Abstract
Background and Objectives
Narcolepsy type 1 (NT1) is still largely underdiagnosed or diagnosed too late in children. Difficulties in obtaining rapid and reliable diagnostic evaluations of the condition in clinical practice partially explain this problem. Predictors of NT1 include cataplexy and sleep-onset REM periods (SOREMPs), documented during nocturnal polysomnography (N-PSG) or through the multiple sleep latency test (MSLT), although low CSF hypocretin-1 (CSF hcrt-1) is the definitive biological disease marker. Obtaining reliable MSLT results is not always feasible in children; therefore, this study aimed to validate daytime continuous polysomnography (D-PSG) as an alternative diagnostic tool.
Methods
Two hundred consecutive patients aged younger than 18 years (112 with NT1; 25 with other hypersomnias, including narcolepsy type 2 and idiopathic hypersomnia; and 63 with subjective excessive daytime sleepiness) were randomly split into 2 groups: group 1 (n = 133) for the identification of diagnostic markers and group 2 (n = 67) for the validation of the detected markers. The D-PSG data collected included the number of spontaneous naps, total sleep time, and the number of daytime SOREMPs (d-SOREMP). D-PSG data were tested against CSF hcrt-1 deficiency (NT1 diagnosis) as the gold standard using receiver operating characteristic (ROC) curve analysis in group 1. ROC diagnostic performances of single and combined D-PSG parameters were tested in group 1 and validated in group 2.
Results
In group 1, the areas under the ROC curve (AUCs) were 0.91 (95% CI 0.86–0.96) for d-SOREMPs, 0.81 (95% CI 0.74–0.89) for the number of spontaneous naps, and 0.70 (95% CI 0.60–0.79) for total sleep time. A d-SOREMP count ≥1 (sensitivity of 95% and specificity of 72%), coupled with a diurnal total sleep time above 60 minutes (sensitivity of 89% and specificity of 91%), identified NT1 in group 1 with high reliability (area under the ROC curve of 0.93, 95% CI 0.88–0.97). These results were confirmed in the validation group with an AUC of 0.88 (95% CI 0.79–0.97).
Discussion
D-PSG recording is an easily performed, cost-effective, and reliable tool for identifying NT1 in children. Further studies should confirm its validity with home D-PSG monitoring. These alternative procedures could be used to confirm NT1 diagnosis and curtail diagnostic delay.
Introduction
Narcolepsy type 1 (NT1) is a chronic central disorder of hypersomnolence that often arises during childhood. However, unfortunately it is frequently diagnosed many years after the onset of symptoms,1 although diagnostic delay has been reported to be shorter (i.e., a mean of 2 years) in recent case series of children in our center.2,3 The 2 core symptoms of NT1 are excessive daytime sleepiness (EDS) and cataplexy, with the latter being pathognomonic for the disease and characterized by episodes of muscle weakness during wakefulness, typically evoked by positive emotions.4 Confirmatory diagnosis is currently based on objective polysomnographic (PSG) criteria obtained through a widely accepted protocol that includes nocturnal polysomnography (N-PSG), followed by the multiple sleep latency test (MSLT), or on the evidence of reduced or absent hypocretin-1 in the CSF (CSF hcrt-1).4 A positive MSLT typically includes multiple sleep-onset REM periods (SOREMPs) and a reduced mean sleep latency (i.e., <8 minutes).4
Childhood narcolepsy exhibits a peculiar phenotype compared with the adult form. First, EDS in children can manifest differently, with symptoms ranging from prolonged napping to behavioral changes,5 the latter often mimicking psychiatric or attention-deficit hyperactivity disorders.6 Second, cataplexy can seem as a complex movement disorder with spontaneous facial (cataplectic facies) and generalized hypotonia intermixed with hyperactive movements, even in the absence of emotional triggers.2,3 As the child grows, this phenotype evolves into the classical picture of transient hypotonia triggered by emotions.5,7
The high disease burden of NT1 calls for disease awareness campaigns among specialists to improve early referral of patients to specialized centers.8,9 Furthermore, it is necessary to search for alternative diagnostic approaches that could complement the invasive lumbar puncture procedure that must be performed on children under analgesic procedures.10 Because long-term EEG recording is frequently used in the diagnostic work-up at many pediatric neurology centers in the study of epilepsy, we simply added electro-oculogram (EOG) and chin EMG channels to the continuous EEG monitoring and used this recording procedure to identify objective daytime PSG (D-PSG) diagnostic markers of narcolepsy by means of conventional visual sleep scoring.
Methods
The study followed recommendations of the STARD 2015 guidelines for reporting diagnostic accuracy studies.11 Consecutive patients, aged up to 18 years, and referred to the narcolepsy center of the IRCCS Istituto delle Scienze Neurologiche di Bologna between January 2014 and December 2019 for suspected narcolepsy were prospectively studied. The diagnostic work-up included the following procedures: (1) clinical evaluation conducted by neurologists specialized in sleep disorders (G.P. and F.P.) with systematic assessment of symptoms and anthropometric features, including the calculation of body mass index (BMI) Z-score according to our previous work12; (2) subjective sleepiness assessment using an adapted version of the Epworth Sleepiness Scale2; (3) 48-hour continuous PSG (type 2 comprehensive portable polysomnography) under free-running conditions, followed by a fixed 5-nap MSLT13; and (4) blood drawn to test for HLA-DQB1*0602 allele positivity, and, whenever possible, lumbar puncture to measure CSF hcrt-1 levels. The procedures were performed in drug-free conditions (either drug-naive or after at least 3 weeks of drug withdrawal).
As an index test, the D-PSG results were used as single and combined parameters. As previously reported,13,14 our video-PSG assessment included 48 hours of continuous recording under “free-running” conditions with a wireless device. Each patient was hospitalized in a single room, with an additional bed available for the accompanying parent (as per Italian regulations requiring the presence of a parent during all medical procedures). During the recording, patients were allowed to sleep “ad libitum” during both daytime and night-time, according to their individual needs. Lunch and dinner were served at 12:00 and 18:30, and breakfast was provided upon awakening. Patients (and their accompanying parents) were also requested to fill in a sleep diary to indicate the timing of daytime naps, occurrence of symptoms, and lights-off/lights-on time that defined the major nocturnal sleep episode. Nocturnal sleep was analyzed separately from daytime recording by merging the information from the diary with the evidence from video-PSG.
