Abstract
Background
Anastomotic strictures occur in up to 38% of patients after ileal pouch–anal anastomosis (IPAA). We sought to compare the safety, effectiveness, and durability of mechanical dilation using a Hegar dilator to endoscopic through-the-scope balloon dilation (EBD) among IPAA patients with a rectal or ileoanal anastomotic stricture.
Methods
We identified adult patients with an IPAA for ulcerative colitis (UC) who underwent a pouchoscopy between January 1, 2015, and December 31, 2019, at a single institution. We compared the effectiveness (median maximum diameter of dilation [MMD]), safety, and durability of mechanical and balloon dilation using standard statistical comparisons.
Results
A total 74 patients had a stricture at the ileoanal anastomosis and underwent at least 1 mechanical or balloon dilation. The MMD with mechanical dilation was 19 (interquartile range [IQR], 18-20) mm for the first dilation and 20 (IQR, 18-20) mm for the second and third dilations. With balloon dilation, the MMD was 12 (IQR, 12-18) mm for the first dilation, 15 (IQR, 12-16.5) mm for the second dilation, and 18 (IQR, 15-18.5) mm for the third dilation. Patients undergoing mechanical dilation experienced a longer duration to second dilation (median 191 days vs 53 days: P < .001), with no difference in complications such as bleeding or perforation noted.
Conclusions
Among patients with ileoanal and rectal strictures, mechanical and balloon approaches to dilation demonstrated similar safety profiles and effectiveness. Mechanical dilation with Hegar dilators appears to be an effective and safe approach to the treatment of distal strictures after IPAA.
Keywords: J-pouch, stenosis, Hegar dilator, proctocolectomy
What is already known
Distal strictures at the ileoanal anastomosis are common in patients after ileal pouch–anal anastomosis and can be managed using a variety of techniques including endoscopic through-the-scope balloon dilation and mechanical dilation using Hegar dilators.
What is new here
In this study, we demonstrated comparable safety using Hegar dilators in comparison with balloon dilation. Additionally, patients treated with Hegar dilation achieved a greater median maximum diameter of dilation and a longer time to second dilation compared with those patients treated with balloon dilation.
How can this study help patient care
This study demonstrates the safety and effectiveness of Hegar dilation and could promote the increased use of this dilation modality in patients with distal strictures after ileal pouch–anal anastomosis.
Introduction
Restorative proctocolectomy with ileal pouch–anal anastomosis (IPAA) is the preferred surgical procedure in the treatment of patients with ulcerative colitis (UC) who fail to respond to medical therapy, or for those patients who develop UC-related dysplasia.1 The use of an IPAA is not limited to UC, and can also be a feasible surgical intervention for patients with inflammatory bowel disease (IBD) unclassified or Crohn’s disease (CD) in select cases.2 Restorative proctocolectomy with IPAA has been shown to result in significant improvement of quality of life for a majority of patients with IBD, across all histopathological categories.2,3 However, multiple pouch-related complications can occur after IPAA, including chronic inflammation in the setting of chronic pouchitis (17% of patients)2 and Crohn’s-like disease of the pouch (10% of patients).4 Among patients with chronic inflammatory conditions of the pouch, complications such as strictures are observed frequently and can lead to a significant burden of disease.
While the prevalence of ileoanal strictures after IPAA is not well established, the management of strictures, when they are observed, has evolved to include a variety of manual, endoscopic, and in some cases surgical approaches. Symptomatic stricture at the ileoanal anastomosis can be treated with mechanical dilation with either a bougie (Hegar) dilator or digital dilation depending on the severity of the stenosis, or endoscopic through-the-scope balloon dilator. Surgical stricturoplasty has also been proposed and used in limited cases.5 All of these approaches have been shown to be effective for relieving symptoms and restoring patency of the IPAA; however, concerns have been raised regarding the perforation risk associated with endoscopic through-the-scope balloon dilation (EBD) in this setting.6 Additionally, although nonfibrotic strictures may be easily treated with 1 dilation, up to 45% of fibrotic strictures require repeat dilation.7
Despite the relatively widespread use of dilation techniques for treating symptomatic strictures in patients with an IPAA, comprehensive analyses of the safety and durability of stricture dilation with these various dilation technique are lacking. Particularly, there is a paucity of data for use of Hegar dilators in IPAA patients with ileoanal anastomotic or rectal strictures. Furthermore, patient and procedural factors that may influence outcomes in this population remain unknown. With these gaps in mind, we performed a retrospective study to compare the safety, effectiveness, and durability of mechanical dilation using a Hegar dilator and EBD among patients with a rectal or ileoanal stricture after IPAA.
