | 2.6 | 0.0240 | Arabinose-5-phosphate isomerase | M | M |
| Q92KG3 | - | SMc00607 | 2.5 | 0.0018 | Uncharacterized protein | S | M |
| Q92R60 | - | SMc02396 | 2.5 | 0.0178 | Porin | M | M |
| Q92NJ1 | - | SMc01582 | 2.4 | 0.0024 | Putative alcohol dehydrogenase | C | M |
| Q92M47 | - | SMc04024 | 2.4 | 0.0086 | Membrane-bound lytic murein transglycosylase | M | M |
| Q92UQ4 | AmpC | SMb21600 | 2.4 | 0.0100 | Beta-lactamase | V | M |
| Q92PB7 | Gph1 | SMc00151 | 2.4 | 0.0428 | Phosphoglycolate phosphatase | G | M |
| Q92S89 | - | SMc02226 | 2.4 | 0.0418 | Uncharacterized protein | S | C |
| Q92MJ0 | - | SMc02451 | 2.3 | 0.0372 | Peptidylprolyl isomerase | M | M |
| Q92RP0 | ilvD1 | SMc00884 | 2.3 | 0.0242 | Dehydratase | E | C |
| Q92RW0 | DppA1 | SMc00786 | 2.3 | 0.0007 | ABC transporter system. | E | M |
| Q92RB2 | SelO | SMc00453 | 2.3 | 0.0232 | Uncharacterized protein | S | C |
| Q92PF4 | - | SMc00548 | 2.3 | 0.0400 | Conserved hypothetical signal peptide protein | S | M |
| Q9Z3S1 | Pgl | SMc03069 | 2.3 | 0.0264 | 6-phosphogluconolactonase | G | C |
| Q7ANR9 | ThuK | SMb20328 | 2.3 | 0.0465 | Putative transporter, ATP-binding protein | P | M |
| P33702 | ExoW | SMb21690 | 2.2 | 0.0063 | Exopolysaccharide production protein | E | M |
| Q92QD4 | - | SMc01267 | 2.2 | 0.0278 | AAA-PrkA domain-containing protein | T | M |
| Q92Q71 | AapJ | SMc02118 | 2.2 | 0.0042 | ABC transporter system | E | M |
| Q92Q81 | - | SMc01052 | 2.2 | 0.0423 | CMP/dCMP-type deaminase domain-containing protein | FJ | M |
| Q52953 | MurD | SMc01864 | 2.2 | 0.0365 | UDP-N-acetylmuramoylalanine-D-glutamate ligase | M | M |
| Q7APB7 | SqdD | SMc04081 | 2.2 | 0.0319 | Glycosyltransferase | M | M |
| Q92UU7 | ThtR | SMb21549 | 2.2 | 0.0052 | Thiosulfate sulfurtransferase | P | C |
| Q92S50 | RkpU | SMc02274 | 2.2 | 0.0455 | Capsule polysaccharide exporter | M | M |
| Q02728 | ExoF | SMb20945 | 2.1 | 0.0004 | Exopolysaccharide production protein | M | C |
| Q02636 | TatA | SMc00387 | 2.1 | 0.0143 | Tyrosine aminotransferase | E | M |
| Q92N45 | - | SMc02723 | 2.1 | 0.0101 | Uncharacterized protein | S | C |
| Q92NK3 | Mltb2 | SMc01845 | 2.1 | 0.0199 | Lytic murein transglycosylase B | M | C |
| Q92NM7 | RecN | SMc01877 | 2.1 | 0.0040 | DNA repair protein | L | C |
| Q92QZ8 | RpsR | SMc00567 | 2.0 | 0.0294 | 30S ribosomal protein S18 | J | C |
| P33701 | ExoV | SMb20949 | 2.0 | 0.0359 | Exopolysaccharide production protein | E | M |
| Q92Q67 | - | SMc02114 | 2.0 | 0.0417 | Uncharacterized protein | S | C |
n.s.: not significant.
Table 2.
Table 2. Downregulated proteins of Sinorhizobium meliloti ΔactJ cells grown in GSM minimal medium at pH 5.6 related to S. meliloti 2011 wild type (wt). Three biological replicates were used to perform relative quantification (in fold change) between strains. The univariate Student t test was conducted between samples. Only proteins detected in the membrane (M) or cytoplasmatic (C) fraction with a p-value<0.05 are indicated. Off proteins are those not detected in any ΔactJ biological replicates but detected in all wt replicates. Proteins were classified according to the Cluster Orthologous Group (COG): energy production and conversion (C), amino acid metabolism and transport (E), carbohydrate metabolism and transport (G), lipid metabolism (I), translation (J), transcription (K), membrane biogenesis (M), posttranslational modification (O), inorganic ion transport and metabolism (P), intracellular trafficking and secretion (U), and unknown function (S).