To compare our assessment data with those obtained using ambulatory PSG recording, we analyzed only the data from the continuous PSG recording of the first night and of the following daytime hours (D-PSG), those preceding the second night. The following measures were obtained from N-PSG: nocturnal sleep latency (n-SL), latency to REM sleep (n-REML), total sleep time (n-TST), time in bed (n-TIB), sleep efficiency (SE), percentage of TST spent in non-REM sleep stages 1, 2, and 3 (N1%, N2%, and N3%), and in REM sleep (R%). The following measures were obtained from the D-PSG recordings (i.e., period from morning awakening until 18:30): the number of naps (dn-NAP), daytime TST (d-TST), and number of SOREMPs (d-SOREMPs). In addition, from the MSLT, we collected the mean sleep latency to the first epoch of sleep (MSLT SL) and the number of SOREMPs (MSLT SOREMPs) according to conventional protocol. Sleep stages were assessed daily by a board-certified sleep technician (S.V.) using current scoring criteria. The sleep technician was blinded to clinical features and final diagnoses.13
Patients were ultimately diagnosed according to the current International Classification of Sleep Disorders, third edition,4 as having NT1, narcolepsy type 2 (NT2), idiopathic hypersomnia (IH), or another sleep disorder, based on clinical, N-PSG, MSLT, and biological findings (reference standard). Individuals with a subjective complaint of EDS who had normal N-PSG sleep features, a normal sleep latency on the MSLT, and a total sleep time in the 24 hours lower than 11 hours were labeled “subjective EDS (sEDS).” The final diagnosis was established by 2 sleep experts (G.P., F.P.) who were blinded to index test results.
Statistical Analyses
For descriptive purposes, 3 groups were analyzed based on their final diagnosis: NT1, other central disorders of hypersomnolence (CNS HS), and sEDS. Data in the different patient groups were described as mean and SDs for continuous variables and as absolute numbers (n) and percentages (%) for categorical variables. Clinical, polysomnographic, and biological data were compared between the different groups using the Kruskal-Wallis test, followed by Dunns post hoc test with Bonferroni adjustment, or Mann-Whitney tests for continuous variables and χ2 test for frequencies.
The whole population was randomly divided into 2 groups (2/3 development data set, group 1, n = 133; 1/3 validation data set and group 2, n = 67), and patients were further categorized into 2 diagnostic groups for the purposes of the study (NT1 and non-NT1). Receiver operating characteristic (ROC) curve analysis was used to establish the best D-PSG diagnostic markers in group 1, and the Youden index was applied to extract the best diagnostic cut-off for each parameter. Diagnostic performance of each parameter was explored in group 1 by estimating sensitivity, specificity (with 95% CI), positive likelihood ratio, and negative likelihood ratios. Positive and negative likelihood ratios express the probability that a person testing positive or negative is truly affected or not. The De Long test was used to compare ROC curves, with a significant level shown by a p value of <0.05. Akaike information criterion (AIC) and Bayesian information criterion (BIC), in addition to the information regarding “correctly classification patients (%)” and area under the ROC curve (AUC), were calculated to choose the best predictive logistic model using the D-PSG markers as independent variables (individually and in combination) and the reference standard as a dependent variable. Eventually, a ROC curve analysis combining the different D-PSG markers was applied to group 1 to describe which combination of multiple parameters can improve sensibility and specificity. This analysis was then replicated in group 2 for validation. Statistical analysis was performed using Stata SE 14.2 and SPSS 23.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by our local ethics committee (Comitato Etico Interaziendale Bologna-Imola, CE-BI, protocol number 17009). Written informed consent was signed by patients' parents, and assent was provided by patients.
Data Availability
Anonymized data not published within this article will be made available upon request from any qualified investigator.
Results
Clinical and Neurophysiologic Description in Patient Subgroups
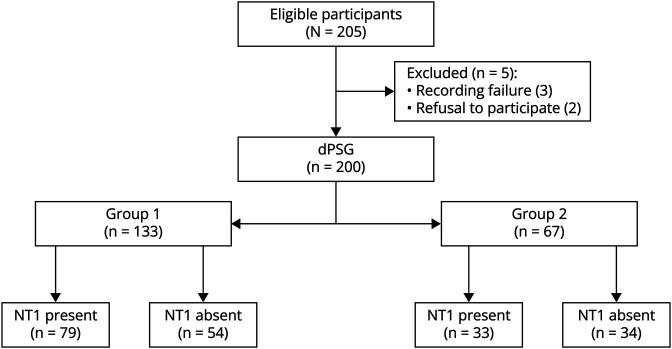
The sample consisted of 200 participants who were prospectively evaluated after excluding 5 subjects for technical reasons (3 because of a failure of the daytime recording procedure) or refusal to participate (n = 2). The final diagnoses were sEDS (n = 63), NT1 (n = 112), and other CNS HS (n = 25; more in detail: 12 IH, and 13 NT2).
The clinical features of the different groups are reported in Table 1. Patient groups differed in gender (fewer males in CNS HS), age (younger in NT1 and sEDS), and age at disease onset (younger in NT1 and sEDS) but had comparable disease duration from the onset of the first symptom to assessment. As expected, core narcolepsy symptoms, including a complaint of disturbed nocturnal sleep, were represented in greater measure in NT1. Patients with NT1 showed higher levels of subjective sleepiness than sEDS subjects, and the highest BMI Z-score. Patients with NT1 most frequently carried the HLA DQB1*06:02 allele, and all had low CSF hcrt-1 levels when tested.
Table 1.
