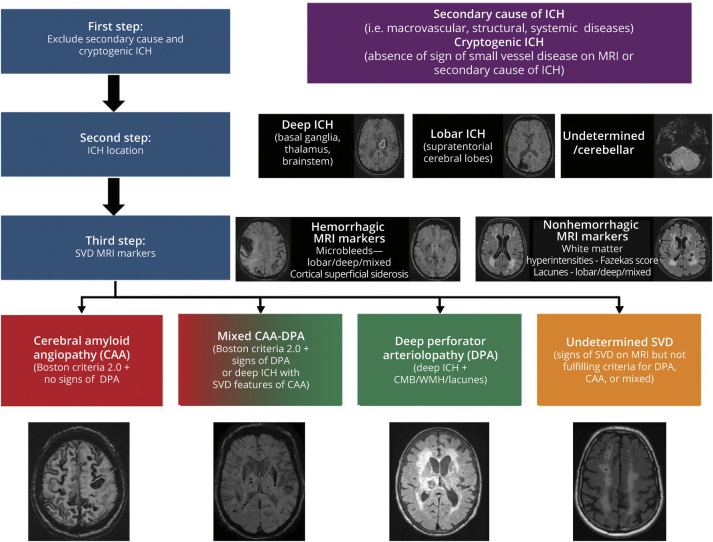
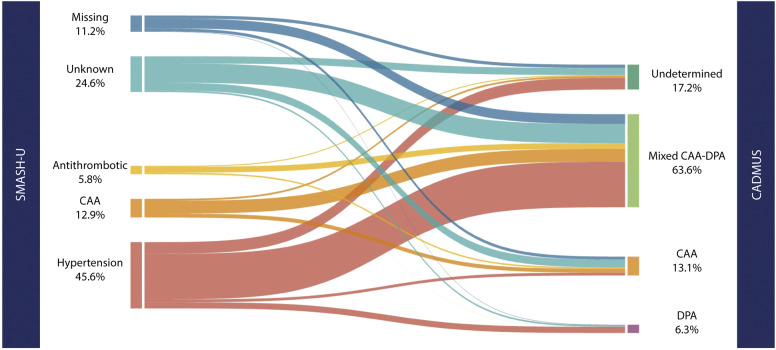
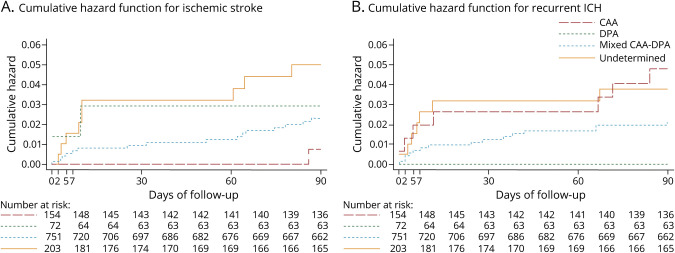
Martina B Goeldlin
Martina B Goeldlin, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Madlaine Mueller
Madlaine Mueller, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Bernhard M Siepen
Bernhard M Siepen, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Wenpeng Zhang
Wenpeng Zhang, MSc
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Hatice Ozkan
Hatice Ozkan, MPhil
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Martina Locatelli
Martina Locatelli, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Yang Du
Yang Du
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Waldo Valenzuela
Waldo Valenzuela, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Piotr Radojewski
Piotr Radojewski, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Arsany Hakim
Arsany Hakim, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Johannes Kaesmacher
Johannes Kaesmacher, MD, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Thomas R Meinel
Thomas R Meinel, MD, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Leander Clénin
Leander Clénin, MMed
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Mattia Branca
Mattia Branca, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Davide Strambo
Davide Strambo, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Tim Fischer
Tim Fischer, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Friedrich Medlin
Friedrich Medlin, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Nils Peters
Nils Peters, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Emmanuel Carrera
Emmanuel Carrera, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Karl-Olof Lovblad
Karl-Olof Lovblad, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Grzegorz M Karwacki
Grzegorz M Karwacki, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Carlo W Cereda
Carlo W Cereda, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Julien Niederhauser
Julien Niederhauser, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Marie-Luise Mono
Marie-Luise Mono, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Achim Mueller
Achim Mueller, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Susanne Wegener
Susanne Wegener, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Sabine Sartoretti
Sabine Sartoretti, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Alexandros A Polymeris
Alexandros A Polymeris, MD, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Valerian Altersberger
Valerian Altersberger, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Mira Katan
Mira Katan, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Marios Psychogios
Marios Psychogios, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Rolf Sturzenegger
Rolf Sturzenegger, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Claude Nauer
Claude Nauer, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Michael Schaerer
Michael Schaerer, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Carlos Buitrago Tellez
Carlos Buitrago Tellez, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Susanne Renaud
Susanne Renaud
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Katharina Minkner Klahre
Katharina Minkner Klahre
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Werner J Z'Graggen
