Abstract
The number of γδ T cells in the peritoneal cavity was increased after an intraperitoneal (i.p.) infection with Escherichia coli in lipopolysaccharide (LPS)-responsive C3H/HeN mice but not in LPS-hyporesponsive C3H/HeJ mice. The γδ T cells preferentially expressed invariant Vγ6 and Vδ1 chains and proliferated to produce a large amount of gamma interferon in the presence of LPS. Mice depleted of γδ T cells by T-cell receptor δ gene mutation showed impaired resistance against E. coli as assessed by bacterial growth. Macrophages from C3H/HeN mice infected with E. coli expressed higher levels of interleukin-15 (IL-15) mRNA than those from the infected C3H/HeJ mice. Administration of anti-IL-15 monoclonal antibody inhibited, albeit partially, the appearance of γδ T cells in C3H/HeN mice after E. coli infection and diminished the host defense against the infection. These results suggest that LPS-stimulated γδ T cells play an important role in the host defense against E. coli infection and that IL-15 may be partly involved in the protection via an increase in the γδ T cells.
T-cell receptor (TCR)-γδ T cells are present in only small numbers in peripheral lymphoid tissues but respond against infection by intracellular bacteria such as Mycobacterium tuberculosis (24), Listeria monocytogenes (43), Salmonella choleraesuis (9), and other pathogens (16, 26). The contribution of the γδ T cells to protection against infection with intracellular bacteria has been tested in mice depleted of the cells. We have previously reported that pretreatment with anti-TCR-γδ monoclonal antibody (MAb) impaired the host defense early after infection with L. monocytogenes (17). Mice rendered deficient in γδ T cells by homologous recombination of the TCR-δ chain gene showed an impaired host defense against M. tuberculosis (29). Thus, γδ T cells may play important roles in the host defense against infection by intracellular parasites. On the other hand, TCR-δ-deficient mice showed exaggerated intestinal damage after oral infection with Eimeria verformis, suggesting that some γδ T cells, such as intraepithelial γδ T cells, play a role in resolution of the inflammatory process (45). γδ T cells may be heterogeneous in function during the course of infectious diseases.
γδ T cells are reported to respond to various bacterial products, such as tetanus toxoid (28), staphylococcal enterotoxin A (46), heat shock protein 65 (HSP65) (4), and isopentenyl pyrophosphate (34, 56), through a TCR-dependent mechanism. On the other hand, it has been reported that a significant fraction of the γδ T-cell population is stimulated by lipopolysaccharide (LPS) (30, 41, 51), a cell wall component of gram-negative bacteria, through an apparently TCR-independent mechanism. We have recently found by using TCR-δ-deficient mice that γδ T cells play an important role in the priming of macrophages for tumor necrosis factor alpha (TNF-α) production in response to LPS (38). Takada et al. have reported prominent increases in γδ T cells in the peritoneal cavities of some strains of mice infected with Escherichia coli, a gram-negative extracellular bacterium (55). Protection against extracellular bacteria is thought to depend mainly on neutrophils and antibody (Ab) (58). Therefore, it is of interest to elucidate whether LPS-stimulated γδ T cells are involved in the host defense against infection with E. coli.
Interleukin-15 (IL-15) is a novel cytokine that uses β and γ chains of IL-2 receptor for signal transduction and shares many properties with, despite having no sequence homology to, IL-2 (14, 15). IL-2 is produced mainly by activated T cells, whereas IL-15 is produced by a wide variety of tissues, including placenta, skeletal muscle, kidney, and macrophages, upon stimulation with LPS (15, 54). IL-15 has stimulatory activities for natural killer (NK) cells, αβ T cells, and B cells (1, 5, 6, 15). We have recently reported that γδ T cells appearing after Salmonella infection or in intestinal intraepithelial lymphocytes can proliferate in response to exogenous IL-15 or IL-15 derived from infected macrophages (18, 39). A significant number of γδ T cells, which emerge at the early stage of infection well before the appearance of IL-2-producing αβ T cells, may preferentially use IL-15 from stimulated macrophages as a growth factor. However, the role of IL-15 in the host defense against bacterial infection remains to be elucidated.
In the present study, to elucidate the roles of γδ T cells and IL-15 in protection against infection, we examined the host defense against E. coli infection in mice depleted of γδ T cells or IL-15. Mice depleted of γδ T cells by TCR-δ gene targeting showed exaggerated bacterial growth after E. coli infection. Administration of an anti-IL-15 MAb inhibited the appearance of γδ T cells after infection and impaired the host defense against E. coli. The implications of these findings for the roles of γδ T cells and IL-15 in the host defense against E. coli infection are discussed.
MATERIALS AND METHODS
Mice.
C3H/HeJ, C3H/HeN, and C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). Eight- to 10-week-old female mice were used for the experiments. TCR-δ−/− mice, which lack the TCR-δ gene, have previously been described (23). Briefly, chimeric mice were produced by injecting ES clones into C57BL/6 mice. A homogeneous TCR-δ+/− population was established by backcrossing δ heterozygotes to C57BL/6 mice more than five times. The resultant heterozygotes (TCR-δ+/−) were bred to obtain the TCR-δ−/− homozygotes. Mice were housed under specific-pathogen-free conditions and offered food and water ad libitum.
Microorganisms and reagents.
E. coli (ATTC 26; American Type Culture Collection, Rockville, Md.) grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) was washed repeatedly, resuspended in phosphate-buffered saline (PBS), and stored at −80°C in small aliquots until used. LPS derived from E. coli O26:B6 or Salmonella typhimurium was purchased from Sigma Chemical Co. (St. Louis, Mo.). Mitomycin C (MMC) was purchased from Kyowa Hakko Kogyo Co. (Tokyo, Japan).