Clinical and Polysomnographic Features in Different Diagnostic Groups
| Variable | sEDS (n = 63) | CNS HS (n = 25) | NT1 (n = 112) | p Value | ||||
| % | n | % | n | % | n | |||
| Sex, male | 68.3 | 43 | 40.0 | 10 | 56.3 | 63 | 0.045a | |
| HLA-DQB1*0602 | 10.0 | 4 | 16.0 | 4 | 94.5 | 104 | <0.001a | |
| 23 missing | 2 missing | |||||||
| Cataplexy | 0 | 0 | 0 | 0 | 98.2 | 108 | <0.001a | |
| 2 missing | ||||||||
| Sleep paralyses | 13.0 | 7 | 16.0 | 4 | 30.0 | 33 | 0.034a | |
| 2 missing | ||||||||
| Hallucinations | 7.4 | 4 | 16.0 | 4 | 46.4 | 51 | <0.001a | |
| 2 missing | ||||||||
| Disturbed nocturnal sleep | 17.0 | 9 | 12.0 | 3 | 47.7 | 51 | <0.001a | |
| 5 missing | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Post hocc | ||
| Age | 11.8 | 4.1 | 14.88 | 2.45 | 11.75 | 3.38 | 0.003b | 1: <0.001 2: 1.00 3: <0.001 |
| CSF hcrt-1 | 346.4 | 51.8 | 336.97 | 40.25 | 30.02 | 53.89 | <0.001b | 1: 1.00 2: <0.001 3: <0.001 |
| Disease onset age | 10.2 | 4.6 | 12.74 | 3.85 | 9.22 | 3.00 | <0.001b | 1: 0.011 2: 0.084 3: <0.001 |
| Disease duration | 2.0 | 3 | 2.14 | 2.5 | 2.5 | 2.9 | 0.610b | |
| aESS | 10.0 | 4.8 | 12.7 | 5.7 | 14.6 | 3.4 | 0.003b | 1: 0.104 2: <0.001 3: 0.111 |
| BMI Z-score | 0.61 | 1.05 | 0.31 | 1.29 | 1.24 | 1.02 | <0.001b | 1: 0.76 2: 0.001 3: 0.001 |
| N-PSG | Mean | SD | Mean | SD | Mean | SD | ||
| n-SL (min) | 17.3 | 16.7 | 10.0 | 15.1 | 5.5 | 7.3 | <0.001 | 1: 0.152 2: <0.001 3: 0.002 |
| n-REML (min) | 84.9 | 35.9 | 73.2 | 32.7 | 26.1 | 48.0 | <0.001 | 1: 0.469 2: <0.001 3: <0.001 |
| n-TST (min) | 479.4 | 64.0 | 434.1 | 123.3 | 471.5 | 75.0 | 0.099 | |
| n-TIB (min) | 520.1 | 68.5 | 467.6 | 125.6 | 528.7 | 76.5 | 0.067 | |
| SE (%) | 92.5 | 4.7 | 91.8 | 12.5 | 89.7 | 7.7 | 0.012 | 1: 0.790 2: 0.044 3: 0.039 |
| N1% (% of TST) | 5.5 | 3.5 | 7.6 | 6.6 | 10.7 | 4.7 | 0.000 | 1: 0.221 2: <0.001 3: <0.001 |
| N2% (% of TST) | 41.0 | 10.6 | 41.1 | 10.8 | 39.5 | 8.3 | 0.116 | |
| N3% (% of TST) | 29.3 | 10.2 | 29.2 | 10.8 | 26.1 | 9.2 | 0.553 | |
| REM% (% of TST) | 23.9 | 4.3 | 22.2 | 6.6 | 23.5 | 5.7 | 0.564 | |
| MSLT | ||||||||
| MSLT SL (min) | 16.5 | 2.5 | 10.6 | 4.2 | 3.6 | 2.9 | <0.001 | 1: 0.005 2: <0.001 3: <0.001 |
| SOREMP (n) | 0.08 | 0.28 | 1.00 | 1.26 | 4.25 | 0.99 | <0.001 | 1: 0.094 2: <0.001 3: <0.001 |
| D-PSG | ||||||||
| dn-Nap (n) | 1.06 | 0.97 | 1.56 | 1.29 | 2.81 | 1.82 | <0.001 | 1: 0.128 2: <0.001 3: 0.001 |
| d-TST (min) | 78.5 | 76.0 | 98.0 | 88.3 | 137.2 | 73.2 | 0.003 | 1: 0.613 2: <0.001 3: 0.017 |
| d-SOREMPs (n) | 0.06 | 0.25 | 0.44 | 0.77 | 2.00 | 1.48 | <0.001 | 1: 0.151 2: <0.001 3: <0.001 |
Abbreviations: CNS HS = central disorders of hypersomnolence; d = daytime; dn-NAP = number of daytime naps; MSLT = multiple sleep latency test; n = nocturnal; N1, N2, and N3 = non-REM sleep stage 1, 2, and 3; NT1 = narcolepsy type 1; REML = REM sleep latency; SE = sleep efficiency; sEDS = subjective excessive daytime sleepiness; SL = sleep latency; SOREMP = sleep-onset REM period; TIB = time in bed; TST = total sleep time.
χ2 test.
Kruskal-Wallis test.
Post hoc: 1 = sEDS vs CNS HS; 2 = sEDS vs NT1; 3 = CNS HS vs NT1.
Nocturnal and daytime sleep features (including MSLT) are reported in Table 1. Patients with NT1 had lower nSL, nREML, and SE, whereas nTST and nTIB did not differ between groups. In addition, patients with NT1 spent more time in N1, with no difference in the distribution of the other sleep stages. As expected according to the diagnostic criteria, the 3 groups differed in MSLT SL (sEDS > CNS HS > NT1) and in MSLT SOREMPs (NT1 > CNS HS > sEDS). Daytime sleep features also showed significant differences between groups. Specifically, patients with NT1 took more naps, slept longer, and displayed more d-SOREMPs than both CNS HS and sEDS.
Search and Validation of Optimal D-PSG Markers for NT1 Diagnosis
Clinical and neurophysiologic data of NT1 and all subjects without NT1 are reported in Table 2. Patients with NT1 presented the same pattern of differences described above, except for gender distribution and age, that were comparable with subjects without NT1.
Table 2.