Methods
Data source and patient population
To identify eligible patients, we used the PROVATION software Version 5.0.520.18 for endoscopy documentation (PROVATION Medical). All adult patients (≥18 years of age at the time of procedure) who underwent a pouchoscopy at UNC Health between January 1, 2015, and December 31, 2019, were eligible. Once identified, chart review was performed to confirm the following additional eligibility criteria: the patient underwent a pouchoscopy with a dilation for an ileoanal or rectal stenosis and the patient had a preoperative diagnosis of UC. Patients with a preoperative diagnosis of CD or familial adenomatous polyposis or who previously underwent IPAA revision were excluded. All patients underwent either mechanical dilation (use of Hegar dilators) or EBD. To standardize the assessment among all patients undergoing dilation during the study period, a maximum of 3 pouchoscopies after January 1, 2015, was recorded for each patient. All pouchoscopies and dilations (Hegar and balloon dilations) in this study were performed by gastroenterologists.
The study protocol was approved by the Institutional Review Board at the University of North Carolina, Chapel Hill.
Outcomes
The effectiveness of mechanical and balloon dilation for ileoanal/rectal strictures was analyzed based on the median maximum diameter of dilation (MMD) and change in diameter of stricture after dilation (with accompanying interquartile range [IQR]). As a secondary analysis, we also analyzed the durability of dilations and the time between the first and subsequent dilations (where applicable). The safety of both approaches was analyzed based on event rates for the following complications or events: perforation, bleeding requiring admission or repeat procedure, emergent hospitalization, emergent surgery after procedure, and nonemergent surgery after procedure.
Covariates
Using a standard diagnostic algorithm of the UNC Multidisciplinary Pouch Clinic, we reviewed the medical record to determine diagnoses of acute pouchitis, chronic pouchitis, and Crohn’s-like disease of the pouch.1,4 Clinical variables that may increase the risk of development of pouchitis, including primary sclerosing cholangitis,8-10 smoking,11,12 the use of nonsteroidal anti-inflammatory drugs,8,11 colectomy indication,13 stages involved in IPAA surgery,14 extraintestinal manifestations of IBD,1,15 and therapy prior to colectomy,16 were documented. The use of IBD-specific medications was evaluated preoperatively (3 months prior to colectomy) and post-IPAA, including the duration of the study period. Disease activity at the time of pouchoscopy was assessed using the endoscopic subscore of the Pouchitis Disease Activity Index.17
Statistical analysis
Categorical variables are presented with raw values and corresponding percentages and were analyzed using Fisher exact and chi-square testing as appropriate. Continuous variables are summarized using median and IQR and were compared using Wilcoxon rank sum testing. Kaplan-Meier testing was used in all time-to-event analyses. For all analyses, 2-sided P values of .05 or less were considered statistically significant. All analyses were performed using SAS statistical software (version 9.4; SAS Institute); Figure 1 was generated using R software version 4.2.0 (R Foundation for Statistical Computing)18 with ggplot2 package version 3.4.0.19
Figure 1.
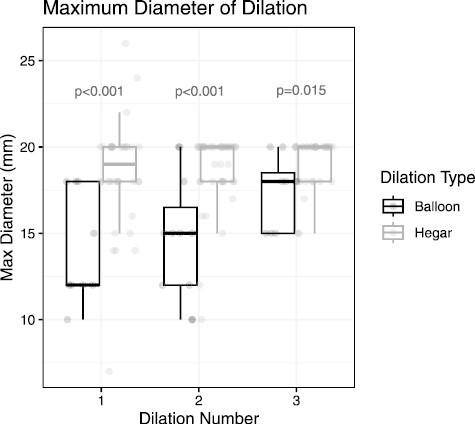
Comparison of median maximum diameter of dilation between patients undergoing Hegar dilation and balloon dilation for distal pouch strictures.
