Abstract
Background and Objectives
Corticobasal syndrome (CBS) with underlying 4-repeat tauopathy is a progressive neurodegenerative disease characterized by declining cognitive and motor functions. Biomarkers for assessing pathologic brain changes in CBS including tau-PET, 18 kDa translocator protein (TSPO)-PET, structural MRI, neurofilament light chain (NfL), or glial fibrillary acidic protein (GFAP) have recently been evaluated for differential diagnosis and disease staging, yet their association with disease trajectories remains unclear. Therefore, we performed a head-to-head comparison of neuroimaging (tau-PET, TSPO-PET, structural MRI) and plasma biomarkers (NfL, GFAP) as prognostic tools for longitudinal clinical trajectories in β-amyloid (Aβ)–negative CBS.
Methods
We included patients with clinically diagnosed Aβ-negative CBS with clinical follow-up data who underwent baseline structural MRI and plasma-NfL analysis for assessing neurodegeneration, [18F]PI-2620-PET for assessing tau pathology, [18F]GE-180-PET for assessing microglia activation, and plasma-GFAP analysis for assessing astrocytosis. To quantify tau and microglia load, we assessed summary scores of whole-brain, cortical, and subcortical PET signal. For structural MRI analysis, we quantified subcortical and cortical gray matter volume. Plasma NfL and GFAP values were assessed using Simoa-based immunoassays. Symptom progression was determined using a battery of cognitive and motor tests (i.e., Progressive Supranuclear Palsy Rating Scale [PSPRS]). Using linear mixed models, we tested whether the assessed biomarkers at baseline were associated with faster symptom progression over time (i.e., time × biomarker interaction).
Results
Overall, 21 patients with Aβ-negative CBS with ∼2-year clinical follow-up data were included. Patients with CBS with more widespread global tau-PET signal showed faster clinical progression (PSPRS: B/SE = 0.001/0.0005, p = 0.025), driven by cortical rather than subcortical tau-PET. By contrast, patients with higher global [18F]GE-180-PET readouts showed slower clinical progression (PSPRS: B/SE = −0.056/0.023, p = 0.019). No association was found between gray matter volume and clinical progression. Concerning fluid biomarkers, only higher plasma-NfL (PSPRS: B/SE = 0.176/0.046, p < 0.001) but not GFAP was associated with faster clinical deterioration. In a subsequent sensitivity analysis, we found that tau-PET, TSPO-PET, and plasma-NfL showed significant interaction effects with time on clinical trajectories when tested in the same model.
Discussion
[18F]PI-2620 tau-PET, [18F]GE-180 TSPO-PET, and plasma-NfL show prognostic potential for clinical progression in patients with Aβ-negative CBS with probable 4-repeat tauopathy, which can be useful for clinical decision-making and stratifying patients in clinical trials.
Introduction
Corticobasal syndrome (CBS) with underlying 4-repeat (4R) tau pathology, characterized by intracellular neuronal and glial 4R tau aggregates, is a progressive neurodegenerative disorder characterized by declining cognitive and motor functions.1-3 4R tauopathies are subclassified mainly as corticobasal degeneration (CBD) or progressive supranuclear palsy (PSP),1 which most commonly manifest as atypical Parkinson syndrome CBS2,3 or PSP Richardson syndrome (PSP-RS), depending on the expression of cortical and subcortical symptoms.3,4 The clinical phenotypes of 4R tauopathies are potentially driven by heterogeneous 4R tau deposition patterns, with predominant brainstem and subcortical tau accumulation and only late-stage cortical tau in PSP-RS5 vs more widespread cortical tau aggregation in patients presenting as CBS.6,7 Clinically, β-amyloid peptide (Aβ)–negative CBS can be diagnosed using the Movement Disorders Society (MDS) criteria for PSP3 or CBD criteria.1
Various biomarkers for assessing the underlying pathologic brain changes in patients with Aβ-negative CBS have recently been evaluated for differential diagnosis and disease staging, yet the prognostic accuracy of these biomarkers for predicting future disease trajectories remains unclear. The next-generation tau-PET tracer [18F]PI-2620 has shown a discrimination of CBS and PSP vs healthy controls as imaging biomarker to detect 4R tauopathies in vivo.4-7 The [18F]PI-2620 PET signal patterns were congruent with the histopathologically expected 4R tau accumulation, showing a shift toward cortical tau in CBS.4,6 Microglial activation, measured with [18F]GE-180 PET tracer targeting the 18 kDa translocator protein (TSPO), has been apparent in 4R tauopathies PSP and Aβ-negative CBS8 as well as in Alzheimer disease (AD).9 As fluid biomarker, levels of neurofilament light chain (NfL) have been identified to be a surrogate for neuroaxonal injury in various neurologic diseases,10-12 besides structural MRI as an imaging-based marker of neurodegeneration. Belonging to the family of class IV intermediate filaments, NfL is a component of the neuronal cytoskeleton in both central and peripheral neurons. In case of neurodegeneration or axonal damage, NfL can be released from neurons into the CSF and blood.13 Elevation of glial fibrillary acid protein (GFAP), a structural component of fibrillary astrocytes, has been suggested as a marker for reactive astrogliosis in blood and CSF in various neurodegenerative diseases.14 In cohorts with clinical diagnosis of 4R tauopathies, levels of NfL and GFAP in both blood and CSF have been shown to be elevated in comparison with idiopathic Parkinson disease (PD) and healthy controls.15-17 High NfL levels have been associated with shorter survival in a retrospective study with patients with PSP-RS 18 and with predicting disease progression in PSP.19,20 However, the role of NfL for predicting disease progression in CBS is still unclear. The identification of prognostic biomarkers is crucial both for patient care itself and for the development of interventional trials. To address this, we performed a head-to-head comparison of neuroimaging (i.e., tau-PET, TSPO-PET, structural MRI) and plasma (i.e., NfL, GFAP) biomarkers to evaluate their impact on future clinical trajectories in 21 patients with Aβ-negative CBS with clinical diagnosis of a probable 4R tauopathy.3
Methods
Participants and Clinical Evaluation
Patients were recruited and clinically tracked at the Department of Neurology at Ludwig-Maximilians-Universität (LMU) Munich between February 2018 and March 2022. They were diagnosed by a movement disorder specialist as PSP-CBS phenotype with probable underlying 4R tauopathy according to the MDS-PSP criteria.3 They also fulfilled the Armstrong criteria of probable or possible CBD-CBS.1 Inclusion criteria were (1) stable pharmacotherapy for at least 1 week before PET examination; (2) a negative family history for PD, frontotemporal dementia, and AD; (3) no severe neurologic or psychiatric disorders other than CBS; and (4) negative Aβ status as determined using standardized diagnostic procedures at the LMU to rule out confounding AD pathology. Specifically, negative Aβ status was determined using CSF (i.e., Aβ42/40 ratio >5.5% or Aβ1-42 >375 pg/mL) or negative [18F]flutemetamol-PET visual read.5,6,8
At baseline, all patients underwent detailed clinical assessment, MRI, blood sampling, and [18F]PI-2620 PET. A subset also underwent baseline [18F]GE-180 TSPO-PET. Because binding properties of this TSPO tracer have been found to depend on genetic polymorphism of the TSPO gene,21 all individuals underwent rs6971 single-nucleotide polymorphism genotyping as described previously.8
Clinical assessments at baseline and follow-up were performed by a movement disorder specialist at the LMU outpatient's clinic for movement disorders. Two experts performed baseline visits, whereas all follow-up visits were conducted by the same movement disorder specialist to reduce interrater variability. All experts were specifically trained for all study procedures before patient inclusion. Assessments included the PSP Rating Scale (PSPRS),22 Unified Parkinson's Disease Rating Scale—Motor Part including the modified Hoehn and Yahr score,23 and the PSP-Clinical Deficits Scale.24 Functional independence was measured using the Schwab and England Activities of Daily Living (SEADL) scale.25 Global cognitive status was assessed with Montreal Cognitive Assessment (MoCA) scale.26 For follow-up visits, different versions of the MoCA were used to avoid training effects. Disease duration was classified as the time between reported symptom onset and baseline clinical assessment. For all participants, clinical diagnosis was reviewed and confirmed at follow-up visits. By the time of the data cutoff for this study, 8 patients had died. Autopsies were not available.
