Abstract
Background and Objectives
Oculopharyngeal muscular dystrophy (OPMD) is a rare progressive neuromuscular disease. MRI is one of the techniques that is used in neuromuscular disorders to evaluate muscle alterations. The aim of this study was to describe the pattern of fatty infiltration of orofacial and leg muscles using quantitative muscle MRI in a large national cohort and to determine whether MRI can be used as an imaging biomarker of disease progression in OPMD.
Methods
Patients with OPMD (18 years or older) were invited from the national neuromuscular database or by their treating physicians and were examined twice with an interval of 20 months, with quantitative MRI of orofacial and leg muscles to assess fatty infiltration which were compared with clinical measures.
Results
In 43 patients with genetically confirmed OPMD, the muscles that were affected most severely were the tongue (mean fat fraction: 37.0%, SD 16.6), adductor magnus (31.9%; 27.1), and soleus (27.9%; 21.5) muscles. The rectus femoris and tibialis anterior muscles were least severely affected (mean fat fractions: 6.8%; SD 4.7, 7.5%; 5.9). Eleven of 14 significant correlations were found between fat fraction and a clinical task in the corresponding muscles (r = −0.312 to −0.769, CI = −0.874 to −0.005). At follow-up, fat fractions had increased significantly in 17 of the 26 muscles: mean 1.7% in the upper leg muscles (CI = 0.8–2.4), 1.7% (1.0–2.3) in the lower leg muscles, and 1.9% (0.6–3.3) in the orofacial muscles (p < 0.05). The largest increase was seen for the soleus (3.8%, CI = 2.5–5.1). Correlations were found between disease duration and repeat length vs increased fat fraction in 7 leg muscles (r = 0.323 to −0.412, p < 0.05).
Discussion
According to quantitative muscle MRI, the tongue, adductor magnus and soleus show the largest fat infiltration levels in patients with OPMD. Fat fractions increased in several orofacial and leg muscles over 20 months, with the largest fat fraction increase seen in the soleus. This study supports that this technique is sensitive enough to show worsening in fat fractions of orofacial and leg muscles and therefore a responsive biomarker for future clinical trials.
Introduction
Oculopharyngeal muscular dystrophy (OPMD) is a rare, autosomal dominant, progressive neuromuscular disease, with an onset usually around the fifth decade.1 The early features are ptosis combined with dysphagia, with limb weakness becoming a progressive feature as the disease progresses.2,3 OPMD is the result of a trinucleotide repeat expansion in the PABPN1 gene; an alanine expansion of the GCN[10] expansion is pathogenic.4 In addition, the size of PABPN1 genotype seems to influence the disease severity and progression. A large French study showed that patients with OPMD with longer expansion of PABPN1 were diagnosed at an earlier age compared with patients with shorter expansions of PABPN1.5 There is no curative treatment for OPMD.
Studies in various neuromuscular disorders6,7 have used MRI to evaluate muscle alterations, showing that muscle MRI can be a sensitive measure to show disease progression, even before changes in strength and functional tests can be identified.8 The most affected muscles in OPMD detected by MRI are the tongue, hamstrings, hip adductor, and calf muscles.3,9-14 Most of the studies used semiquantitative scores to determine the amount of fatty infiltration patients with in OPMD. Only one small (semi)quantitative MRI study in patients with OPMD (n = 8) has been published showing changes in muscle fat fraction in 7 of 18 leg muscles over the 13-month follow-up.10 This study showed no disease progression in semiquantitative scores. Therefore, studies assessing the natural history in OPMD with more precise measurements and a high sensitivity to change in fat infiltration are needed.
The aim of this study was to describe the pattern of fatty infiltration of orofacial and leg muscles using quantitative muscle MRI in a national large cohort of OPMD and to determine whether MRI can be used as an imaging biomarker of disease progression in OPMD.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The medical ethics committee (CMO Arnhem-Nijmegen gave approval for the study (NL54606.091.15) and all patients gave their approval by signing their informed consent.
Patients
Patients with OPMD (older than 18 years) were recruited from the national neuromuscular database (CRAMP database: Computer Registry of All Myopathies and Polyneuropathies)15 or by their treating physicians (distributed evenly across the country). We are the recognized national OPMD reference center, part of the “Muscle center of the Netherlands.” In addition, family members of patients with OPMD who enrolled in this study were asked to participate through an information letter to find asymptomatic carriers or patients with subclinical signs of OPMD. All participants gave permission for genetic testing. Exclusion criteria were nonoral food intake and any other cause of dysphagia.
Muscle MRI Acquisition
Muscle MRI was performed using a scanning protocol as published previously.16-18 In short, the head-neck region and the lower extremities were examined with a 3T MRI (TIM Trio, Siemens, Erlangen, Germany). Patients were positioned supine and head first. Imaging stacks were positioned with the most proximal slice at the trochanter major for the upper legs, at the head of the fibula for the lower legs, and the center of the forehead for the orofacial muscles. Axial T1-weighted turbo-spin echo images were collected of the upper and lower legs (repetition time = 646–735 ms, echo times = 12 ms, field of view = 434 × 262 mm, in-plane resolution = 0.97 × 0.97 mm, slice gap/thickness = 5/5 mm, number of slices = 32). Sagittal T1-weighted 3D MP RAGE images with a resolution of 1 × 1 × 1 were collected for the head-neck region to allow semiquantitative assessment of fat infiltration (repetition time = 1,900 ms, echo time = 12 ms, field of view = 250 × 250 mm, in-plane resolution = 0.98 × 0.98 mm, slice thickness/gap = 1/0 mm, number of slices = 192). To quantitatively assess fatty infiltration, we acquired a 3D 2-point Dixon sequence (repetition time = 10 ms, echo times = 2.45 and 3.67 ms, field of view = 299 × 435 mm, in-plane resolution = 1.36 × 1.36 mm, slice thickness/gap = 5/0 mm). The Dixon settings were the same for all 3 regions, with the exception for the number of slices (upper legs/lower legs: 72 slices, head-neck: 64 slices).
