Abstract
Background:
Manganese (Mn) is both an essential and toxic metal, and associations with neurodevelopment depend on exposure timing. Prospective data examining early life Mn with adolescent cognition are sparse.
Methods:
We enrolled 140 Italian adolescents (10–14 years old) from the Public Health Impact of Metals Exposure study. Mn in deciduous teeth was measured using laser ablation-mass spectrometry to represent prenatal, postnatal and early childhood exposure. The California Verbal Learning Test for Children (CVLT-C) was administered to assess adolescent verbal learning and memory. Multivariable regression models estimated changes in CVLT-C scores and the odds of making an error per doubling in dentine Mn in each exposure period. Multiple informant models tested for differences in associations across exposure periods.
Results:
A doubling in prenatal dentine Mn levels was associated with lower odds of making an intrusion error (OR = 0.23 [95% CI: 0.09, 0.61]). This beneficial association was not observed in other exposure periods. A doubling in childhood Mn was beneficially associated with short delay free recall: (β=0.47 [95% CI: −0.02, 0.97]), which was stronger in males (β = 0.94 [95% CI: 0.05, 1.82]). Associations were null in the postnatal period.
Conclusion:
Exposure timing is critical for understanding Mn-associated changes in cognitive function.
Keywords: manganese, memory, neurodevelopment, early-life, critical periods, susceptibility
1. Introduction
Manganese (Mn) is an essential nutrient and established neurotoxicant [1,2], whereby both insufficient and excess levels have been associated with neurotoxicity [3–7]. As a nutrient, Mn is involved in several key enzymatic processes during neurotransmitter synthesis and metabolism [1–3,8]. At both low and excess levels of Mn, these enzymatic processes may be disrupted, resulting in inadequate development of multiple cell types such as glial cells, which are responsible for healthy brain development [3,9]. The mechanisms of Mn neurotoxicity from overexposure include oxidative stress, disruption of homeostatic pathways, and disruption of multiple neurotransmitters, including dopamine [2,3,10–12]. Further, evidence suggests Mn can disrupt the endocrine system through changes to hormone synthesis, secretion, binding, and transport, including hormones important for thyroid function and pubertal development like thyroxine (T4), triiodothyronine (T3), and prolactin [1–4]. As a result, suboptimal Mn levels may adversely impact homeostasis of hormones, leading to neurodevelopmental decrements. Mn is naturally present in the human diet, but it is also commonly used in ferroalloy (steel) production, as well as in battery production for electric vehicles [14], and was historically used as an agricultural fungicide and gasoline additive [1]. Residential proximity to ferroalloy industry has been shown to result in higher levels of Mn in environmental media (e.g., air or soil) [15–19], thus putting nearby communities at risk of neurotoxicity from Mn overexposure.
Susceptibility to Mn neurotoxicity may change throughout development, and whether Mn is beneficial or detrimental can depend not only on dose, but also on the developmental period in which exposure occurs [20,21]. Recent epidemiological studies have reported that the timing of Mn exposure (e.g., prenatal, early postnatal, or early childhood) impacts the directionality and strength of association with functional connectivity measured using brain imaging [21], as well as with neuropsychological assessments of intelligence, visual spatial learning, and motor function in children [21–26]. Differential cognitive effects of Mn by exposure timing have also been reported in animal studies [27–29] and may be related to changing morphology of the brain during development or varying demands of Mn for healthy growth [24]. The brain, especially cortical and subcortical regions, rapidly develops during gestation [30]; thus, gestation may be a time of increased demand for Mn as an essential nutrient [3,31]. This is consistent with studies that have reported increasing levels of maternal Mn throughout pregnancy [32,33], and higher Mn biomarker concentrations among pregnant people versus non-pregnant people [34–36]. The beneficial role of Mn in pregnancy is further exemplified by studies that have reported the highest Mn levels in deciduous teeth during the prenatal period that steadily decline before birth [24,37]. In contrast, during the postnatal period, multiple neural and glial developmental processes occur, which have been shown to be sensitive to Mn neurotoxicity [38–40], supporting the hypothesis that excess Mn in the postnatal exposure period could disrupt cellular function. Thus, to comprehensively understand the effects of early-life Mn on cognitive function, it is critical to evaluate exposure during multiple periods of development.
Previous pediatric epidemiological studies have reported both beneficial and adverse associations between Mn and cognitive functions, including verbal learning and memory [9,20,24,26,41–46]. However, most prior studies have been limited, with few exceptions [20,24,26], to cross-sectional analyses in late childhood or early adolescence. These prior studies have found that Mn exposure is associated with structural and functional impairment in the hippocampus and prefrontal cortex, two brain regions involved in verbal learning and memory [11,29,47–49]. Verbal learning and memory are critical for overall academic performance and cognitive function of children and adolescents. Therefore, identifying periods of susceptibility in this neurocognitive domain following early-life exposure to Mn is critical to children’s health.
The objective of this study was to evaluate Mn-associated changes in adolescent verbal learning and memory across three early-life exposure periods: prenatal, postnatal and early childhood. We examined prospective associations using data from participants of the Public Health Impact of Metals Exposure (PHIME) study, a cohort designed to investigate health effects of Mn exposure from nearby ferroalloy production. Given previous findings of sexually dimorphic Mn associations with neurodevelopment in PHIME and other cohorts [5,23,26,42,45,50], we also explored sex-specific effects of Mn.
2. Methods
2.1. Study Population
The PHIME study was designed to investigate associations between Mn exposure from ferroalloy emissions and neurodevelopment. The study design has been described in detail previously [52]. Briefly, adolescents were enrolled from three areas in the Brescia province of northern Italy: Bagnolo Mella (BM), an area with active ferroalloy production since 1974; Valcamonica (VA), an area with historical ferroalloy production that ceased in 2001; and Garda Lake (GL), an area with no history of ferroalloy production. Children ages 10–14 years were recruited from public schools if they were born to families residing in the study area since 1970 and had lived in the study area since birth. Children were excluded if they (1) had clinically diagnosed neurological, metabolic, hepatic, or endocrine diseases, or clinically evident hand/finger motor deficits; (2) were currently taking any prescription psychoactive drugs; (3) had visual deficits not adequately corrected; or (4) had ever received total parenteral nutrition.
Recruitment took place in two phases: 311 adolescents were enrolled in the first phase (2007–2010) and 410 were enrolled in the second phase (2010–2014) for a total of 721 participants. The recruitment phases reflect two waves of funding, but with identical study protocols, questionnaires, and procedures. In 2013, we obtained supplemental funding to collect and measure Mn in naturally shed (i.e., deciduous) baby teeth. Teeth were available from a subset of the PHIME cohort that included participants from both phases (n = 195); one tooth was collected per participant. Adolescent verbal learning and memory was only measured in the second phase (N=410). Of the participants in the second phase who had available teeth for analysis (N=145), 140 also had complete outcome data on adolescent verbal learning and memory, which comprises the final sample for this analysis.
Eligible participants and their parents received a detailed description of the study procedures before consenting to participate. The Institutional Review Boards at the Ethical Committee of Brescia, the Icahn School of Medicine at Mount Sinai, and the University of California, Santa Cruz approved all PHIME study protocols.