Fluorescein isothiocyanate (FITC)-conjugated anti-CD3ɛ MAb (145-2C11), phycoerythrin (PE)-conjugated anti-TCR-αβ MAb (H57-597), PE-conjugated anti-TCR-γδ MAb (GL-3), and biotin-conjugated anti-CD3ɛ MAb were purchased from Pharmingen (San Diego, Calif.). Red-613-conjugated streptavidin was obtained from Life Technologies (Gaithersburg, Md.). Anti-IL-15 MAb (G277-3588) was purchased from Pharmingen, and isotype control antibody (rat immunoglobulin G) was from Inter-Cell Technologies, Inc. (Hopewell, N.J.).
Preparation of lymphocytes.
Mice were intraperitoneally (i.p.) inoculated with E. coli at a dose of one-fifth the 50% lethal dose (LD50) (108 CFU/mouse) in 1.0 ml of PBS on day 0. Peritoneal exudate cells (PEC) were harvested on days 0, 1, 3, 5, and 7 after inoculation by centrifugation at 110 × g for 5 min, washed twice, and resuspended at optimal concentrations in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 10% serum. Smear specimens for differential counts were stained with Giemsa solution. PEC were spread on plastic plates and incubated for 1 h in a CO2 incubator at 37°C to obtain nonadherent cells. For liver lymphocytes, fresh liver was immediately perfused with sterile Hanks balanced salt solution through the portal vein to wash out all remaining peripheral blood and then meshed with a stainless steel mesh. After the coarse pieces were removed by centrifugation at 50 × g for 1 min, the cell suspensions were again centrifuged, resuspended in 8 ml of 45% Percoll (Sigma), and layered on 5 ml of 67.5% Percoll. The gradients were centrifuged at 600 × g at 20°C for 20 min. Lymphocytes at the interface were harvested and washed twice with Hanks balanced salt solution.
Bacterial growth.
Mice were inoculated i.p. with 108 CFU of E. coli in 1.0 ml of PBS. The peritoneal contents were lavaged with 3 ml of PBS and harvested after gentle massage. Samples were serially diluted with PBS. The livers and spleens were removed and separately placed in homogenizers containing 5 ml of PBS. Samples were spread on Tripto-Soya agar (Nissui Pharmaceutical, Tokyo, Japan) plates, and colonies were counted after incubation for 24 h at 37°C.
Flow cytometry.
Non-plastic-adherent PEC and liver lymphocytes were incubated with saturating amounts of FITC-, PE-, and biotin-conjugated Abs for 30 min at 4°C. To detect biotin-conjugated MAb, cells were stained with Red-613-conjugated streptavidin after incubation with a primary MAb. Cells were analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). The liver lymphocytes were carefully gated by forward and side light scattering. The data were analyzed with FACScan Research software (Becton Dickinson).
Proliferation assay.
The γδ T cells were purified by cell sorting with an EPICS ELITE (Coulter, Hialeah, Fla.) electric cell sorter from the non-plastic-adherent cells and liver lymphocytes on day 3 after E. coli infection. The purity of sorted cells was more than 97% (data not shown). Ninety-six-well tissue culture plates were incubated overnight at 4°C with 100 μg of anti-TCR-γδ MAb per ml. The plates were then washed thoroughly and incubated for 1 h at 37°C with RPMI 1640 medium containing 10% fetal calf serum. The sorted γδ T cells (5 × 104/well) were incubated in the 96-well plates for 48 h with or without 1, 10, or 100 μg of LPS per ml in the presence or absence of MMC-treated spleen cells (3 × 104/well). During the last 8 h of incubation, 1.0 μCi of [3H]thymidine/well was added. The cells were then harvested, and the amount of [3H]thymidine incorporated was determined by scintillation counting.
Cytokine assays.
The IL-2, IL-4, and gamma interferon (IFN-γ) levels in supernatants were determined by enzyme-linked immunosorbent assay (ELISA). ELISA for IFN-γ was performed in triplicate with Genzyme (Cambridge, Mass.) MAb according to the manufacturer’s instructions, and ELISAs for IL-2 and IL-4 were performed with Biotrak MAbs (Amersham, Buckinghamshire, England).
V gene segment usage analysis.
Total RNA was extracted by the acid-guanidium-phenol-chloroform method from γδ T cells purified by cell sorting. cDNA synthesis and PCR were performed as described by Saiki et al. (47) with a cDNA cycle kit (Invitrogen Corp., San Diego, Calif.). RNA was primed with either 20 pmol of γ chain C region (Cγ) primers (5′ CTT ATG GAG GAT TTG TTT CAG C 3′) or 6.7 pmol of δ chain J region (Jδ) primers (5′ TTG GTT CCA CAG TCA CTT GG 3′) in 21-μl reaction mixtures for reverse transcription. The PCR was performed with a PCR thermal cycler (Takara Corp., Tokyo, Japan). PCR cycles were run for 1 min at 94°C, 1 min at 54°C, and 30 s at 72°C. Before the first cycle, a denaturation step for 7 min at 94°C was included, and after 35 cycles, the extension was prolonged for 4 min at 72°C. The 5′ V primers were as follows: Vγ1/2, 5′ ACA CAG CTA TAC ATT GGT AC 3′; Vγ2, 5′ CGG CAA AAA ACA AAT CAA CAG 3′; Vγ4, 5′ TGT CCT TGC AAC CCC TAC CC 3′; Vγ5, 5′ TGT GCA CTG GTA CCA ACT GA 3′; Vγ6, 5′ GGA ATT CAA AAG AAA ACA TTG TCT 3′; Vγ7, 5′ AAG CTA GAG GGG TCC TCT GC 3′; Vδ1, 5′ ATT CAG AAG GCA ACA ATG AAA G 3′; Vδ2, 5′ AGT TCC CTG CAG ATC CAA GC 3′; Vδ3, 5′ TTC CTG GCT ATT GCC TCT GAC 3′; Vδ4, 5′ CCG CTT CTC TGT GAA CTT CC 3′; Vδ5, 5′ CAG ATC CTT CCA GTT CAT CC 3′; Vδ6, 5′ TCA AGT CCA TCA GCC TTG TC 3′; Vδ7, 5′ CGC AGA GCT GCA GTG TAA CT 3′; and Vδ8, 5′ AAG GAA GAT GGA CGA TTC AC 3′ (9).