Results
Patient characteristics
Among 477 eligible patients, a total of 74 (16%) patients had an ileoanal or rectal stenosis that required dilation and thus were included in this retrospective study. Among these individuals, 27 patients underwent digital dilation alone at the time of pouchoscopy and were not further analyzed, leaving 38 patients who underwent Hegar dilation and 9 patients who underwent EBD.
We assessed basic demographic factors, including sex, race, and age at pouchoscopy. There were no significant differences between patients who underwent Hegar dilation compared with EBD. There were also no significant differences in key disease and surgery-specific factors, including disease extent at the time of colectomy, family history of IBD, history of primary sclerosing cholangitis, or stage of surgery (Table 1). Pouch-related factors including IPAA diagnosis, presence of inflammation at the site of stricture, and use of medications prior to dilation, including anti-tumor necrosis factor alpha and systemic steroids, were not different between groups.
Table 1.
Comparison of demographics and clinical characteristics of patients undergoing mechanical dilation or balloon dilation for rectal or ileoanal stricture after ileal pouch–anal anastomosis.
| Variable | Patients undergoing mechanical dilation (n = 38) | Patients undergoing balloon dilation (n = 9) | P value |
|---|---|---|---|
| Male | 16 (42) | 1 (11) | .127 |
| Race | |||
| White | 32 (82) | 6 (75) | .638 |
| Non-White | 7 (18) | 2 (25) | |
| Age at Pouchoscopy (median, IQR) | 44 (37-54) | 38 (31-51) | .368 |
| Disease extent at time of colectomya | 1.000 | ||
| Proctitis | 0 (0) | 0 (0) | |
| Left-sided | 3 (10) | 0 (0) | |
| Extensive | 26 (90) | 5 (100) | |
| Unknown | 9 (N/A) | 4 (N/A) | |
| Family history of IBD | 7 (18) | 1 (11) | .600 |
| Primary sclerosing cholangitis | 1 (3) | 0 (0) | 1.000 |
| Indication for surgery | |||
| Medically refractory | .175 | ||
| Dysplasia/cancer | 28 (78) | 6 (67) | |
| Other | 3 (8) | 0 (0) | |
| Multiple | 4 (11) | 1 (11) | |
| indications | 1 (3) | 2 (22) | |
| Stage of surgery | .944 | ||
| I | 2 (6) | 1 (11) | |
| II | 11 (35) | 3 (33) | |
| III | 9 (29) | 2 (22) | |
| Modified II | 9 (29) | 2 (22) | |
| IPAA diagnosis | .847 | ||
| Normal | 5 (13) | 2 (22) | |
| Acute pouchitis | 2 (5) | 0 (0) | |
| Chronic antibiotic-dependent pouchitis | 17 (45) | 3 (33) | |
| Chronic antibiotic-refractory pouchitis | 5 (13) | 1 (11) | |
| Crohn’s-like disease of the pouch | 9 (24) | 3 (33) | |
| Inflammation present at stricture | 28 (43) | 5 (56) | .480 |
| Anti-TNF predilation | 4 (11) | 2 (22) | .322 |
| Systemic steroids predilation | 16 (42) | 3 (33) | .720 |
Values are n (%) or median (interquartile range).
Abbreviations: IBD, inflammatory bowel disease; IPAA, ileal pouch–anal anastomosis; IQR, interquartile range; N/A, not applicable; TNF, tumor necrosis factor alpha.
aFisher exact test calculated on patients with known disease extent at the time of colectomy.
Efficacy
For those that underwent Hegar dilation, 17 (45%) patients had only 1 dilation, while 4 (11%) patients and 17 (45%) patients required 2 and 3 total dilations within the study period, respectively. In comparison, all patients in the EBD group required more than 1 dilation, with 4 (44%) patients requiring a second and 5 (56%) requiring a third. The MMD of dilation with mechanical dilation with Hegar dilators was 19 (IQR, 18-20) mm for the first dilation and 20 (IQR, 18-20) mm for the second and third dilations. With balloon dilation, the MMD was 12 (IQR, 12-18) mm for the first dilation, 15 (IQR, 12-16.5) mm for the second dilation, and 18 (IQR, 15-18.5) mm for the third dilation; these differences between groups were significant between groups across number of dilations (P < .001 for the first and second dilations, P = .015 for third dilation) (Figure 1).