2.2. Tooth collection and Mn exposure
The aim of this analysis was to examine the association between early-life Mn exposure and adolescent neurodevelopment. W used deciduous teeth, which are a retrospective marker of early-life exposure,[24,37,51,52] because no other early-life exposure biomarkers were collected in the PHIME cohort. Deciduous teeth (incisors, canines, and molars) that were free of defects (e.g., caries) were analyzed for Mn. Detailed analytical methods have been described elsewhere [23,51,53]. In brief, concentrations of Mn in dentine were determined using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). The neonatal line (NL), a histological feature formed in teeth at birth, was used as a reference point to distinguish between dentine formed during the prenatal period versus dentine formed after birth. Mn concentrations were measured in three distinct exposure periods: the prenatal (2nd trimester to birth), early postnatal (birth to ~1 year), and early childhood (~1.5 to 6 years) periods. Forty measurements were taken from each tooth; 30 measurements were taken from the primary dentine, reflecting prenatal and early postnatal exposure up to 3 months for incisors and ~1 year for molars, and an additional 10 measurements were taken from secondary circumpulpal dentine, which begins to form at the time of tooth root completion (at ~1.5 years). The area under the curve (AUC) of Mn levels across all sampling points was calculated to estimate cumulative Mn exposure during the prenatal and early postnatal periods [23,53]. The prenatal AUC represents measures taken from dentine before the NL formed (i.e., exposure before birth), and the postnatal AUC represents dentine sampled after the NL formed (i.e., exposure after birth). Mn tooth concentrations measured from the 10 sampling points in secondary circumpulpal dentine were averaged to represent exposure that occurred from ~1 year of age until ~6 years of age; thus, we consider this a measure of integrated childhood Mn exposure. Measurements taken from secondary circumpulpal dentine represent a longer exposure period because the formation of this tooth matrix proceeds at a slower rate compared to primary dentine (as long as the tooth remains viable) [54].
All dentine Mn levels were normalized to dentine calcium (Ca) levels to account for individual variations in tooth mineral density. Final dentine Mn levels are represented as ratios of Mn to Ca and are unitless: 55Mn:43Ca AUC x 10,000 for prenatal and postnatal periods, and average 55Mn:43Ca for the childhood period [23]. Values below the limit of detection (LOD) (n=2 for postnatal period) were assigned a value of half the minimum value among all other samples above the LOD (i.e., 0.015 55Mn:43Ca AUC x 10,000). The limit of detection was 0.03 AUC Mn55:Ca43 × 104. Laboratory technicians were blinded to participant neurobehavioral and demographic information.
2.3. Neurobehavioral assessment
All neurobehavioral assessments were administered by trained neuropsychologists prior to measurement of tooth Mn levels. Adolescent verbal learning and memory was evaluated using an Italian translation of the California Verbal Learning Test, Children’s Edition (CVLT-C) [55]. Briefly, the CVLT-C consisted of five trials of recall of a list of 15 verbally presented words belonging to three semantic categories (5 words per category). The first five trials assessed learning and were followed by one recall trial of an interference list (i.e., a list of 15 different words that share one common semantic category from the first list). Short delay recall (recall immediately following the interference list) and long delay recall (recall after a 20-minute delay) trials were also administered, where short and long delay recall trials were used to assess memory storage (i.e., retention) and retrieval. Short and long delay recall trials were conducted as free recall (i.e., without prompts) and cued recall (i.e., with prompts). Primary outcomes from the CVLT-C evaluated in this study include the number of correct words recalled on the first and last learning trials (i.e., trial 1 and trial 5), short delay free and cued recall, and long delay free and cued recall. In addition, two types of errors were calculated: intrusions and perseverations. Intrusions reflect the number of non-target words recalled by the participant within a trial, summed across all trials (i.e., when a participant states a word in a recall trial that is not from the CVLT-C target list); perseverations reflect the number of target words repeated within a trial, summed across all trials (i.e., when a participant states a word more than once in a recall trial that is from the CVLT-C target list). Higher scores on learning and recall trials indicate more words recalled and thus better verbal learning and memory, whereas higher scores on error outcomes (i.e., intrusions and perseverations) indicate less accurate cognitive performance. CVLT-C outcomes are summarized in Table 1.
Table 1.
Description of outcomes of the California Verbal Learning Test (CVLT-C)
| CVLT-C Outcome | Description | Direction of beneficial effect |
|---|---|---|
| Trial 1 | Number of correct target words recalled on Trial 1 | Higher score |
| Trial 5 | Number of correct target words recalled on Trial 5 | |
| Short delay free recall | Number of correct target words recalled immediately following the interference list without semantic cues | |
| Short delay cued recall | Number of correct target words recalled immediately following the interference list with semantic cues | |
| Long delay free recall | Number of correct target words recalled after a long delay (~20 minutes) without semantic cues | |
| Long delay cued recall | Number of correct target words recalled after a long delay (~20 minutes) with semantic cues | |
| Intrusions | Total number of responses not from the target word list summed across all trials | Lower score |
| Perseverations | Total number of target words repeated within a trial summed across all trials |
2.4. Covariates
Sociodemographic information was collected at enrollment using a standardized questionnaire administered by trained researchers. Information included biological sex, age (in years), birth order (first, second, third or higher), and area of residence (Bagnolo Mella, Valcamonica, Garda Lake). Socioeconomic status (SES) was categorized into low, medium, or high based on methodology developed in Italy that combines information on parental education and occupation [56,57]. In addition, an abbreviated version of the Home Observation Measurement of the Environment (HOME) Short Form (National Longitudinal Surveys,1979) was used to estimate cognitive and emotional stimulation in the home. Other biological specimens were collected at the time of neurobehavioral assessment, including blood samples for measurement of other metals (e.g., lead [Pb] and iron [Fe]). Whole blood samples (4 mL) were collected using a 19- gauge butterfly catheter into a Lithium Heparin Sarstedt Monovette Vacutainer. Analysis of blood lead concentrations was performed at the University of California, Santa Cruz, using inductively coupled plasma-mass spectrometry, as previously described [15,57].
2.5. Multiple imputation
Data were missing on several important covariates (range of missingness: 0 – 6%); therefore, we used Monte Carlo Markov Chain (MCMC) imputation to impute 20 datasets using all variables potentially related to covariates with missing values, including measures of Mn in biological and environmental samples, CVLT-C outcomes, and potential confounders (Table S1) [59]. Data were assumed to be missing at random; that is, we assumed that we could describe the probability of missingness using information from other covariates, but that missingness was not dependent on unobserved data. We imputed missing values for all participants (N=721) but restricted the analysis to participants who had complete exposure and outcome data (N=140). To calculate summary statistics on demographic information in Table 2 and Table S2, we averaged across imputed datasets.
Table 2.
Demographics of PHIME study participants, stratified by child biological sex.
| All Participants (N=140) | Females (N=78) | Males (N=62) | |
|---|---|---|---|
| n (%) or median (25th, 75th percentile) | |||
| Age (years): | 12.0 (11.0, 13.0) | 12.0 (11.0, 13.0) | 12.0 (11.0, 12.8) |
| Child SES status: | |||
| Low | 29 (20.7%) | 23 (29.5%) | 6 (9.7%) |
| Medium | 80 (57.1%) | 37 (47.4%) | 43 (69.4%) |
| High | 31 (22.1%) | 18 (23.1%) | 13 (21.0%) |
| Study Areaa | |||
| Bagnolo Mella | 77 (55.0%) | 43 (55.1%) | 34 (54.8%) |
| Garda Lake | 32 (22.9%) | 20 (25.6%) | 12 (19.4%) |
| Valcamonica | 31 (22.1%) | 15 (19.2%) | 16 (25.8%) |
| Adolescent Blood Pb (μg/dL) | 1.3 (1.0, 1.8) | 1.2 (0.9, 1.5) | 1.3 (1.1, 1.9) |
| Adolescent Ferritin (ng/mL) | 31.0 (21.5, 43.3) | 30.2 (19.9, 43.0) | 31.7 (22.2, 44.6) |
| HOME Scoreb | 6.1 (5.0, 7.0) | 6.0 (5.0, 7.0) | 7.0 (5.8, 7.0) |
| Dentine Mnc | |||
| Prenatal AUC x 104 | 0.43 (0.33, 0.53) | 0.42 (0.34, 0.50) | 0.44 (0.32, 0.56) |
| Postnatal AUC x 104 | 0.13 (0.09, 0.16) | 0.13 (0.11, 0.17) | 0.12 (0.08, 0.15) |
| Childhood Average | 0.0007 (0.0005, 0.0009) | 0.0007 (0.0005, 0.0009) | 0.0007 (0.0005, 0.0009) |
| Tooth attrition | |||
| No attrition | 74 (52.9%) | 46 (59.0%) | 28 (45.2%) |
| Attrition in <1/3 of tooth | 54 (38.6%) | 28 (35.9%) | 26 (41.9%) |
| Attrition in >1/3 of tooth | 12 (8.6%) | 4 (5.1%) | 8 (12.9%) |
Bagnolo Mella: active ferroalloy exposure since 1974; Garda Lake – no current or historical ferroalloy exposure; Valcamonica - historical ferroalloy production that ceased in 2001
HOME Score: Home Observation Measurement of the Environment, values range from 0–9
Dentine Mn levels are presented as ratio of Mn to Ca and are unitless. Prenatal and postnatal Mn are presented as area under the curve (AUC) x 104.