Werner J Z'Graggen, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
David Bervini
David Bervini
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Leo H Bonati
Leo H Bonati, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Roland Wiest
Roland Wiest, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Marcel Arnold
Marcel Arnold, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Robert J Simister
Robert J Simister, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Duncan Wilson
Duncan Wilson, MD, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Hans Rolf Jäger
Hans Rolf Jäger, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,
Urs Fischer
Urs Fischer, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,*,
David J Werring
David J Werring, MD, PhD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,*,
David J Seiffge
David J Seiffge, MD
1From the Departments of Neurology (M.B.G., M.M., B.M.S., T.R.M., L.C., M.A., U.F., D.J.S., W.J.Z.G.) and Neurosurgery (D.B., W.J.Z.G.), and the University Institute for Diagnostic and Interventional Neuroradiology (W.V., P.R., A.H., J.K., R.W.), Inselspital Bern University Hospital and University of Bern; Graduate School for Health Sciences (M.B.G., B.M.S.) and CTU Bern (M.B.), University of Bern, Switzerland; Stroke Research Centre (M.B.G., W.Z., H.O., M.L., Y.D., D.J.W.), University College London Queen Square Institute of Neurology, United Kingdom; Service of Neurology (D.S.), Department of Clinical Neurosciences, Lausanne University Hospital and University of Lausanne; Department of Radiology (T.F.), Cantonal Hospital St. Gallen; Department of Internal Medicine (F.M.), Stroke Unit and Division of Neurology, HFR Fribourg–Cantonal Hospital; Stroke Center Hirslanden (N.P.), Klinik Hirslanden Zurich; Stroke Research Group (E.C.), Department of Clinical Neurosciences, University Hospital and Faculty of Medicine, Geneva; Department of Radiology and Medical Informatics (K.-O.L.), University of Geneva; Department of Radiology and Nuclear Medicine (G.M.K.), Luzerner Kantonsspital; Stroke Center EOC (C.W.C.), Neurocenter of Southern Switzerland; Stroke Unit (J.N.), GHOL, Hôpital de zone de Nyon; Stadtspitäler Triemli und Waid (M.-L.M.), Zurich; Department of Neurology (A.M., S.W.), University Hospital and University of Zurich; Department of Radiology and Nuclear Medicine (S.S.), Kantonsspital Winterthur; Department of Neurology and Stroke Center (A.A.P., V.A., M.K., U.F., L.H.B.) and Center for Rehabilitation Rheinfelden (L.H.B.), University Hospital Basel and University of Basel; Diagnostic and Interventional Neuroradiology (M.P.), Department of Radiology and Nuclear Medicine, University Hospital Basel; Department of Neurology (R.S.), Kantonsspital Graubünden, Chur; Department of Radiology/Neuroradiology (C.N.), Kantonsspital Graubünden, Chur; Department of Neurology (M.S.), and Department of Radiology (C.B.T.), Buergerspital Solothurn; Division of Neurology (S.R.), Pourtalès Hospital, Neuchâtel; Department of Radiology (K.M.K.), Réseau Hospitalier Neuchâtelois, Switzerland; Comprehensive Stroke Service (R.J.S., D.J.W.) and Neuroradiological Academic Unit (H.R.J.), Department of Brain Repair & Rehabilitation, University College London Hospital, United Kingdom; and New Zealand Brain Research Institute (D.W.), Christchurch.
1,*,✉;
for Swiss Stroke Registry Investigators and SIGNAL Investigators1