Muscle MRI Analysis
A fat fraction map, with voxel values ranging from 0% to 100%, was calculated from the Dixon images. The Dixon sequences acquire in-phase and out-phase images that were reconstructed with the vendors software into fat and water images. These fat and water images were used to create a quantitative fat fraction map using MATLAB (2018a) by dividing fat image by the sum of the fat and water image. Those fat and water images were reconstructed using the scanner software. Regions of interests (ROI's) were drawn around 12 upper leg muscles, 8 lower leg muscles, and 3 orofacial muscles on the Dixon images (Figure 1). The fat fraction map was used to draw the regions of interest (ROI's) of the muscles. Fat fractions were calculated per ROI. Four medical imaging students were trained to draw the ROI's of the leg muscles. These drawn muscles were checked by one investigator (R.K.). All orofacial muscles were drawn by one investigator (R.K.). For the upper legs, ROIs were drawn on 3 slices, being at 1/3, 1/2, and 2/3 of the femur bone, starting from the trochanter major. The mean fat fraction of each muscle was calculated by the mean of the 3 slices. For the lower legs, ROIs were drawn at single slice, that is, the slice with the greatest periphery of the lower leg. The orofacial muscles were also drawn on a single slice, at the height where the inferior alveolar nerve was visible. The intrinsic tongue muscles (transversus and superior longitudinal) were measured together and named as tongue in this paper.
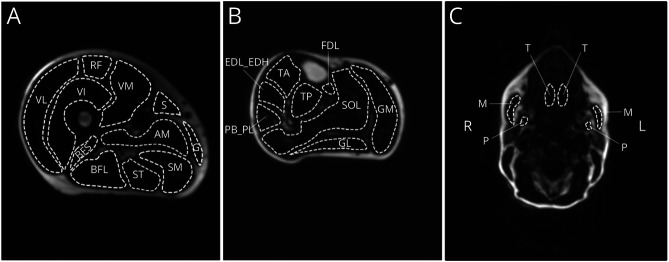
Figure 1. Typical Examples of a Dixon Fat Fraction Image of the Right Upper Leg, Right Lower Leg, and Head-Neck.
(A) Regions of interest per muscle of the upper leg. AM = adductor magnus; BFL = biceps femoris long head; BFS = biceps femoris short head; G = gracilis; RF = rectus femoris; S = sartorius; SM = semimembranosus; ST = semitendinosus; VI = vastus intermedius; VL = vastus lateralis; VM = vastus medialis. (B) Regions of interest per muscle of the lower leg. EDL_EDH = extensor digitorum/hallucis longus; FDL = flexor digitorum longus; GL = gastrocnemius lateralis; GM = gastrocnemius medialis; PB_PL = peroneus brevis and longus; SOL = soleus; TA = tibialis anterior; TP = tibialis posterior. (C) Regions of interest per muscle of the orofacial muscles. M = masseter, P = pterygoid medialis and T = tongue. R = right, L = left.
Semiquantitative Muscle MRI
A modified version of the Lamminen scale was used to semiquantitatively assess muscle fatty infiltration of the orofacial muscles (temporal, masseter, pterygoid medialis and lateralis, and tongue muscles) on T1-weighted images.19 All available images were used to determine the degree of fat infiltration. An experienced musculoskeletal radiologist (J.d.R.) performed all analyses at baseline and follow-up. Fatty infiltration was scored as: “0 = normal, 1 = mild with only traces of fatty infiltration, 2 = moderate with fatty infiltration in less than 50% of the muscle tissue, 3 = severe with fatty infiltration in more than 50% of the muscle tissue, and 4 = the entire muscle replaced by abnormal signal.”19,20 We did not analyze the leg muscles with the Lamminen scale because other studies already showed moderate to strong correlations between quantitative muscle fat fractions and semiquantitative Lamminen scores in other neuromuscular diseases.21,22 R.K. and J.d.R. analyzed all MRI images at baseline and follow-up blinded for each other's judgments.
Clinical Measures
To ensure consistency, all measurements were performed by one investigator (R.K.). Muscle strength and functional capacity of the oropharyngeal region and of the upper and lower extremities were measured as described previously23 and are explained below in short.
Muscle Strength
For manual muscle testing, the scale of the Medical Research Council (MRC) was used, ranging from 0 (no muscle contraction) to 5 (maximal muscle strength), applied to the neck (extension and flexion), elbows (extension and flexion), the knees (extension and flexion), the hips (abduction and flexion), the feet (dorsal flexion and plantar flexion), handgrip, the wrists (extension and flexion), and shoulder abduction bilaterally (score 0–130).24
Fixed dynamometry (Newton, N) was performed with the use of the strength transducer KAP-S 2 kN (Angewandte System Technik GmbH) for measuring the maximal isometric contraction of the shoulder abduction (deltoid muscle), the hip flexion (iliopsoas muscle), and the knee extension (quadriceps muscle).
Maximum bite force (MBF) of the frontal teeth was measured with the use of a Bite Force Gauge (Vrije Universiteit, Amsterdam).
Maximum isometric anterior tongue pressure (MITP, kilopascal, kPa) was measured with the Iowa Oral Performance Instrument.25
For all force measurements, the best performance of 3 trials was used for further analysis.
Functional Capacity
The timed stair walking test (10-steps) measured in seconds.26
The motor function measure (MFM) consists of 3 parts: D1 includes stance and transfer tasks, D2 includes tasks of axial and proximal muscles, and D3 includes tasks of distal muscles. The total score ranges from 0%–100%, where a score of 100% indicates no functional motor deficits.27
Swallowing and chewing capacity was measured by the maximum swallowing volume (MSV), maximum swallowing speed (MSS), and the test of masticating and swallowing solids (TOMASS). The MSV is the maximal amount of water (mL) a patient can swallow in one swallowing attempt.28 MSS is the result of drinking 150 mL water as quick as possible.29,30 The TOMASS (one part) is the time needed to eat a standardized cracker as fast as possible, in seconds.31
The speech capacity was measured by the maximum repetition rate (MRR) and maximum phonation time (MPT). The MRR is performed by producing the syllable sequences (/pa/, /ta/, /ka/, /pataka/) as fast as possible (syllables/second), measured during the first 5 seconds.32 For the MPT, it requires the phonation of an/a/as long as possible (seconds).33
Statistical Analysis
All statistical calculations were performed with IBM SPSS Statistics (version 25), and p values of <0.05 were considered statistically significant. At baseline, the pattern of fatty infiltration (%) was analyzed in each patient in comparison with age to realize a heatmap. The relation between the Lamminen scores and quantitative MRI fat fractions was examined with Spearman rho correlation coefficients. Whether and how strong clinical measures and fat fractions of the corresponding muscles are associated was also tested with Spearman rho correlation coefficients, including 95% confidence intervals. The fat fractions at baseline and follow-up were compared using the paired samples t tests with 95% confidence intervals. Apart from these absolute differences, relative changes in fat fractions were calculated in percentages (where the lower and upper limits of the 95% confidence intervals were also converted into percentages). Responsiveness was assessed by calculating the standardized response mean analysis (SRM; meanchange/SDchange). Responsiveness is considered small (SRM values of 0.2), moderate (SRM >0.5), or large (SRM >0.8).34 The Lamminen scores at baseline and follow-up were compared using the related samples Wilcoxon signed-rank test. The relation between the changes in fat fraction with the changes in clinical measures was tested with Spearman rho correlation coefficients, including 95% confidence intervals. Spearman rho correlation coefficients were also used to test whether the changes in fat fractions (%) are associated with disease duration and repeat length.