Clinical and Polysomnographic Data in NT1 and Non-NT1 Subjects
| Variable | Non-NT1 (n = 88) | NT1 (n = 112) | p Value | ||
| Clinical data | % | n | % | n | |
| Male sex | 60.2 | 53 | 56.3 | 63 | 0.572a |
| HLA-DQB1*0602 | 12.3 | 94.5 | <0.001a | ||
| Cataplexy | 0 | 0 | 98.2 | 108 | <0.001a |
| Sleep paralyses | 13.9 | 11 | 30.0 | 33 | 0.010a |
| Hallucinations | 10.1 | 8 | 46.4 | 51 | <0.001a |
| Disturbed nocturnal sleep | 15.4 | 12 | 47.7 | 51 | <0.001a |
| Mean | SD | Mean | SD | ||
| Age | 12.6 | 4.0 | 11.8 | 3.4 | 0.052b |
| CSF hcrt-1 (pg/mL) | 342.6 | 47.2 | 30.0 | 53.9 | <0.001b |
| Disease onset age | 11.0 | 4.5 | 9.2 | 3.0 | <0.001b |
| Disease duration | 2.0 | 2.9 | 2.5 | 2.9 | 0.681b |
| aESS | 10.9 | 5.2 | 14.6 | 3.4 | 0.002b |
| BMI Z-score | 0.5 | 1.1 | 1.2 | 1.0 | <0.001b |
| Neurophysiological data | |||||
| N-PSG | Mean | SD | Mean | SD | |
| n-SL (min) | 15.2 | 16.5 | 5.5 | 7.3 | <0.001b |
| n-REML (min) | 81.6 | 35.2 | 26.1 | 48.0 | <0.001b |
| n-TST (min) | 466.5 | 86.8 | 471.5 | 75.0 | 0.863b |
| n-TIB (min) | 505.2 | 90.9 | 528.7 | 76.5 | 0.349b |
| SE (%) | 92.3 | 7.7 | 89.7 | 7.7 | 0.013b |
| N1% (% of TST) | 6.1 | 4.6 | 10.7 | 4.7 | <0.001b |
| N2% (% of TST) | 41.0 | 10.6 | 39.5 | 8.3 | 0.055b |
| N3% (% of TST) | 29.2 | 10.3 | 26.1 | 9.2 | 0.358b |
| REM% (% of TST) | 23.4 | 5.1 | 23.5 | 5.7 | 0.937b |
| MSLT | |||||
| MSLT SL (min) | 14.8 | 4.1 | 3.6 | 2.9 | <0.001b |
| SOREMP (n) | 0.35 | 0.82 | 4.25 | 0.99 | <0.001b |
| D-PSG | |||||
| dn-NAP (n) | 1.20 | 1.08 | 2.81 | 1.82 | <0.001b |
| d-TST (min) | 84.0 | 79.6 | 137.2 | 73.2 | 0.001b |
| d-SOREMPs (n) | 0.17 | 0.48 | 2.00 | 1.48 | <0.001b |
Abbreviations: aESS = adapted Epworth Sleepiness Scale; BMI = body mass index; CSF hcrt-1 = CSF hypocretin-1; d = daytime; dn-NAP = number of daytime naps; D-PSG = daytime polysomnography; MSLT = multiple sleep latency test; n = nocturnal; N1, N2, N3 = non-REM sleep stage 1, 2, 3; non-NT1 = non-narcolepsy type 1; NT1 = narcolepsy type 1; REML = REM sleep latency; SE = sleep efficiency; SL = sleep latency; SOREMP = sleep-onset REM period; TIB = time in bed; TST = total sleep time.
χ2 test.
Mann-Whitney test.
The 2 randomly generated groups (Figure 1) did not differ in age (12.1 ± 3.8 vs 12.3 ± 3.5, p = 0.7), sex (61% vs 52% of males, p = 0.2), HLADQB*0602 positivity (65.3% vs 61.4%, p = 0.6), or final NT1 diagnosis (59.4% vs 49.3%, p = 0.1).
Figure 1. Flow Diagram of Patient Distribution in 2 Randomly Generated Groups.
NT1 = narcolepsy type 1; D-PSG = daytime polysomnography.
ROC curve analysis in group 1 (Figure 2) showed the following AUCs in percentages: d-SOREMPs (91%, 95% CI 86%–96%), dn-NAP (81%, 95% CI 74%–89%), and d-TST (70% 95% CI 60%–79%). The Youden index identified the following optimal cut-offs to differentiate NT1 from other conditions: at least 1 for d-SOREMP, at least 2 for dn-NAPs, and more than 60 minutes for d-TST.
Figure 2. ROC Curves of D-PSG Data in Group 1.
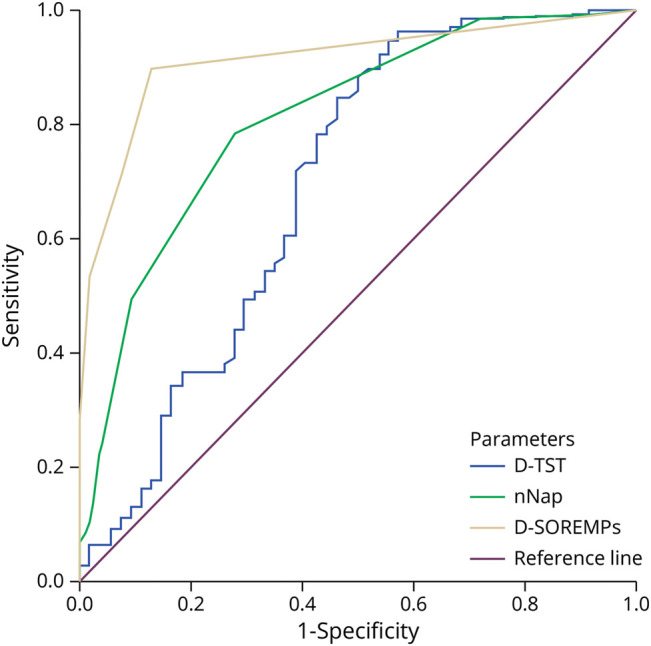
D-PSG = daytime polysomnography; D-TST = total sleep time; D-SOREMP = daytime sleep onset REM period; ROC = receiver operating characteristic.
Table 3 reports the diagnostic performances of the different parameters in group 1. At least 1 d-SOREMP showed the best profile of positive and negative likelihood ratios, followed by at least 2 dn-NAPs, and by more than 60 minutes of d-TST. When further considering the possibility of at least 2 d-SOREMPs, there was a dramatic increase in specificity but decreased sensitivity. We also compared the ROC curves of d-SOREMP ≥1 with current MSLT criteria (i.e., a mean sleep latency of below 8 minutes coupled with at least 2 SOREMP in the test), and with recently proposed criteria for children (having a mean sleep latency of below 8.2 minutes or at least 2 SOREMPs in the test)15; no statistically significant differences were noted (p = 0.08 vs current MSLT criteria, and p = 0.85 vs recently proposed criteria).
Table 3.
Diagnostic Performances of Single D-PSG Parameters
| Single positive variable | NT1 | Non-NT1 | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Positive likelihood ratio | Negative likelihood ratio | ||
| Pos | Neg | Pos | Neg | |||||
| Group 1 (n = 133) | n = 79 | n = 54 | ||||||
| dn-NAP ≥2 | 62 | 17 | 15 | 39 | 78 (68–87) | 72 (58–84) | 2.83 | 0.30 |
| d-TST >60 min | 75 | 4 | 30 | 24 | 95 (88–99) | 44 (31–59) | 1.71 | 0.11 |
| d-SOREMP ≥1 | 71 | 8 | 7 | 47 | 90 (81–96) | 87 (75–95) | 6.93 | 0.12 |
| d-SOREMP ≥2 | 42 | 37 | 1 | 53 | 53 (42–64) | 98 (90–100) | 28.71 | 0.48 |
| Group 2 (n = 67) | n = 33 | n = 34 | ||||||
| dn-NAP ≥2 | 25 | 8 | 12 | 22 | 76 (58–89) | 65 (46–80) | 2.15 | 0.37 |
| d-TST >60 min | 28 | 5 | 19 | 15 | 85 (68–95) | 44 (27–62) | 1.52 | 0.34 |
| d-SOREMP ≥1 | 27 | 6 | 5 | 29 | 82 (65–93) | 85 (69–95) | 5.56 | 0.21 |
| d-SOREMP ≥2 | 33 | 10 | 1 | 33 | 77 (61–88) | 97 (85–100) | 26.09 | 0.24 |
Abbreviations: d = daytime; dn-NAP = number of daytime naps; D-PSG = daytime polysomnography; non-NT1 = non-narcolepsy type 1; NT1 = narcolepsy type 1; SOREMP = sleep-onset REM period; TST = total sleep time.