PCR products (2 μl) were subjected to electrophoresis on a 1.5% agarose gel (GIBCO) and transferred to a GeneScreen Plus filter (New England Nuclear, Boston, Mass.). The Southern blots of γ PCR products were hybridized with MNG6 cDNA containing the Cγ2 gene, and those of δ PCR products were hybridized with a Jδ1 probe (5′ TTG GTT CCA CAG TCA CTT GG 3′) or Jδ2 probe (5′ CTC CAC AAA GAG CTC TAT GCC CA 3′). The Cγ2 probe was labeled with [α-32P]dCTP by using the Megaprime DNA labeling system (Amersham International, Amersham, United Kingdom) according to the manufacturer’s instructions. The Jδ1 and Jδ2 probes were labeled with [γ-32P]ATP by using the Megalabel 5′-labeling kit (Takara Shuzo Co. Ltd., Kyoto, Japan) according to the manufacturer’s instructions. After hybridization, the filters were incubated in 1 M NaCl–1% sodium dodecyl sulfate (SDS)–10% dextran sulfate–100 μg of heat-denatured salmon sperm DNA per ml for 18 h at 60°C, and then the filters were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% SDS for 15 min at 60°C. The radioactivity of each band of PCR product was analyzed with a Fujix BAS2000 Bio-image analyzer (Fuji Photo Film Co., Ltd., Tokyo, Japan).
For nucleotide sequencing, reverse transcription-PCR products were resolved in low-melting-point agarose gels, isolated, and cloned into TA vector PCR II (Invitrogen). Purified double-stranded DNAs were sequenced by using the Taq Dye Primer Cycle sequencing kit and an ABI 373A DNA sequencer (Applied Biosystems, Foster City, Calif.).
Expression of cytokine genes.
C3H/HeJ and C3H/HeN mice were killed 3 days after i.p. inoculation with E. coli (108 CFU/mouse). Extraction of total RNA from sorted γδ T cells and cDNA synthesis were performed as described above. PEC were spread in plastic plates and incubated for 1 h in a CO2 incubator at 37°C. After nonadherent cells were washed away with PBS, adherent cells were used in in vitro experiments. Of the adherent cells, >95% were macrophages, as assessed by morphological findings. Extraction of total RNA from macrophages and cDNA synthesis were performed as described above. Serial dilutions of total RNA were primed with 20 pmol of random primer in 21-μl reaction mixtures for reverse transcription. Synthesized cDNAs were amplified by PCR with primers derived from the murine cDNA. The specific primers were as follows: IL-2 sense, 5′ TGA TGG ACC TAC AGG AGC TCC TGA G 3′; IL-2 antisense, 5′ GAG TCA AAT CCA GAA CAT GCC GCA G 3′; IL-4 sense, 5′ CGA AGA ACA CCA CAG AGAGTG AGC T 3′; IL-4 antisense, 5′ GAC TCA TTC ATG GTG CAG CTT ATC G 3′; IL-6 sense, 5′ TGG AGT CAC AGA AGG AGT GGC TAA G 3′; IL-6 antisense, 5′ TCT GAC CAC AGT GAG GAA TGT CCA C 3′; IL-10 sense, 5′ TAC CTG GTA GAA GTG ATG CC 3′; IL-10 antisense, 5′ CAT CAT GTA TGC TTC TAT GC 3′; IL-12 sense, 5′ GGA GAC CCT GCC CAT TGA ACT 3′; IL-12 antisense, 5′ CAA CGT TGC ATC CTA GGA TCG 3′; IL-15 sense, 5′ GTG ATG TTC ACC CCA GTT GC 3′; IL-15 antisense, 5′ TCA CAT TCT TTG CAT CCA GA 3′; IFN-γ sense, 5′ AGC GGC TGA CTG AAC TCA GAT TGT AG 3′; IFN-γ antisense, 5′ GTC ACA GTT TTC AGC TGT ATA GGG 3′; TNF-α sense, 5′ GGC AGG TCT ACT TTG GAG TCA TTG C 3′; TNF-α antisense, 5′ ACA TTC GAG GCT CCA GTG AAT TCG G 3′; transforming growth factor β (TGF-β) sense, 5′ CTT TAG GAA GGA CCT GGG TT 3′; and TGF-β antisense, 5′ CAG GAG CGC ACA ATC ATG TT 3′.
The PCR product was subjected to electrophoresis on a 1.5% agarose gel (Nakarai Tesque) and transferred to a GeneScreen Plus filter (New England Nuclear), and probes were labeled with [γ-32P]ATP by using the Megalabel 5′-labeling kit (Takara Shuzo Co. Ltd.) according to the manufacturer’s instructions. Oligonucleotide probes were as follows: IL-2, 5′ GAG ACA TCC TGG GGA GTT TCA 3′; IL-4, 5′ GAG TCT CTG CAG CTC CAT GA 3′; IL-6, 5′ TAG AAA TTC TTC AAG GAT T 3′; IL-10, 5′ GGT CTT CAG CTT CTC ACC CA 3′; IL-12, 5′ TCT GTC TGC AGA GAA GGT CAC A 3′; IL-15, 5′ GCA ATG AAC TGC TTT CTC CT 3′; IFN-γ, 5′ GGT CAC TGC AGC TCT GAA TG 3′; TNF-α, 5′ CCA GGT CAC TGT CCC AGC AT 3′; and TGF-β, 5′ ACC TTG CTG TAC TGT GTG TC 3′.
After hybridization, the filters were incubated in 1 M NaCl–1% SDS–10% dextran sulfate–100 μg of heat-denatured salmon sperm DNA per ml for 18 h at 60°C, and then the filters were washed in 2× SSC–1% SDS for 15 min at 60°C. The radioactivity of each band of PCR product was analyzed with the Fujix BAS2000 Bio-image analyzer (Fuji Photo Film Co., Ltd.).