2.6. Statistical Analysis
Univariate analyses, including histograms and boxplots, were conducted for exposures and outcomes. Dentine Mn levels were log2-transformed to reduce the impact of outliers. CVLT-C error outcomes (i.e., intrusions and perseverations) were non-normally distributed and not improved by transformation. In addition, we aimed to model the association between Mn in each time period with error outcomes so that results reflected a meaningful change that is relevant to our population. Thus, to satisfy model assumptions and improve interpretation, these outcomes were dichotomized and modeled as binary outcome variables as above vs. below the median score for all participants (median=1 for intrusions, median=5 for perseverations; Table S3). Pairwise correlations between dentine Mn levels in different exposure periods (prenatal, postnatal, childhood) and between CVLT-C outcomes were calculated using Spearman correlation coefficients.
Table 3:
P-values from multiple informant models (N=140).
| CVLT-C outcome | P-value |
|---|---|
|
| |
| Trial 1 Recall | 0.97 |
| Trial 5 Recall | 0.36 |
| Short Delay Free Recall | 0.07* |
| Short Delay Cued Recall | 0.52 |
| Long Delay Free Recall | 0.52 |
| Long Delay Cued Recall | 0.24 |
|
| |
| CVLT-C errors | P-value |
|
| |
| Intrusions | 0.03* |
| Perseverations | 0.80 |
P-value ≤ 0.10
CVLT-C = California Verbal Learning Test for Children;
Confounders were chosen a priori based on prior literature [20,23,24,26,58] and a directed acyclic graph (DAG) (Figure S1). Covariates included in the final models were biological sex (female vs. male), child age (years, continuous), socioeconomic status (categorical: medium, high vs. low), HOME score (continuous), ln-transformed adolescent blood Pb (μg/dl, continuous), and tooth attrition, a measure of tooth wear (ordinal: attrition in less than one-third of tooth, attrition in more than one-third but less than two-thirds of tooth, vs. no attrition). We did not control for smoking status as a precision variable although this may be associated with adolescent neurodevelopment, because all participants reported being non-smokers. Given the low correlations (0.03 – 0.29) between Mn levels in different exposure periods, we were able to also adjust regression models for Mn levels in all other exposure periods (e.g., the childhood Mn association was adjusted for Mn levels in the prenatal and postnatal periods).
Since Mn is both an essential nutrient and a neurotoxicant [2,3], we evaluated potential nonlinearity in the associations between Mn and verbal learning and memory outcomes by visually inspecting Mn modeled as a smoothed term (i.e., spline) in fully adjusted generalized additive models (GAMs). There was no evidence of non-linear associations in GAM models; therefore, Mn levels were modeled as continuous variables in multivariable and logistic regression models. We used multivariable regression to estimate associations between a doubling of dentine Mn in each exposure period (prenatal, early postnatal, childhood) with CVLT-C outcomes, in both crude and covariate-adjusted models. Adjusted linear regression models were fit for each of the 20 imputed datasets, and we obtained pooled beta (β) estimates and 95% confidence intervals (95% CIs) across fits using Rubin’s rule with the miceadds package in R [57]. Beta estimates in final models represent the covariate-adjusted change in CVLT-C learning or recall trial score per doubling in dentine Mn level in each exposure period. To estimate associations between Mn with dichotomized CVLT-C error outcomes, intrusions and perseverations, adjusted logistic regression models were fit for each of the 20 imputed datasets and pooled using the same methods as above. Results from logistic models are the covariate-adjusted odds ratio (OR) of making more than the median number of CVLT-C errors per doubling in dentine Mn level in each period.
Next, to supplement findings from regression models, we used multiple informant models to test whether associations between Mn and CVLT-C outcome scores were statistically different across exposure periods [20,60,61]. Multiple informant models test whether the information relayed by different informants (in this case, Mn during different exposure periods) relates in the same manner to an outcome (in this case, CVLT-C outcomes) [60]; thus, we used this approach to test for exposure period-specific associations of Mn with verbal learning and memory. Generalized estimating equations (GEEs), with either an identity link for the continuous outcomes or a logit link for the binary outcomes, were fit for each of the 20 imputed datasets for each CVLT-C outcome. Model fits were pooled using Rubin’s rule with proc mianalyze in SAS, using adapted code from Sanchez et al. 2011[60] and Bauer et al. 2021[20]. We considered associations to be significantly different across exposure periods when the interaction term between Mn level and exposure period p-value was < 0.10.
We explored the possibility of sex-specific effects by fitting sex-stratified linear and logistic regression models and, separately, including sex*Mn interaction terms in main (i.e., non-stratified) models. We considered sex*Mn interactions to be significant if the interaction term p-value was <0.10. We also conducted two sensitivity analyses. First, we estimated associations between Mn in each exposure period and CVLT-C scores among participants with complete data on all covariates (i.e., complete case analysis). In addition, we explored potential confounding by iron (Fe) status by adding adolescent ferritin, a sensitive measure of Fe status [62–64], as a covariate in our models. Iron (Fe) is an essential nutrient involved in many biologic functions that support cognition, including neurotransmitter synthesis and cellular oxygen transport [65,66], and iron levels have been associated with Mn levels; as such, Fe status may act as a potential confounder of the Mn-neurodevelopment associations [25,66–68].
All statistical analyses were conducted using R version 3.6.1 and SAS version 9.4.
3. Results
About half (56%) of the participants in this analysis were female. The median age of participants at the time of neurobehavioral assessment was 12 years (Table 2), which was similar for males and females. Most adolescents were from a middle SES family (57%) and resided in the Bagnolo Mella study area (55%). Compared to males, a higher proportion of females were from families designated as low SES (females: 30% vs. males: 10%) (Table 2). We compared characteristics of participants included in this analysis to those in the full cohort that did not have available dentine Mn levels or complete outcome data; those included in this analysis were more likely to reside in Bagnolo Mella (Table S2).
Median (25th, 75th percentile) dentine Mn levels, as the ratio of 55Mn:43Ca AUC x 104, in the prenatal period was 0.43 (0.33, 0.53), which was higher than levels in the postnatal period (median: 0.13; 25th, 75th percentile: 0.09, 0.16). The median childhood average dentine Mn level was 0.0007 (25th, 75th percentile: 0.0005, 0.0009). Levels of dentine Mn were similar between females and males in all three exposure periods (Table 2). Prenatal, postnatal and childhood Mn levels were weakly correlated (ρ = 0.03 – 0.29). CVLT-C scores were weakly to strongly correlated (ρ = 0.05 – 0.83), with the largest correlations between short delay free, short delay cued recall, long delay free recall, and long delay cued recall trials (Figure S2). The median and range of CVLT-C scores were similar between sexes, except for intrusions (Table S3), where females committed fewer intrusion errors on average than males (median: females=0, males=2).