Table 4 presents the diagnostic performances of D-PSG markers (individually and in combination) and of the reference standard to identify NT1 in logistic regression models. The most efficient model included only the variable “d-SOREMP ≥1” that correctly classified 89% of subjects, with AUC = 88% (95% CI = 83%–92%), AIC = 96.7, and BIC = 102.5. The addition of dn-NAP ≥2 and d-TST >60 minutes, both individually and in combination, showed no significant differences for any of the considered parameters (maximization for “correctly classification” and “AUC”; minimization for AIC and BIC). For comparison we provided, again, in Table 4, information regarding the recently proposed and current MSLT criteria, with the latter resulting as the best predictive parameters because they correctly classified 93% of subjects, with AUC = 93%, AIC = 67.2, and BIC = 72.9.
Table 4.
Diagnostic Performances of Single and Combined D-PSG and MSLT Parameters
| Parameters | Correctly classification, % | AUC, % (95% CI) | AIC | BIC |
| d-TST >60 min | 74 | 70 (56–82) | 152.6 | 158.4 |
| dn-NAP ≥2 | 76 | 75 (61–87) | 148.7 | 154.5 |
| d-SOREMP ≥2 | 71 | 76 (62–88) | 135.4 | 141.2 |
| d-SOREMP ≥1 | 89 | 88 (76–96) | 96.7 | 102.5 |
| d-SOREMP ≥1 + dn-NAP ≥2 | 89 | 90 (81–96) | 96.1 | 104.7 |
| d-SOREMP ≥1 + d-TST >60 min | 89 | 91 (82–96) | 93.7 | 102.4 |
| d-SOREMP ≥1 + d-TST >60 min + dn-NAP ≥2 | 89 | 91 (82–96) | 94.9 | 106.4 |
| MSLT-ICSD | 93 | 93 (86–97) | 67.2 | 72.9 |
| MSLT-Neurology | 91 | 89 (80–95) | 80.0 | 85.8 |
Abbreviations: AIC = Akaike information criterion; AUC = area under the ROC curve; BIC = Bayesian information criterion; d = daytime; dn-NAP = number of daytime naps; D-PSG = daytime polysomnography; ICSD = International Classification of Sleep Disorder; MSLT = multiple sleep latency test; ROC = receiver operating characteristic; SOREMP = sleep-onset REM period; TST = total sleep time.
“Correctly classified patients (%),” AUC with 95% CI (%), AIC and BIC in the predictive logistic models using the D-PSG markers (individually and in combination) for group 1, MSLT-ICSD and MSLT-neurology criteria14 as independent variables, and the reference standard as dependent variable for the whole data set.
To describe which combination of the above-mentioned parameters could increase sensibility and specificity, positivities for these parameters were grouped and ROC curve analysis was performed. The AUC was 93% (95% CI 88%–97%), with sensitivity/specificity profiles of the different combinations as reported in Table 5. The combination (n ≥ 4) of both dn-NAP and d-TST showed a high sensitivity (with lower specificity). Conversely, the presence of at least 1 d-SOREMP combined with d-TST exhibited a good sensitivity/specificity profile. The other combinations (with both d-TST and dn-NAP or with d-SOREMPs ≥2) optimized specificity but provided low sensitivity.
Table 5.
Sensitivity and Specificity (With 95% CIs) of Different Combinations of Positive Parameters in Group 1 and Group 2
| Combination | Group 1 | Group 2 | ||||
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | AUC, % | Sensitivity, % | Specificity, % | AUC, % | |
| ≥0 (d-SOREMPs = 0; dn-NAP = 0; d-TST = 0) | 100 | 0 | 93 (88–97) | 100 | 0 | 88 (79–97) |
| ≥1 (d-SOREMPs = 0; dn-NAP = 1; d-TST = 0) | 97 (89–100) | 37 (25–53) | 94 (82–100) | 38 (22–56) | ||
| ≥2 (d-SOREMPs = 0; dn-NAP = 0; d-TST = 1) | 97 (89–100) | 41 (28–56) | 91 (79–99) | 41 (26–59) | ||
| ≥3 (d-SOREMPs = 1; dn-NAP = 0; d-TST = 0) | 95 (88–99) | 72 (58–84) | 88 (72–98) | 62 (43–77) | ||
| ≥4 (d-SOREMPs = 0; dn-NAP = 1; d-TST = 1) | 94 (87–98) | 76 (62–88) | 88 (72–98) | 65 (46–80) | ||
| ≥6 (d-SOREMPs = 1; dn-NAP = 0; d-TST = 1) | 89 (80–95) | 91 (82–96) | 84 (68–94) | 88 (72–98) | ||
| ≥8 (d-SOREMPs = 1; dn-NAP = 1; d-TST = 1) | 73 (59–85) | 91 (82–96) | 72 (56–87) | 91 (79–99) | ||
| ≥9 (d-SOREMPs = 2; dn-NAP = 1; d-TST = 0) | 53 (42–64) | 98 (90–100) | 69 (50–84) | 97 (85–100) | ||
| ≥11 (d-SOREMPs = 2; dn-NAP = 1; d-TST = 1) | 52 (41–63) | 98 (90–100) | 66 (47–81) | 97 (85–100) | ||
| >11 | 0 | 100 | 0 | 100 | ||
Abbreviations: d = daytime; dn-NAP = number of daytime naps; SOREMP = sleep-onset REM period; TST = total sleep time.
The best cut-offs obtained from sample 1 were further tested for validation in sample 2 (Table 3). The d-SOREMP ≥1 showed a sensitivity of 82% (95% CI 65%–93%) and a specificity of 85% (95% CI 69%–95%). In contrast, the d-SOREMP ≥2 presented a sensitivity of 77% (95% CI 67–88) and a specificity of 97% (95% CI 85%–100%). In the combination analysis, the AUC was 88% (95% CI 79%–97%; Table 5). The combination of parameters that maximized sensitivity (d-SOREMP ≥1 or d-SOREMP = 0, but with d-TST >60 minutes and dn-NAP ≥2) showed a sensitivity of 88% (95% CI 77%–96%) and a specificity of 62% (95% CI 51%–71%). The combination of parameters that maximized the specificity (d-SOREMP ≥1 and at least one of d-TST >60 minutes or dn-NAP ≥2) showed a sensitivity of 84% (95% CI 67%–94%) and a specificity of 88% (95% CI 76%–95%).