Statistical analysis.
The statistical significance of the data was determined by the Student t test. A P value of less than 0.05 was taken as significant.
RESULTS
Kinetics of bacterial growth in organs after i.p. inoculation with E. coli.
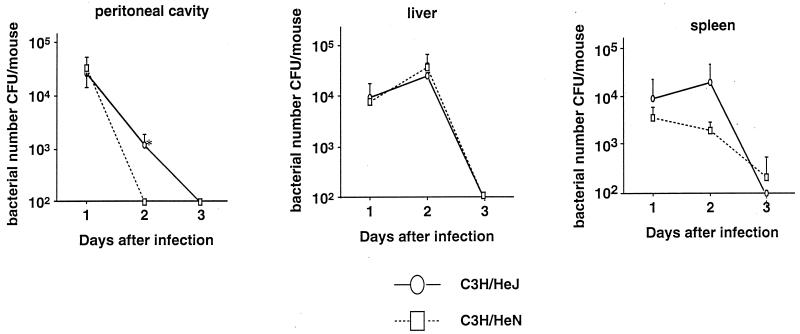
C3H/HeN and C3H/HeJ mice were inoculated i.p. with E. coli at a dose of 108 CFU (one-fifth the LD50)/mouse, and the kinetics of bacterial growth in the peritoneal cavity, liver, and spleen were examined. As shown in Fig. 1, the numbers of bacteria in the peritoneal cavity and spleen had decreased by day 3 of infection in both mouse strains. However, on day 2, the number of bacteria in C3H/HeN mice was significantly smaller than that in C3H/HeJ mice (P < 0.05). There was no difference in the bacterial number in the liver between the strains of mice at any stage after E. coli infection.
FIG. 1.
Kinetics of bacterial growth in the peritoneal cavities, livers, and spleens of C3H/HeJ and C3H/HeN mice after i.p. inoculation with 108 CFU of E. coli. Means and standard errors for five mice are shown. An asterisk indicates a significant difference from the value for C3H/HeN mice (P < 0.05).
Kinetics of γδ T cells in the peritoneal cavity and liver after E. coli infection.
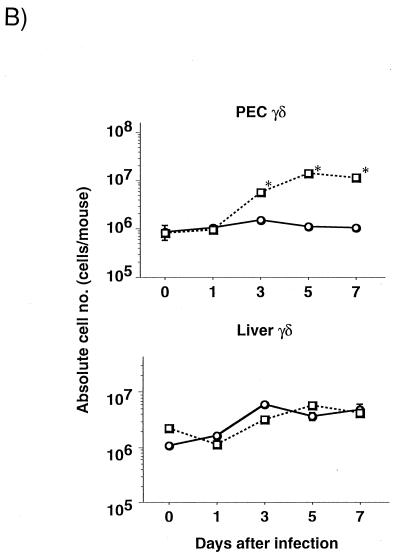
To examine the cell influx in the peritoneal cavity and liver after E. coli infection, we analyzed the kinetics of PEC from C3H/HeJ and C3H/HeN mice inoculated i.p. with 108 CFU of E. coli. The numbers of polymorphonuclear leukocytes (PMN) and macrophages in the peritoneal cavity were smaller in C3H/HeJ than in C3H/HeN mice on day 3 after E. coli infection (PMN, [2.5 ± 0.6] × 106 versus [32.3 ± 5.5] × 106; macrophages, [18.3 ± 3.6] × 106 versus [87.5 ± 16.0] × 106 [n = 5]). The absolute numbers of lymphocytes did not differ significantly for the two strains at any stage after E. coli infection (data not shown). Flow cytometry analysis of the expression of CD3 and TCR-γδ was carried out with the nonadherent PEC and liver lymphocytes from both strains of mice on days 0, 1, 3, 5, and 7 of infection. A representative result from three independent experiments is shown in Fig. 2A. γδ T cells in the nonadherent PEC of C3H/HeN mice increased, constituting more than 30% of all cells on day 3 after E. coli inoculation, whereas the percentage of γδ T cells in the PEC of C3H/HeJ mice was only 4.0% at this stage of infection. No significant difference in the proportions of γδ T cells in livers of C3H/HeJ and C3H/HeN mice was observed at any stage of infection (data not shown). The proportion of αβ T cells in the peritoneal cavity changed little (from 10.9 to 11.6%) and that in the liver decreased (from 22.2 to 14.9%) after E. coli infection in C3H/HeN mice (data not shown). The kinetics of the absolute numbers of peritoneal and liver γδ T cells after i.p. E. coli inoculation are shown in Fig. 2B. The absolute number of γδ T cells in the peritoneal cavity was significantly increased 3 days after E. coli infection in C3H/HeN mice compared with C3H/HeJ mice (P < 0.05), while no significant difference in the numbers of γδ T cells in the livers of C3H/HeN and C3H/HeJ mice was detected.
FIG. 2.
Kinetics of peritoneal γδ T cells after i.p. E. coli inoculation. C3H/HeJ or C3H/HeN mice were inoculated with 108 CFU of E. coli (one-fifth the LD50) on day 0. (A) Non-plastic-adherent PEC were stained with anti-CD3ɛ and anti-TCR-γδ MAbs. The number in each panel indicates the percentage of γδ T cells in whole nonadherent peritoneal cells. (B) Kinetics of the absolute numbers of peritoneal and liver γδ T cells after i.p. inoculation with E. coli. ○, C3H/HeJ mice; □, C3H/HeN mice. The number of γδ T cells was calculated from the percentage of the cells among nonadherent PEC or liver lymphocytes. Means and standard errors for five mice are shown. Asterisks indicate significant differences from the values for C3H/HeJ mice (P < 0.05).
Proliferation and cytokine production of γδ T cells in the peritoneal cavity induced by E. coli infection.