Standard Protocol Approvals, Registrations, and Patient Consents
All patients were recruited within the Activity of Cerebral Networks, Amyloid and Microglia in Aging and Alzheimer's Disease (ActiGliA) study, a prospective cohort study of the Munich Cluster for Systems Neurology (SyNergy) at LMU. The study and data analyses were approved by the local ethics committee LMU (ethics-applications: 17-569, 17-755 and 19-022) and the German radiation protection authorities (BfS-application: Z 5 − 22464/2017-047-K-G). Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Patients did not receive compensation for study participation.
Neuroimaging, Acquisition, and Processing
All PET procedures, including radiochemistry, acquisition and preprocessing, were conducted using established standardized protocols.5,8,27-29 All patients were scanned at the Department of Nuclear Medicine, LMU, using a Biograph 64 PET/CT scanner or a harmonized Biograph mCT (Siemens, Erlangen, Germany). For detection of microglial activation, [18F]GE-180 TSPO-PET recordings (average dose: 179 ± 13 megabecquerels [MBq]) with an emission window of 60–80 minutes after injection were performed.8 Dynamic [18F]PI-2620 tau-PET (average dose: 188 ± 15 MBq) with emission recording 0–60 minutes after injection was obtained to assess tau aggregation. Static frames of the late phase (20–40 minutes)30 were reconstructed for assessing tau binding.
All PET data analyses were performed using PMOD (version 3.9; PMOD Technologies LLC, Zurich, Switzerland). For primary analysis, static emission recordings which were coregistered to the Montreal Neurology Institute (MNI) space using nonlinear warping (16 iterations, frequency cutoff 25, transient input smoothing 8 × 8 × 8 mm3) to tracer-specific templates acquired in previous in-house studies were used.5,8,27,28 For TSPO-PET, a late-phase template, consisting of cognitively unimpaired controls with intact motor function was used (nt = 11, 60–80 minutes p.i.). Tau-PET images were coregistered to a late-phase template, consisting of a mixed population of healthy controls and patients with 4R-tauopathies without distinction of tau-PET positivity (nt = 28, 20–40 minutes p.i.). Intensity normalization of all PET images was assessed by calculation of standardized uptake value ratios (SUVRs). Therefore, the cerebellum was used as a pseudo-reference tissue for microglia-PET.31 The cerebellum was chosen as a unified pseudo-reference tissue because it was also used for tau-PET analysis. The dentate nucleus and superior and posterior layers of the cerebellum were excluded to account for potential tau-PET positivity in cerebellar areas and in adjacent extracerebral structures. Using the Brainnetome atlas,32 the brain was divided into 210 cortical and 36 subcortical volume of interests (VOIs), and SUVRs were calculated. Per patient, the standardized regional deviation of SUVR (z-score) was computed vs the readouts of already established age-matched and sex-matched control cohorts for [18F]GE-180 (nc = 1333) and [18F]PI-2620 (nc = 146).
Because patterns of abnormal tau-PET or TSPO-PET can be heterogeneous in patients with Aβ-negative CBS, we computed summary measures of brain-wide PET abnormality. Specifically, we determined number of VOIs of the Brainnetome atlas in which PET SUVRs fell above a z-score of 1.5. This cutoff of 1.5 SD is based on experience on a sample of healthy controls without any evidence for neurologic disease or cognitive deficits, increasing the sensitivity to detect early PET abnormalities in patients with suspected 4R tauopathies. Summary measures of PET abnormality were determined for the whole brain, as well as for the cortex and subcortex separately, to determine whether subcortical and/or cortical tau-PET or TSPO-PET abnormalities were associated with clinical CBS trajectories.
For structural MRI, 3-dimensional T1 MRI data (repetition time = 2,060 milliseconds, 0.8 mm isotropic voxel size) recorded on a 3T SIEMENS Magnetom Prisma system (Siemens Healthineers, Erlangen, Germany) were nonlinearly spatially normalized to MNI standard space using PNEURO tool (version 3.9; PMOD Technologies LLC) and segmented into tissue-specific probability maps. Regional gray matter density as a proxy of gray matter volume was extracted for each of the Brainnetome VOI.
Assessment of a CBS Clinical Composite Score
Because there was no consensus on a single clinical scale that captures CBS symptom severity at the beginning of the study in 2018, we composed a CBS clinical composite across available clinical scales including PSPRS, MoCA, and SEADL. We specifically selected these scales to summarize global clinical status, including cognitive symptom severity (i.e., MoCA), subcortical symptom severity (i.e., PSPRS), and impaired activities of daily living (i.e., SEADL). Specifically, MoCA, PSPRS, and SEADL scores were fed into principal component analyses (i.e., prcomp command of the stats package in R statistical software). The first principal component that captured most of the variance across MoCA, PSPRS, and SEADL scores was extracted as a summary measure of clinical disease severity (i.e., CBS clinical composite). The variance explained for the first principal component was 89.11%, suggesting that this component captures a large proportion of the variance across the clinical measures. For future clinical studies in CBS, the Cortical Basal Ganglia Functional Scale, published in 2020,34,35 may be implemented as an additional single clinical scale for CBS symptoms.
Plasma Biomarker Assessment
NfL and GFAP levels were quantitatively determined in plasma samples using a commercial SIMOA kit (#103345; Quanterix, Billerica, MA) following the manufacturer's instructions. All samples were analyzed in the same plate blinded to clinical information and were measured on the same day. The used aliquot underwent only 1 thaw/freeze cycle. NfL concentrations were measured using the Simoa HD-X analyzer (Quanterix).
Statistical Analyses
First, we assessed whether patients with Aβ-negative CBS declined during the follow-up period. To this end, we used linear mixed-effects models using the time from baseline as a predictor of the clinical composite or PSPRS, controlling for age, sex, education, body mass index (BMI), disease duration, and random slope and intercept. We then determined whether baseline tau-PET, TSPO-PET, structural MRI, NfL, or GFAP levels moderated clinical trajectories. Specifically, we tested the interaction effect of each baseline biomarker (i.e., tau-PET, TSPO-PET, structural MRI abnormality, NfL, GFAP) with time on the clinical composite or PSPRS. Again, models were controlled for age, sex, education, BMI, disease duration, and random slope and intercept. For tau-PET or TSPO-PET abnormality, analyses were conducted using global PET abnormality scores and subcortical and cortical PET abnormality scores, to determine the effect of either cortical or subcortical PET abnormality on clinical trajectories. In addition, we performed simulated interventions to determine whether baseline PET or fluid biomarkers can help select patients at high risk of clinical progression to reduce sample sizes for potential intervention effects. To this end, we determined patient-specific annual change rates in the clinical composite and PSPRS using linear mixed-effects models. Using these subject-specific change rates, we ran simulated interventions with hypothetical intervention effects of 20%/30%/40% using the R-package pwr (settings: 2-sample t test, 2-tailed, type I error rate = 0.05, power = 0.8). Simulated interventions were performed for the whole CBS sample and stratified by high and low biomarker groups (i.e., tau-PET, TSPO-PET, structural MRI, NfL, GFAP) determined by median split. All analyses were computed using R statistical software. Linear mixed models were run using the lmer package.