Discussion
Our study indicates that D-PSG can be used to identify NT1 in the differential diagnosis of suspected narcolepsy among children and adolescents. We found that the occurrence of at least 1 d-SOREMP, with or without more than 60 minutes of d-TST, is an appropriate threshold with accuracy that is not significantly different from the gold standard MSLT. Although no statistically significant differences were noted in the comparison of this potential diagnostic modality and the current PSG-MSLT standard, our approach offers the benefit of expanding diagnostic prospects with a simplified procedure that is applicable outside the sleep laboratory and that could be added to the current diagnostic standard. In clinical settings with high pretest probability, these parameters confirm the presence of NT1, whereas in clinical settings with low pretest probability, they can rule out NT1. This indicates that the procedure could be valuable for cases of patients who are not willing or able to comply with in-laboratory evaluation. Moreover, considering the increased miniaturization of hardware, at home D-PSG recordings, with or without night-time recordings, are likely to be increasingly adopted instead of the MSLTs because they provide a more naturalistic assessment of daytime sleepiness, the major symptom of hypersomnia.
The onset of NT1 at a young age has a tremendous impact on the well-being, social interactions, and school achievements. It is also associated with severe behavioral disturbances that have detrimental effects on the quality of life.8,16 The overall impact of the disease on academic achievements cannot be explained by lower cognitive abilities because patients with NT1 do not usually show consistent impairment.15 More strikingly, young patients with NT1 often suffer from severe mood disturbances, resulting in behavioral abnormalities such as temper tantrums, emotional instability, or psychiatric complications.8,17 Similarly, data from adult case series strongly suggest that diagnosis at an early age is a key factor in improving disease outcome regarding the overall patient well-being, in social interactions and stable family life.18 Unfortunately, an early occurrence of symptoms is associated with delayed diagnosis.19 Furthermore, the onset of symptoms before the age of 18, or a longer time gap between EDS and cataplexy onset in adults who subsequently received a correct diagnosis, predicts a poor outcome in adults, especially in patients aged older than 35 years at the time of the interview.20 Although disease awareness campaigns are directed at the general population, the use of “red flag” calls for further evaluation by specialists and can improve the situation; this also needs to be accompanied by a corresponding increase in the ability to diagnose.9
Once a clinical suspicion of narcolepsy is raised, objective neurophysiologic and biological evaluations are required according to the current diagnostic criteria.4 There is presently an open scientific debate on the role of different polysomnographic and biological markers to better distinguish different diseases with distinct underlying biological bases.21,22 In adults, the role of the MSLT is well established, and at least 2 SOREMPs (including the one in the N-PSG before the MSLT) and a mean MSLT SL below 8 minutes is a reliable diagnostic marker for NT1.4 Limited data are currently available regarding children and adolescents, especially below 6 years of age, with a single study validating the use of the MSLT in young patients and showing that either a short mean MSLT sleep latency (below 8.2 minutes) or MSLT SOREMPs ≥2 are equally useful to identify NT1 in children, with AUC of 0.98 and of 0.97.15
Only a few studies have evaluated daytime PSG sleep features in adults. We previously found that D-PSG data under “free-running” conditions can identify patients with positive MSLT by counting the number of spontaneous daytime naps and, more importantly, the number of spontaneous d-SOREMPs,13 the latter being as effective as the number of SOREMPs in the MSLT in identifying narcolepsy. Other protocols of continuous PSG recording have been proposed to diagnose IH, with the possibility of 2 invited ad libitum naps,23 or in the condition of continuous bed rest.24 Other innovative approaches applied in adults include actigraphic home monitoring to identify periods of daytime inactivity (i.e., sleep episodes) and night-time hyperactivity (i.e., disturbed nocturnal sleep).25 Disregarding the differences in sleep requirements in different age groups and variations induced by these variable protocols, the current International Classification of Sleep Disorders has now adopted a cut-off of 11/24 hours of sleep time to confirm a diagnosis of idiopathic hypersomnia, a statement that needs replication in children.4
Other potentially useful NT1 markers have been proposed by analyzing N-PSG features in adults. Specifically, the presence of a SOREMP at night was shown in 50% of patients with NT1,26 and in NT1, it most often occurred with a direct transition from wakefulness (W) or N1 to REM sleep.14 Patients with NT1 also present a nocturnal overrepresentation of N1 and W,14 along with significant sleep-wake state instability,14,27 consistent with subjective disturbed nocturnal sleep complaint. A machine learning approach using a quantitative signal analysis of different PSG channels (EEG, EMG, and EOG) allowed the identification of a peculiar state dissociation that is intrinsic to NT1.28 To date, only a few studies have focused on the nocturnal sleep features of pediatric NT1. REM behavior disorder (RBD), an intrinsic feature of NT1,29 also seemed to be frequent in children with NT1, and its presence was correlated with the severity of cataplexy.30 RBD can be the first symptom of the disease during childhood, with cataplexy occurring a few years later.31 Several studies have confirmed the presence of REM sleep without atonia in children with NT1,29 and the visual quantification of REM sleep without atonia was even proposed as a potential disease marker with an AUC of 0.87 in a small cohort of 40 children with various central disorders of hypersomnolence.32 However, this approach can be limited by issues of high interscorer variability. In addition, frequent periodic limb movements during sleep are found in children with NT1, but their features did not allow for the identification of pediatric patients with NT1 vs children and adolescents with restless legs syndrome.33 Recently, disturbed nocturnal sleep (evaluated by the number of transitions to W or N1 per hour of TST) was confirmed as a reliable diagnostic marker for NT1 diagnosis when combined with n-SOREMP occurrence (AUC of 0.91) in a large multicenter study in children.34 The features of nocturnal sleep bouts may contribute to the differential diagnosis, despite the lack of analyses on their diagnostic performances.35 The combination of REM sleep latency and quantitative analysis of muscle tone showed further promising results in identifying children with NT1 (AUC of 0.99 and of 0.94 in test and validation cohorts), partially overcoming the issues related to visual sleep scoring.36 Last, a peculiar profile of motor activity documented by actigraphy was also confirmed in children with NT1 as a possible screening tool.25
In this study, we extended our previous observation concerning the diagnostic utility of D-PSG13 to children; this finding is highly useful for the following reason: (1) it expands our prospects of making the diagnosis with a simplified approach that is possibly useable outside the sleep laboratory; (2) it captures neurophysiologic disease markers (SOREMPs, possibly coupled with time spent asleep during the daytime) in a setting that is more comfortable and realistic for young patients; and (3) it can also be applied to pediatric neurology/epilepsy settings, by extending the use of ambulatory EEG monitoring with a few additional recording channels and being applicable to patients not complying with the in-laboratory procedures. D-PSG has several advantages compared with the traditional N-PSG-MSLT protocol because it does not require a dedicated room and personnel during the daytime but simply requires the application of visual sleep scoring to modified ambulatory EEG monitoring.37 However, despite this approach showing reliable results in the clinical arena of differential diagnosis of NT1 in pediatrics, we suggest its use as a complementary tool alongside the gold standard MSLT or CSF hcrt-1 assessments.