To investigate the functions of γδ T cells induced by E. coli infection, we first examined the expression of cytokine genes in freshly isolated γδ T cells from C3H/HeN and C3H/HeJ mice infected with E. coli 3 days previously by cell sorting with an electric cell sorter. The γδ T cells in the peritoneal cavities of E. coli-infected mice expressed high levels of mRNAs specific for IFN-γ, TNF-α, and TGF-β but not IL-2, IL-4, or IL-6 (Fig. 3).
FIG. 3.
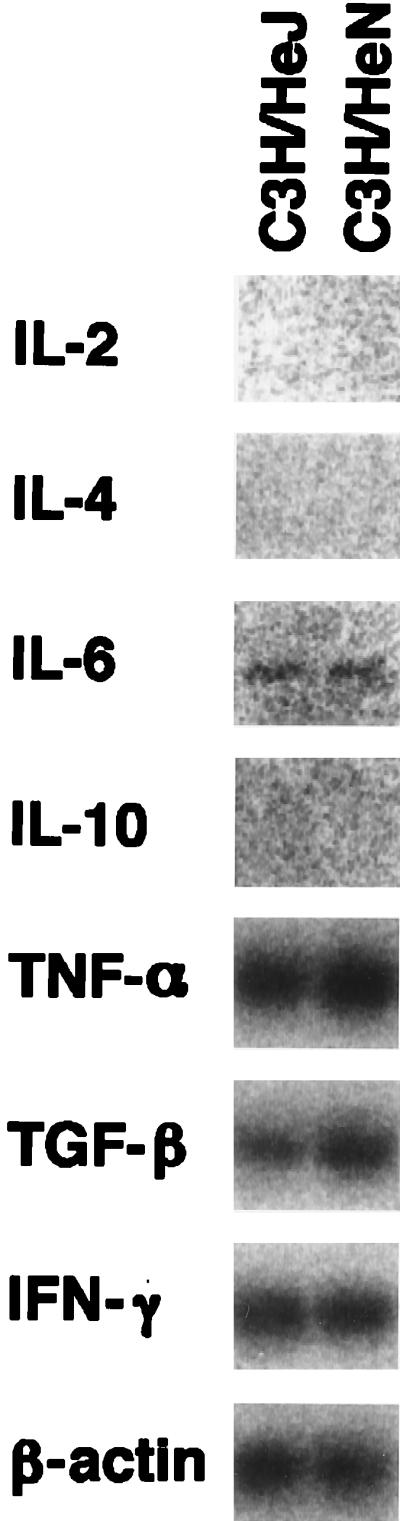
Expression of cytokine mRNAs in peritoneal γδ T cells sorted from C3H/HeJ and C3H/HeN mice infected with E. coli 3 days previously. γδ T cells were sorted from nonadherent PEC pooled from five mice of each strain, and total RNA was reverse transcribed into cDNA and amplified by PCR. The results are representative of those from three independent experiments.
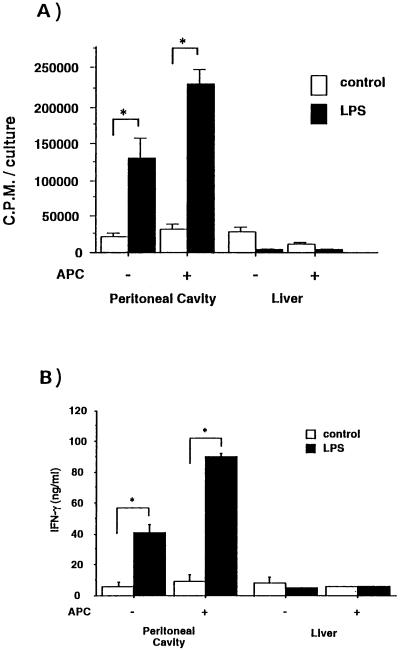
We next examined the proliferative response and cytokine production of the γδ T cells induced by E. coli infection in the peritoneal cavities and livers of C3H/He mice. γδ T cells were purified by cell sorting from the nonadherent peritoneal cells on day 3 of E. coli infection. The γδ T cells were incubated for 48 h on anti-TCR-γδ MAb-coated dishes with an optimum dose (10 μg/ml) of LPS in the presence or absence of MMC-treated spleen cells. Figure 4A shows that E. coli-induced γδ T cells in the peritoneal cavity exhibited a strong proliferative response to LPS even in the absence of MMC-treated spleen cells, whereas γδ T cells from liver showed no proliferation in response to LPS.
FIG. 4.
Proliferative response and cytokine production of γδ T cells from the peritoneal cavities or livers of C3H/HeN mice in the presence of LPS. (A) Purified populations of γδ cells were incubated (5 × 104/well) in anti-TCR-γδ MAb-coated 96-well plates for 48 h in the presence or absence of MMC-treated spleen cells (3 × 104/ml), with or without 10 μg of LPS per ml. During the last 8 h of incubation, 1.0 μCi of [3H]thymidine per well was added. The cells were then harvested, and the amount of [3H]thymidine incorporated was determined by scintillation counting. The data are representative of those from two separate experiments and are expressed as the means of triplicates ± standard deviations. Asterisks indicate significant differences from the values for the control (P < 0.05). (B) Purified γδ T cells (5 × 104 cells) were cultured similarly in the presence or absence of MMC-treated spleen cells with or without LPS for 24 h at 37°C, and the culture supernatant was collected. The cytokine activity in the culture supernatant was tested for the presence of IFN-γ by ELISA. The data are representative of two separate experiments and are expressed as the means of triplicates ± standard deviations. Asterisks indicate significant differences from the values for the control (P < 0.05).
To assess whether the γδ T cells produced IFN-γ at the protein level in response to LPS, we examined the production of cytokines with or without LPS. Figure 4B shows that γδ T cells stimulated with LPS produced a large amount of IFN-γ, whereas neither IL-2 nor IL-4 was detected in the supernatant. The γδ T cells from the liver did not produce either IFN-γ or IL-4 in the presence of LPS. These results suggest that the peritoneal γδ T cells induced by E. coli infection produce IFN-γ in response to LPS.
Vγ and Vδ gene expression in γδ T cells in the peritoneal cavity and liver in mice infected with E. coli.