| Acc. Number | Protein Name | Locus tag | Fold Change | p-value | Annotation/Possible function | Functional Classification (COG) | Fraction |
|---|---|---|---|---|---|---|---|
| Q92YS3 | - | SMa1447 | Off | - | Major Facilitator Superfamily (MFS) permease | G | M |
| Q92NV0 | MacA | SMc04350 | Off | - | Multidrug Resistance (MDR) efflux pump | M | M |
| Q92NU9 | MacB | SMc04351 | Off | - | Multidrug Resistance (MDR) efflux pump | M | M |
| Q92RJ4 | McpU | SMc00975 | Off | - | Methyl-accepting chemotaxis protein | S | M |
| Q92N35 | ChoV | SMc02739 | Off | - | ABC transporter system. Choline transport | E | C |
| Q92NP6 | AcrA | SMc01458 | Off | - | Multidrug efflux pump | M | C |
| Q92RZ2 | FlgK | SMc03048 | Off | - | Flagellar hook-associated protein | N | C |
| Q92R88 | ActJ | SMc02366 | Off | - | Response regulator | T | C |
| Q52883 | CheB1 | SMc03010 | Off | - | Protein-glutamate methylesterase | T | C |
| Q52894 | DegP1 | SMc02365 | 10.4 | 0.0001 | Serine endoprotease | O | C |
| Q92RE2 | McsK | SMc00028 | 8.8 | 0.0465 | Transmembrane mechanosensitive channel | M | M |
| Q92NP7 | AcrB | SMc01457 | 7.9 | 0.0026 | Multidrug Resistance (MDR) efflux pump | P | M |
| Q92ZA8 | Nex18 | SMa1077 | 7.3 | 0.0265 | Symbiotically-induced uncharacterized protein | S | C |
| Q92RX8 | McpZ | SMc00765 | 5.3 | 0.0067 | Methyl-accepting chemotaxis protein | S | M |
| Q92LS5 | Pgi | SMc03139 | 5.1 | 0.0043 | Phosphosugar isomerase | M | C |
| Q92JX8 | - | SMc03977 | 4.3 | 0.0229 | Uncharacterized protein | S | C |
| Q930X2 | - | SMa0137 | 3.6 | 0.0391 | Diguanylate cyclase/phosphodiesterase | T | M |
| Q52977 | NtrB | SMc01042 | 3.4 | 0.0376 | Sensory histidine kinase/phosphatase | T | C |
| Q7APE0 | PssA | SMc00552 | 3.3 | 0.0275 | Phosphatidylserine synthase | I | M |
| Q92QF0 | SecY | SMc01289 | 3.2 | 0.0195 | Member of the protein translocation channel SecYEG | U | M |
| Q7APF1 | CheY2 | SMc03011 | 3.1 | 0.0269 | Response regulator | T | C |
| O54239 | FliF | SMc03014 | 3.0 | 0.0092 | Flagellar M-ring protein | N | M |
| Q92RR2 | - | SMc00910 | 2.8 | 0.0035 | Uncharacterized protein | G | M |
| Q92QF2 | RpmD | SMc01291 | 2.8 | 0.0169 | 50S ribosomal protein L30 | J | M |
| Q92N15 | HmuT | SMc01512 | 2,5 | 0,0406 | Putative hemin binding protein | P | C |
| Q9X7L1 | ClpP3 | SMc02720 | 2.5 | 0.0041 | ATP-dependent protease of misfolded proteins. Subunit 3 | O | C |
| Q92LW4 | EmrA | SMc03168 | 2.5 | 0.0172 | Multidrug Resistance (MDR) efflux pump | V | M |
| Q92V53 | - | SMb21199 | 2.4 | 0.0118 | ABC transporter. Unknown function | P | M |
| Q92XI7 | RhtX | SMa2337 | 2.3 | 0.0119 | RhtX family siderophore transporter | S | M |
| Q92V36 | - | SMb21214 | 2.2 | 0.0341 | 2-deoxyglucose-6-phosphatase | S | C |
| Q92TY9 | MinC | SMb21524 | 2.1 | 0.0111 | Septum site-determining protein | D | C |
| Q92PZ6 | TrxB | SMc01224 | 2.0 | 0.0250 | Thioredoxin reductase | C | M |
| P56893 | CysN | SMc00090 | 2.0 | 0.0292 | Sulfate adenylyltransferase. subunit 1 | P | C |
n.s.: not significant.
Moreover, we also considered proteins detected in all the biological replicates of the mutant that could not be detected in any biological replicate of the wt strain (proteins on) or the reverse pattern (proteins off) for each condition. By this criterion, 3 proteins on and 9 proteins off in ΔactJ cells cultured at pH 5.6 were detected (Tables 1 and 2), whereas ΔactJ cells cultured at pH 7.0 showed 10 proteins on and 22 proteins off (Tables S3 and S4).
3.2. Overall analysis of the ActJ-related proteomic profiles
In order to explore possible functional correlations related to ActJ absence under both growth conditions, the predicted biochemical function for each of the differential protein markers was analyzed. The DEPs were grouped into 4 main categories of COGs, as proposed by Tatusov and collaborators: Cellular processes and signaling (D, M, N, O, T, U, V, W, Y and Z), information storage and processing (A, B, J, K and L), metabolism (C, E, F, G, H, I, P and Q), and poorly characterized (R and S) 27,28.
We also analyzed the overall distribution of the COG categories in which the differential markers were grouped (Figure 3 A and B).
Figure 3.
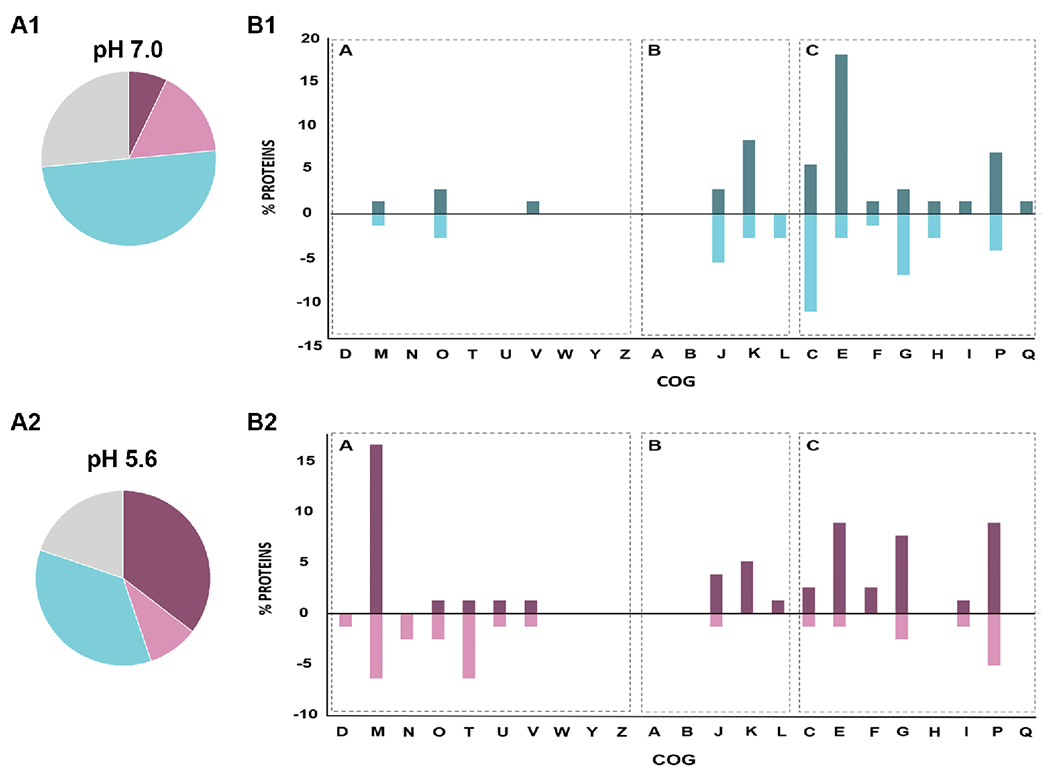
A. Clusters of Orthologous Groups (COGs) of differentially expressed proteins obtained in GSM cultures at pH 7.0 (A1) or pH 5.6 (A2). In the panel, the two pie charts indicate the relative distribution of the cellular functions: violet represents Cellular Processes and Signaling (A), pink represents Information Storage and Processing (B), light blue represents Metabolism (C) and grey represents Poorly Characterized Proteins. B. Up- and downregulated ΔactJ vs. wt differential proteins were grouped into COG categories, B1 shows the results at pH 7.0 and B2 shows the results at pH 5.6. In each bar graph, the percent proteins is plotted on the ordinate for each of the COGs listed on the abscissa and the graph is divided in 3 zones according to the distribution of cellular functions as was mentioned before (see “A”, “B” and “C”). Percentages greater than zero correspond to upregulated proteins and those with percentages lower than zero correspond to downregulated proteins. 100% corresponds to all proteins found to be differential in each condition, excluding those belonging to the uncharacterized category of COGs. COG categories: RNA processing and modification (A), chromatin structure and dynamics (B), energy production and conversion (C), cell cycle control and mitosis (D), amino acid metabolism and transport I, nucleotide metabolism and transport (F), carbohydrate metabolism and transport (G), coenzyme metabolism (H), lipid metabolism (I), translation (J), transcription (K), replication and repair (L), membrane biogenesis (M), cell motility (N), posttranslational modification, protein turnover, chaperone functions (O), inorganic ion transport and metabolism (P), secondary structure (Q), signal transduction (T) intracellular trafficking and secretion (U), nuclear structure (Y), cytoskeleton (Z).