Data Availability
On reasonable request, the anonymized data that support the findings of this study are available from the corresponding author.
Results
Patients
Forty-four patients with OPMD were recruited through their treating physicians or the national neuromuscular database. In addition, 19 family members of these patients being putative OPMD patients were asked to participate as well. Figure 2 shows the results of the recruitment and inclusion of the study, showing a total of 43 patients with genetically confirmed OPMD participating in this longitudinal study. Ten different families were identified in our study group, geographically distributed approximately evenly across the country.
Figure 2. Patient Recruitment and Inclusion of the Study.
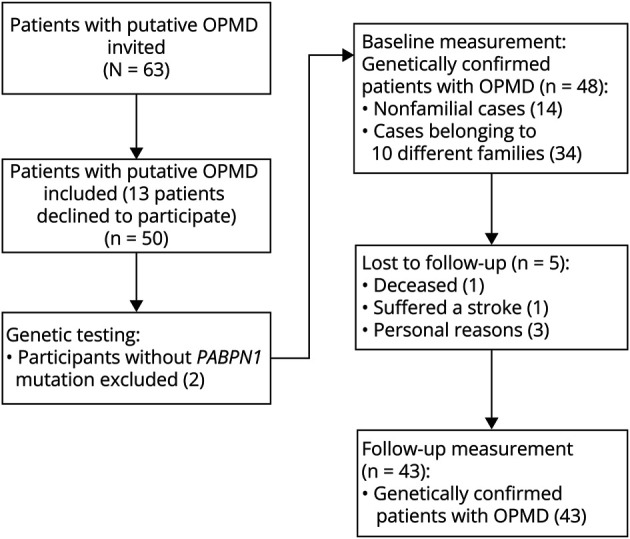
Table 1 summarizes the demographic and genetic details of the patients, including 4 patients without any complaints at baseline which are presented as asymptomatic carriers. The baseline data were collected from February 2016 until January 2017, and the follow-up data were collected from October 2017 until June 2018. The mean interval between the measurements was 20 months (SD 1.8).
Table 1.
Patient Characteristics
| Patient characteristics (n = 43) | |
| Mean age at baseline (range) | 60.2 (44–79 y) |
| Sex (men/women) | 21/22 |
| Mean age at onset (range) | 50.3 (37–73 y) |
| Repeat length (N) | |
| 11/11 | 2 |
| 10/12 | 3 |
| 10/13 | 6 |
| 10/14 | 7 |
| 10/15 | 2 |
| 10/16 | 23 |
Five head-neck scans, 4 upper leg scans, and 6 lower leg scans were missing due to technical errors or patients experienced claustrophobia during the MRI scan.
Pattern of Fatty Infiltration at Baseline
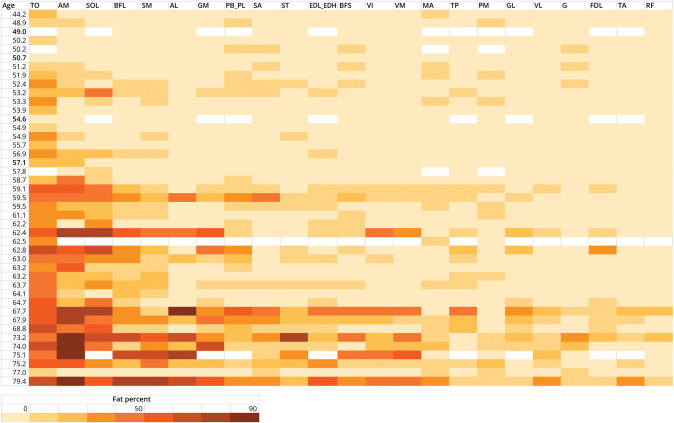
Fat fractions per muscle ranged from 2% to 89%. Figure 3 shows a heatmap of the fat fractions of all muscles with the participants sorted by age in years (top to bottom) and the muscles sorted from highest average fat fraction to lowest average fat fraction (left to right). The 3 most severely affected muscles were the tongue (mean fat fraction: 37.0%, SD 16.6), the adductor magnus (mean: 31.9%, SD 27.1), and soleus (mean: 27.9%, SD 21.5). The rectus femoris and tibialis anterior muscles were least severely affected (mean fat fractions: 6.8%, SD 4.7% and 7.5%, SD 5.9, respectively). Figure 3 shows the fat fractions of all muscles sorted by the participants' age (years). Furthermore, the heatmap reveals that the severely affected muscles at later disease stages are also the muscles that are affected early in the disease.
Figure 3. Heatmap Displaying the Pattern of Fatty Infiltration in OPMD at Baseline.
Each row represents a patient ordered by age. Each column represents a muscle ordered by the degree of fatty infiltration (mean fat fraction of left and right side). AL = adductor longus; AM = adductor magnus; BFL = biceps femoris long head; BFS = biceps femoris short head; EDL_EDH = extensor digitorum longus_extensor hallucis longus; FDL = flexor digitorum longus; G = Gracilis; GL = gastrocnemius lateralis; GM = gastrocnemius medialis; MA = masseter; PB_PL = peroneus brevis_peroneus longus; PM = pterygoid medialis; RF = rectus femoris; SA = sartorius; SM = semimembranosus; SOL = soleus; ST = semitendinosus; TA = tibialis anterior; TO = tongue; TP = tibialis posterior; VI = vastus intermedius; VL = vastus lateralis; VM = vastus medialis. White bar = no MRI scan due to technical errors or claustrophobia. Bold age = asymptomatic carrier.