Data Availability
Ethics approvals do not allow unrestricted and open-source sharing of patient-specific data with third-parties. Anonymized data that support the findings of this study are available on reasonable request from the corresponding author.
Results
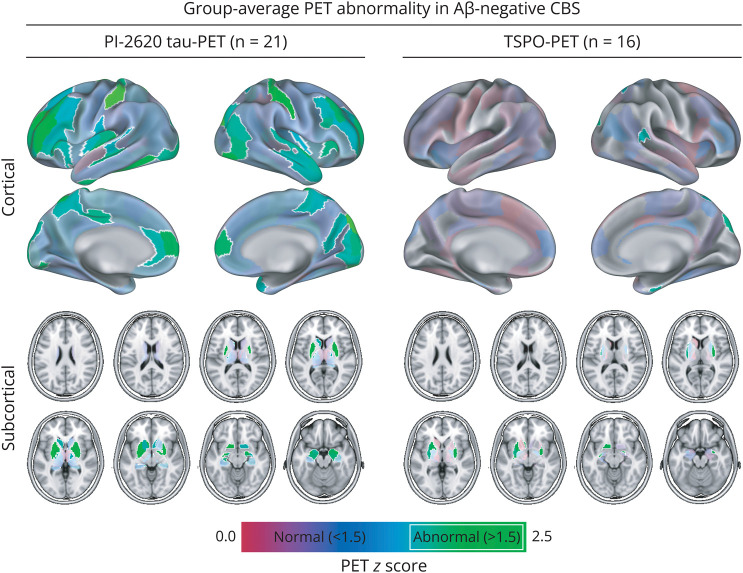
Overall, 21 patients with Aβ-negative CBS with longitudinal clinical data were included in this study (see Table 1 for demographic and clinical characteristics). At baseline, all patients underwent detailed clinical assessment, MRI, blood sampling, and [18F]PI-2620 PET. A subset of n = 16 patients also underwent baseline [18F]GE-180 TSPO-PET. All included patients were medium or high affinity binder for [18F]GE-180 TSPO-PET. Cross-sectional analysis of [18F]PI-2620 PET and [18F]GE-180-PET8 of parts of the cohort has been reported before.4-6 The median clinical follow-up times were 1.95 years ranging between 0.75 and 2.72 years with an average of 3.1 ± 0.7 visits. As expected, we found a significant effect of clinical follow-up time on the CBS clinical composite scores (B/SE = −12.126/2.279, p < 0.001) and PSPRS (B/SE = 7.847/1.240, p < 0.001). Baseline z-score maps of [18F]PI-2620 tau-PET and [18F]GE-180 TSPO-PET are shown in Figure 1, showing widespread elevations of subcortical and cortical [18F]PI-2620 tau-PET signals and limited cortical and moderate subcortical [18F]GE-180 TSPO-PET signal increases. The PET summary scores for global, cortical and subcortical assessments are summarized in Table 1.
Table 1.
Demographic and Clinical Sample Characteristics at Baseline
| Variable | Aβ-negative CBS (n = 21) |
| Sex (male/female) | 9/12 |
| Age, y | 68.7 ± 12.0 |
| Education, y | 12.8 ± 2.8 |
| Disease duration, y | 2.69 ± 1.35 |
| Follow-up, y, median (range) | 1.95 (0.75–2.72) |
| PSPRS scores | 22.2 ± 13.7 |
| SEADL scores | 69.0 ± 19.2 |
| MoCA scores | 23.7 ± 4.1 |
| CBS clinical composite scores | 15.3 ± 22.9 |
| Global PI-2620 tau-PET summary score | 107.28 ± 69.88 (median = 105) |
| Cortical PI-2620 tau-PET summary score | 90.94 ± 63.9 (median = 88) |
| Subcortical PI-2620 tau-PET summary score | 16.34 ± 10.03 (median = 16) |
| Global TSPO-PET summary score | 49.12 ± 36.98 (median = 32) |
| Cortical TSPO-PET summary score | 40.14 ± 31.06 (median = 21) |
| Subcortical TSPO-PET summary score | 8.98 ± 7.51 (median = 7) |
Abbreviations: Aβ = β-amyloid; CBS = corticobasal syndrome; MoCA = Montreal Cognitive Assessment; PSPRS = Progressive Supranuclear Palsy Rating Scale; SEADL = Schwab and England Activities of Daily Living; TSPO = 18 kDa translocator protein.
Data are presented as mean ± SD, unless indicated otherwise. The CBS clinical composite score is defined by a principal component analysis using MoCA, PSPRS, and SEADL scores.
Figure 1. Group-Average PET Abnormality in Aβ-Negative CBS.
Surface and subcortical renderings of elevated group-level for PI-2620 tau-PET z-scores (left panels) and TSPO-PET z-scores (right-panels) that were referenced against PET images obtained in healthy controls. Z-scores greater than 1.5 are considered pathologic and are highlighted by white margins. Aβ = β-amyloid; CBS = corticobasal syndrome; TSPO = 18 kDa translocator protein.