The results indicated that at low pH most of the DEPs were involved in cellular processing and signalling plus metabolism categories, whereas under neutral condition the proteins mostly represented were involved in metabolic functions (Figure 3 A and B). Moreover, the percentage of DEPs from the group performing information storage and processing was greater under the neutral conditions than in the acidic media. This analysis also revealed that about 30% of the DEPs belonged to the poorly characterized group under both pH conditions tested, suggesting that ActJ may also regulate novel pathways that have yet to be discovered.
We also analyzed the COG distribution of up- and downregulated proteins in ΔactJ vs. wt cells under both stressed and neutral conditions (Figure 3B). Proteins associated with cell wall, membrane, and envelope biogenesis (category M) were the most upregulated group at low pH, along with those related to carbohydrates, amino-acids, and inorganic ion metabolism and transport (categories G, E and P). As regards downregulated proteins, the highest DEP percentages were represented by proteins involved in signal transduction mechanisms (T) and cell wall, membrane, and envelope biogenesis (category M). As to the neutral condition, the DEPs were mostly grouped in categories related to amino acid metabolism and energy production (categories C and E). To a lesser extent, we identified upregulated proteins associated with transcription (K) and inorganic ion transport (P); and downregulated proteins associated with translation (J) and carbohydrate metabolism (G).
The DEPs in ΔactJ vs. wt cells in the proteomic analysis at pH 7.0 and at pH 5.6 were found to be distributed in three replicons present in the S. meliloti 2011 genome (Figure S3). To obtain statistically supported data of the possible pathways altered in the absence of ActJ under both pH conditions, we used the STRING application (https://string-db.org) 22 to perform a functional-enrichment analysis (data not shownThe results demonstrated that several pathways listed in KEGG online database were significantly enriched at a low pH, such as EPS production and cell wall biogenesis. By contrast, proteases that participate in the degradation of transiently denatured and unfolded proteins as well as diverse transporters decreased in expression in the stressed ΔactJ cells.
At neutral pH, proteins involved in amino acid transport and metabolism were upregulated whereas those affecting energy production and conversion, ribosomal proteins, and several proteins with unknown functions were downregulated in ΔactJ cells. This finding would suggest that ActJ also plays a role under neutral conditions, one apparently involved in regulating central metabolic functions. . In agreement, we know from previous work that the policystronic transcription of actJ operon (degP1-actJ-actK-glnE) is different at neutral or acid condition. degP1-actJ-actK-glnE mRNA is transcribed mainly at pH 5.6 while actJ-actK-glnE is transcribed at both pH conditions 5.6 and 7.0 18, indicating that ActJ is able to control gene transcription at both pHs. Thus, it is plausible that the non-phosphorylated isoform of ActJ -at pH 7.0- can also binds to DNA promoters and favors transcription. A mechanism like this has been reported for the response regulator PmrA in Salmonella enterica, in which unphosphorylated PmrA binds to PmrA-dependent promoters in vitro, although with lower affinity than PmrA-P 29. This evidence is relevant for this work because, excluding Rhizobiaceae family, S. meliloti ActJK shares some degree of homology with Salmonella enterica PmrAB (39% and 26% identity with the RR and HK, respectively) 18. However, we consider that the most probable scenario is a phosphorylation of ActJ at pH 5.6 since the proteomic profiles obtained at pH 7.0 and pH 5.6 were clearly different.
3.3. Loss of ActJ increased EPS production in acid stress
The composition of the rhizobial outer cell surface includes a variety of polysaccharides. The exopolysaccharides (EPS) are macromolecules weakly-associated with the rhizobial surface that can be released into the environment in large amounts30,31. EPS polymers play a major role in symbiosis, and are also involved in other cellular functions such as nutrient gathering, surface attachment, and protection against environmental stress plus antimicrobial compounds32–35. S. meliloti is able to synthesize two EPS variants: the exopolysaccharide succinoglycan (EPSI) and galactoglucan (EPSII); both polysaccharides can independently support symbiosis with the host 34,36. The products encoded in exo-exs and wgx (formerly exp) clusters are required for the synthesis of EPSI and EPSII, respectively 34,37–41.
The relative abundance of certain proteins involved in EPS biosynthesis increased in acid-grown ΔactJ cultures— such as ExoN, ExoA, ExoF, ExoW and ExoV (Figure S4), and we also observed a 4.6-fold greater abundance of CuxR, an AraC-like transcriptional activator that induces exo cluster expression upon cyclic-di-GMP binding 42. These finding indicated that numerous proteins associated with EPS and cell envelope synthesis depended on the presence and/or activity of ActJ, thus suggesting an involvement of the regulator in modulating EPS biosynthesis. EPS quantification under both pH conditions revealed an exacerbation of EPS production in acid-cultured cells, a trait in S. meliloti that had been previously described 9,43 (Figure 4). The results also demonstrated a significant difference in the amount of polysaccharides in acid-cultured ΔactJ cells from those in wt cells (Figure 4), indicating that EPS production under acidic pH could be directly or indirectly repressed by ActJ. As previously stated, EPS production is necessary for proper symbiosis development. Mutant strains overproducing these polysaccharides have been reported to be defective in root hair colonization 44, and manifested symbiotic defects of varying severity 45–48. As to ActJK, the relevance of this TCS in S. meliloti-M. sativa symbiosis and the system’s involvement in nitrogen fixation, nodule occupation, and terminal bacteroid differentiation has been recently reported 18. When S. meliloti invades the plant tissue and encounters the acidic environment of both the infection thread and the symbiosome 49,50, ActJ might serve to fine tune the regulation of EPS biosynthesis. Therefore, the findings of an actJ-dependent control of EPS biosynthesis might well reflect an altered phenotype in the null actJ isogenic strain in the presence of the Medicago sativa host.
Figure 4.