Durability
Time-to-event analysis using Kaplan-Meier testing was performed for both time to second or third dilation. Time to second dilation of the ileoanal/rectal stricture was significantly longer in the Hegar dilation group (median 191 days) as compared with the EBD group (median 53 days) (P < .001) (Figure 2). There was no significant difference between time to third dilation between groups (median 126 days vs 98 days; P = .117) (Figure 3).
Figure 2.
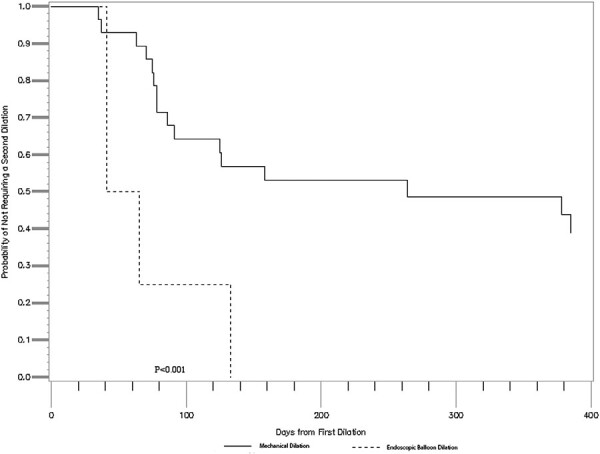
Comparison of time to a second dilation, mechanical vs balloon dilation (median 191 days [mechanical] vs 53 days [endoscopic through-the-scope balloon dilation]; P < .001).
Figure 3.
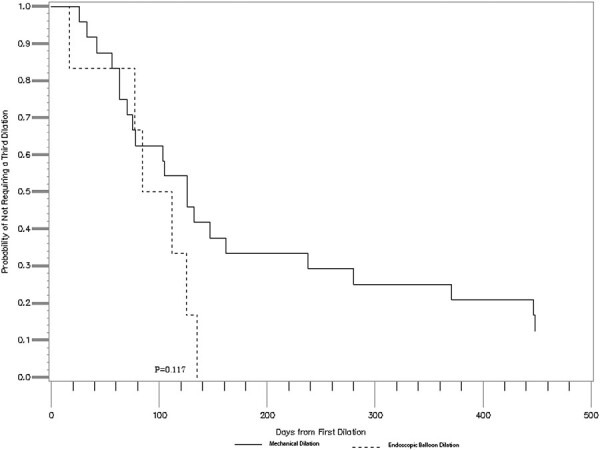
Comparison of time to a third dilation, mechanical vs balloon dilation (median 126 days [mechanical] vs 98 days [endoscopic through-the-scope balloon dilation]; P = .117).
Safety
Overall, there were no significant safety or adverse events observed in both groups evaluated. One patient experienced bleeding after Hegar dilation and 1 experienced bleeding after EBD, with no significant difference in the 2 approaches (P = .709). Both bleeding events occurred on the second dilation. Neither event required hospitalization. There were no perforations, emergent hospitalizations, or emergent or nonemergent surgeries during the study period.
Discussion
In this retrospective study, we observed that mechanical dilation with Hegar dilators is an effective and safe approach for treatment of ileoanal/rectal strictures as compared with EBD. Both mechanical dilation and EBD approaches provided relief of strictures, with Hegar dilation showing increased median MMD and an extended time to second dilation. When examining safety, there were no perforations, hospitalizations, or emergent surgical repairs observed in either balloon or mechanical dilation in our population throughout the study period, indicating that both approaches may be safe methods of treating rectal or ileoanal anastomotic strictures.