More Widespread Cortical Tau-PET Is Associated With Faster Clinical Deterioration
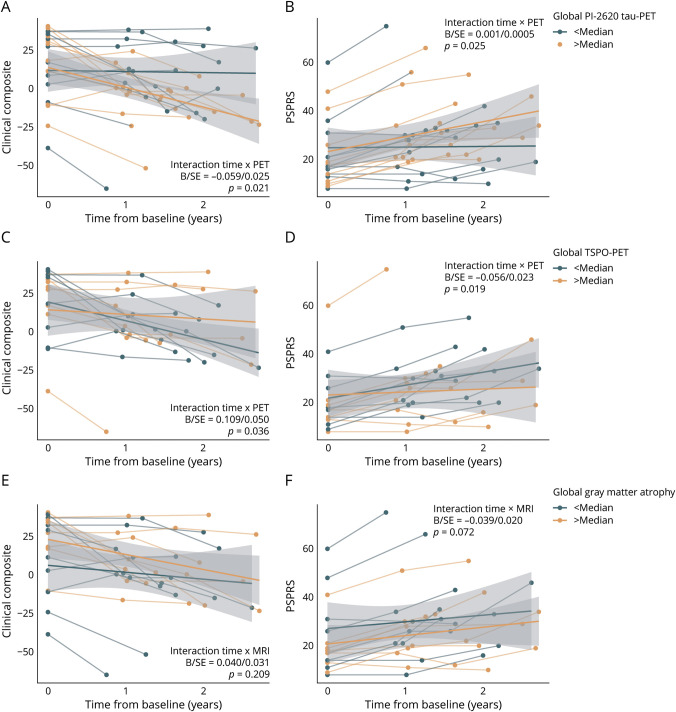
In a first step, we tested whether baseline [18F]PI-2620 tau-PET predicted speed of clinical progression in Aβ-negative CBS. We hypothesized that higher [18F]PI-26260 tau-PET readouts are associated with faster clinical progression. To test this, we assessed the interaction effect between [18F]PI-2620 tau-PET and follow-up time on trajectories in the CBS clinical composite score and PSPRS using linear mixed models adjusting for age, sex, education, BMI, disease duration, and random slope and intercept. For global [18F]PI-2620 tau-PET abnormality (i.e., number of Brainnetome regions of interest with a z-score >1.5), we found a significant time by [18F]PI-2620 tau-PET interaction on the CBS clinical composite score (B/SE = −0.059/0.025, p = 0.021, Figure 2A) and PSPRS (B/SE = 0.001/0.0005, p = 0.025, Figure 2B), supporting the hypothesis that faster clinical deterioration in patients with Aβ-negative CBS is associated with more widespread global [18F]PI-2620 tau-PET signal. When stratifying this analysis by [18F]PI-2620 tau-PET abnormality in the cortex vs subcortex, cortical [18F]PI-2620 tau-PET was associated with faster decline on the CBS clinical composite score (B/SE = −0.063/0.027, p = 0.024) and PSPRS (B/SE = 0.001/0.0005, p = 0.026) while subcortical [18F]PI-2620 tau-PET abnormality was not (CBS clinical composite: B/SE = −0.286/0.184, p = 0.127; PSPRS: B/SE = 0.005/0.004, p = 0.191). This suggests that specifically the elevation of [18F]PI-2620 tau-PET in the cortex is associated with faster clinical progression in Aβ-negative CBS. Linear mixed model statistics are summarized in Table 2.
Figure 2. Neuroimaging-Based Prediction of Clinical Trajectories in Aβ-Negative CBS.
Line plots illustrating clinical trajectories on the CBS clinical composite score (A, C, E) and PSPRS (B, D, F) stratified by abnormality in global PI-2620 tau-PET (A, B), global TSPO-PET (C, D) or global MRI-based gray matter atrophy (E, F). For visualization, regression fits were split into above and below median groups to illustrated disease trajectories relative to imaging signal abnormality; however, interactions were computed using continuous measures. Statistics are based on linear mixed models controlling for age, sex, education, body mass index, disease duration, and random slope and intercept. CBS clinical composite score is defined by a principal component analysis using MoCA, PSPRS, and SEADL scores. A decrease in score value of the CBS clinical composite score indicates a clinical deterioration while in PSPRS an increase in score value indicates clinical worsening. Linear model fits (i.e., least squares line) are indicated together with 95% CIs. Aβ = β-amyloid; CBS = corticobasal syndrome; MoCA = Montreal Cognitive Assessment; PSPRS = Progressive Supranuclear Palsy Rating Scale; SEADL = Schwab and England Activities of Daily Living; TSPO = 18 kDa translocator protein.
Table 2.
Linear Mixed Model Statistics for Time by Biomarker Interactions on Clinical Trajectories
| Dependent variable | Biomarker | No. (subjects/observations) | B/SE | T | p Value |
| CBS clinical composite score | Global PI-2620 tau-PET | 21/64 | −0.059/0.025 | −2.395 | 0.021 |
| Cortical PI-2620 tau-PET | −0.063/0.027 | −2.352 | 0.024 | ||
| Subcortical PI-2620 tau-PET | −0.286/0.184 | −1.577 | 0.127 | ||
| Global TSPO-PET | 16/51 | 0.109/0.050 | 2.185 | 0.036 | |
| Cortical TSPO-PET | 0.118/0.060 | 1.971 | 0.057 | ||
| Subcortical TSPO-PET | 0.660/0.243 | 2.716 | 0.011 | ||
| Global gray matter volume | 17/53 | 0.040/0.031 | 1.282 | 0.209 | |
| Cortical gray matter volume | 0.044/0.033 | 1.336 | 0.190 | ||
| Subcortical gray matter volume | 0.171/0.351 | 0.488 | 0.628 | ||
| NfL | 20/62 | −0.230/0.111 | −2.073 | 0.044 | |
| GFAP | 19/59 | −0.020/0.027 | −0.755 | 0.455 | |
| PSPRS | Global PI-2620 tau-PET | 21/64 | 0.001/0.0005 | 2.329 | 0.025 |
| Cortical PI-2620 tau-PET | 0.001/0.0005 | 2.318 | 0.026 | ||
| Subcortical PI-2620 tau-PET | 0.005/0.004 | 1.331 | 0.191 | ||
| Global TSPO-PET | 16/51 | −0.056/0.023 | −2.462 | 0.019 | |
| Cortical TSPO-PET | −0.059/0.028 | −2.138 | 0.040 | ||
| Subcortical TSPO-PET | −0.369/0.106 | −3.470 | 0.002 | ||
| Global gray matter volume | 17/53 | −0.039/0.020 | −1.950 | 0.072 | |
| Cortical gray matter volume | −0.041/0.021 | −1.925 | 0.076 | ||
| Subcortical gray matter volume | −0.403/0.231 | −1.747 | 0.103 | ||
| NfL | 20/62 | 0.176/0.046 | 3.842 | 0.0004 | |
| GFAP | 19/59 | −0.007/0.013 | −0.567 | 0.574 |
Abbreviations: CBS = corticobasal syndrome; GFAP = glial fibrillary acidic protein; NfL = neurofilament light chain; MoCA = Montreal Cognitive Assessment; PSPRS = Progressive Supranuclear Palsy Rating Scale; SEADL = Schwab and England Activities of Daily Living; TSPO = 18 kDa translocator protein.
Linear mixed model statistics were adjusted for age, sex, education, body mass index, disease duration, as well as random slope and intercept. The CBS clinical composite score is defined by a principal component analysis using MoCA, PSPRS, and SEADL scores.
Plasma NfL but Not GFAP Predicts Clinical Progression
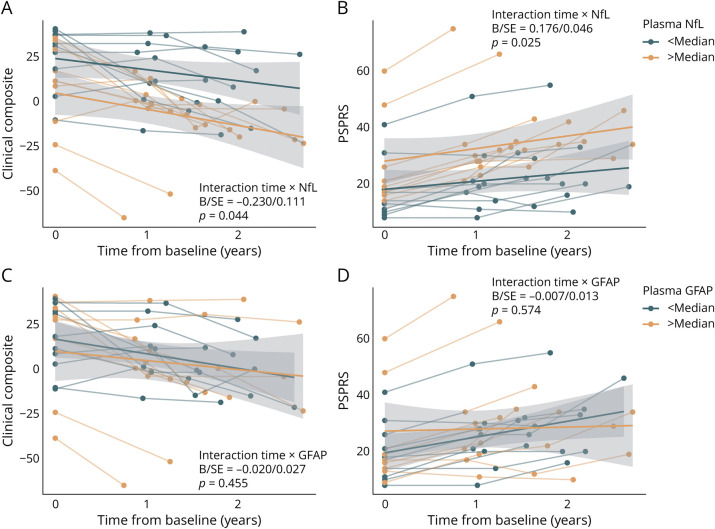
Next, we assessed whether plasma NfL and GFAP are associated with faster clinical worsening. For NfL, we found a significant interaction with time on worsening in the clinical composite (B/SE = −0.230/0.111, p = 0.044, Figure 3A) and PSPRS (B/SE = 0.176/0.046, p < 0.001, Figure 3B), indicating that stronger neurodegeneration is associated with faster clinical worsening. Yet, no time by biomarker abnormality interaction was found for GFAP, neither for the clinical composite (B/SE = −0.020/0.027, p = 0.455, Figure 3C) nor for PSPRS (B/SE = −0.007/0.0127, p = 0.574, Figure 3D). This suggests that GFAP as a marker of abnormal astrocyte function is not associated with subsequent clinical worsening in Aβ-negative CBS. A summary of linear mixed model statistics can be found in Table 2.