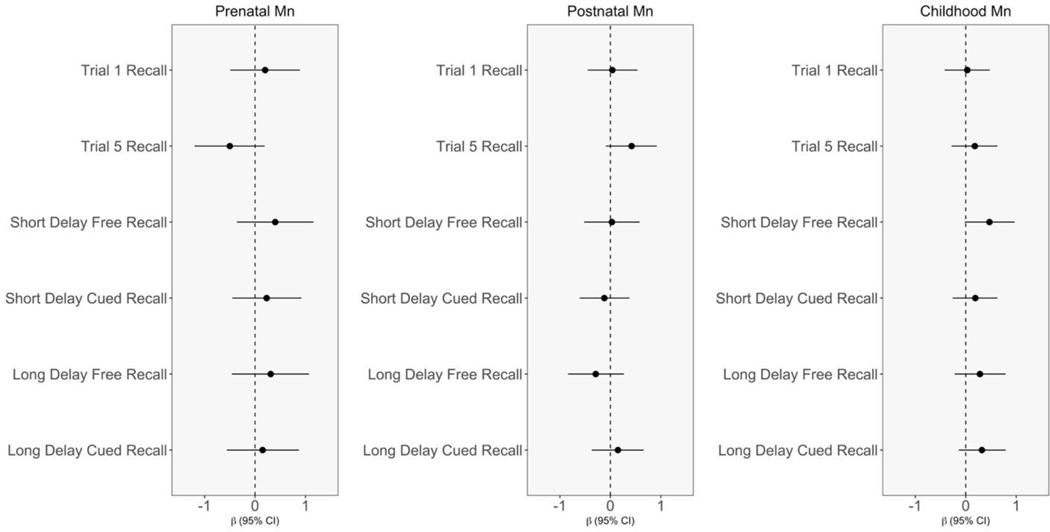
Crude and covariate-adjusted associations of Mn with verbal learning and memory were mostly null across all three exposure periods (Figure 1; Table S4; Table S5). Nonetheless, there was a trend of protective associations between dentine Mn in the prenatal and childhood periods and recall trials, as nearly all effect estimates were positive (Figure 1; Table S5). Among all participants, a doubling in childhood Mn was associated with an increased number of correct words recalled on the short delay free recall condition (β=0.47 [95% CI: −0.02, 0.97]) (Figure 1; Table S5). This association was similar though less precise in the prenatal exposure period (β= 0.40 [95% CI: −0.36, 1.16]), and null in the postnatal period (β= 0.03, [95% CI: −0.52, 0.58]). Results from the multiple informant model suggest that the associations were different across exposure periods (p-value=0.07) (Table 3). In exploratory sex-stratified analyses, the association between childhood dentine Mn and short delay free recall was stronger in males compared to females (males: β = 0.94 [95% CI: 0.05, 1.82] vs. females: β = 0.37 [95% CI: −0.29, 1.03]) (Figure 2; Table S6). Further, in exploratory sex-stratified multiple informant models, results suggest that the associations between dentine Mn and short delay free recall across exposure periods were different among males only (Table S7). There were also beneficial associations between childhood dentine Mn and short delay cued recall, long delay free recall, and long delay cued recall (Figure 1), which were stronger among males (short delay cued recall β= 0.63 [95% CI: −0.11, 1.38]; long delay free recall β= 0.57 [95% CI: −0.29, 1.43]; long delay cued recall β= 0.95 [95% CI: 0.17, 1.73]), compared to females (short delay cued recall β= −0.06 [95% CI: −0.69, 0.57]; long delay free call β= 0.19 [95% CI:−0.52, 0.89]; long delay cued recall β= 0.01 [95% CI: −0.63, 0.64]) (Figure 2, Table S6). In non-stratified models, the sex*Mn interaction term was significant for long delay cued recall in the childhood exposure period only (p-value = 0.05; Figure 2).
Figure 1: Adjusted associations of prenatal, postnatal, and childhood dentine manganese (Mn) with verbal learning and memory recall trials based on multivariable linear regression models.
Beta estimates (dots) represent change in California Verbal Learning Test for Children (CVLT-C) outcome score per doubling in dentine Mn, with 95% confidence intervals (lines). Models were adjusted for biological sex, child age, socioeconomic status, abbreviated HOME score, natural log-transformed blood Pb, tooth attrition, and dentine Mn in other exposure periods.
Figure 2: Sex-stratified adjusted associations of prenatal, postnatal, and childhood dentine manganese (Mn) with verbal learning and memory recall trials based on multivariable linear regression models.
Beta estimates (dots) represent change in California Verbal Learning Test for Children (CVLT-C) outcome score per doubling in Mn, with 95% confidence intervals (lines). Models were adjusted for child age, socioeconomic status, abbreviated HOME score, natural log-transformed blood Pb, tooth attrition, and dentine Mn in other exposure periods. Asterisk indicates that p-value for sex*Mn interaction term in non-stratified models is ≤ 0.10.
Based on covariate-adjusted logistic regression models, protective associations were observed between prenatal dentine Mn with intrusions: a doubling in prenatal dentine Mn concentrations was associated with a 77% decrease in the odds of making greater than the median number of intrusion errors (OR = 0.23 [95% CI: 0.09, 0.61]; Table 4). Associations were similar in crude models (Table S7), though the association between prenatal Mn and intrusions was slightly attenuated (OR = 0.44 [95% CI: 0.21, 0.92)].The association between Mn in the prenatal period and intrusions was different than the associations in other exposure periods, where associations were imprecise and null (postnatal OR = 1.16 [95% CI: 0.64, 2.10]; childhood OR= 1.09, [95% CI: 0.64, 1.89]). This was consistent with findings from multiple informant models, which suggested that Mn-intrusion error associations were statistically different across exposure periods (p-value = 0.03) (Table 3). In exploratory analyses, there was evidence of differences by sex: the protective association between prenatal Mn and intrusions was stronger among males (OR = 0.08 [95% CI: 0.01, 0.63]) than females (OR = 0.40 [95% CI: 0.11, 1.37]), though the sex*Mn interaction term was not significant (p-value = 0.11) (Table 4). Most other associations between dentine Mn and CVLT errors were null. Although ORs for associations between prenatal Mn and perseveration errors were large in magnitude, (e.g., OR=1.50 [95% CI: 0.70, 3.23]), estimates were imprecise.
Table 4.
Adjusted odds ratios between CVLT-C errors following a doubling in dentine Mn level based on logistic regression modelsa,b
| Outcome | Model | N | Prenatal Mn OR (95% CI) | Postnatal Mn OR (95% CI) | Childhood Mn OR (95% CI) |
|---|---|---|---|---|---|
| Intrusions (≥1 vs. 0) | All b | 140 | 0.23 (0.09, 0.61) | 1.16 (0.64, 2.10) | 1.09 (0.64, 1.89) |
| Female | 78 | 0.40 (0.11, 1.37) | 0.85 (0.31, 2.37) | 1.20 (0.58, 2.48) | |
| Male | 62 | 0.08 (0.01, 0.63) | 1.39 (0.59, 3.29) | 1.39 (0.46, 4.19) | |
| Perseverations (≥ vs. <5) | All b | 140 | 1.50 (0.70, 3.23) | 0.92 (0.53, 1.59) | 1.00 (0.61, 1.65) |
| Female | 78 | 2.48 (0.70, 8.76) | 0.53 (0.19, 1.51) | 1.19 (0.58, 2.44) | |
| Male | 62 | 1.22 (0.38, 3.91) | 1.04 (0.48, 2.26) | 1.04 (0.45, 2.42) |
Models were adjusted for child age, socioeconomic status, HOME score, natural log-transformed blood Pb, tooth attrition, and dentine Mn in other exposure periods.
Models were additionally adjusted for biological sex.
CVLT-C = California Verbal Learning Test for Children; Mn = manganese
Asterisk indicates a significant sex*Mn interaction term in non-stratified models (p-value < 0.10).
In sensitivity analyses in which models were restricted to participants with complete data on exposure, outcome, and key covariates (N=127), associations between Mn and adolescent verbal learning and memory were smaller in magnitude but similar to findings from main analyses (Tables S8 and S9). When we considered ferritin as an additional covariate in models, results did not differ meaningfully from main results (data not shown).