Correlation Between Fat Fractions and Lamminen Scores at Baseline
The Lamminen score correlated significantly with the fat fraction of the left masseter (r = 0.412, p = 0.009), the left tongue muscle (r = 0.738, p < 0.001), and the right tongue muscle (r = 0.851, p < 0.001). The fat fraction of the temporalis and pterygoid medialis muscles did not correlate significantly with the Lamminen scores (p > 0.05).
Correlation Between Fat Fractions and Clinical Measures at Baseline
Eleven of 14 correlations between mean fat fractions and corresponding clinical tasks were found to be statistically significant (Table 2).
Table 2.
Correlations Between Fat Fractions (%) and Clinical Measures at Baseline
| Fat fractions (%) | MFM total | MFM D1 | MRC sum score | MRC knee extension | MRC knee flexion | Dynamometry knee extension | 10-Step stair test |
| Mean fat fraction, total legs |
r = −0.722 (−0.847 to −0.519) p < 0.001 (n = 39) |
r = −0.719 (−0.846 to −0.515) p < 0.001 (n = 39) |
r = −0.519 (−0.722 to −0.234) p = 0.001 (n = 39) |
NA | NA | NA |
r = 0.509 (0.196 to 0.728) p = 0.002 (n = 34) |
| Mean fat fraction, quadriceps | NA | NA | NA |
r = −0.644 (−0.796 to −0.415) p < 0.001 (n = 42) |
NA |
r = −0.312 (−0.572 to −0.005) p = 0.047 (n = 41) |
NA |
| Mean fat fraction, hamstrings | NA | NA | NA | NA |
r = −0.659 (−0.810 to −0.426) p < 0.001 (n = 39) |
NA | NA |
| Fat fractions (%) | MITP | Maximum bite force | Maximum chewing time | Maximum swallowing speed | Maximum swallowing volume |
| Mean fat fraction, tongue |
r = −0.769 (−0.874 to −0.596) p < 0.001 (n = 40) |
NA | NA |
r = −0.503 (−0.709 to −0.218) p = 0.001 (n = 40) |
r = −0.364 (−0.613 to −0.050) p = 0.021 (n = 40) |
| Mean fat fraction, masseter | NA |
r = −0.176 (−0.470 to 0.152) p = 0.276 (n = 40) |
r = 0.005 (−0.338 to 0.347) p = 0.976 (n = 35) |
NA | NA |
| Mean fat fraction, pterygoid medialis | NA |
r = −0.456 (−0.677 to −0.159) p = 0.003 (n = 40) |
r = 0.158 (−0.195 to 0.475) p = 0.364 (n = 35) |
NA | NA |
Abbreviations: MFM = motor function measure; MITP = maximum isometric tongue pressure; MRC = medical research council; NA = not applicable.
Mean fat fraction refers to the mean of the left and right side of the muscle. Mean of total legs = mean of muscles of upper and lower legs. Quadriceps = mean of Rectus Femoris, Vastus Intermedius, Vastus Lateralis, and Vastus Medialis. Hamstrings = mean of Biceps Femoris long head, Biceps Femoris short head, Semimembranosus, and Semitendinosus. 95% CI and number of patients are displayed in parentheses.
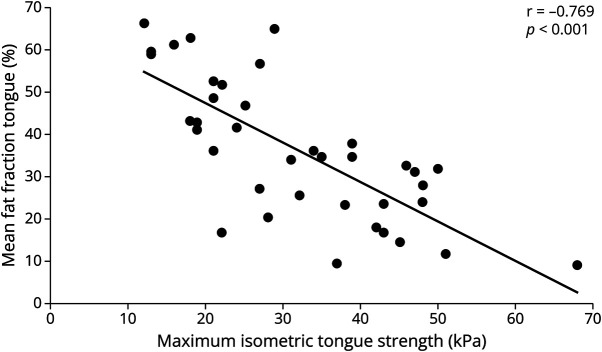
The mean fat fraction of the total legs correlated significantly with the MFM total, MFM part D1, MRC sum score, and the 10-step stair test. MRC scores of knee extension and flexion correlated significantly with the mean fat fraction of the corresponding muscle groups. The mean fat fraction of the orofacial muscles correlated significantly with the maximum swallowing capacity, maximum isometric tongue pressure, and MBF. The strongest correlation was found between the mean fat fraction of the tongue muscle and the maximum isometric tongue pressure (r = −0.769, p < 0.001, Figure 4).
Figure 4. Mean Fat Fraction of the Tongue Muscle Correlates With Maximum Isometric Tongue Strength (kPa).
Longitudinal Changes in MRI Outcome Measures
Quantitative Fat Fraction
Almost all muscles (25 of 26) showed increased fat fraction from baseline to follow-up, but 17 of the 26 muscles reached statistical significance (Table 3). When even more strictly calculated with Bonferroni correction, only 8 remain with a p value <0.001. Per muscle group, an overall increase in absolute fat fraction over time was seen for the orofacial muscles (1.9%, p = 0.005), the upper leg muscles (1.7%, p < 0.001), and the lower leg muscles (1.7%, p < 0.001). Of the orofacial muscles, the right masseter and the tongue muscle showed a significant increase in fat fraction (p < 0.05). Fat fraction also increased in most of the upper and lower leg muscles over time. The largest increase in absolute fat fraction was seen for the soleus muscle (3.8%). Responsiveness of fat fraction was the highest for the soleus (SRM = 0.95), followed by the biceps femoris long head (SRM = 0.91) and adductor magnus (SRM = 0.79).
Table 3.