Strictures among patients with IPAA are common; however, there is no one standard approach to treat these strictures. While medical management has been attempted in the past,20 medical therapy alone is not sufficient to treat strictures once identified. This applies to strictures that are fibrotic or nonfibrotic in nature, though nonfibrotic strictures may have a better response to use of medical therapy alongside procedural intervention.21
The primary goal in managing pouch strictures is to avoid unwanted outcomes including permanent diversion, pouch failure, and pouch excision. Historically, balloon dilation has been the more widely used treatment of choice. Two single-center studies and 1 retrospective case series demonstrated that the use of EBD for treatment of pouch strictures, which included pouch inlet strictures,22-24 afferent limb strictures,23,24 and ileoanal anastomotic strictures,22-24 was safe and effective at maintaining pouch patency. EBD also resulted in significant improvement in quality of life for patients who underwent this procedure.22 However, no studies have directly compared the real-world effectiveness or safety of EBD to mechanical dilation with Hegar dilators in rectal strictures or ileoanal anastomtic strictures. In this study, we demonstrated that Hegar dilation could be performed safely by gastroenterologists, with a larger median MMD and longer time to second dilation being noted. Given that a digital rectal examination alone often provides some degree of mechanical dilation, this study should provide gastroenterologists with the enhanced confidence to safely pursue Hegar dilation at larger diameters than were typically pursued by initial attempts at EBD.
Besides Hegar dilation and EBD, alternative repair methods including surgical stricuroplasty or endoscopic stricturotomy have had limited or no application for distal strictures. Surgical stricturoplasty has been previously investigated in small subset of cases of distal strictures.5 Endoscopic stricturotomy was originally described in pouch inlet and afferent limb strictures6; more recently it has been suggested as a first-line approach in the setting of distal bowel or pouch-anal anastomotic strictures when an experienced endoscopist is available.25,26 Surgical stricturoplasty was initially described in detail in management of small bowel strictures in CD.27,28 In patients who have undergone IPAA, it was hypothesized this technique would be best applied in patients that have failed EBD, or who have strictures that are long or multiple and angulated. With these selection criteria, surgical stricturoplasty can result in a longer time interval between procedure and stricture recurrence or pouch failure.5 However, the results of this small subset have not resulted in larger uptake, likely due to the potential risk of damaging the sphincter complex with this technique. In addition to these approaches, novel techniques, including bougie cap dilators allowing for direct visualization during dilation29 have also been utilized. The continued emergence of alternative treatment presents an ongoing need for re-evaluation of the best approaches to management of these patients.
A remaining important factor to consider is the significant difference in cost between the 2 approaches directly investigated in this study, Hegar dilation and EBD. Hegar dilation involves the use of an apparatus that involves a small one-time cost for the instrument (around $30), that can be used and sterilized for reuse for many subsequent cases. Essentially, the cost for this additional step of the procedure becomes negligible over time. Alternatively, endoscopic balloon dilators are single use; cost estimates start around $189 to $262, and likely have increased.30 Similarly, recently the use of reusable Eder-Puestow metal olive dilators for the dilation of postoperative benign rectal strictures has been shown to be more cost-effective compared with EBD.31 Taken together, per procedure Hegar dilation is more cost effective than EBD.
Although our study offers a comparison of mechanical and EBD approaches to dilation of distal pouch strictures, there are existing limitations. Like many prior studies investigating approaches for management of pouch strictures, this study is limited to a single, tertiary care center.6,22,23,28 Future work may benefit from expanding these data to multiple clinical sites, including a variety of endoscopists with a range of experience, to improve the generalizability. Comparison of clinical and pouch features between those that require single as compared repeat dilation in a larger cohort may also provide insight into the durability in specific populations. Due to the limitations of a retrospective review of endoscopy reports, we were not able to reliably assess any differences in outcome based on a stricture features, such as fibrotic as compared with inflammatory types. Also such a comparison requires a larger sample size but may be informative when assessing any variation in durability of response. We were also unable to reliably capture the length or inner diameter of the strictures prior to dilation, as these features of distal strictures are not routinely recorded in our practice. Because endoscopists are largely responsible for making this selection independently in our current framework, quality-of-life measures would be particularly informative in a larger study in order to understand how patient preference may impact procedure choice, as well as how patient preference may impact the timing between attempts at dilation. Finally, a minority of patients at our center do home self-dilations; however, this is not well documented in the medical record. As such, we could not evaluate the impact of these approaches on durability between pouchoscopy and subsequent dilations in the setting of pouchoscopy.