4. Discussion
Mn is an essential nutrient required for healthy development, but the exposure level at which Mn shifts from beneficial to neurotoxic for the developing brain remains unknown [2,3,31]. In this analysis of dentine Mn measured in multiple exposure periods of early life, most associations with adolescent verbal learning and memory were null. However, associations for prenatal Mn and childhood Mn were suggestive of a beneficial effect, particularly among males. The adolescent CVLT outcomes that were most sensitive to prenatal and childhood Mn, respectively, were intrusion errors and words recalled.
The strong beneficial association between prenatal Mn and intrusions is consistent with Mn being an essential nutrient for early brain development [2,3,9,31]. Most toxicological studies of early life Mn exposure have focused on identifying the effects of overexposure such as reported structural changes to the brain and decreased neurodevelopmental scores later in life [27–29,69]. Mn deficiency, on the other hand, is less studied in the animal literature, limiting the ability to identify specific mechanisms by which early life Mn acts beneficially on brain structure and function. However, it is known that Mn is required for metabolism of amino acids, lipids, proteins, and carbohydrates. For example, multiple enzymes, such as transferases, hydrolases and ligases, cannot function adequately without Mn [2,70,71]. Further, children with induced Mn deficiency, as a result of a genetic mutation to the Mn influx transporter SLC39A8, have been shown to suffer from severe neurological deficits [3,72]. Mn is also actively transported across the placenta, suggesting it is required for fetal development [73,74]. Lastly, studies consistently report that biomarker levels of Mn increase during pregnancy and are higher in pregnant people versus non-pregnant people [24,32–37]. Collectively, this affirms that Mn is necessary for healthy development and suggests that Mn may be upregulated in early life to meet metabolic needs. Our findings are also consistent with other epidemiological studies that have noted beneficial associations of prenatal Mn measured in deciduous teeth [5,20,24,26,58], maternal serum [4], and placental tissue [75], with neurodevelopmental outcomes such as behavior, cognition, visuospatial learning, and memory. Overall, our results support the conclusion that the levels of dentine Mn measured in this population reflecting prenatal exposure are beneficial for adolescent neurodevelopment, consistent with Mn being an essential nutrient.
Memory is a complex cognitive domain that requires input from several structures of the brain, including the hippocampus, prefrontal cortex, and their surrounding structures [76–79]. In the prenatal exposure period, increased dentine Mn was associated with lower odds of committing more than the median number of intrusion errors. An intrusion error (i.e., stating a word in a recall trial that is not from the CVLT-C target list) reflects an inability to inhibit incorrect responses or confusion by the participant during the test, which suggests impaired prefrontal cortex function or impaired coordination between multiple brain regions. Thus, the observed beneficial associations between prenatal Mn and decreased intrusions suggest that, in this exposure period, and at these levels, Mn may beneficially impact prefrontal cortex function and/or intrinsic connectivity. This is consistent with prior literature that has identified the prefrontal cortex as being impacted by early-life Mn exposure, [3,9,29,49] and with studies that have reported changes in intrinsic function connectivity following prenatal Mn exposure [21]. One limitation of our study is the lack of data on specific types of intrusion errors (e.g., whether the error responses were related semantically to correct responses), which is more typically collected in studies of adult populations. The type and frequency of intrusions can help distinguish between types of neuropathological dysfunction mediated by different parts of the brain, such as the medial-temporal or subcortical-frontal structures [80]. As we lack information on the type and frequency of intrusions in each recall trial, we interpret our measure of total intrusion errors (i.e., total number of non-target words recalled during all trials) as a global error measure related to overall brain function, as opposed to a more specific structural aspect of memory function [79].
In the childhood exposure period, there was also evidence of beneficial Mn associations with improved scores on the short delay and long delay recall trials. To date, there are no published studies that examined Mn exposure within the same childhood period that we measured in our study (i.e., between ages 1–6) in relation to verbal learning and memory. Previous cross-sectional studies of Mn exposure in later childhood and early adolescence (6–13 years old), in contrast to our findings, have reported adverse associations between Mn exposure and verbal learning and memory [42,44–46]. For example, associations between hair Mn and decreased verbal learning and memory scores were estimated in several studies: Canadian children assessed with the CVLT-C [44], Brazilian children using the Memory for Designs from the Developmental Neuropsychological Assessment (NEPSY-II) [42], and Mexican children using multiple versions of the Children’s Auditory Verbal Learning Test (CAVLT) [45,81]. The discrepancy in findings between our analysis and prior cross-sectional studies may be due to differences in exposure levels or exposure timing. In other words, the beneficial versus toxic roles of Mn may shift when exposure occurs later in development due to increased susceptibility during specific periods of brain development. Specifically, during adolescence (ages 10–19), and particularly during early years of adolescence (ages 10–14), there is a unique and dramatic maturational process in the prefrontal cortex and dopaminergic neurons of the brain where dopamine axons grow from striatum to the prefrontal cortex [82,83]. Dopaminergic neurons are affected by Mn exposure [2,11,47,84,85] and Mn tends to accumulate in brain areas rich in dopamine (e.g., the basal ganglia and the substantia nigra) [70,86,87]. Dopaminergic neurons have been shown to play a large role in cognitive control in the prefrontal cortex as well as in the processing of incoming sensory signals [88,89], and insult to dopaminergic neurons can adversely impact cognitive function [90,91]. In prior work in the PHIME cohort, we reported an adverse association between adolescent hair Mn and long delay free and cued recall [64]. However, we are unable to directly compare associations as this analysis examined joint associations between multiple metals, examined only a select number of CVLT-C outcomes, and was cross-sectional in design [64]. Thus, the late childhood and early adolescent periods, compared to the early childhood period that our tooth biomarker reflects, still may be a more sensitive exposure period to Mn and result in greater insults to prefrontal and/or dopamine structures. As such, future studies are needed to elucidate sensitive periods of exposure to Mn in relation to verbal learning and memory.
In our data, we observed null associations between postnatal Mn and adolescent verbal learning and memory. Previous studies in the PHIME cohort have similarly estimated null associations between postnatal dentine Mn and IQ scores from the Wechsler Intelligence Scale for Children (WISC) [20], and between postnatal dentine Mn and visuospatial learning measured using the Virtual Radial Arm Maze [5]. In contrast, other cohorts have reported beneficial associations between postnatal dentine Mn and verbal learning and memory measured on the Children’s Auditory Verbal Learning Test (CAVLT) and Memory for Designs from the Developmental Neuropsychological Assessment (NEPSY-II) battery at 9 and 10.5 years old [26]. However, the postnatal dentine Mn measurement used in the aforementioned study by Mora et al. [26] reflects a shorter period of exposure, from birth to the third postnatal month, whereas the postnatal dentine Mn measure in our study reflects exposure from birth through the first year of life [20,23]. The integrated Mn measurement in our study also does not capture changes in Mn exposure at as high of a resolution as prior studies in which dentine Mn levels were measured in weekly increments [24,37,58]. This difference in time resolution of exposure measures between studies could partially explain the null findings in our study compared with other studies of shorter [26] or more finely resolved [24,58] exposure periods. It is also possible that the postnatal period (birth to ~1 year of life) may not be as sensitive to Mn-induced changes in adolescent verbal learning and memory compared to the prenatal or childhood exposure periods. More research, ideally in larger prospective cohorts, is needed to elucidate the effects of postnatal Mn on cognition.