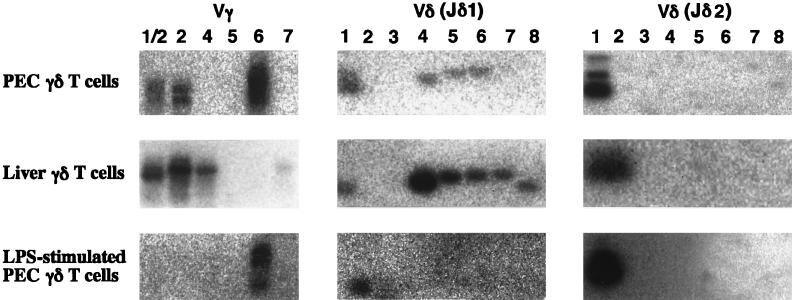
To examine the V gene expression of the γδ T cells in the peritoneal cavity and liver in C3H/HeN mice following E. coli infection, total RNA was extracted from γδ T cells sorted from nonadherent PEC and livers of mice inoculated with E. coli 3 days previously, and V gene expression was analyzed by reverse transcription-PCR. As shown in Fig. 5, the γδ T cells in PEC expressed the Vγ6 and Vδ1 genes preferentially, whereas the γδ T cells in the livers of C3H/HeN mice expressed Vγ1/2, Vγ4, and a diversity of Vδ genes. We further examined the V gene expression of the γδ T cells in the peritoneal cavity after stimulation with LPS in vitro. γδ T cells expressing the Vγ6 and Vδ1 genes were enriched after stimulation with LPS (Fig. 5).
FIG. 5.
Vγ or Vδ usages of γδ T cells in PEC and liver on day 3 after E. coli infection. Total RNA extracted from γδ T cells (5 × 104 cells) sorted from five C3H/HeN mice infected with E. coli 3 days previously or from γδ T cells stimulated with LPS as described in the legend to Fig. 4 was reverse transcribed into cDNA and amplified by PCR with primers for Cγ or Cδ and various Vγ or Vδ segments, respectively. The Southern blot of γ PCR products was hybridized with MNG6. The Southern blot of δ PCR products was hybridized with an oligonucleotide probe for Jδ1 or Jδ2. The results are representative of those from three independent experiments.
To determine the junctional diversity of the Vγ6-Jγ1 and Vδ1-Jδ2 gene rearrangements, we determined the nucleotide sequences of the Vγ6 and Vδ1 transcripts of the peritoneal γδ T cells in E. coli-infected mice. All of 20 Vγ6-Vγ1 transcripts and 18 of 20 Vδ1-Jδ2 transcripts showed no junctional diversity and the same junctional joining (data not shown), resulting in in-frame invariant canonical sequences, which are preferentially expressed in fetal thymocytes at the late stage (approximately day 17) of gestation and in the intraepithelial lymphocytes of reproductive organs such as the uterus (20, 22). We have already reported that most γδ T cells in the peritoneal cavities of naive C3H/He mice express Vγ1/2 and Vδ6 transcripts with junctional diversity (17). Taken together, these results suggest that the Vγ and Vδ expression of the E. coli-induced γδ T cells in the peritoneal cavity is different from that of γδ T cells in the peritoneal cavities of naive mice and in the livers of E. coli-infected mice.
Effects of γδ T-cell-depletion on the eradication of bacteria in mice infected with E. coli.
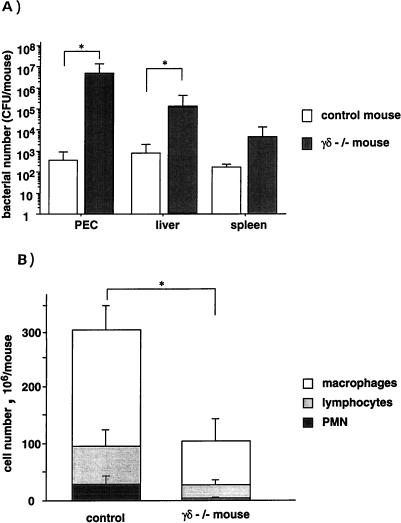
To confirm a protective role for γδ T cells in E. coli infection, TCR-δ−/− mice with a C57BL/6 background were infected with E. coli (108 CFU/mouse) and sacrificed on day 3. Control (TCR-δ+/−) mice showed an increase in γδ T cells on day 3 after E. coli infection (data not shown). As shown in Fig. 6A, a significant increase in the number of E. coli cells was detected in the peritoneal cavities, livers, and spleens of TCR-δ−/− mice compared with control mice. The numbers of PMN, macrophages, and lymphocytes in the peritoneal cavities of TCR-δ−/− mice were smaller than those for control mice (Fig. 6B). These results suggest that an increase of γδ T cells in the peritoneal cavity is one of the factors responsible for the eradication of E. coli.
FIG. 6.
Bacterial growth in the peritoneal cavities and spleens of TCR-δ−/− mice after E. coli infection. TCR-δ−/− mice and their littermate control mice were inoculated i.p. with 2 × 108 CFU of E. coli on day 0. (A) The numbers of E. coli CFU recovered from peritoneal cavities and spleens of infected mice on day 3 were determined by colony formation assay on tryptic soy agar. Values are means ± standard deviations for groups of five mice. Asterisks indicate significant differences from the values for control mice (P < 0.05). (B) Populations of PEC obtained from TCR-δ−/− mice and control mice on day 3 after i.p. inoculation with E. coli. PMN, macrophages, and lymphocytes were judged by morphologic characteristics after staining with Giemsa solution. Values are means ± standard deviations for groups of five mice.
Involvement of IL-15 in the appearance of γδ T cells after infection with E. coli.