Mean Fat Fractions (%) and SD at Baseline and Follow-up, Percentage of Worsening (95%), p Values and Standardized Response Means of All Orofacial Muscles, Upper Leg Muscles, and Lower Leg Muscles
| Baseline fat fraction Mean (SD) |
Follow-up fat fraction Mean (SD) |
Absolute change in fat fraction (95% CI) | Relative change in fat fraction (95% CI) | p Value | SRM | |
| Orofacial muscles | ||||||
| Masseter L | 10.6 (7.1) | 11.6 (8.1) | 1.0 (−0.6 to 2.5) | 9.4 (−5.7 to 23.6) | 0.236 | 0.19 |
| Masseter R | 10.6 (6.1) | 12.2 (7.4) | 1.6 (0.1 to 3.1) | 15.0 (0.9 to 29.2) | 0.043 | 0.35 |
| Pterygoid medialis | 9.6 (4.7) | 11.4 (9.8) | 1.8 (−0.5 to 4.0) | 18.8 (−5.2 to 41.7) | 0.122 | 0.26 |
| Tongue | 37.0 (17.0) | 39.8 (19.2) | 2.8 (1.0 to 4.7) | 7.6 (2.7 to 12.7) | 0.003 | 0.50 |
| Mean orofacial muscles | 19.1 (7.6) | 21.0 (9.3) | 1.9 (0.6 to 3.3) | 9.9 (3.1 to 17.3) | 0.005 | 0.50 |
| Upper leg muscles | ||||||
| Adductor Longus | 13.7 (19.4) | 15.8 (21.9) | 2.1 (0.4 to 3.8) | 15.3 (2.9 to 27.7) | 0.015 | 0.40 |
| Adductor Magnus | 30.3 (26.4) | 33.6 (27.7) | 3.3 (1.9 to 4.6) | 10.9 (6.3 to 15.2) | <0.001 | 0.79 |
| Biceps femoris long head | 18.1 (16.6) | 21.1 (18.0) | 3.0 (1.9 to 4.0) | 16.6 (10.5 to 22.1) | <0.001 | 0.91 |
| Biceps femoris short head | 11.3 (10.5) | 13.5 (12.4) | 2.2 (0.7 to 3.6) | 19.5 (6.2 to 31.9) | 0.004 | 0.49 |
| Gracilis | 8.1 (5.8) | 8.3 (5.9) | 0.2 (−0.4 to 0.8) | 2.5 (−4.9 to 9.9) | 0.487 | 0.11 |
| Rectus femoris | 6.8 (4.8) | 7.0 (4.9) | 0.2 (−0.3 to 0.7) | 2.9 (−4.4 to 10.3) | 0.384 | 0.12 |
| Sartorius | 12.8 (10.5) | 13.8 (11.9) | 1.0 (0.1 to 2.0) | 7.8 (0.8 to 15.6) | 0.036 | 0.33 |
| Semimembranosus | 17.5 (15.5) | 20.3 (17.1) | 2.8 (1.4 to 4.1) | 16.0 (8.0 to 23.4) | <0.001 | 0.67 |
| Semitendinosus L | 11.5 (10.9) | 13.2 (12.0) | 1.7 (0.6 to 2.7) | 14.8 (5.2 to 23.5) | 0.002 | 0.52 |
| Semitendinosus R | 11.4 (11.4) | 11.9 (11.4) | 0.5 (−0.3 to 1.2) | 4.4 (−2.6 to 10.5) | 0.243 | 0.21 |
| Vastus Intermedius | 11.0 (10.8) | 12.7 (13.5) | 1.7 (0.7 to 2.8) | 15.5 (6.4 to 25.5) | 0.002 | 0.53 |
| Vastus Lateralis | 7.8 (6.1) | 8.4 (7.1) | 0.6 (0.1 to 1.3) | 7.7 (1.3 to 16.7) | 0.091 | 0.29 |
| Vastus Medialis | 10.3 (11.1) | 11.7 (12.5) | 1.4 (0.5 to 2.3) | 13.6 (4.9 to 22.3) | 0.004 | 0.50 |
| Mean upper leg muscles | 13.2 (10.9) | 14.9 (11.9) | 1.7 (0.8 to 2.4) | 12.9 (6.1 to 18.2) | <0.001 | 0.64 |
| Lower leg muscles | ||||||
| Extensor Digitorum/Hallucis Longus | 13.2 (11.4) | 15.1 (13.2) | 1.9 (0.8 to 3.0) | 14.4 (6.1 to 22.7) | 0.001 | 0.58 |
| Flexor Digitorum Longus | 7.4 (4.4) | 7.3 (4.5) | −0.1 (−0.8 to 0.7) | −1.4 (−10.8 to 9.5) | 0.877 | −0.05 |
| Gastrocnemius Lateralis | 9.5 (8.0) | 12.0 (10.9) | 2.5 (1.3 to 3.9) | 26.3 (13.7 to 41.1) | <0.001 | 0.68 |
| Gastrocnemius Medialis | 15.5 (16.6) | 17.5 (17.2) | 2.0 (1.1 to 2.9) | 12.9 (7.1 to 18.7) | <0.001 | 0.71 |
| Peroneus Brevis/Peroneus Longus | 15.2 (10.3) | 16.9 (11.8) | 1.7 (0.8 to 2.6) | 11.2 (5.3 to 17.1) | 0.001 | 0.63 |
| Soleus | 28.8 (21.7) | 32.6 (23.2) | 3.8 (2.5 to 5.1) | 13.2 (8.7 to 17.7) | <0.001 | 0.95 |
| Tibialis Anterior L | 7.3 (5.4) | 7.9 (6.4) | 0.6 (−0.1 to 1.4) | 8.2 (−1.4 to 19.2) | 0.098 | 0.27 |
| Tibialis Anterior R | 7.9 (6.7) | 8.6 (6.8) | 0.7 (0.1 to 1.4) | 8.9 (1.3 to 17.7) | 0.034 | 0.35 |
| Tibialis Posterior | 10.7 (8.7) | 12.6 (10.7) | 1.9 (0.8 to 3.0) | 17.8 (7.5 to 28.0) | 0.001 | 0.59 |
| Mean lower leg muscles | 13.7 (9.6) | 15.4 (11.0) | 1.7 (1.0 to 2.3) | 12.4 (7.3 to 16.8) | <0.001 | 0.89 |
Abbreviation: SRM = standardized response mean.
When no significant difference is present between left and right side, the muscle is represented in mean. p values in bold are statistically significant after Bonferroni correction.
As previously published,23 in this cohort, 9 clinical measures changed over time (strength of the deltoid muscle, quadriceps, iliopsoas and tongue, plus the MRC sum score, the 10-step stair test, MFM part D1, MFM total score, and the maximum repetition rate of/pa/). However, when comparing changes in fat fraction with these changes in clinical measures, we only found a significant correlation between the deterioration of mean fat fractions of the total legs with the decrease of the MFM total score (r = −0.447, p = 0.006) and with part D1 of the MFM (r = −0.435, p = 0.007).
Lamminen Scale
The Lamminen score increased significantly over 20 months' time for the left masseter (baseline: median 2.0, range 1–2; follow-up: median 2.0, range 1–3; p < 0.046), left pterygoid medialis (baseline: median 2.0, range 1–2; follow-up: median 2.0, range 1–3; p < 0.025), and right tongue muscles (baseline: median 2.0, range 1–4; follow-up: median 2.0, range 1–4; p < 0.046), but not for the other orofacial muscles.