Conclusions
Overall, the data from our study have important clinical implications. We have shown mechanical dilation may be offered as an equally safe and potentially more effective method as compared with through-the-scope EBD for treating distal strictures that occur after IPAA. We also observed that mechanical dilation may improve the durability of dilations, at least between the first and second dilations. Importantly, Hegar dilation is likely a more cost-effective approach as compared with EBD. Considered together, the use of mechanical dilation, including Hegar dilators, should be considered in the treatment of ileoanal or rectal strictures in patients who have undergone IPAA for a history of UC, especially for those patients who require repeat dilations.
Contributor Information
Kimberly Darlington, Division of Gastroenterology and Hepatology, University of North Carolina, Chapel Hill, NC, USA.
Annmarie Wang, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Hans H Herfarth, Division of Gastroenterology and Hepatology, University of North Carolina, Chapel Hill, NC, USA; Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC, USA; Multidisciplinary Center for Inflammatory Bowel Diseases, University of North Carolina, Chapel Hill, NC, USA.
Edward L Barnes, Division of Gastroenterology and Hepatology, University of North Carolina, Chapel Hill, NC, USA; Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC, USA; Multidisciplinary Center for Inflammatory Bowel Diseases, University of North Carolina, Chapel Hill, NC, USA.
Funding
This research was supported by a grant from the National Institutes of Health (K23DK127157-01 [to E.L.B.]).
Conflict of Interest
E.L.B. has served as a consultant for AbbVie, Bristol-Meyers Squibb, Lilly, and Target RWE. H.H.H. has served as a consultant for Alivio, AMAG, BMS, ExeGI, Finch, Fresenius Kabi, Gilead, Janssen, Lycera, Merck, Otsuka, Pfizer, PureTech, and Ventyx; and has received research support from Allakos, Artizan Biosciences, Novo Nordisk, and Pfizer. K.D. and A.W. have no relevant disclosures.
Data Availability
Raw data may be available for further analysis. Please direct any questions regarding data availability and analytic methods to edward_barnes@med.unc.edu.
REFERENCES
- 1. Barnes EL, Lightner AL, Regueiro M.. Perioperative and postoperative management of patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(6):1356-1366. [DOI] [PubMed] [Google Scholar]
- 2. Fazio VW, Kiran RP, Remzi FH, et al. . Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257(4):679-685. [DOI] [PubMed] [Google Scholar]
- 3. Marcello PW, Roberts PL, Schoetz DJ Jr, Coller JA, Murray JJ, Veidenheimer MC.. Long-term results of the ileoanal pouch procedure. Arch Surg. 1993;128(5):500-3; discussion 503. discussion 5034. [DOI] [PubMed] [Google Scholar]
- 4. Barnes EL, Kochar B, Jessup HR, Herfarth HH.. The incidence and definition of Crohn’s disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis. 2019;25(9):1474-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu XR, Mukewar S, Kiran RP, Remzi FH, Shen B.. Surgical stricturoplasty in the treatment of ileal pouch strictures. J Gastrointest Surg. 2013;17(8):1452-1461. [DOI] [PubMed] [Google Scholar]
- 6. Lan N, Wu JJ, Wu XR, Hull TL, Shen B.. Endoscopic treatment of pouch inlet and afferent limb strictures: stricturotomy vs. balloon dilation. Surg Endosc. 2021;35(4):1722-1733. [DOI] [PubMed] [Google Scholar]
- 7. Lewis WG, Kuzu A, Sagar PM, Holdsworth PJ, Johnston D.. Stricture at the pouch-anal anastomosis after restorative proctocolectomy. Dis Colon Rectum. 1994;37(2):120-125. [DOI] [PubMed] [Google Scholar]
- 8. Lepisto A, Karkkainen P, Jarvinen HJ.. Prevalence of primary sclerosing cholangitis in ulcerative colitis patients undergoing proctocolectomy and ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2008;14(6):775-779. [DOI] [PubMed] [Google Scholar]
- 9. Hata K, Watanabe T, Shinozaki M, Nagawa H.. Patients with extraintestinal manifestations have a higher risk of developing pouchitis in ulcerative colitis: multivariate analysis. Scand J Gastroenterol. 2003;38(10):1055-1058. [DOI] [PubMed] [Google Scholar]