Total EPS quantification performed on cells obtained from mid-exponential cultures grown in GSM media at pH 7.0 or pH 5.6. In the figure, the EPS production in mg of glucose per mg of protein is plotted on the ordinate for each of the two S. meliloti strains indicated on the abscissa at the two respective pHs. The data are the means of three independent experiments ± standard deviations. The error bars represent standard deviations of the mean. The Tukey test was used to analyze the replicates. Common letters denote no significant difference (p > 0.05).
3.4. Positive modulation of multidrug efflux pump expression during acid stress by ActJ
Multidrug resistance efflux pumps are known to provide bacteria with a practical way to tolerate harmful insults through relying on a high efficiency of drug extrusion and the associated broad range of substrate specificities 51–53. Although well studied in E. coli, the roles of multidrug-resistance pumps in rhizobia are largely unexplored, in spite of being one of the bacterium with the largest number of those types of proteins encoded in its genome 54,55. In our proteomic approach, several efflux pumps belonging to the ATP-binding cassette (ABC) 56, major facilitator 57, and resistance-nodulation-division structural superfamilies 58,59 were found to be differentially expressed (Table 2). To support this evidence, we analyzed promoter activities under neutral and acid conditions by introducing promoter-eGFP transcriptional fusions into S. meliloti wt and ΔactJ backgrounds. EmrA was found to be down-regulated in ΔactJ at a 2.5-fold decrease below the levels present in wild-type cells at pH 5.6 (Table 2), and transcriptional fusions demonstrated that ActJ regulated emrA transcription under acid stress (Figure 5A and 5B). EmrA exhibits characteristics of a membrane fusion protein and belongs to the multidrug efflux pumps family 60. The locus emrA is adjacent to the major facilitator transport protein emrB; together they form the operon emrAB. The former’s expression was found to be increased in thermal shock 61 and at acid pH 62. As these abiotic stresses have a negative impact on the cellular envelope, EmrAB could well be involved in establishing a tolerance response phenotype. We also found additional efflux pump components differentially expressed by wt vs. ΔactJ cells under acid stress (Table 2)—namely, AcrB and AcrA, which form a resistance-nodulation-division multidrug-resistent pump superfamily63,64; and MacA and MacB, belonging to multidrug-resistent-pump-like proteins 65. The former is well studied in Escherichia coli, where it is constitutively expressed 59: TolC is the outer membrane protein component, AcrA is the periplasmic adaptor protein, and AcrB is the inner membrane transporter. Together, they can transport a wide variety of molecules 66. Under the acid stress condition, AcrB was found downregulated in ΔactJ cells (an almost 8-fold decrease), while AcrA was shifted to off (Table 2). Consistent with these observations, acrAB transcription was enhanced in acid pH when ActJ was present (Figure 5A and 5B). A similar result was found for MacAB-TolC. In this instance, we found both MacA and MacB in an off state in ΔactJ cells in the stressed condition (Table 2), whereas macAB transcription became increased in acid stress when ActJ was present (Figure 5A and 5B). These data strongly suggest that ActJ positively modulates the major-facilitator pump EmrAB, and the multidrug-resistance pumps ArcAB and MacAB when S. meliloti encounters an acid environment. We are tempted to speculate regarding a scenario where rhizobia express efflux pumps in order to secrete basic metabolites as a strategy to counteract extracellular acidity. Such mechanism was observed in Mycobacterium tuberculosis, in which the OmpATb porin is overexpressed in acid condition and secretes ammonia to neutralize the pH 67.
Figure 5.

eGFP transcriptional fusions and fluorescence assays performed on cells obtained from mid-exponential cultures grown in GSM media at neutral pH (7.0, Panel A) or acid pH (5.6, Panel B). In each panel the relative fluorescence units (RFUs) per OD600 are plotted on the ordinate for each of the transcribed loci in wild-type (wt) and mutant (ΔactJ) cells indicated on the abscissa. Strains containing the promotorless pHU231eGFP plasmid are indicated as PΦ. Error bars represent the standard deviations of the mean of three replicates. ANOVA Tukey’s multiple comparison test was performed to analyze significance. Bars with same letters are not significantly different (p> 0.05).
3.5. ActJ regulated DegP1 transcription
Under both pH conditions, DEPs in the mutant cells included proteases involved in protein turnover. These results indicated that DegP1 and ClpP3 were downregulated in ΔactJ cells in acid pH (at a 10.4 and 2.5-fold decrease in abundance, respectively). At neutral pH DegP1 was also downregulated (3-fold change), whereas ClpP2 was upregulated (2-fold change). DegP1 is a periplasmic serine endoprotease, where the degP1 locus has been described as the most strongly induced gene in S. meliloti under acidic conditions10. Draghi and collaborators reported an increase in both transcription and translation of this protease in S. meliloti cells grown in continuous cultures at acidic pH 9. DegP1, encoded upstream from actJ (Figure S1), was found to be downregulated in ΔactJ cells under both the pH conditions of the study, with a greater decrease at acid pH (Tables 1 and 2). In our previous study, PdegP1-eGFP fusion assays had documented an increase in relative fluorescence only at pH 5.6, confirming that ActJ regulates degP1 transcription during acid stress18. A shift in external pH can denature proteins present in the periplasm, since the Gram-negative outer membrane is permeable to hydrogen ions6 68. In agreement with previous studies 69,70, we hypothesize here that DegP1 upregulation at acid pH could be related to the degradation of misfolded periplasmic proteins, thus, contributing to the adaptation by S. meliloti to this stressful condition. Our results suggest that ActJ-related transcriptional regulation is a critical biologic process that enables cells to respond to low pH and is closely associated with proteome quality control proteins.
3.6. Further ActJ targets identifed
The proteomic data revealed additional proteins that contribute to our understanding of S. meliloti ActJK-associated physiology. For instance, we wondered if ActJ had any role in motility and chemotaxis at low pH, since smaller amounts of FlgK, FliF, the chemotaxis protein McpU, and the response regulator CheY2 were detected in the mutant strain (Table 2). CheY2 is involved in a mechanism that operates in the S. meliloti chemotactic signalling chain involving the CheY1 and CheY2 response regulators. CheY2 is phosphorylated and activated by its cognate histidine kinase, CheA, which controls the flagellar motor. In the absence of CheZ phosphatase activity, the concentration of CheY2-P is rapidly reset, first by a phosphotransfer from CheY2-P first back to CheA, and next to CheY1, which acts as a phosphate sink.
Neither the wt nor the ΔactJ mutant exhibited swimming phenotypes, and no flagellins were detected under the acid condition (data not shown). These absences are probably a result of the acid-related EPS overproduction of the wt and ΔactJ strains cited above, since we had observed an arrest in motility in S. meliloti and Agrobacterium tumefaciens when EPS production was enhanced 71,72. Probably, motility repression, which have been implied as part of a general stress response not exclusively related with low pH10, is a way to save energy when cells are coping with a stressful situation.