Asymptomatic Carriers
At baseline, one asymptomatic carrier showed elevated fat fractions in the adductor magnus (mean fat fraction of left and right side: 20.4%) and tongue (24.1%). The fat fraction of the adductor magnus was at the follow-up 20.7% and 26.7% for the tongue. At follow-up, fat fraction had increased from 8.6% to 10.9% for the biceps femoris longus and from 9.4% to 14.0% for the semimembranosus. The other 3 asymptomatic carriers presented a fat fraction in the adductor magnus of 5.8%, 6.3%, and 4.6%. In the tongue, 2 asymptomatic carriers presented a fat fraction of 8.9% and 9.3%; one asymptomatic carrier had a missing value for the fat fraction in the tongue. The muscles in these 3 asymptomatic carriers all had fat fractions less than 10%.
Natural History
There was a significant, but small correlation between disease duration (y) and change in fat fraction (%) for the biceps femoris longus (r = 0.323, p = 0.045), vastus intermedius (r = 0.337, p = 0.036), and extensor digitorum/hallucis longus (r = 0.331, p = 0.046) muscles. Repeat length showed a significant, but small negative relation with change in fat fraction (%) for the biceps femoris short head (r = −0.339, p = 0.037), extensor digitorum/hallucis longus (r = −0.412, p = 0.011), gastrocnemius lateralis (r = −0.352, p = 0.038), gastrocnemius medialis (r = −0.337, p = 0.041), and peroneus brevis/peroneus longus (r = −0.392, p = 0.016) muscles. No relation was found between these patient characteristics (disease duration or repeat length) and the change in fat fraction (%) for the orofacial muscles and other upper and lower leg muscles (p > 0.05).
Discussion
This longitudinal quantitative muscle MRI study shows that progression of fat infiltration can be detected in the orofacial and lower extremity muscles in patients with OPMD within a period of 20 months. At baseline, the rectus femoris and tibialis anterior muscles were least affected by fat infiltration, while the tongue, the adductor magnus, and the soleus muscles showed the highest levels of fat infiltration, which is consistent with the international study by Alonso-Jimenez et al.12 Almost all muscles (25 of 26) showed an increase in fat fraction over time, 17 of 26 orofacial and lower extremity muscles showed a statistical significant increase in fat fraction from baseline to follow-up, with the largest increase seen in the soleus muscle (3.8%). A progression in fat fraction over time was detected in all muscle groups, for example, the upper leg, lower leg, and orofacial muscles. In addition, fat infiltration was also detected with the semiquantitative Lamminen score in the masseter, pterygoid medialis, and tongue muscles. These findings confirm that muscle MRI can be used as a longitudinal biomarker in OPMD within a timeframe of 20 months.
Longitudinal muscle MRI studies are still rare in OPMD. There is only one other study consisting of 8 patients showing that 39% (7/18) of the leg muscles worsened over 13 months. In our study with 43 patients, 65% (17/26) of the orofacial and leg muscles worsened, being nearly twice as much, which can also be explained by the longer follow-up period of 20 months and the larger sample size.
In this study, muscle MRI correlates strongly with clinical measures. Similar findings were reported by our group on facioscapulohumeral muscular dystrophy and supports MRI as biomarker for disease severity.16 A significant correlation between the MRI quantitative and semiquantitative assessments was only seen for the right masseter and tongue muscles. No correlation was found for the left masseter, temporal, and pterygoid medialis muscles, probably because the Lamminen score is less sensitive to detect small changes in fatty infiltration.35 Other MRI studies in OPMD showed moderate to strong correlations between fat fractions and Lamminen scores of leg muscles.21,22 Further research is needed to assess the fatty infiltration and the relationship between fat fractions and Lamminen scores of the orofacial muscles to determine whether the Lamminen score is a sensitive outcome measure to detect fatty infiltration in orofacial muscles too. We found limited significant correlations between the changes in fat fraction with changes in clinical measures over time. A possible cause for this finding is that the changes over time are not that large yet. However, we were able to find significant changes over time with clinical measures, quantitative muscle ultrasound, and now with muscle MRI.23,36 In this study, the leg muscles showed the largest deterioration and the highest responsiveness, for example, the soleus muscle. Further longitudinal research is needed to better understand the correlation between increasing fat fractions and worsening clinical performances to observe which measure is the most sensitive in designing future clinical trials. Our results showed that change in fat fraction of several leg muscles had a weak, although statistically significant relation with disease duration and repeat length, but clinically only relevant when larger cohorts of patients have settled this point. Patients with OPMD with a long disease duration showed larger changes in fat fractions compared with patients with OPMD with shorter disease durations. However, we did not expect the results, and this is in contrast with our previous findings,23,36 where fast and slow rates of disease progression, according to clinical and ultrasound measurements, were found in patients with short and with long disease durations and irrespective of repeat lengths. Obviously, larger research groups are needed to better understand how disease duration influences disease progression in OPMD.
Remarkably, the rectus femoris muscle appeared normal on MRI, while it was one of the most affected muscles in our muscle ultrasound study.36 In addition, no significant deterioration was seen for the rectus femoris muscle. A previous study has shown that MRI detects fatty infiltration and edema, but not fibrosis, while ultrasound does.21 This suggests that muscle involvement in OPMD starts before fatty infiltration is detectable on MRI.
Unique in this study is that we were able to include some asymptomatic carriers. One of the 4 asymptomatic carriers already presented elevated fat fractions (adductor magnus 20.4% and tongue 24.1%) compared with fat fractions of less than 10% found in lower extremity muscles of healthy controls.22,37 The normal fat fraction levels of the bulbar muscles are less examined and might show more variation in healthy controls.38 Both the adductor magnus and tongue fat fractions levels increased at follow-up. This emphasizes the ability of muscle MRI to detect structural muscle changes and disease progression before functional decline and justifies the need for further research with larger groups of asymptomatic carriers and healthy controls.