In exploratory sex-stratified analyses, the beneficial associations between childhood Mn and recall trials were stronger among males than females. Sex-specific effects of Mn on neurodevelopment have been reported previously in multiple cohorts across exposure periods and neurodevelopmental domains [5,23,26,42,45,50]. In our data, dentine Mn levels were similar between sexes, suggesting that any sex-dependent associations are related to the sexually dimorphic susceptibility of Mn and not to differences in measured Mn biomarker concentrations. Sex-specific associations could be related to anatomical differences in the central nervous system between males and females, as estrogen, a sex hormone, has been documented to play a key role in neuronal structure and brain function [92]. There is also growing evidence that brain areas rich in dopamine, the target neurotransmitter for Mn toxicity, are sexually dimorphic, where females tend to be more sensitive to neurotoxic effects [93]. There may also be sex-specific differences in the striatal response to dopamine [94,95], which means that cognitive and motor functions may be differentially regulated by dopamine systems between sexes [93]. Therefore, given equal exposure, neurotoxic effects of Mn may differ by biological sex as a result of a) inherent anatomical differences related to biological sex hormones, b) greater toxicity related to disruption of neurotransmitters, particularly with dopamine for females, and/or c) the role of specific brain regions impacted by Mn exposure, as cognitive function may be differently regulated or carried out by different brain regions between sexes. Future studies in other cohorts measuring exposure during the early childhood period (1–6 years old) are warranted to understand the sex-dependent susceptibility of insult to the brain related to Mn exposure during this time period.
This study has both strengths and limitations. Given the small sample size (n=140), we had limited statistical power, particularly in sex-stratified analyses. The use of the tooth biomarker is rapidly evolving, and as such, the resolution with which exposure can be measured using teeth has changed over time. In this present study, our exposure metric represents earlier technology in tooth analytic chemistry that averaged the area under the curve over longer exposure periods (40 total spot measurements) to derive the prenatal, postnatal, and childhood measurements, compared to more recent studies that have finer resolution (i.e., weekly) measurements [24,37]. Regardless, the tooth biomarker allowed us to measure retrospective exposure during multiple exposure periods spanning distinct developmental stages, thus allowing us to evaluate associations in a prospective manner and to establish temporality between exposure and outcome. Further, it is unclear which proportion of Mn in teeth reflect endogenous versus exogenous exposure. However, dentine Mn has been previously associated with environmental Mn sources in epidemiological [51,96–98], exposure [37], and experimental studies [99], suggesting that tooth levels do reflect environmental exposure to Mn. Lastly, tooth metal measurements were taken from a mix of incisors, molars and canines, which develop over slightly different time ranges and may thus represent exposure during slightly different time periods. We lack information on tooth type for the majority of participants and were thus unable to account for such potential differences. This may result in exposure misclassification, although we expect this misclassification to be non-differential with respect to the outcome. We used the CVLT-C to assess verbal learning and memory among adolescents, an understudied age group in relation to environmental exposures. We lacked data on the types of learning strategies used by participants during administration of the CVLT-C (i.e., semantic or serial clustering of responses) or on specific types of errors (i.e., when intrusions and perseverations occurred), which marginally limits the ability to make inferences about the parts of the brain impacted by Mn. On the other hand, prior studies have used the CVLT-C to discern between exposure groups (e.g., studies of early-life exposure to alcohol), suggesting that the CVLT-C can be a sensitive tool for measuring general brain changes following early-life exposures [100,101].
Selection bias may be possible if those selected into the analytical sample differ by variables that are related to both dentine Mn levels and adolescent verbal learning and memory scores (e.g., SES). However, subjects who had complete exposure and outcome data (N=140) were similar to the full cohort (N=721) on all measured characteristics except study area and abbreviated HOME score. Most children in our analysis were from a single study area, Bagnolo Mella. This likely reflects this area being added during the second phase of the study, which was contemporaneous with deciduous teeth collection and the addition of the abbreviated version of the HOME Short Form. Participants were also unaware of Mn exposure levels or outcome scores and children with diagnosed neurological deficits were excluded, limiting concern for selection bias into this study. Although we adjusted for adolescent Pb exposure, we were unable to adjust for Pb or other essential metals (e.g., copper) during the exposure periods in our study. We were also unable to adjust for other potentially neurotoxic co-exposures such as arsenic, cadmium, or mercury at any exposure time point; thus, we cannot rule out unmeasured confounding by earlier exposures and/or modification by co-exposures, which have been reported in other studies [20,24,46,68]. In addition, no participants in this analytic sample reported smoking and we therefore did not further consider smoking as potential confounder, though there may be misclassification of this variable if participants did not accurately report their smoking status. We also lack data on current hormone levels or puberty stage for all participants, which may be related to both metal exposure and neurodevelopment. Lastly, we lacked data on iron (Fe) status concurrent with Mn exposure, which may result in unmeasured confounding or effect measure modification, as low maternal Fe (a proxy for prenatal exposure) has previously been associated with worse neurodevelopmental outcomes in children [25,67,68]. However, in sensitivity models where we adjusted for adolescent ferritin, a sensitive measure of Fe status [62–64], results were similar to main models).,
5. Conclusion
In this study of Italian adolescents, we observed beneficial associations of prenatal and childhood Mn with adolescent verbal learning and memory, which were not evident for early postnatal Mn. The strongest beneficial associations were estimated between intrusion errors and prenatal Mn, and between short- and long delay recall and childhood Mn, particularly among males. Results support the conclusions that exposure timing is important for understanding Mn-associated changes in neurodevelopment and that further studies among adolescents are needed to better understand sex-specific neurobehavioral effects of early-life Mn exposure.
Supplementary Material
Highlights.
We examined associations between early-life manganese (Mn) and adolescent memory.
Prenatal tooth Mn was associated with fewer memory and learning errors.
Childhood tooth Mn was associated with better memory recall.
Postnatal tooth Mn was not associated with adolescent memory.
Exposure timing is important for understanding manganese-cognition associations.
Acknowledgments
We are grateful for all participants of the PHIME cohort for their participation and time.
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIEHS): F31-ES033558 (AF), F31-ES033507 (SS), F31-ES029010 (JAB), T32-ES014562 (AF & SS), R01-ES019222 (MH, ROW, BCH)
Footnotes
Institutional Review Board Statement
Eligible participants received a detailed description of the study procedures before consenting to participate. The Institutional Review Boards at the Ethical Committee of Brescia, the Icahn School of Medicine at Mount Sinai, and the University of California, Santa Cruz approved all PHIME study protocols.
Conflicts of Interest/Disclaimer
Authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Data are confidential.