We also found that the cold shock proteins CspA3 and CspA7 were upregulated in ΔactJ low pH cultures, which were previously associated with general stress responses in Bacillus subtilis and Brucella melitensis 73,74. Similar results were recently observed recently in S. meliloti counterparts as well 75 and have been also suggested to be involved in the development of symbiosis by S. meliloti 76, which findings raise the question of whether the arrested symbiosis observed in an actJ mutant18 is due to CspA3 and CspA7 overexpression in an acidic symbiotic niche.
4. CONCLUSIONS
Within their respective niches, microorganisms are subjected to extreme changes in environmental conditions such as fluctuations in light, temperature, nutrient levels, osmolarity or pH. S. meliloti is a model microorganism for a relevant group of alpha-proteobacteria capable of fixing nitrogen during symbiosis with legumes. To initiate symbiosis by infecting plant roots, rhizobia need to be able to survive in the soil. Cell viability and symbiosis in S. meliloti and several other related rhizobia species are strongly affected by increased hydrogen-ion concentrations in the environment. In view of the global distribution of acid agronomic soils, an enhancement in the understanding of the mechanisms involved in the rhizobial response to low pH becomes a central issue for the rational improvement of inoculants.
The present comparative proteomic study was conducted on batch cultures with the aim at identifying the various ActJ-regulated targets in order to gain a better comprehension of ActJK involvement in S. meliloti free-living acid stress adaptation. Considering the data presented here and summarized in Figure 6, we propose that S meliloti utilizes ActJK to mount an acid tolerance phenotype by overexpressing DegP1 in the periplasm and the EmrAB, MacAB and AcrAB pumps in the membrane. In addition, we speculate that the impaired symbiosis observed in ΔactJ might be the result of an exacerbation of EPS biosynthesis, since we also observed an increase of EPS biosynthetic proteins and EPS production when ΔactJ cells were grown in acid medium. Future studies are needed to clarify the role of ActJK role in modulating EPS metabolism.
Figure 6.

Summary of most relevant pathways linked to an ActJ activity in S. meliloti. The figure constitutes an overall scheme for the current model depicting the adaptation of S. meliloti to acid stress, involving the key functional pathways operating in the different subcellular compartments at either pH 7.0 (left panel) or pH 5.6 (right panel). The intact arrows denote upregulated pathways and the blocked arrows downregulated pathways in the presence of ActJ. The putative regulations are represented by dashed arrows and the experimentally confirmed regulation by solid arrows. This bacterium exhibits a global response at low pH resulting from the cellular deterioration caused by acid stress. In S. meliloti, ActJ could trigger the various mechanisms illustrated to cope specifically with acid stress, such as an increase in the degP1 and actJK expression, a downregulation of EPS synthesis, and changes in the cell envelope, all of which modulations would contribute to the resistance to an acidic challenge.
Our results also revealed that ActJ regulates multiple genes, and its function influences on the transcriptome, proteome and therefore in the physiology of S. meliloti. The participation of ActJ in the biology of S. meliloti grown under optimal pH conditions includes determinants of energy production, such as reductases and oxidoreductases, proteins involved in the glutathione hydrolysis essential for the maintenance of a cellular redox state, transporters, efflux pumps, and transcriptional regulators (which latter functions are up and downregulated in ΔactJ cells compared to wt cells). At neutral pH (Tables S3 and S4), in the absence of ActJ, more than 40 uncharacterized DEPs were detected in our analyses. These proteins with unknown functions may mediate additional as-yet-uncharacterized roles for ActJ. Therefore, further studies involving these proteins are needed to determine their biologic role and their relationship to ActJ.
In summary, the present work provides new insights into the role of ActJ, and consequently into the ActJK system during acid-tolerance in S. meliloti and in rhizobia carrying orthologs of this TCS. Altered amounts of the DEPs in the ΔactJ mutant at pH 5.6 could indicate that the mutant does not react normally to stress because of direct disruption of stress signal transduction. Thus, the comprehensive information gained in this work provides several starting points for new studies aimed at fully unravelling the mechanism of action of ActJK in S. meliloti and related bacteria. Additional studies are in progress to shed light on the physiologic and metabolic significance of early ActJ-induced molecular changes at acid pH that activate the downstream biochemical alterations described here, and to elucidate the connection between the ActJK TCS role and the cell’s internal pH status.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Paula Giménez, Silvana Tongiani (both members of CPA CONICET at IBBM), and to Dr. María Pia Valacco and Dr. Silvia Margarita Moreno for the excellent support at the CEQUIBIEM. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Funding
This work was partially supported by CONICET, Argentina Grant/Award PIP2015-0700 and MinCyT, Argentina Grant/Award PICT2017-2833 and PICT2020-1475. J.F.A., C.V., J.H.C. are fellows of CONICET. M.C.M.., A.L., W.O.D. and M.F.D. are members of the Research Career of CONICET.
ABBREVIATIONS
- DEP
differentially expressed protein
- CDS
coding sequences
- M
log2(fold change)
- COG
cluster of orthologous groups
- GSM
glutamate–sucrose minimal medium
Footnotes
Supporting Information
Table with strains (Table S1) and primers (Table S2) used in this work, upregulated proteins of S. meliloti ΔactJ cells grown in GSM minimal media pH 7.0 related to wild-type S. meliloti 2011 (Table S3), down-regulated proteins of S. meliloti ΔactJ cells grown in GSM minimal media pH 7.0 related to wild-type S. meliloti 2011 (Table S4). (PDF)
Genetic elements located on the actJ coding region (Figure S1), principal component analysis (PCA) score plots of the four studied groups and samples studied (Figure S2), genomic representation of the differentially expressed proteins in ΔactJ vs. wt cells in the proteomic assay at pH 7.0 and at pH 5.6 (Figure S3), differentially expressed proteins involved in exopolysaccharide biosynthesis in response to acid stress in S. meliloti ΔactJ mutant (Figure S4) (PDF).
The authors declare no competing financial interest.
The mass spectrometry proteomics raw data sets generated for this study have been deposited in the Mass Spectrometry Interactive Virtual Environment (MassIVE) (https://massive.ucsd.edu/ProteoSAFe/help.jsp) with the data set identifier MassIVE MSV000090690.