Our study has some limitations. First, we analyzed data from a single center; our methodology needs to be confirmed in larger multicenter studies. Second, despite D-PSG recording in “free-running” conditions being more naturalistic than conventional in-laboratory procedures, the hospital setting is different from everyday life and a comparison with home monitoring is needed before extending the application of our results to the habitual setting of children.
To conclude, we have demonstrated the usefulness of D-PSG for the diagnosis of NT1 in pediatric patients. This approach expands the diagnostic prospects in difficult cases, potentially in the home setting, in pediatric neurology services, and in settings in which a dedicated sleep laboratory is not available. Extending sleep monitoring techniques to in-field assessment will allow for a faster recognition of this challenging and disabling disease, will mean that less time will pass before children with NT1 and adolescents are able to access disease-modifying treatments, and ultimately improve patient-centered interventions. It may also have more naturalistic value in the evaluation of daytime sleepiness in free-running conditions. Moreover, deep learning methods applied to daytime and night-time sleep obtained using 24-hour PSG recordings will lead to a combination of diagnostic parameters that are useful to diagnose NT1 and to increase our knowledge of central disorders of hypersomnolence in children and adolescents.
Glossary
- AIC
Akaike information criterion
- AUC
area under the ROC curve
- BIC
Bayesian information criterion
- BMI
body mass index
- CNS HS
central disorders of hypersomnolence
- CSF hcrt-1
CSF hypocretin-1 level
- D-PSG
daytime polysomnography
- d-SOREMP
daytime SOREMP
- EDS
excessive daytime sleepiness
- EOG
electro-oculogram
- IH
idiopathic hypersomnia
- MSLT
multiple sleep latency test
- n
nocturnal
- N1, N2, N3
non-REM sleep stage 1, 2, 3
- non-NT1
non-narcolepsy type 1
- N-PSG
nocturnal polysomnography
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- RBD
REM behavior disorder
- REML
REM sleep latency
- SE
sleep efficiency
- sEDS
subjective EDS
- SL
sleep latency
- SOREMP
sleep-onset REM period
- ROC
receiver operating characteristic
- SOREMP
sleep-onset REM period
- TIB
time in bed
- TST
total sleep time
Appendix. Authors
| Name | Location | Contribution |
| Fabio Pizza, MD, PhD | Department of Biomedical and Neuromotor Sciences (DIBINEM), University of Bologna; IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Luca Vignatelli, MD | IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy | Drafting/revision of the manuscript for content, including medical writing for content; and analysis or interpretation of data |
| Stefano Vandi, RPSGT | IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy | Major role in the acquisition of data |
| Corrado Zenesini, MSc | IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy | Analysis or interpretation of data |
| Francesco Biscarini, MD | Department of Biomedical and Neuromotor Sciences (DIBINEM), University of Bologna, Italy | Drafting/revision of the manuscript for content, including medical writing for content, and major role in the acquisition of data |
| Christian Franceschini, PsyD, PhD | Department of Medicine and Surgery, University of Parma, Italy | Study concept or design |
| Elena Antelmi, MD, PhD | Neurology Unit, Movement Disorders Division, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Italy | Major role in the acquisition of data |
| Francesca Ingravallo, MD, PhD | Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Italy | Drafting/revision of the manuscript for content, including medical writing for content |
| Emmanuel Mignot, MD, PhD | Tanford University Center for Sleep Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, CA | Major role in the acquisition of data |
| Oliviero Bruni, MD | Department of Developmental and Social Psychology, Sapienza University, Rome, Italy | Major role in the acquisition of data |
| Lino Nobili, MD, PhD | IRCCS Istituto Giannina Gaslini; Dipartimento di Neuroscienze, Riabilitazione, Oftalmologia, Genetica e Scienze Materno-Infantili, DINOGMI, University of Genoa, Genoa, Italy | Major role in the acquisition of data |
| Pierangelo Veggiotti, MD | University of Milan, Italy | Major role in the acquisition of data |
| Raffaele Ferri, MD, PhD | Clinical Neurophysiology Research Unit, Oasi Research Institute-IRCCS, Troina, Italy | Major role in the acquisition of data and study concept or design |
| Giuseppe Plazzi, MD, PhD | IRCCS Istituto delle Scienze Neurologiche di Bologna; Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio-Emilia, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; and study concept or design |
Study Funding
The publication of this article was supported by the “Ricerca Corrente” funding from the Italian Ministry of Health.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Luca G, Haba-Rubio J, Dauvilliers Y, et al. ; European Narcolepsy Network. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22(5):482-495. doi: 10.1111/jsr.12044 [DOI] [PubMed] [Google Scholar]
- 2.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134(pt 12):3477-3489. doi: 10.1093/brain/awr244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponziani V, Gennari M, Pizza F, Balsamo A, Bernardi F, Plazzi G. Growing up with type 1 narcolepsy: its anthropometric and endocrine features. J Clin Sleep Med. 2016;12(12):1649-1657. doi: 10.5664/jcsm.6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders, Third Edition (ICSD-3). American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.Postiglione E, Antelmi E, Pizza F, Lecendreux M, Dauvilliers Y, Plazzi G. The clinical spectrum of childhood narcolepsy. Sleep Med Rev. 2018;38:70-85. doi: 10.1016/j.smrv.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Lecendreux M, Lavault S, Lopez R, et al. Attention-deficit/hyperactivity disorder (ADHD) symptoms in pediatric narcolepsy: a cross-sectional study. Sleep. 2015;38(8):1285-1295. doi: 10.5665/sleep.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136(pt 12):3787-3795. doi: 10.1093/brain/awt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plazzi G, Clawges HM, Owens JA. Clinical characteristics and burden of illness in pediatric patients with narcolepsy. Pediatr Neurol. 2018;85:21-32. doi: 10.1016/j.pediatrneurol.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 9.Vignatelli L, Antelmi E, Ceretelli I, et al. Red Flags for early referral of people with symptoms suggestive of narcolepsy: a report from a national multidisciplinary panel. Neurol Sci. 2019;40(3):447-456. doi: 10.1007/s10072-018-3666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testoni C, Sallemi G, Pizza F, et al. Use and safety of nitrous oxide during lumbar puncture for the diagnosis of childhood narcolepsy. Sleep Med. 2019;59:120-122. doi: 10.1016/j.sleep.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 11.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. doi: 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponziani V, Pizza F, Zenesini C, Vignatelli L, Pession A, Plazzi G. BMI changes in pediatric type 1 narcolepsy under sodium oxybate treatment. Sleep. 2021;44(7):zsaa295. doi: 10.1093/sleep/zsaa295 [DOI] [PubMed] [Google Scholar]