Figure 3. Fluid Biomarker-Based Prediction of Clinical Trajectories in Aβ-Negative CBS.
Line plots illustrating clinical trajectories on the clinical composite (A, C) and PSPRS (B, D) stratified by abnormality in plasma NfL (A, B) and plasma GFAP (C, D). For visualization, regression fits were split into above and below median groups to illustrated disease trajectories relative to NfL and GFAP levels; however, interactions were computed using continuous measures. Statistics are based on linear mixed models controlling for age, sex, education, body mass index, disease duration, and random slope and intercept. CBS clinical composite score is defined by a principal component analysis using MoCA, PSPRS, and SEADL scores. A decrease in score value of the CBS clinical composite score indicates a clinical deterioration, whereas in PSPRS an increase in score value indicates clinical worsening. Aβ = β-amyloid; CBS = corticobasal syndrome; GFAP = glial fibrillary acidic protein; MoCA = Montreal Cognitive Assessment; NfL = neurofilament light chain; PSPRS = Progressive Supranuclear Palsy Rating Scale; SEADL = Schwab and England Activities of Daily Living.
More Widespread TSPO-PET Readout Is Associated With Slower Clinical Progression
For TSPO-PET, we found that more widespread global signal was associated with slower worsening both on the clinical composite (B/SE = 0.109/0.050, p = 0.037, Figure 3C) and PSPRS (B/SE = −0.056/0.023, p = 0.019, Figure 3D). In contrast to tau-PET, this effect was stronger for subcortical TSPO-PET (clinical composite: B/SE = 0.660/0.243, p = 0.011; PSPRS: B/SE = −0.369/0.106, p = 0.002) rather than cortical TSPO-PET abnormality (clinical composite: B/SE = 0.118/0.060, p = 0.057; PSPRS: B/SE = −0.059/0.028, p = 0.040). These findings suggest that more widespread microglial activation is associated with attenuated clinical progression in Aβ-negative CBS. For summary statistics, refer to Table 2.
MRI-Based Volumetry Is Not Predictive of Clinical Progression
When assessing the number of VOIs falling below an atrophy z-score cutoff of −1.5 on structural MRI, we did not find any association with faster decline in the clinical CBS composite score or PSPRS, neither for global (clinical composite: B/SE = 0.040/0.031, p = 0.209; Figure 3E; PSPRS: B/SE = −0.039/0.020, p = 0.072, Figure 3F) nor for cortical (clinical composite: B/SE = 0.044/0.033, p = 0.190; PSPRS: B/SE = −0.041/0.021, p = 0.076) or subcortical VOIs (clinical composite: B/SE = 0.171/0.351, p = 0.628; PSPRS: B/SE = −0.403/0.231, p = 0.103). Detailed statistics are summarized in Table 2. As we did not find that pronounced brain atrophy was associated with faster clinical disease progression, we do not expect systematic confounding effects of gray matter atrophy on the respective PET study results.
Sample Size Estimation for Clinical Trials
Finally, we assessed whether [18F]PI-2620 tau-PET, [18F]GE-180 TSPO-PET, or NfL, all of which have been shown to predict clinical trajectories in Aβ-negative CBS individually, can help identifying patients at risk of clinical progression. This would help in the future with reducing (as compared with solely relying on the clinical diagnosis) sample sizes required for detecting intervention effects in clinical trials. To this end, we performed simulated interventions (i.e., 10/20/30% attenuation of annualized change rates on the clinical composite or PSPRS) using either the whole CBS sample or patients above or below median biomarker abnormalities (i.e., global [18F]PI-2620 tau-PET, global [18F]GE-180 TSPO-PET, NfL). Based on these samples, we determined the minimum sample size required for detecting intervention effects at a significance level of 0.05 and a power of 80%. We found that selecting patients with Aβ-negative CBS with [18F]PI-2620 tau-PET signals above median reduced the number of patients required to detect simulated intervention effects by 50%. Similarly, selecting patients with [18F]GE-180 TSPO-PET signals below-median reduced required sample sizes by 24%. Strongest sample size reductions capacities were found for plasma NfL: Selecting patients with above-median plasma NfL levels reduced sample sizes by 60%. Detailed sample size estimates for different intervention strengths and biomarker selection criteria are summarized in Table 3.
Table 3.
Sample Size Estimation for Detecting Simulated Intervention Effects Using Biomarker-Based Patient Stratification Strategies
| Primary end point: CBS clinical composite score | Primary end point: PSPRS | |||||||
| Intervention effect | Intervention effect | |||||||
| Median annualized change rate | 10% | 20% | 30% | Median annualized change rate | 10% | 20% | 30% | |
| PI-2620 tau-PET | ||||||||
| Pooled (N = 21) | 481 | 110 | 45 | 708 | 196 | 96 | ||
| >Median | −14.77 | 234 | 54 | 22 | 9.44 | 295 | 82 | 41 |
| <Median | −8.28 | 833 | 189 | 77 | 6.68 | 1,279 | 353 | 173 |
| TSPO-PET | ||||||||
| Pooled (N = 16) | 796 | 181 | 74 | 879 | 243 | 119 | ||
| >Median | −6.65 | 971 | 221 | 90 | 4.02 | 1,264 | 348 | 171 |
| <Median | −16.52 | 604 | 138 | 56 | 9.95 | 548 | 152 | 75 |
| NfL | ||||||||
| Pooled (N = 21) | 481 | 110 | 45 | 708 | 196 | 96 | ||
| >Median | −14.06 | 189 | 44 | 18 | 10.0 | 255 | 71 | 35 |
| <Median | −7.38 | 1,049 | 283 | 97 | 3.41 | 1,141 | 315 | 154 |
Abbreviations: CBS = corticobasal syndrome; MoCA = Montreal Cognitive Assessment; PSPRS = Progressive Supranuclear Palsy Rating Scale; SEADL = Schwab and England Activities of Daily Living; TSPO = 18 kDa translocator protein.
Numbers are N required per study arm (i.e., placebo vs verum). CBS clinical composite score is defined by a principal component analysis using MoCA, PSPRS, and SEADL scores.