The γδ T cells that appear during the course of infection with E. coli produce IFN-γ, but not IL-2, after LPS stimulation. We have previously shown that IL-15 produced by the macrophages is involved in the stimulation of the γδ T cells during salmonellosis (39). We therefore examined whether IL-15 produced by the infected macrophages is involved in the stimulation of the γδ T cells during E. coli infection. To determine whether IL-15 was induced in macrophages after E. coli infection, we tried to detect the IL-15 mRNA in the peritoneal macrophages of C3H/HeJ and C3H/HeN mice 3 days after E. coli infection. The levels of expression of the IL-15 gene in macrophages after E. coli infection are presented in Fig. 7. Consistent with previous reports (11, 36), macrophages of C3H/HeN mice expressed TNF-α and IL-6 more abundantly after E. coli infection than did those of C3H/HeJ mice. The level of expression of IL-15 mRNA, especially the longer message, which is translated most efficiently (40), in macrophages after infection with E. coli was higher in C3H/HeN mice than in C3H/HeJ mice.
FIG. 7.
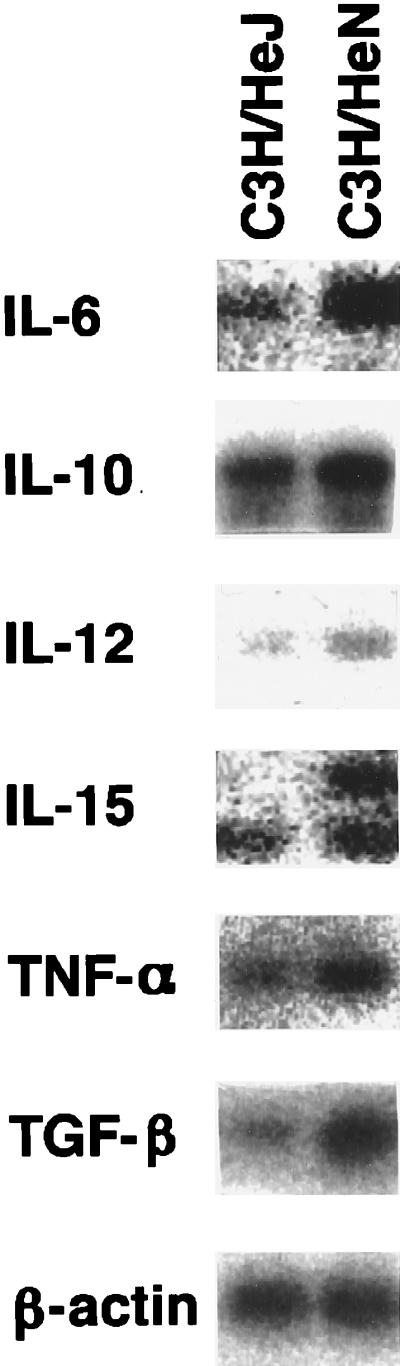
Expression of monokine mRNAs in peritoneal macrophages of mice infected with E. coli. The peritoneal macrophages were obtained from five C3H/HeJ or C3H/HeN mice 3 days after inoculation with E. coli (108 CFU/mouse). Total RNA extracted from the pooled macrophages was reverse transcribed into cDNA and amplified by PCR with each cytokine-specific primer. The results are representative of those from three independent experiments.
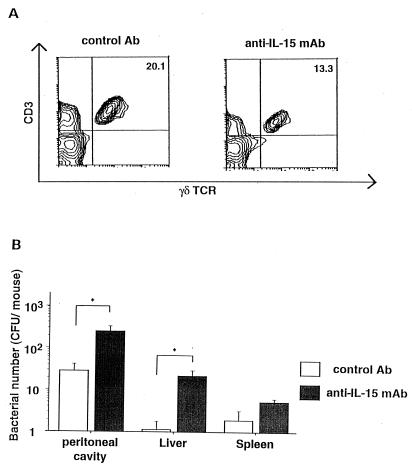
To elucidate the role of IL-15 in the defense against E. coli infection, we examined the effect of in vivo administration of anti-IL-15 MAb on the appearance of γδ T cells and the eradication of E. coli in C3H/HeN mice after infection. C3H/HeN mice were injected i.p. with anti-IL-15 neutralizing MAb (200 μg) or isotype control Ab at 2 h before E. coli challenge, and 3 days later, the numbers of PEC, γδ T cells, and bacteria were determined. The absolute number of peritoneal cells in anti-IL-15 MAb-treated mice was much the same as that in control mice at that stage of E. coli infection (data not shown). A typical three-color profile is shown in Fig. 8A. The appearance of γδ T cells was partly inhibited in the peritoneal cavity in anti-IL-15 MAb-treated mice after the infection. Bacterial numbers were significantly increased in the peritoneal cavities, livers, and spleens of anti-IL-15 MAb-treated mice compared with control mice (Fig. 8B). These results suggested that endogenous IL-15 is at least partly responsible for the γδ T-cell proliferation after E. coli infection and plays an important role in host defense against the infection.
FIG. 8.
Effects of in vivo administration of anti-IL-15 MAb on recovery of bacteria from the peritoneal cavity and spleen and appearance of γδ T cells in the peritoneal cavity after E. coli infection. C3H/HeN mice were inoculated i.p. with 200 μg of anti IL-15 MAb, or control Ab was injected i.p. 2 h before challenge with 2 × 108 CFU of E. coli. (A) Non-plastic-adherent PEC from infected mice on day 3 were stained with anti-TCR-αβ and anti-TCR-γδ MAbs. (B) The numbers of E. coli CFU recovered from the peritoneal cavities, livers, and spleens of infected mice on day 3 were determined by colony formation assay on tryptic soy agar. Values are means ± standard errors for groups of three mice. Asterisks indicate significant differences from the values for control mice (P < 0.05).
DISCUSSION
It is generally accepted that the host defense against infection with E. coli, an extracellular bacterium, is almost exclusively dependent on neutrophils and Ab (58). We show here the possibility that γδ T cells are also involved in the host defense against E. coli infection. γδ T-cell numbers were remarkably increased in the peritoneal cavity after an i.p. infection with E. coli in LPS-responsive C3H/HeN mice but not in LPS-hyporesponsive C3H/HeJ mice. The γδ T cells appearing in the peritoneal cavity after i.p. infection with E. coli produced a large amount of IFN-γ in the presence of LPS. Mice depleted of γδ T cells by TCR-δ gene mutation showed an impaired host defense against E. coli. These results suggest that LPS-stimulated γδ T cells help to protect against E. coli infection.