Although highly important for swallowing, no pharyngeal muscles were assessed because these small muscles were too hard to detect on the Dixon fat fraction images due to the level of resolution. Meanwhile, MRI scanners have been updated and now have improved sequences with higher resolutions, which will make it possible to study the pharyngeal muscles in the future.39 In addition, only one slice of the head-neck region was analyzed, while observing more slices through the whole head-neck region would give more insights in the fatty infiltration of the muscles of the head and neck area. Nevertheless, this study gives insight in the pattern of fatty infiltration of the orofacial muscles over time. Discrepancy between muscles when comparing both sides was observed, for example, in the masseter muscle. The left masseter showed increase as well, however not large enough to gain statistical significance. This may be due to natural left-right differences in people (e.g. a favorite chewing side) but also to the sample size. Furthermore, owing to the enrollment of family members who lived together in their childhood, environmental factors cannot be ruled out. However, these are probably small in comparison with genetic and possible epigenetic factors. Finally, although asymptomatic carriers are difficult to identify and recruit, further research with larger groups of asymptomatic carriers is needed to confirm our findings and better understand this disease and its consequences from the start.
In conclusion, this study demonstrates the pattern of fatty infiltration in the orofacial and lower extremity muscles in patients with OPMD and the progression of this fat infiltration within 20 months. Quantitative muscle MRI is a sensitive biomarker that might be useful for future clinical trials of sufficient duration.
Acknowledgment
We thank the medical imaging students, Sietske Otten, Dominique Roost, Sigrid Glerum, and Lonneke Holthuysen for their help in drawing all ROI's for the leg muscles. We also thank Sjaak van Asten for his help with data acquisition and processing.
Glossary
- MFM
motor function measure
- MPT
maximum phonation time
- MRC
Medical Research Council
- MRR
maximum repetition rate
- MSS
maximum swallowing speed
- MSV
maximum swallowing volume
- OPMD
oculopharyngeal muscular dystrophy
- ROI
regions of interest
- SRM
standardized response mean
- TOMASS
test of masticating and swallowing solids
Appendix. Authors
| Name | Location | Contribution |
| Rosemarie H.M.J.M. Kroon, MA | Radboud University Medical Center, The Netherlands | Acquisition, analysis and interpretation of data, drafting of the manuscript and tables/figures |
| Johanna G. Kalf, PhD | Radboud University Medical Center, The Netherlands | Study concept and design, acquisition, analysis, interpretation of data, revision of the manuscript |
| Bert J.M. de Swart, PhD | Radboud University Medical Center, The Netherlands | Study concept and design, revision of the manuscript |
| Linda Heskamp, PhD | University Medical Centre Utrecht, The Netherlands | Setting up MR protocol and MR analysis software, revision of the manuscript |
| Jacky W.J. de Rooy, MD | Radboud University Medical Center, The Netherlands | Analysis and interpretation of data, revision of the manuscript |
| Baziel G.M. van Engelen, MD, PhD | Radboud University Medical Center, The Netherlands | Grant writing, study concept and design, interpretation of data, revision of the manuscript |
| Corinne G.C. Horlings, MD, PhD | Radboud University Medical Center, The Netherlands Medical University of Innsbruck, Austria | Study concept and design, analysis and interpretation of data, revision of the manuscript |
Study Funding
This project is funded by the Association Française contre les Myopathie (AFM) Téléthon (Project No. 17110).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Brais B, Bouchard JP, Xie YG, et al. . Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18(2):164-167. doi: 10.1038/ng0298-164 [DOI] [PubMed] [Google Scholar]
- 2.Bilgen C, Bilgen IG, Sener RN. Oculopharyngeal muscular dystrophy: clinical and CT findings. Comput Med Imaging Graph. 2001;25(6):527-529. doi: 10.1016/s0895-6111(01)00021-0 [DOI] [PubMed] [Google Scholar]
- 3.King MK, Lee RR, Davis LE. Magnetic resonance imaging and computed tomography of skeletal muscles in oculopharyngeal muscular dystrophy. J Clin Neuromuscul Dis. 2005;6(3):103-108. doi: 10.1097/01.cnd.0000152060.57673.25 [DOI] [PubMed] [Google Scholar]
- 4.de Leeuw RH, Garnier D, Kroon R, et al. . Diagnostics of short tandem repeat expansion variants using massively parallel sequencing and componential tools. Eur J Hum Genet. 2019;27(3):400-407. doi: 10.1038/s41431-018-0302-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard P, Trollet C, Stojkovic T, et al. . Correlation between PABPN1 genotype and disease severity in oculopharyngeal muscular dystrophy. Neurology. 2017;88(4):359-365. doi: 10.1212/WNL.0000000000003554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis TA, Hollingsworth KG, Coombs A, et al. . Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One. 2013;8:e70993. doi: 10.1371/journal.pone.0070993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wokke BH, Bos C, Reijnierse M, et al. . Comparison of dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J Magn Reson Imaging. 2013;38(3):619-624. doi: 10.1002/jmri.23998 [DOI] [PubMed] [Google Scholar]
- 8.Andersen G, Dahlqvist JR, Vissing CR, Heje K, Thomsen C, Vissing J. MRI as outcome measure in facioscapulohumeral muscular dystrophy: 1-year follow-up of 45 patients. J Neurol. 2017;264(3):438-447. doi: 10.1007/s00415-016-8361-3 [DOI] [PubMed] [Google Scholar]
- 9.Fischmann A, Gloor M, Fasler S, et al. . Muscular involvement assessed by MRI correlates to motor function measurement values in oculopharyngeal muscular dystrophy. J Neurol. 2011;258(7):1333-1340. doi: 10.1007/s00415-011-5937-9 [DOI] [PubMed] [Google Scholar]
- 10.Fischmann A, Hafner P, Fasler S, et al. . Quantitative MRI can detect subclinical disease progression in muscular dystrophy. J Neurol. 2012;259(8):1648-1654. doi: 10.1007/s00415-011-6393-2 [DOI] [PubMed] [Google Scholar]
- 11.van der Sluijs BM, Lassche S, Knuiman GJ, et al. . Involvement of pelvic girdle and proximal leg muscles in early oculopharyngeal muscular dystrophy. Neuromuscul Disord. 2017;27(12):1099-1105. doi: 10.1016/j.nmd.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Jimenez A, Kroon HMJM, Alejaldre-Monforte A, et al. . Muscle MRI in a large cohort of patients with oculopharyngeal muscular dystrophy. J Neurol Neurosurg Psychiatry. 2019;90(5):576-585. doi: 10.1136/jnnp-2018-319578 [DOI] [PubMed] [Google Scholar]