References
- 1.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Manganese [Internet]. 2012. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp151.pdf [PubMed]
- 2.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M, et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295:6312–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung SE, Cheong H-K, Ha E-H, Kim B-N, Ha M, Kim Y, et al. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect. 2015;123:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer J, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environ Int. 2017;108:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claus Henn B, Ettinger AS, Schwartz J, Téllez-Rojo MM, Lamadrid-Figueroa H, Hernández-Avila M, et al. Early Postnatal Blood Manganese Levels and Children’s Neurodevelopment. Epidemiology. 2010;21:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X-D, Zhang J, Yan C-H, Shen X-M. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–8. [DOI] [PubMed] [Google Scholar]
- 8.Bouabid S, Tinakoua A, Lakhdar-Ghazal N, Benazzouz A. Manganese neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J Neurochem. 2016;136:677–91. [DOI] [PubMed] [Google Scholar]
- 9.Vollet K, Haynes EN, Dietrich KN. Manganese Exposure and Cognition Across the Lifespan: Contemporary Review and Argument for Biphasic Dose-Response Health Effects. Curr Environ Health Rep. 2016;3:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015;2:315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal AP, Guilarte TR. Mechanisms of lead and manganese neurotoxicity. Toxicol Res. 2013;2:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinkov AA, Paoliello MMB, Mazilina AN, Skalny AV, Martins AC, Voskresenskaya ON, et al. Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update. Int J Mol Sci. 2021;22:4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou S-Y, Luo H-L, Mailman RB, Li Z-C, Zhang Y-W, Cai M, et al. Effect of manganese on neural endocrine hormones in serum of welders and smelters. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2018;50:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Quan J, Zhao S, Song D, Wang T, He W, Li G. Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci Total Environ. 2022;819:153105. [DOI] [PubMed] [Google Scholar]
- 15.Butler L, Gennings C, Peli M, Borgese L, Placidi D, Zimmerman N, et al. Assessing the contributions of metals in environmental media to exposure biomarkers in a region of ferroalloy industry. J Expo Sci Environ Epidemiol. 2019;29:674–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes EN, Ryan P, Chen A, Brown D, Roda S, Kuhnell P, et al. Assessment of personal exposure to manganese in children living near a ferromanganese refinery. Sci Total Environ. 2012;0:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas EL, Bertrand P, Guazzetti S, Donna F, Peli M, Jursa TR, et al. Impact of Ferromanganese Alloy Plants on Household Dust Manganese Levels: Implications for Childhood Exposure. Environ Res. 2015;138:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menezes-Filho JA, Paes CR, de C. Pontes ÂM, Moreira JC, Sarcinelli PN, Mergler D. High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. Neurotoxicology. 2009;30:1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavilonis BT, Lioy PJ, Guazzetti S, Bostick BC, Donna F, Peli M, et al. Manganese concentrations in soil and settled dust in an area with historic ferroalloy production. J Expo Sci Environ Epidemiol. 2015;25:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer J, White RF, Coull BA, Austin C, Oppini M, Zoni S, et al. Critical windows of susceptibility in the association between manganese and neurocognition in Italian adolescents living near ferro-manganese industry. NeuroToxicology. 2021;87:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rechtman E, Navarro E, de Water E, Tang CY, Curtin P, Papazaharias DM, et al. Early-Life Critical Windows of Susceptibility to Manganese Exposure and Sex-Specific Changes in Brain Connectivity in Late Adolescence. Biol Psychiatry Glob Open Sci. 2022;S2667174322000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer J, Fruh V, Howe CG, White RF, Claus Henn B. Associations of metals and neurodevelopment: a review of recent evidence on susceptibility factors. Curr Epidemiol Rep. 2020;7:237–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu Y-HM, Claus Henn B, Hsu H-HL, Pendo MP, Coull BA, Austin C, et al. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res. 2017;159:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claus Henn B, Austin C, Coull BA, Schnaas L, Gennings C, Horton MK, et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. Manganese in Teeth and Neurodevelopment in Young Mexican-American Children. Environ Res. 2015;142:688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernández-Bonilla D, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaudin SA, Nisam S, Smith DR. Early life versus lifelong oral manganese exposure differently impairs skilled forelimb performance in adult rats. Neurotoxicol Teratol. 2013;38:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaudin SA, Strupp BJ, Strawderman M, Smith DR. Early Postnatal Manganese Exposure Causes Lasting Impairment of Selective and Focused Attention and Arousal Regulation in Adult Rats. Environ Health Perspect. 2017;125:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conley TE, Beaudin SA, Lasley SM, Fornal CA, Hartman J, Uribe W, et al. Early Postnatal Manganese Exposure Causes Arousal Dysregulation and Lasting Hypofunctioning of the Prefrontal Cortex Catecholaminergic Systems. J Neurochem [Internet]. 2019. [cited 2019 Dec 14]; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jnc.14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiles J, Jernigan TL. The Basics of Brain Development. Neuropsychol Rev. 2010;20:327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbuckle TE, Liang CL, Morisset A-S, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere. 2016;163:270–82. [DOI] [PubMed] [Google Scholar]
- 33.Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res. 2004;95:119–25. [DOI] [PubMed] [Google Scholar]
- 34.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, et al. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ Res. 2020;183:109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong L, Yang Q, Liu C-W-B, Wang X, Zeng H-L. Assessment of 12 Essential and Toxic Elements in Whole Blood of Pregnant and Non-pregnant Women Living in Wuhan of China. Biol Trace Elem Res. 2021;199:2121–30. [DOI] [PubMed] [Google Scholar]
- 36.Guy M, Accrombessi M, Fievet N, Yovo E, Massougbodji A, Le Bot B, et al. Toxics (Pb, Cd) and trace elements (Zn, Cu, Mn) in women during pregnancy and at delivery, South Benin, 2014–2015. Environ Res. 2018;167:198–206. [DOI] [PubMed] [Google Scholar]
- 37.Friedman A, Bauer J, Austin C, Downs TJ, Tripodis Y, Heiger-Bernays W, et al. Multiple metals in children’s deciduous teeth: results from a community-initiated pilot study. J Expo Sci Environ Epidemiol [Internet]. 2021. [cited 2021 Nov 8]; Available from: https://www.nature.com/articles/s41370-021-00400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. NeuroToxicology. 2018;64:204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem Int. 2002;41:271–7. [DOI] [PubMed] [Google Scholar]
- 40.Chen C-J, Liao S-L. Oxidative Stress Involves in Astrocytic Alterations Induced by Manganese. Exp Neurol. 2002;175:216–25. [DOI] [PubMed] [Google Scholar]
- 41.Bouchard MF, Surette C, Cormier P, Foucher D. Low level exposure to manganese from drinking water and cognition in school-age children. NeuroToxicology. 2018;64:110–7. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho CF de, Oulhote Y, Martorelli M, Carvalho CO de, Menezes-Filho JA, Argollo N, et al. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. NeuroToxicology. 2018;69:253–9. [DOI] [PubMed] [Google Scholar]
- 43.Iyare PU. The effects of manganese exposure from drinking water on school-age children: A systematic review. NeuroToxicology. 2019;73:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur M-È, et al. Neurobehavioral Function in School-Age Children Exposed to Manganese in Drinking Water. Environ Health Perspect. 2014;122:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, Solís-Vivanco R, Montes S, Riojas-Rodríguez H, et al. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res. 2013;121:39–44. [DOI] [PubMed] [Google Scholar]
- 46.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. NeuroToxicology. 2006;27:210–6. [DOI] [PubMed] [Google Scholar]
- 47.Kern C, Smith DR. Pre-weaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synap N Y N. 2011;65:532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peres TV, Eyng H, Lopes SC, Colle D, Gonçalves FM, Venske DKR, et al. Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. NeuroToxicology. 2015;50:28–37. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Fu H, Liu B, Liu X, Chen W, Yu X. The effect of postnatal manganese exposure on the NMDA receptor signaling pathway in rat hippocampus. J Biochem Mol Toxicol. 2017;31:e21969. [DOI] [PubMed] [Google Scholar]
- 50.Rechtman E, Curtin P, Papazaharias DM, Renzetti S, Cagna G, Peli M, et al. Sex-specific associations between co-exposure to multiple metals and visuospatial learning in early adolescence. Transl Psychiatry [Internet]. 2020. [cited 2020 Nov 30];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7578810/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining Fetal Manganese Exposure from Mantle Dentine of Deciduous Teeth. Environ Sci Technol. 2012;46:5118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morishita H, Arora M. Tooth-matrix biomarkers to reconstruct critical periods of brain plasticity. Trends Neurosci. 2017;40:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–9. [DOI] [PubMed] [Google Scholar]