Discussion
Our major aim was to test the impact of neuroimaging and plasma biomarkers on disease progression in patients with Aβ-negative CBS of the clinical category “with probable underlying 4R tauopathy.” Our head-to-head-comparison included biomarkers concerning neurodegeneration (NfL and structural MRI), pathology (tau-PET), neuroinflammation (TSPO-PET), and astrogliosis (GFAP). To our knowledge, this study is the first to investigate the impact of potential biomarkers on clinical trajectories in patients with Aβ-negative CBS. For the clinical scores PSPRS and the CBS clinical composite score (consisting of PSPRS, SEADL, and MoCA), we observed a highly significant effect of follow-up time in patients with CBS over a ∼2-year follow-up period, suggesting worsening of symptoms over time. In our mixed linear model, the PSPRS rate of change in patients with CBS was 7.8 ± 1.2 points per year. In 1 study assessing longitudinal changes in PSPRS in 9 histopathologically proven cases of 4R-tauopathy with CBS phenotype, the average decline of PSPRS was ∼10 points per year,36 hence slightly above our observed clinical decline. This may be partially due to our study's inclusion criteria requiring our patients to undergo 3 brain scans at baseline, possibly rendering them as less clinically affected.
Assessing the interaction effect between biomarkers and time on clinical trajectories, we showed that more widespread [18F]PI-2620 tau PET signal and higher levels of NfL in plasma at baseline are associated with disease progression in our Aβ-negative CBS cohort. The impact of [18F]PI-2620 tau load on clinical progression was specifically driven by cortical rather than subcortical elevation of [18F]PI-2620 tau-PET signal. By contrast, we found that higher levels of [18F]GE-180-PET tracer uptake were associated with a slower disease course. In our sample, GFAP in plasma and structural MRI were not associated with clinical progression. Finally, we assessed whether the biomarkers with prognostic value (i.e., [18F]PI-2620 tau-PET, [18F]GE-180 TSPO-PET, and plasma-NfL) could help identifying patients at risk of clinical progression to reduce sample sizes required for detecting intervention effects in clinical trials. Strongest sample size reduction capacities were found for plasma NfL, followed by [18F]PI-2620 tau-PET.
Concerning neuroimaging biomarkers, our findings align with studies of AD, a 3-repeat/4R tauopathy and PSP patients with Richardson syndrome phenotype, in which in vivo PET markers of tau burden were associated with the disease course at baseline.34-36 In our Aβ-negative CBS cohort, the effect of [18F]PI-2620 tau-PET on disease progression is driven by cortical rather than subcortical tracer signal. For [18F]PI-2620 tau-PET, the reason for the observed limited value of subcortical tau tracer enhancement concerning clinical progression may be a ceiling effect with high subcortical binding in predilection sites, like the basal ganglia, with high tracer uptake independently from disease duration and disease severity.5
Regarding TSPO-PET, we found that less widespread microglial activity levels were associated with faster disease progression, particularly in the subcortex. Speculatively, microglial activation occurs early during the disease course. In our previous cross-sectional TSPO-PET study in Aβ-negative CBS, we found higher TSPO-PET signal in early stages of disease.8 Microglial activation might, thus, be protective in terms of disease progression, with low TSPO-PET readouts in some patients reflecting a functional burnout of microglia indicative of a faster disease course. In keeping with this, a potential protective effect of microglia activation during early disease course has been shown in patients with AD.37,38 On the contrary, in a study of 17 patients with PSP-RS with a rate of change in PSPRS similar to that in this study (∼6–7 points per year), a PCA-based analysis of the TSPO-PET tracer data as obtained by the first-generation tracer [11C]PK11195 revealed that subcortical neuroinflammation was associated with faster clinical progression.39 The difference between our and the result of the above study may be due to differences in distribution of pathologic brain changes in PSP-RS vs CBS, with a shift toward cortical brain regions in case of CBS,40 and due to differences in disease duration and hence disease dynamics (∼4.7 years vs 2.7 years in our cohort). The latter might affect study results because microglial activation associated with progressive neuronal injury reflecting disease progression may occur in later disease stages.38 Moreover, [11C]PK11195 has a relatively low brain uptake41 and may only be partially comparable with the tracer used in our study.
When assessing regional brain atrophy on structural MRI, no association was found between gray matter volume and clinical progression. This suggests that the degree of brain atrophy does not forecast future clinical trajectories, similar to previous findings regarding 4R-tauopathy PSP with Richardson Syndrome phenotype, showing that tau-PET outperforms MRI regarding the prognostic value.39
Concerning fluid biomarkers, we found that stronger neurodegeneration measured by NfL in plasma is associated with faster clinical worsening. NfL has been associated with survival in a retrospective study with patients with PSP-RS18 and with a prognostic value concerning disease progression in PSP-RS,19,20 but not yet in patients with CBS. The range and variation rate of plasma/CSF NfL concentrations in neurodegenerative diseases are still not understood in detail, and further studies with longitudinal NfL measurements in patients with CBS as well as other phenotypes of 4R tauopathies are warranted. In our Aβ-negative CBS cohort, GFAP in plasma as a potential marker of astrogliosis was not associated with disease progression. CSF/plasma GFAP has been identified as a possible diagnostic and disease course monitoring biomarker in various neurodegenerative diseases14-17 while, so far, a prognostic value has only been shown for clinical outcome in stroke patients.42
Overall, our findings on the relevance of imaging and blood biomarkers on clinical progression in patients with Aβ-negative CBS suggest an important role for tau burden, microglial activation, and neuronal damage on prediction clinical progression in CBS. In future investigations, the question of whether and to what degree clinical progression is paralleled by progression of the above biomarkers needs to be addressed. Particularly, longitudinal imaging and blood analysis would allow to investigate potential changes in the interplay between these biomarkers over time and to study the influence of potential biomarker interplay changes over time on the clinical phenotype course.
We acknowledge several limitations to this study. Our cohort was diagnosed using clinical criteria without neuropathologic verification. To reduce the risk of clinical misdiagnosis, all patients have been seen in a specialized outpatient clinic for movement disorders, diagnosis was reconfirmed at each follow-up visit, and all patients fulfilled diagnostic criteria for a probable 4R tauopathy according to the MDS-PSP criteria.3 A further clinical investigation of the cohort will be conducted to follow-up on natural disease course and mortality and to, eventually, evaluate postmortem histopathology in the context of antemortem biomarker profile and in-depth clinical characterization. This will allow us to validate sensitivity and specificity of the PET, MRI, and blood biomarkers studied against the diagnostic gold standard and to further assess clinical scores for patient stratification for potential therapeutic studies. A further limitation of our study is the relatively small number of patients. This might mask effects such as age dependency of tracer binding and of fluid biomarker levels. Moreover, despite strong correlations between plasma and CSF-derived NfL (r ∼0.743), plasma NfL may be influenced by confounding factors such as renal function or neuropathies in comparison with CSF-derived NfL. However, we decided to use clinically easily accessible plasma measures in our study to maximize the sample size for our main analysis as not all participants underwent CSF sampling. The replication of our findings with larger clinical cohorts will, thus, be important to determine generalizability of our results and to evaluate a potential synergistic effect of tau-PET, TSPO-PET, and plasma-NfL as baseline biomarkers on sample size reductions for clinical trials designs. Moreover, future studies should also include other subtypes of primary tauopathies to compare the biomarkers' potential prognostic role in different phenotypes of suspected 4R-tauopathies and patients with Aβ-positive CBS to evaluate a potential generalizability of our results for CBS in the course of AD.
In conclusion, tau-PET, TSPO-PET, and plasma NfL show effect on future clinical progression in Aβ-negative CBS. Thus, this can be useful for future clinical decision-making and for stratifying patients for clinical trials. Moreover, our power calculation data demonstrate that clinical trials in patients with Aβ-negative CBS are feasible and that these baseline biomarkers have potential to reduce sample sizes when designing interventional trials in CBS.