- 10. White E, Melmed GY, Vasiliauskas EA, et al. . A prospective analysis of clinical variables, serologic factors, and outcome of ileal pouch-anal anastomosis in patients with backwash ileitis. Dis Colon Rectum. 2010;53(7):987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Achkar JP, Al-Haddad M, Lashner B, et al. . Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3(1):60-66. [DOI] [PubMed] [Google Scholar]
- 12. Shen B, Fazio VW, Remzi FH, et al. . Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4(1):81-9; quiz 2. quiz23. [DOI] [PubMed] [Google Scholar]
- 13. Yanai H, Ben-Shachar S, Mlynarsky L, et al. . The outcome of ulcerative colitis patients undergoing pouch surgery is determined by pre-surgical factors. Aliment Pharmacol Ther. 2017;46(5):508-515. [DOI] [PubMed] [Google Scholar]
- 14. Kochar B, Barnes EL, Peery AF, et al. . Delayed ileal pouch anal anastomosis has a lower 30-day adverse event rate: analysis from the National Surgical Quality Improvement Program. Inflamm Bowel Dis. 2018;24(8):1833-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnes EL, Kochar B, Herfarth HH, et al. . Creation of a Case-finding definition for identifying patients with acute pouchitis in administrative claims data. Clin Gastroenterol Hepatol. 2021;19(4):842-844.e1. [DOI] [PubMed] [Google Scholar]
- 16. Barnes EL, Herfarth HH, Kappelman MD, et al. . Incidence, risk factors, and outcomes of pouchitis and pouch-related complications in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1583-1591.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen B, Achkar JP, Connor JT, et al. . Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003;46(6):748-753. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. [Google Scholar]
- 19. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 20. Shen B. Diagnosis and management of postoperative ileal pouch disorders. Clin Colon Rectal Surg 2010;23(4):259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prudhomme M, Dozois RR, Godlewski G, Mathison S, Fabbro-Peray P.. Anal canal strictures after ileal pouch-anal anastomosis. Dis Colon Rectum. 2003;46(1):20-23. [DOI] [PubMed] [Google Scholar]
- 22. Shen B, Fazio VW, Remzi FH, et al. . Endoscopic balloon dilation of ileal pouch strictures. Am J Gastroenterol. 2004;99(12):2340-2347. [DOI] [PubMed] [Google Scholar]
- 23. Shen B, Lian L, Kiran RP, et al. . Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis. 2011;17(12):2527-2535. [DOI] [PubMed] [Google Scholar]
- 24. Fumery M, Patel NS, Boland BS, Dulai PS, Singh S, Sandborn WJ.. Efficacy and safety of endoscopic balloon dilatation of ileoanal pouch strictures. Inflamm Bowel Dis. 2018;24(6):1316-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee KE, Shen B.. Endoscopic therapy for pouch leaks and strictures: a systematic review. Dis Colon Rectum. 2022;65(S1):S92-S104. [DOI] [PubMed] [Google Scholar]
- 26. Shen B, Kochhar G, Navaneethan U, et al. . Practical guidelines on endoscopic treatment for Crohn’s disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol 2020;5(4):393-405. [DOI] [PubMed] [Google Scholar]
- 27. Roy P, Kumar D.. Strictureplasty for active Crohn’s disease. Int J Colorectal Dis. 2006;21(5):427-432. [DOI] [PubMed] [Google Scholar]
- 28. Tonelli F, Fedi M, Paroli GM, Fazi M.. Indications and results of side-to-side isoperistaltic strictureplasty in Crohn’s disease. Dis Colon Rectum. 2004;47(4):494-501. [DOI] [PubMed] [Google Scholar]
- 29. Lafeuille P, Yzet C, Bonniaud P, et al. . Use of a bougie-shaped cap for dilation with direct visual control for an esophageal stricture induced by radiation therapy. Endoscopy. 2023;55(suppl 1):E18-E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Committee AT, Siddiqui UD, Banerjee S, et al. . Tools for endoscopic stricture dilation. Gastrointest Endosc. 2013;78(3):391-404. [DOI] [PubMed] [Google Scholar]
- 31. Xinopoulos D, Kypreos D, Bassioukas SP, et al. . Comparative study of balloon and metal olive dilators for endoscopic management of benign anastomotic rectal strictures: clinical and cost-effectiveness outcomes. Surg Endosc. 2011;25(31):756-763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data may be available for further analysis. Please direct any questions regarding data availability and analytic methods to edward_barnes@med.unc.edu.