- 54.Berkovitz BKB, Holland GR, Moxham BJ. Oral Anatomy, Histology and Embryology - 5th Edition. 5th ed. Elsevier; 2017. [Google Scholar]
- 55.Fine EM, Delis DC. California Verbal Learning Test – Children’s Version. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encycl Clin Neuropsychol [Internet]. New York, NY: Springer; 2011. [cited 2023 Aug 4]. p. 476–9. Available from: 10.1007/978-0-387-799483_1538 [DOI] [Google Scholar]
- 56.Cesana GC, Ferrario M, De Vito G, Sega R, Grieco A. [Evaluation of the socioeconomic status in epidemiological surveys: hypotheses of research in the Brianza area MONICA project]. Med Lav. 1995;86:16–26. [PubMed] [Google Scholar]
- 57.Lucchini RG, Zoni S, Guazzetti S, Bontempi E, Micheletti S, Broberg K, et al. Inverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescents. Environ Res. 2012;118:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robitzsch A, Grund S. miceadds: Some Additional Multiple Imputation Functions, [Internet]. 2023. Available from: https://CRAN.R-project.org/package=miceadds [Google Scholar]
- 60.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical Methods to Study Timing of Vulnerability with Sparsely Sampled Data on Environmental Toxicants. Environ Health Perspect. 2011;119:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu J, Zhao H, Braun JM, Zheng T, Zhang B, Xia W, et al. Associations of Trimester-Specific Exposure to Bisphenols with Size at Birth: A Chinese Prenatal Cohort Study. Environ Health Perspect. 2019;127:107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schildroth S, Friedman A, Bauer J, Claus Henn B. Associations of a metal mixture with iron status in U.S. adolescents: Evidence from the National Health and Nutrition Examination Survey. New Dir Child Adolesc Dev. 2022;cad.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schildroth S, Friedman A, White RF, Kordas K, Placidi D, Bauer JA, et al. Associations of an industry-relevant metal mixture with verbal learning and memory in Italian adolescents: The modifying role of iron status. Environ Res. 2023;224:115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCann S, Perapoch Amadó M, Moore SE. The Role of Iron in Brain Development: A Systematic Review. Nutrients. 2020;12:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schildroth S, Kordas K, Bauer JA, Wright RO, Claus Henn B. Environmental Metal Exposure, Neurodevelopment, and the Role of Iron Status: a Review. Curr Environ Health Rep. 2022;9:758–87. [DOI] [PubMed] [Google Scholar]
- 67.Iglesias L, Canals J, Arija V. Effects of prenatal iron status on child neurodevelopment and behavior: A systematic review. Crit Rev Food Sci Nutr. 2018;58:1604–14. [DOI] [PubMed] [Google Scholar]
- 68.Kupsco A, Estrada-Gutierrez G, Cantoral A, Schnaas L, Pantic I, Amarasiriwardena C, et al. Modification of the effects of prenatal manganese exposure on child neurodevelopment by maternal anemia and iron deficiency. Pediatr Res [Internet]. 2020. [cited 2020 May 2]; Available from: http://www.nature.com/articles/s41390-020-0754-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lasley SM, Fornal CA, Mandal S, Strupp BJ, Beaudin SA, Smith DR. Early Postnatal Manganese Exposure Reduces Rat Cortical and Striatal Biogenic Amine Activity in Adulthood. Toxicol Sci. 2020;173:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci [Internet]. 2013. [cited 2020 Nov 2];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3690350/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeda A, Ishiwatari S, Okada S. Manganese uptake into rat brain during development and aging. J Neurosci Res. 1999;56:93–8. [DOI] [PubMed] [Google Scholar]
- 72.Wahlberg KE, Guazzetti S, Pineda D, Larsson SC, Fedrighi C, Cagna G, et al. Polymorphisms in Manganese Transporters SLC30A10 and SLC39A8 Are Associated With Children’s Neurodevelopment by Influencing Manganese Homeostasis. Front Genet [Internet]. 2018. [cited 2019 Dec 14];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6307466/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bałasz M, Szkilnik R, Brus R, Malinowska-Borowska J, Kasperczyk S, Nowak D, et al. Perinatal Manganese Exposure and Hydroxyl Radical Formation in Rat Brain. Neurotox Res. 2015;27:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC, Andersen ME, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicol Sci. 2011;122:297–316. [DOI] [PubMed] [Google Scholar]
- 75.Freire C, Amaya E, Gil F, Fernández MF, Murcia M, Llop S, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ. 2018;621:340–51. [DOI] [PubMed] [Google Scholar]
- 76.Mızrak E, Bouffard NR, Libby LA, Boorman ED, Ranganath C. The hippocampus and orbitofrontal cortex jointly represent task structure during memory-guided decision making. Cell Rep. 2021;37:110065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annu Rev Psychol. 2016;67:105–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Squire LR, Dede AJO. Conscious and Unconscious Memory Systems. Cold Spring Harb Perspect Biol. 2015;7:a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strauss E, Sherman EMS, Spreen O. Memory. Compend Neuropsychol Tests Adm Norms Comment 3rd Ed. New York, NY, US: Oxford University Press; 2006. p. xvii, 1216. [Google Scholar]
- 80.Graves LV, Holden HM, Van Etten EJ, Delano-Wood L, Bondi MW, Salmon DP, et al. New Intrusion Analyses on the CVLT-3: Utility in Distinguishing the Memory Disorders of Alzheimer’s versus Huntington’s Disease. J Int Neuropsychol Soc JINS. 2019;25:878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García-Chimalpopoca Z, Hernández-Bonilla D, Cortez-Lugo M, Escamilla-Núñez C, Schilmann A, Riojas-Rodríguez H, et al. Verbal Memory and Learning in Schoolchildren Exposed to Manganese in Mexico. Neurotox Res. 2019;36:827–35. [DOI] [PubMed] [Google Scholar]
- 82.Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoops D, Flores C. Making Dopamine Connections in Adolescence. Trends Neurosci. 2017;40:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim H, Lee D, Kim K. Combined Exposure to Metals in Drinking Water Alters the Dopamine System in Mouse Striatum. Int J Environ Res Public Health. 2021;18:6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilcox JM, Consoli DC, Paffenroth KC, Spitznagel BD, Calipari ES, Bowman AB, et al. Manganese-induced hyperactivity and dopaminergic dysfunction depend on age, sex and YAC128 genotype. Pharmacol Biochem Behav. 2022;173337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dorman DC, Struve MF, Wong BA, Dye JA, Robertson ID. Correlation of Brain Magnetic Resonance Imaging Changes with Pallidal Manganese Concentrations in Rhesus Monkeys Following Subchronic Manganese Inhalation. Toxicol Sci. 2006;92:219–27. [DOI] [PubMed] [Google Scholar]
- 87.Saritha K, Celia DA, Shahryar HK, Nikolay FM. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol [Internet]. 2014. [cited 2020 Jun 29];88. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3859803/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ott T, Nieder A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn Sci. 2019;23:213–34. [DOI] [PubMed] [Google Scholar]
- 89.Stalter M, Westendorff S, Nieder A. Dopamine Gates Visual Signals in Monkey Prefrontal Cortex Neurons. Cell Rep. 2020;30:164–172.e4. [DOI] [PubMed] [Google Scholar]
- 90.Jokinen P, Brück A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15:88–93. [DOI] [PubMed] [Google Scholar]
- 91.Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–302. [DOI] [PubMed] [Google Scholar]
- 92.Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. [DOI] [PubMed] [Google Scholar]
- 93.Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience. 2014;282:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental Exposure to Manganese Increases Adult Susceptibility to Inflammatory Activation of Glia and Neuronal Protein Nitration. Toxicol Sci. 2009;112:405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. [DOI] [PubMed] [Google Scholar]
- 96.Johnston J, Franklin M, Roh H, Austin C, Arora M. Lead and Arsenic in Shed Deciduous Teeth of Children Living Near a Lead-Acid Battery Smelter. Environ Sci Technol. 2019;53:6000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, et al. Determinants of Manganese in Prenatal Dentin of Shed Teeth from CHAMACOS Children Living in an Agricultural Community. Environ Sci Technol [Internet]. 2013; Available from: https://pubs.acs.org/sharingguidelines [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gunier RB, Mora AM, Smith D, Arora M, Austin C, Eskenazi B, et al. Biomarkers of Manganese Exposure in Pregnant Women and Children Living in an Agricultural Community in California. Environ Sci Technol. 2014;48:14695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Austin C, Richardson C, Smith D, Arora M. Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res. 2017;155:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of Verbal Learning and Memory in Children with Heavy Prenatal Alcohol Exposure or Attention-Deficit/Hyperactivity Disorder. Alcohol Clin Exp Res. 2011;35:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Riggins T, Cacic K, Buckingham-Howes S, Scaletti LA, Jo Salmeron B, Black MM. Memory ability and hippocampal volume in adolescents with prenatal drug exposure. Neurotoxicol Teratol. 2012;34:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are confidential.