- 13.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22(1):32-40. doi: 10.1111/j.1365-2869.2012.01032.x [DOI] [PubMed] [Google Scholar]
- 14.Pizza F, Vandi S, Iloti M, et al. Nocturnal sleep dynamics identify narcolepsy type 1. Sleep. 2015;38(8):1277-1284. doi: 10.5665/sleep.4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizza F, Barateau L, Jaussent I, et al. Validation of multiple sleep latency test for the diagnosis of pediatric narcolepsy type 1. Neurology. 2019;93(11):e1034-e1044. doi: 10.1212/WNL.0000000000008094 [DOI] [PubMed] [Google Scholar]
- 16.Blackwell JE, Alammar HA, Weighall AR, Kellar I, Nash HM. A systematic review of cognitive function and psychosocial well-being in school-age children with narcolepsy. Sleep Med Rev. 2017;34:82-93. doi: 10.1016/j.smrv.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Ludwig B, Smith S, Heussler H. Associations between neuropsychological, neurobehavioral and emotional functioning and either narcolepsy or idiopathic hypersomnia in children and adolescents. J Clin Sleep Med. 2018;14(4):661-674. doi: 10.5664/jcsm.7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingravallo F, Gnucci V, Pizza F, et al. The burden of narcolepsy with cataplexy: how disease history and clinical features influence socio-economic outcomes. Sleep Med. 2012;13(10):1293-1300. doi: 10.1016/j.sleep.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 19.Levy S, McArthur I, Crow B, Zuberi S. Factors influencing time to diagnosis in childhood narcolepsy type 1. J Child Neurol. 2019;34(8):440-445. doi: 10.1177/0883073819836548 [DOI] [PubMed] [Google Scholar]
- 20.Maski K, Steinhart E, Williams D, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13(3):419-425. doi: 10.5664/jcsm.6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fronczek R, Arnulf I, Baumann CR, Maski K, Pizza F, Trotti LM. To split or to lump? Classifying the central disorders of hypersomnolence. Sleep. 2020;43(8):zsaa044. doi: 10.1093/sleep/zsaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammers GJ, Bassetti CLA, Dolenc-Groselj L, et al. Diagnosis of central disorders of hypersomnolence: a reappraisal by European experts. Sleep Med Rev. 2020;52:101306. doi: 10.1016/j.smrv.2020.101306 [DOI] [PubMed] [Google Scholar]
- 23.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32(6):753-759. doi: 10.1093/sleep/32.6.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evangelista E, Lopez R, Barateau L, et al. Alternative diagnostic criteria for idiopathic hypersomnia: a 32-hour protocol. Ann Neurol. 2018;83(2):235-247. doi: 10.1002/ana.25141 [DOI] [PubMed] [Google Scholar]
- 25.Filardi M, Pizza F, Bruni O, Natale V, Plazzi G. Circadian rest-activity rhythm in pediatric type 1 narcolepsy. Sleep. 2016;39(6):1241-1247. doi: 10.5665/sleep.5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70(7):891-902. doi: 10.1001/jamaneurol.2013.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen GL, Knudsen S, Jennum P. Sleep transitions in hypocretin-deficient narcolepsy. Sleep. 2013;36(8):1173-1177. doi: 10.5665/sleep.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephansen JB, Olesen AN, Olsen M, et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat Commun. 2018;9(1):5229. doi: 10.1038/s41467-018-07229-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antelmi E, Pizza F, Franceschini C, Ferri R, Plazzi G. REM sleep behavior disorder in narcolepsy: a secondary form or an intrinsic feature? Sleep Med Rev. 2020;50:101254. doi: 10.1016/j.smrv.2019.101254 [DOI] [PubMed] [Google Scholar]
- 30.Antelmi E, Pizza F, Vandi S, et al. The spectrum of REM sleep-related episodes in children with type 1 narcolepsy. Brain. 2017;140(6):1669-1679. doi: 10.1093/brain/awx096 [DOI] [PubMed] [Google Scholar]
- 31.Nevsimalova S, Prihodova I, Kemlink D, Lin L, Mignot E. REM behavior disorder (RBD) can be one of the first symptoms of childhood narcolepsy. Sleep Med. 2007;8(7-8):784-786. doi: 10.1016/j.sleep.2006.11.018 [DOI] [PubMed] [Google Scholar]
- 32.Bin-Hasan S, Videnovic A, Maski K. Nocturnal REM sleep without atonia is a diagnostic biomarker of pediatric narcolepsy. J Clin Sleep Med. 2018;14(2):245-252. doi: 10.5664/jcsm.6944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferri R, DelRosso LM, Aricò D, et al. Leg movement activity during sleep in school-age children and adolescents: a detailed study in normal controls and participants with restless legs syndrome and narcolepsy type 1. Sleep. 2018;41(4):zsy010. doi: 10.1093/sleep/zsy010 [DOI] [PubMed] [Google Scholar]
- 34.Maski K, Pizza F, Liu S, et al. Defining disrupted nighttime sleep and assessing its diagnostic utility for pediatric narcolepsy type 1. Sleep. 2020;43(10):zsaa066. doi: 10.1093/sleep/zsaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maski KP, Colclasure A, Little E, et al. Stability of nocturnal wake and sleep stages defines central nervous system disorders of hypersomnolence. Sleep. 2021;44(7):zsab021. doi: 10.1093/sleep/zsab021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvani A, Vandi S, Pizza F, Antelmi E, Ferri R, Plazzi G. Combining information on nocturnal rapid eye movement sleep latency and atonia to facilitate diagnosis of pediatric narcolepsy type 1. Sleep. 2021;44(3):zsaa203. doi: 10.1093/sleep/zsaa203 [DOI] [PubMed] [Google Scholar]
- 37.Seneviratne U, D'Souza WJ. Ambulatory EEG. Handb Clin Neurol. 2019;160:161-170. doi: 10.1016/B978-0-444-64032-1.00010-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available upon request from any qualified investigator.