REFERENCES
- (1).Evans JR; von Caemmerer S Enhancing Photosynthesis. Plant Physiol. 2011, 155 (1), 19 LP – 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Buckley DH; Schmidt T Exploring the Biodiversity of Soil—a Microbial Rain Forest. Biodivers. Microb. life Found. Earth’s Biosph 2001, 183–208. [Google Scholar]
- (3).Kallenbach CM; Wallenstein MD; Schipanksi ME; Grandy AS Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol 2019, 10, 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bouton JH; Sumner ME Alfalfa Medicago Sativa L., in Highly Weathered, Acid Soils. Plant Soil 1983, 74 (3), 431–436. [Google Scholar]
- (5).Fierer N; Jackson RB The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (3), 626 LP – 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Glenn AR; Dilworth MJ The Life of Root Nodule Bacteria in the Acidic Underground. FEMS Microbiol. Lett 1994, 123 (1–2), 1–9. [Google Scholar]
- (7).Hungria M; Vargas M Environmental Factors Affecting N2 Fixation in Grain Legumes in the Tropics, with an Emphasis on Brazil. F. Crop. Res - 2000, 65, 151–164. [Google Scholar]
- (8).Vassileva V; Milanov G; Ignatov G; Nikolov B Effect of Low PH on Nitrogen Fixation of Common Bean Grown at Various Calcium and Nitrate Levels. J. Plant Nutr 1997, 20 (2–3), 279–294. [Google Scholar]
- (9).Draghi WO; Papa M. F. Del; Hellweg C; Watt SA; Watt TF; Barsch A; Lozano MJ. A Consolidated Analysis of the Physiologic and Molecular Responses Induced under Acid Stress in the Legume-Symbiont Model-Soil Bacterium Sinorhizobium meliloti. Nat. Publ. Gr 2016, No. April, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hellweg C; Pühler A; Weidner S The Time Course of the Transcriptomic Response of Sinorhizobium meliloti 1021 Following a Shift to Acidic pH. BMC Microbiol. 2009, 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kiss E; Huguet T; Poinsot V; Batut J The TypA Gene Is Required for Stress Adaptation as Well as for Symbiosis of Sinorhizobium meliloti 1021 with Certain Medicago Truncatula Lines. Mol. Plant. Microbe. Interact 2004, 17 (3), 235–244. [DOI] [PubMed] [Google Scholar]
- (12).Reeve WG; Tiwari RP; Guerreiro N; Stubbs J; Dilworth MJ; Glenn AR; Rolfe BG; Djordjevic MA; Howieson JG Probing for PH-Regulated Proteins in Sinorhizobium medicae Using Proteomic Analysis. J. Mol. Microbiol. Biotechnol 2004, 7 (3), 140–147. [DOI] [PubMed] [Google Scholar]
- (13).Tiwari RP; Reeve WG; Fenner BJ; Dilworth MJ; Glenn AR; Howieson JG Probing for PH-Regulated Genes in Sinorhizobium medicae Using Transcriptional Analysis. J. Mol. Microbiol. Biotechnol 2004, 7 (3), 133–139. [DOI] [PubMed] [Google Scholar]
- (14).Vinuesa P; Neumann-Silkow F; Pacios-Bras C; Spaink HP; Martinez-Romero E; Werner D Genetic Analysis of a PH-Regulated Operon from Rhizobium tropici CIAT899 Involved in Acid Tolerance and Nodulation Competitiveness. Mol. Plant. Microbe. Interact 2003, 16 (2), 159–168. [DOI] [PubMed] [Google Scholar]
- (15).Tiwari RP; Reeve WG; Dilworth MJ; Glenn AR Acid Tolerance in Rhizobium meliloti Strain WSM419 Involves a Two-Component Sensor-Regulator System. Microbiology 1996, 142 (Pt 7 (7), 1693–1704. [DOI] [PubMed] [Google Scholar]
- (16).Tiwari RP; Reeve WG; Dilworth MJ; Glenn AR An Essential Role for ActA in Acid Tolerance of Rhizobium meliloti. Microbiology 1996, 142 (Pt 3, 601–610. [DOI] [PubMed] [Google Scholar]
- (17).Hoch JA; Silhavy TJ Two-Component Signal Transduction; Washington, D.C, 1995. [Google Scholar]
- (18).Albicoro FJ; Draghi WO; Martini MC; Salas ME; Torres Tejerizo GA; Lozano MJ; López JL; Vacca C; Cafiero JH; Pistorio M; Bednarz H; Meier D; Lagares A; Niehaus K; Becker A; Del Papa MF The Two-Component System ActJK Is Involved in Acid Stress Tolerance and Symbiosis in Sinorhizobium meliloti. Journal of biotechnology. 2021, 329:80–91. [DOI] [PubMed] [Google Scholar]
- (19).Beringer JE R Factor Transfer in Rhizobium Leguminosarum. J. Gen. Microbiol 1974, 84 (1), 188–198. [DOI] [PubMed] [Google Scholar]
- (20).Del Papa MF; Balagué LJ; Sowinski SC; Wegener C; Segundo E; Martinez Abarca F; Toro N; Niehaus K; Pühler A; Aguilar OM; Martínez-Drets G; Lagares A Isolation and Characterization of Alfalfa-Nodulating Rhizobia Present in Acidic Soils of Central Argentina and Uruguay. Appl. Environ. Microbiol 1999, 65 (4), 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sambrook J; Fritsch EF; Maniatis T Molecular Cloning: A Laboratory Manual.; Cold spring harbor laboratory press, 1989. [Google Scholar]
- (22).Szklarczyk D; Morris JH; Cook H; Kuhn M; Wyder S; Simonovic M; Santos A; Doncheva NT; Roth A; Bork P; Jensen LJ; von Mering C The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017, 45 (D1), D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Trevelyan WE; Forrest RS; Harrison JS Determination of Yeast Carbohydrates with the Anthrone Reagent. Nature 1952, 170 (4328), 626–627. [DOI] [PubMed] [Google Scholar]
- (24).McIntosh M; Krol E; Becker A Competitive and Cooperative Effects in Quorum-Sensing-Regulated Galactoglucan Biosynthesis in Sinorhizobium meliloti. J. Bacteriol 2008, 190 (15), 5308–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Del Papa MF; Pistorio M; Draghi WO; Lozano MJ; Giusti MA; Medina C; van Dillewijn P; Martinez-Abarca F; Moron Flores B; Ruiz-Sainz JE; Megías M; Pühler A; Niehaus K; Toro N; Lagares A Identification and Characterization of a NodH Ortholog from the Alfalfa-Nodulating Or191-like Rhizobia. Mol Plant Microbe Interact 2007, 20 (2), 138–145. [DOI] [PubMed] [Google Scholar]
- (26).Sallet E; Roux B; Sauviac L; Jardinaud M-F; Carrere S; Faraut T; de Carvalho-Niebel F; Gouzy J; Gamas P; Capela D; Bruand C; Schiex T Next-Generation Annotation of Prokaryotic Genomes with EuGene-P: Application to Sinorhizobium Meliloti 2011. DNA Res. 2013, 20 (4), 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Tatusov RL; Koonin EV; Lipman DJ A Genomic Perspective on Protein Families. Science 1997, 278 (5338), 631–637. [DOI] [PubMed] [Google Scholar]