Glossary
- 4R
4-repeat
- Aβ
β-amyloid
- AD
Alzheimer disease
- BMI
body mass index
- CBD
corticobasal degeneration
- CBS
corticobasal syndrome
- GFAP
glial fibrillary acidic protein
- LMU
Ludwig-Maximilians-Universität
- MBq
megabecquerel
- MDS
Movement Disorders Society
- MNI
Montreal Neurology Institute
- MoCA
Montreal Cognitive Assessment
- NfL
neurofilament light chain
- PD
Parkinson disease
- PSP
progressive supranuclear palsy
- PSP-RS
PSP Richardson syndrome
- PSPRS
PSP Rating Scale
- SEADL
Schwab and England Activities of Daily Living
- SUVR
standardized uptake value ratio
- TSPO
18 kDa translocator protein
- VOI
volume-of-interest
Appendix. Authors
| Name | Location | Contribution |
| Carla Palleis, MD | Department of Neurology, University Hospital, LMU Munich; Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Nicolai Franzmeier, PhD | Munich Cluster for Systems Neurology, SyNergy; Institute for Stroke and Dementia Research, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Endy Weidinger, MD | Department of Neurology, University Hospital, LMU Munich; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Alexander M. Bernhardt, MD | Department of Neurology, University Hospital, LMU Munich; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Sabrina Katzdobler | Department of Neurology, University Hospital, LMU Munich; Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Stephan Wall | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Christian Ferschmann | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Stefanie Harris | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Julia Schmitt | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sebastian Schuster | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Johannes Gnörich, MD | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Anika Finze | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Gloria Biechele, MD | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Simon Lindner, MD | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Nathalie L. Albert, MD | Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Peter Bartenstein, MD | Munich Cluster for Systems Neurology, SyNergy; Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Osama Sabri, MD | Department of Nuclear Medicine, Leipzig University Medical Centre, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Henryk Barthel, MD | Department of Nuclear Medicine, Leipzig University Medical Centre, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Rainer Rupprecht, MD | Department of Psychiatry and Psychotherapy, University of Regensburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Brigitte Nuscher | German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Andrew W. Stephens | Life Molecular Imaging GmbH, Berlin, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Boris-Stephan Rauchmann, MD | German Center for Neurodegenerative Diseases, DZNE-Munich; Institute of Neuroradiology, University Hospital, LMU Munich; Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany; Sheffield Institute for Translational Neuroscience (SITraN), University of Sheffield, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Robert Perneczky, MD, PhD, MBA | Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich; Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany; Sheffield Institute for Translational Neuroscience (SITraN), University of Sheffield; Ageing Epidemiology Research Unit (AGE), School of Public Health, Imperial College, London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Christian Haass, PhD | Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich; Chair of Metabolic Biochemistry, Biomedical Center (BMC), Ludwig-Maximilians-Universität LMU, Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Matthias Brendel, MD | Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich; Department of Nuclear Medicine, University Hospital, LMU Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Johannes Levin, MD | Department of Neurology, University Hospital, LMU Munich; Munich Cluster for Systems Neurology, SyNergy; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Günter U. Höglinger, MD | Department of Neurology, University Hospital, LMU Munich; German Center for Neurodegenerative Diseases, DZNE-Munich, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) to P. Bartenstein, R. Rupprecht and N.L. Albert (project numbers 421887978, 422182557), and to M. Brendel (project numbers BR4580/1-1/RO5194/1-1). The recruitment of the ActiGliA cohort was supported by the presidential fond of the Helmholtz society (to C. Haass). This project was also supported by the German Center for Neurodegenerative Diseases (DZNE, DescribePSP Study), the German Parkinson's Association (DPG, ProPSP Study) and the Hirnliga e.V. (Manfred-Strohscheer-Stiftung). Tau-PET imaging was funded by the Alzheimer Forschung Initiative e.V. (grant number #19063p). C. Palleis, P. Bartenstein, G.U. Höglinger, C. Haass, J. Levin, M. Brendel and R. Perneczky were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy: ID 390857198). G.U. Höglinger and C. Haass were also funded by the NOMIS foundation (FTLD project), Volkswagen Stiftung/Lower Saxony Ministry for Science/Petermax-Müller Foundation (Etiology and Therapy of Synucleinopathies and Tauopathies), European Joint Programme on Rare Diseases (Improve-PSP). The Lüneburg Heritage has supported the work of C. Palleis, S. Katzdobler and J. Levin. The Friedrich-Baur-Stiftung has supported the work of C. Palleis.
Disclosure
C. Palleis is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies” (EP 23 156 122.6) filed by LMU Munich. N. Franzmeier served as a consultant for MSD, has received speaker honoraria from LMI and has funded research collaborations with Avid Radiopharmaceuticals. E. Weidinger reports no disclosures relevant to the manuscript. A. Bernhardt reports no disclosures relevant to the manuscript. S. Katzdobler reports no disclosures relevant to the manuscript. S. Wall reports no disclosures relevant to the manuscript. C. Ferschmann reports no disclosures relevant to the manuscript. S. Harris reports no disclosures relevant to the manuscript. J. Schmitt reports no disclosures relevant to the manuscript. S. Schuster reports no disclosures relevant to the manuscript. J. Gnörich reports no disclosures relevant to the manuscript. A. Finze reports no disclosures relevant to the manuscript. G. Biechele reports no disclosures relevant to the manuscript. S. Lindner reports no disclosures relevant to the manuscript. N. L. Albert reports no disclosures relevant to the manuscript. P. Bartenstein reports no disclosures relevant to the manuscript. O. Sabri received research support from Life Molecular Imaging. H. Barthel received reader honoraria from Life Molecular Imaging and speaker honoraria from Novartis/AAA. R. Rupprecht is on the advisory board for Biogen, and has consulted for GABA Therapeutics. B. Nuscher reports no disclosures relevant to the manuscript. A.W. Stephens is employee of Life Molecular Imaging. B.-S. Rauchmann reports no disclosures relevant to the manuscript. R. Perneczky is on the advisory board for Biogen and has consulted for Eli Lilly, he is a grant recipient from Janssen Pharmaceutica and Boehringer Ingelheim, and has received speaker honoraria from Janssen-Cilag, Pfizer and Biogen. C. Haass collaborates with DENALI therapeutics. M. Brendel received speaker honoraria from GE healthcare and Life Molecular Imaging and is an advisor of Life Molecular Imaging. J. Levin reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA and Roche, consulting fees from Axon Neuroscience and Biogen and author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers. J. Levin is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies” (EP 23 156 122.6) filed by LMU Munich. In addition, he reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. G.U. Höglinger serves as a consultant for Abbvie, Alzprotect, Aprineua, Asceneuron, Bial, Biogen, Biohaven, Kyowa Kirin, Lundbeck, Novartis, Retrotope, Roche, Sanofi, UCB; received honoraria for scientific presentations from Abbvie, Bayer Vital, Bial, Biogen, Bristol Myers Squibb, Kyowa Kirin, Roche, Teva, UCB, Zambon; and holds a patent on Treatment of Synucleinopathies (US 10,918,628 B2; EP 3 525 788). Go to Neurology.org/N for full disclosures.