- 13.Gloor M, Fasler S, Fischmann A, et al. . Quantification of fat infiltration in oculopharyngeal muscular dystrophy: comparison of three MR imaging methods. J Magn Reson Imaging. 2011;33(1):203-210. doi: 10.1002/jmri.22431 [DOI] [PubMed] [Google Scholar]
- 14.Melkus G, Sampaio IC, Smith JC, et al. Quantitative vs qualitative muscle MRI: imaging biomarker in patients with oculopharyngeal muscular dystrophy (OPMD). Neuromuscul Disord. 2023;33(1):24-31. doi: 10.1016/j.nmd.2022.09.010 [DOI] [PubMed] [Google Scholar]
- 15.van Engelen BG, van Veenendaal H, van Doorn PA, et al. . The Dutch neuromuscular database CRAMP (Computer Registry of All Myopathies and Polyneuropathies): development and preliminary data. Neuromuscul Disord. 2007;17(1):33-37. doi: 10.1016/j.nmd.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 16.Mul K, Vincenten SCC, Voermans NC, et al. . Adding quantitative muscle MRI to the FSHD clinical trial toolbox. Neurology. 2017;89(20):2057-2065. doi: 10.1212/WNL.0000000000004647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen BH, Voet NBM, Geurts AC, van Engelen B, Heerschap A. Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients. Neurology. 2016;86(18):1700-1707. doi: 10.1212/WNL.0000000000002640 [DOI] [PubMed] [Google Scholar]
- 18.Janssen BH, Voet NBM, Nabuurs CIHC, et al. . Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. 2014;9(1):e85416. doi: 10.1371/journal.pone.0085416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamminen AE. Magnetic resonance imaging of primary skeletal muscle diseases: patterns of distribution and severity of involvement. Br J Radiol. 1990;63(756):946-950. doi: 10.1259/0007-1285-63-756-946 [DOI] [PubMed] [Google Scholar]
- 20.Ortolan P, Zanato R, Coran A, Beltrame V, Stramare R. Role of radiologic imaging in genetic and acquired neuromuscular disorders. Eur J Transl Myol. 2015;25(2):5014. doi: 10.4081/ejtm.2015.5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mul K, Horlings GC, Vincenten SCC, Voermans NC, van Engelen BGM, van Alfen N. Quantitative muscle MRI and ultrasound for facioscapulohumeral muscular dystrophy: complementary imaging biomarkers. J Neurol. 2018;265(11):2646-2655. doi: 10.1007/s00415-018-9037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heskamp L, van Nimwegen M, Ploegmakers MJM, et al. . Lower extremity muscle pathology in myotonic dystrophy type 1 assessed by quantitative MRI. Neurology. 2019;92(24):e2803-e2814. doi: 10.1212/WNL.0000000000007648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroon R, Kalf JG, de Swart BJM, et al. . Longitudinal assessment of strength, functional capacity, oropharyngeal function, and quality of life in oculopharyngeal muscular dystrophy. Neurology. 2021;97(15):e1475–e1483. doi: 10.1212/WNL.0000000000012640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Council MR. Aids to the Investigation of Peripheral Nerve Injuries. Her Majesty’s Stationary Office; 1976. [Google Scholar]
- 25.Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. J Speech Hear Res. 1992;35(6):1239-1245. doi: 10.1044/jshr.3506.1239 [DOI] [PubMed] [Google Scholar]
- 26.Nightingale EJ, Pourkazemi F, Hiller CE. Systematic review of timed stair tests. J Rehabil Res Dev. 2014;51(3):335-350. doi: 10.1682/JRRD.2013.06.0148 [DOI] [PubMed] [Google Scholar]
- 27.Bérard C, Payan C, Hodgkinson I, Fermanian J; MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005;15(7):463-470. doi: 10.1016/j.nmd.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Paepens M, Stepman G, DeHeyder E, Beeckman A, Kalf J. Maximum swallowing volume: comparison between Flemish and Dutch normal values. Dysphagia. 2020;35:155-156. [Google Scholar]
- 29.Nathadwarawala KM, Nicklin J, Wiles CM. A timed test of swallowing capacity for neurological patients. J Neurol Neurosurg Psychiatry. 1992;55(9):822-825. doi: 10.1136/jnnp.55.9.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepman G, Paepens M, DeHeyder E, Beeckman A, Kalf J. Maximum swallowing speed: comparison between Flemish and Dutch normal values. Dysphagia. 2020;35:154-155. [Google Scholar]
- 31.Huckabee ML, McIntosh T, Fuller L, et al. . The Test of Masticating and Swallowing Solids (TOMASS): reliability, validity and international normative data. Int J Lang Commun Disord. 2018;53(1):144-156. doi: 10.1111/1460-6984.12332 [DOI] [PubMed] [Google Scholar]
- 32.Gadesmann M, Miller N. Reliability of speech diadochokinetic test measurement. Int J Lang Commun Disord. 2008;43(1):41-54. doi: 10.1080/13682820701234444 [DOI] [PubMed] [Google Scholar]
- 33.Speyer R, Bogaardt HC, Passos VL, et al. . Maximum phonation time: variability and reliability. J Voice. 2010;24(3):281-284. doi: 10.1016/j.jvoice.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459-468. doi: 10.1016/s0895-4356(99)00206-1 [DOI] [PubMed] [Google Scholar]
- 35.Heskamp L, Okkersen K, van Nimwegen M, et al. . Quantitative muscle MRI depicts increased muscle mass after a behavioral change in myotonic dystrophy type 1. Radiology. 2020;297:132-142. doi: 10.1148/radiol.2020192518 [DOI] [PubMed] [Google Scholar]
- 36.Kroon R, Kalf JG, Meijers RL, et al. . Muscle ultrasound is a sensitive biomarker in oculopharyngeal muscular dystrophy. Muscle Nerve. 2022;66(4):453-461. doi: 10.1002/mus.27679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra MA, Heskamp L, Mul K, et al. . Specific muscle strength is reduced in facioscapulohumeral dystrophy: an MRI based musculoskeletal analysis. Neuromuscul Disord. 2018;28(3):238-245. doi: 10.1016/j.nmd.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 38.Klickovic U, Zampedri L, Sinclair CDJ, et al. . Skeletal muscle MRI differentiates SBMA and ALS and correlates with disease severity. Neurology. 2019;93(9):e895-e907. doi: 10.1212/WNL.0000000000008009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakao Y, Uchiyama Y, Honda K, Yamashita T, Saito S, Domen K. Age-related composition changes in swallowing-related muscles: a Dixon MRI study. Aging Clin Exp Res. 2021;33(12):3205-3213. doi: 10.1007/s40520-021-01859-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On reasonable request, the anonymized data that support the findings of this study are available from the corresponding author.