- (28).Tatusov RL; Fedorova ND; Jackson JD; Jacobs AR; Kiryutin B; Koonin EV; Krylov DM; Mazumder R; Mekhedov SL; Nikolskaya AN; Rao BS; Smirnov S; Sverdlov AV; Vasudevan S; Wolf YI; Yin JJ; Natale DA The COG Database: An Updated Version Includes Eukaryotes. BMC Bioinformatics 2003, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wösten MM; Groisman EA Molecular Characterization of the PmrA Regulon. J. Biol. Chem 1999, 274 (38), 27185–27190. [DOI] [PubMed] [Google Scholar]
- (30).Fraysse N; Couderc F; Poinsot V Surface Polysaccharide Involvement in Establishing the Rhizobium-Legume Symbiosis. Eur. J. Biochem 2003, 270 (7), 1365–1380. [DOI] [PubMed] [Google Scholar]
- (31).Skorupska A; Janczarek M; Marczak M; Mazur A; Król J. Rhizobial Exopolysaccharides: Genetic Control and Symbiotic Functions. Microb. Cell Fact 2006, 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Downie JA The Roles of Extracellular Proteins, Polysaccharides and Signals in the Interactions of Rhizobia with Legume Roots. FEMS Microbiol. Rev 2010, 34 (2), 150–170. [DOI] [PubMed] [Google Scholar]
- (33).Mendis HC; Madzima TF; Queiroux C; Jones KM Function of Succinoglycan Polysaccharide in Sinorhizobium meliloti Host Plant Invasion Depends on Succinylation, Not Molecular Weight. MBio 2016, 7 (3), e00606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pellock BJ; Cheng H-P; Walker GC Alfalfa Root Nodule Invasion Efficiency Is Dependent on <Em>Sinorhizobium Meliloti</Em>Polysaccharides. J. Bacteriol 2000, 182 (15), 4310 LP – 4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Urzainqui A; Walker GC Exogenous Suppression of the Symbiotic Deficiencies of Rhizobium meliloti Exo Mutants. J. Bacteriol 1992, 174 (10), 3403–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).González LJ; Bahr G; Nakashige TG; Nolan EM; Bonomo RA; Vila AJ Membrane Anchoring Stabilizes and Favors Secretion of New Delhi Metall²-Lactamase. Nat. Chem. Biol 2016, No. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Becker A; Kleickmann A; Keller M; Arnold W; Pühler A Identification and Analysis of the Rhizobium meliloti ExoAMONP Genes Involved in Exopolysaccharide Biosynthesis and Mapping of Promoters Located on the ExoHKLAMONP Fragment. Mol. Gen. Genet. MGG 1993, 241 (3), 367–379. [DOI] [PubMed] [Google Scholar]
- (38).Becker A; Kleickmann A; Arnold W; Pühler A Analysis of the Rhizobium meliloti ExoH/ExoK/ExoL Fragment: ExoK Shows Homology to Excreted End²-1,3-1,4-Glucanases and ExoH Resembles Membrane Proteins. Mol. Gen. Genet. MGG 1993, 238 (1), 145–154. [DOI] [PubMed] [Google Scholar]
- (39).Glucksmann MA; Reuber TL; Walker GC Family of Glycosyl Transferases Needed for the Synthesis of Succinoglycan by Rhizobium meliloti. J. Bacteriol 1993, 175 (21), 7033–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Long S; Reed JW; Himawan J; Walker GC Genetic Analysis of a Cluster of Genes Required for Synthesis of the Calcofluor-Binding Exopolysaccharide of Rhizobium meliloti J. Bacteriol. 1988, 170 (9), 4239–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Gonzalez JE; Reuhs BL; Walker GC Low Molecular Weight EPS II of Rhizobium Meliloti Allows Nodule Invasion in Medicago sativa. Proc. Natl. Acad. Sci. U. S. A 1996, 93 (16), 8636–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Schaper S; Steinchen W; Krol E; Altegoer F; Skotnicka D; Sogaard-Andersen L; Bange G; Becker A AraC-like Transcriptional Activator CuxR Binds c-Di-GMP by a PilZ-like Mechanism to Regulate Extracellular Polysaccharide Production. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (24), E4822–E4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hawkins JP; Ordonez PA; Oresnik IJ Characterization of Mutations That Affect the Nonoxidative Pentose Phosphate Pathway in Sinorhizobium Meliloti. J. Bacteriol 2017, 200 (2), e00436–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Cheng HP; Walker GC Succinoglycan Production by Rhizobium meliloti Is Regulated through the ExoS-ChvI Two-Component Regulatory System. J. Bacteriol 1998, 180 (1), 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Doherty D; Leigh JA; Glazebrook J; Walker GC Rhizobium meliloti Mutants That Overproduce the R. Meliloti Acidic Calcofluor-Binding Exopolysaccharide. J. Bacteriol 1988, 170 (9), 4249–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ozga DA; Lara JC; Leig JA The Regulation of Exopolysaccharide Production Is Important at Two Levels of Nodule Development in Rhizobium meliloti. Mol. Plant. Microbe. Interact 1994, 7 (6), 758–765. [DOI] [PubMed] [Google Scholar]
- (47).Wells DH; Chen EJ; Fisher RF; Long SR ExoR Is Genetically Coupled to the ExoS-ChvI Two-Component System and Located in the Periplasm of Sinorhizobium meliloti. Mol. Microbiol 2007, 64 (3), 647–664. [DOI] [PubMed] [Google Scholar]
- (48).Yao S-Y; Luo L; Har KJ; Becker A; Ruberg S; Yu G-Q; Zhu J-B; Cheng H-P Sinorhizobium meliloti.ExoR and ExoS Proteins Regulate Both Succinoglycan and Flagellum Production. J. Bacteriol 2004, 186 (18), 6042–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Geddes BA; Gonzalez JE; Oresnik IJ Exopolysaccharide Production in Response to Medium Acidification Is Correlated with an Increase in Competition for Nodule Occupancy. Mol. Plant. Microbe. Interact 2014, 27 (12), 1307–1317. [DOI] [PubMed] [Google Scholar]
- (50).Pierre O; Engler G; Hopkins J; Brau F; Boncompagni E; Herouart D Peribacteroid Space Acidification: A Marker of Mature Bacteroid Functioning in Medicago truncatula Nodules. Plant. Cell Environ 2013, 36 (11), 2059–2070. [DOI] [PubMed] [Google Scholar]
- (51).Du D; van Veen HW; Luisi BF Assembly and Operation of Bacterial Tripartite Multidrug Efflux Pumps. Trends Microbiol. 2018, 23 (5), 311–319. [DOI] [PubMed] [Google Scholar]