References
- 1.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503. doi: 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rösler TW, Tayaranian Marvian A, Brendel M, et al. Four-repeat tauopathies. Prog Neurobiol. 2019;180:101644. doi: 10.1016/j.pneurobio.2019.101644 [DOI] [PubMed] [Google Scholar]
- 3.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853-864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzmeier N, Brendel M, Beyer L, et al. Tau spreading is driven by neuronal connectivity in primary tauopathies: evidence from tau-PET and histopathology. medRxiv. 2021. doi: 10.1101/2021.08.16.21261523 [DOI] [Google Scholar]
- 5.Brendel M, Barthel H, van Eimeren T, et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 2020;77(11):1408-1419. doi: 10.1001/jamaneurol.2020.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palleis C, Brendel M, Finze A, et al. Cortical [(18) F]PI-2620 binding differentiates corticobasal syndrome subtypes. Mov Disord. 2021;36(9):2104-2115. doi: 10.1002/mds.28624 [DOI] [PubMed] [Google Scholar]
- 7.Song M, Beyer L, Kaiser L, et al. Binding characteristics of [(18)F]PI-2620 distinguish the clinically predicted tau isoform in different tauopathies by PET. J Cereb Blood Flow Metab. 2021;41(11):2957-2972. doi: 10.1177/0271678X211018904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palleis C, Sauerbeck J, Beyer L, et al. In vivo assessment of neuroinflammation in 4-repeat tauopathies. Mov Disord. 2021;36(4):883-894. doi: 10.1002/mds.28395 [DOI] [PubMed] [Google Scholar]
- 9.Rauchmann BS, Brendel M, Franzmeier N, et al. Microglial activation and connectivity in Alzheimer disease and aging. Ann Neurol. 2022;92(5):768-781. doi: 10.1002/ana.26465 [DOI] [PubMed] [Google Scholar]
- 10.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 11.Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930-937. doi: 10.1212/WNL.0000000000003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 13.Gafson AR, Barthélemy NR, Bomont P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143(7):1975-1998. doi: 10.1093/brain/awaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364-374. doi: 10.1016/j.tins.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord. 2010;16(2):142-145. doi: 10.1016/j.parkreldis.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 16.Schulz I, Kruse N, Gera RG, et al. Systematic assessment of 10 biomarker candidates focusing on α-synuclein-related disorders. Mov Disord. 2021;36(12):2874-2887. doi: 10.1002/mds.28738 [DOI] [PubMed] [Google Scholar]
- 17.Baiardi S, Quadalti C, Mammana A, et al. Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res Ther. 2022;14(1):153. doi: 10.1186/s13195-022-01093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donker Kaat L, Meeter LH, Chiu WZ, et al. Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat Disord. 2018;56:98-101. doi: 10.1016/j.parkreldis.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 19.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3:216-225. doi: 10.1002/acn3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology. 2018;90(4):e273-e281. doi: 10.1212/WNL.0000000000004859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen DR, Gunn RN, Rabiner EA, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52(1):24-32. doi: 10.2967/jnumed.110.079459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130(pt 6):1552-1565. doi: 10.1093/brain/awm032 [DOI] [PubMed] [Google Scholar]
- 23.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 24.Piot I, Schweyer K, Respondek G, et al. The progressive supranuclear palsy clinical deficits scale. Mov Disord. 2020;35(4):650-661. doi: 10.1002/mds.27964 [DOI] [PubMed] [Google Scholar]
- 25.Schwab R, England A. Projection technique for evaluating surgery in Parkinson's disease. In: Gillingham FJ, Donaldson IML, eds. Third Symposium on Parkinson's Disease. E&S Livingstone; 1969:152-157. [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27.Wollenweber FA, Därr S, Müller C, et al. Prevalence of amyloid positron emission tomographic positivity in poststroke mild cognitive impairment. Stroke. 2016;47(10):2645-2648. doi: 10.1161/STROKEAHA.116.013778 [DOI] [PubMed] [Google Scholar]
- 28.Beyer L, Nitschmann A, Barthel H, et al. Early-phase [(18)F]PI-2620 tau-PET imaging as a surrogate marker of neuronal injury. Eur J Nucl Med Mol Imaging. 2020;47(12):2911-2922. doi: 10.1007/s00259-020-04788-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finze A, Biechele G, Rauchmann B-S, et al. Individual regional associations between Aβ-, tau- and neurodegeneration (ATN) with microglial activation in patients with primary and secondary tauopathies. medRxiv. 2022. doi: 10.1101/2022.11.12.22282082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M, Scheifele M, Barthel H, et al. Feasibility of short imaging protocols for [(18)F]PI-2620 tau-PET in progressive supranuclear palsy. Eur J Nucl Med Mol Imaging. 2021;48(12):3872-3885. doi: 10.1007/s00259-021-05391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyoo CH, Ikawa M, Liow JS, et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med. 2015;56(5):701-706. doi: 10.2967/jnumed.114.146027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L, Li H, Zhuo J, et al. The human Brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508-3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolabas ZI, Kuemmerle LB, Perneczky R, et al. Multi-omics and 3D-imaging reveal bone heterogeneity and unique calvaria cells in neuroinflammation. bioRxiv. 2021. doi: 10.1101/2021.12.24.473988 [DOI] [Google Scholar]
- 34.Lang AE, Stebbins GT, Wang P, et al. The Cortical Basal ganglia Functional Scale (CBFS): development and preliminary validation. Parkinsonism Relat Disord. 2020;79:121-126. doi: 10.1016/j.parkreldis.2020.08.021 [DOI] [PubMed] [Google Scholar]
- 35.Street D, Jabbari E, Costantini A, et al. Progression of atypical parkinsonian syndromes: PROSPECT-M-UK study implications for clinical trials. Brain. 2023;146(8):3232-3242. doi: 10.1093/brain/awad105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabbari E, Holland N, Chelban V, et al. Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol. 2020;77(3):377-387. doi: 10.1001/jamaneurol.2019.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ewers M, Biechele G, Suárez-Calvet M, et al. Higher CSF sTREM2 and microglia activation are associated with slower rates of beta-amyloid accumulation. EMBO Mol Med. 2020;12(9):e12308. doi: 10.15252/emmm.202012308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain. 2017;140(3):792-803. doi: 10.1093/brain/aww349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malpetti M, Passamonti L, Jones PS, et al. Neuroinflammation predicts disease progression in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2021;92(7):769-775. doi: 10.1136/jnnp-2020-325549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling H, de Silva R, Massey LA, et al. Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol Appl Neurobiol. 2014;40(2):149-163. doi: 10.1111/nan.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banati RB. Visualising microglial activation in vivo. Glia. 2002;40(2):206-217. doi: 10.1002/glia.10144 [DOI] [PubMed] [Google Scholar]
- 42.Liu G, Geng J. Glial fibrillary acidic protein as a prognostic marker of acute ischemic stroke. Hum Exp Toxicol. 2018;37(10):1048-1053. doi: 10.1177/0960327117751236 [DOI] [PubMed] [Google Scholar]
- 43.Alagaratnam J, von Widekind S, De Francesco D, et al. Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol Open. 2021;3(1):e000143. doi: 10.1136/bmjno-2021-000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Ethics approvals do not allow unrestricted and open-source sharing of patient-specific data with third-parties. Anonymized data that support the findings of this study are available on reasonable request from the corresponding author.