- (52).Sun J; Deng Z; Yan A Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun 2014, 453 (2), 254–267. [DOI] [PubMed] [Google Scholar]
- (53).Tang G; Wang S; Lu D; Huang L; Li N; Luo L Tiwari. Microbiol. Res 2017, 198, 1–7. [DOI] [PubMed] [Google Scholar]
- (54).Konstantinidis KT; Tiedje JM Trends between Gene Content and Genome Size in Prokaryotic Species with Larger Genomes. Proc. Natl. Acad. Sci 2004, 101 (9), 3160–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Martinez JL; Sánchez MB; Martínez-Solano L; Hernandez A; Garmendia L; Fajardo A; Alvarez-Ortega C Functional Role of Bacterial Multidrug Efflux Pumps in Microbial Natural Ecosystems. FEMS Microbiol. Rev 2009, 33 (2), 430–449. [DOI] [PubMed] [Google Scholar]
- (56).Lubelski J; Konings WN; Driessen AJM Distribution and Physiology of ABC-Type Transporters Contributing to Multidrug Resistance in Bacteria. Microbiol. Mol. Biol. Rev 2007, 71 (3), 463 LP – 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Pao SS; Paulsen IT; Saier MH Jr Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev 1998, 62 (1), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Nikaido H. Structure and Mechanism of RND-Type Multidrug Efflux Pumps. Adv. Enzymol. Relat. Areas Mol. Biol 2011, 77, 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Nikaido H; Takatsuka Y Mechanisms of RND Multidrug Efflux Pumps. Biochim. Biophys. Acta 2009, 1794 (5), 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Rossbach S; Kunze K; Albert S; Zehner S; Gottfert M The Sinorhizobium meliloti EmrAB Efflux System Is Regulated by Flavonoids through a TetR-like Regulator (EmrR). Mol. Plant. Microbe. Interact 2014, 27 (4), 379–387. [DOI] [PubMed] [Google Scholar]
- (61).Barnett MJ; Bittner AN; Toman CJ; Oke V; Long SR Dual RpoH Sigma Factors and Transcriptional Plasticity in a Symbiotic Bacterium. J. Bacteriol 2012, 194 (18), 4983–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Santos MR; Marques AT; Becker JD; Moreira LM The Sinorhizobium Meliloti EmrR Regulator Is Required for Efficient Colonization of Medicago sativa Root Nodules. Mol. Plant-Microbe Interact 2014, 27 (4), 388–399. [DOI] [PubMed] [Google Scholar]
- (63).Du D; Wang Z; James NR; Voss JE; Klimont E; Ohene-Agyei T; Venter H; Chiu W; Luisi BF Structure of the AcrAB–TolC Multidrug Efflux Pump. Nature 2014, 509 (7501), 512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Franssen HJ; Vijn I; Yang WC; Bisseling T Developmental Aspects of the Rhizobium-Legume Symbiosis BT - 10 Years Plant Molecular Biology; Schilperoort RA, Dure L, Eds.; Springer Netherlands: Dordrecht, 1992; pp 89–107. [DOI] [PubMed] [Google Scholar]
- (65).Eda S; Mitsui H; Minamisawa K Involvement of the SmeAB Multidrug Efflux Pump in Resistance to Plant Antimicrobials and Contribution to Nodulation Competitiveness in Sinorhizobium meliloti. Appl. Environ. Microbiol 2011, 77 (9), 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Okusu H; Ma D; Nikaido H AcrAB Efflux Pump Plays a Major Role in the Antibiotic Resistance Phenotype of Escherichia Coli Multiple-Antibiotic-Resistance (Mar) Mutants. J. Bacteriol 1996, 178 (1), 306 LP – 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Song H; Huff J; Janik K; Walter K; Keller C; Ehlers S; Bossmann SH; Niederweis M Expression of the OmpATb Operon Accelerates Ammonia Secretion and Adaptation of Mycobacterium tuberculosis to Acidic Environments. Mol. Microbiol 2011, 80 (4), 900–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Wilks JC; Slonczewski JL PH of the Cytoplasm and Periplasm of Escherichia coli: Rapid Measurement by Green Fluorescent Protein Fluorimetry. J. Bacteriol 2007, 189 (15), 5601–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Fu X; Wang Y; Shao H; Ma J; Song X; Zhang M; Chang Z DegP Functions as a Critical Protease for Bacterial Acid Resistance. FEBS J. 2018, 285 (18), 3525–3538. [DOI] [PubMed] [Google Scholar]
- (70).He D; Zhang M; Liu S; Xie X; Chen PR Protease-Mediated Protein Quality Control for Bacterial Acid Resistance. Cell Chem. Biol 2019, 26 (1), 144–150.e3. [DOI] [PubMed] [Google Scholar]
- (71).Hoang HH; Gurich N; González JE Regulation of Motility by the ExpR/Sin Quorum-Sensing System in Sinorhizobium meliloti. J. Bacteriol 2008, 190 (3), 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Yuan Z-C; Haudecoeur E; Faure D; Kerr KF; Nester EW Comparative Transcriptome Analysis of Agrobacterium Tumefaciens in Response to Plant Signal Salicylic Acid, Indole-3-Acetic Acid and Gamma-Amino Butyric Acid Reveals Signalling Cross-Talk and Agrobacterium--Plant Co-Evolution. Cell. Microbiol 2008, 10 (11), 2339–2354. [DOI] [PubMed] [Google Scholar]
- (73).Graumann P; Wendrich TM; Weber MH; Schröder K; Marahiel MA A Family of Cold Shock Proteins in Bacillus Subtilis Is Essential for Cellular Growth and for Efficient Protein Synthesis at Optimal and Low Temperatures. Mol. Microbiol 1997, 25 (4), 741–756. [DOI] [PubMed] [Google Scholar]
- (74).Wang Z; Liu W; Wu T; Bie P; Wu Q RNA-Seq Reveals the Critical Role of CspA in Regulating Brucella Melitensis Metabolism and Virulence. Sci. China. Life Sci 2016, 59 (4), 417–424. [DOI] [PubMed] [Google Scholar]
- (75).Hagberg KL; Price JP; Yurgel SN; Kahn ML The Sinorhizobium Meliloti Nitrogen Stress Response Changes Radically in the Face of Concurrent Phosphate Stress. Front. Microbiol 2022, 13, 800146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).O’Connell KP; Thomashow MF Transcriptional Organization and Regulation of a Polycistronic Cold Shock Operon in Sinorhizobium meliloti RM1021 Encoding Homologs of the Escherichia Coli Major Cold Shock Gene CspA and Ribosomal Protein Gene RpsU. Appl. Environ. Microbiol 2000, 66 (1), 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