Similar to αβ T cells, γδ T cells secrete various cytokines and express cytolytic functions (27). Most γδ T cells appearing after infection with intracellular bacteria are reported to produce Th1-type cytokines, in particular IFN-γ (2, 11, 12, 33, 57), whereas γδ T cells during infection with a helminth, Nippostrongylus brasiliensis, preferentially produced Th2-type cytokines, mostly IL-4 (11). Furthermore, γδ T cells, especially in the epithelium, produce TGF-β for immunoregulation and/or immunoglobulin A production (7, 13, 53). We have previously observed, with mice depleted of γδ T cells by treatment with anti-TCR-γδ MAb, that γδ T cells contribute to defense early after Listeria infection via IFN-γ production (17). Mice with mutated TCR-δ genes showed impaired TNF-α production in response to LPS (38). Our results reveal that the γδ T cells accumulating in the peritoneal cavity after E. coli infection expressed IFN-γ mRNA and produced a significant amount of IFN-γ in the presence of LPS under TCR triggering. We speculate that γδ T cells produce IFN-γ in response to E. coli and their cell component LPS and activate macrophages which consequently eliminate the E. coli. We have previously reported that γδ T cells appearing during the course of listeriosis produced macrophage chemotactic factor, in addition to IFN-γ (17). Our present results reveal that accumulation of PMN and macrophages in the peritoneal cavity following E. coli infection was impaired in TCRδ−/− mice. Therefore, it is also possible that γδ T cells produce chemokines for neutrophils and monocytes in addition to IFN-γ and protect mice from E. coli infection. Although E. coli was completely eliminated in the peritoneal cavity by day 3, γδ T cells were still increased on days 5 and 7 after infection. Mukasa et al. have recently suggested that γδ T cells expressing invariant Vγ6 and Vδ1 chains have a function in controlling influences on the host inflammatory responses (35). Our data indicated that the γδ T cells also expressed mRNA specific for TGF-β, which can modulate immune and inflammatory responses. γδ T cells appearing after E. coli infection may have different functions at distinct time points during the course of E. coli infection. Further analysis of cytokine production by γδ T cells is required to clarify their roles in E. coli infection.
It has been described that a significant number of γδ T cells are stimulated by LPS through an apparently TCR-independent mechanism (30, 41, 51). Leclercq and Plum have reported that TCR Vγ5 cells, which are preferentially present in the epidermis, are activated to produce cytokines upon interaction with LPS via TCR-independent pathways (30). Nitta et al. have reported that γδ T cells in the peritoneal cavity proliferate in response to a TCR triggering in synergy with LPS (41). Similarly, we found that the γδ T cells in the peritoneal cavity of E. coli-infected mice proliferate and produce a significant amount of IFN-γ in the presence of LPS when their γδ TCRs are stimulated with anti-TCR MAb. Stimulation of the γδ T cells by LPS was indeed accessory cell independent, excluding the possibility that LPS induced expression of ligands for γδ TCR on accessory cells or production of growth factors from accessory cells. Thus, it appears that LPS may have a costimulatory activity for γδ T-cell stimulation upon TCR triggering. The peritoneal γδ T cells induced by E. coli infection expressed the Vγ6 gene, which is expressed by γδ T cells in the uterus and tongue, together with the Vδ1 gene, rearranged to Jδ2, similar to Vγ5 T cells in the epidermis (22). All Vγ6-Jγ1 and Vδ1-Jδ2 mRNAs from the γδ T cells we sequenced have no junctional diversity, similar to those from the γδ T cells in the fetal thymus and uterus. On the other hand, the liver γδ T cells expressed Vγ1/2 and did not respond to LPS. Thus, it would appear that only γδ T cells with particular V genes such as Vγ5 and Vδ1 or Vγ6 and Vδ1 are stimulated with LPS from gram-negative bacteria through a TCR-independent mechanism. Further analysis is required to clarify which receptor of the γδ T cells recognizes LPS.
Although LPS from E. coli is apparently involved in γδ T-cell stimulation, the ligand for the γδ TCR during E. coli infection is not known. It has been reported that Vγ6 and Vδ1 T cells expand at sites of inflammation in the absence of pathogen-derived antigens in Listeria infection and in Listeria-induced autoimmune orichitis (35, 44). This suggests that Vγ6 and Vδ1 T cells do not respond to foreign antigens but rather respond to a host-derived antigen that is conserved between the host and bacteria. In mice, a high proportion of γδ T cells have been found to respond to unique peptides of mycobacterial and mammalian HSP65 (4). The HSP65-reactive γδ T cells characteristically express Vγ1 and Vδ6 with junctional diversity (42). We have previously reported that the peritoneal γδ T cells appearing during infection with intracellular bacteria such as S. choleraesuis (9), L. monocytogenes (17), and Mycobacterium bovis BCG (19) preferentially expressed Vγ1 and Vδ6 genes. However, the present study revealed that the γδ T cells appearing in E. coli infection preferentially expressed Vγ6 and Vδ1 and thus differ from those capable of responding to HSP65. In fact, Takada et al. have reported that γδ T cells from E. coli-infected mice did not proliferate in response to purified protein derivative or HSP65 derived from M. tuberculosis (55). A number of murine γδ T-cell clones are reported to recognize major histocompatibility complex molecules or major histocompatibility complex-related gene products such as TL and Qa in a manner quite different from the antigen recognition shown by αβ T cells (3, 21, 31, 48). Similarly, a herpesvirus protein was found to directly stimulate γδ T cells independent of antigen processing and presentation (25, 50). Human γδ T cells are stimulated by apparently nonproteinaceous low-molecular-weight ligands, including isopentenyl pyrophosphate, which represents a ubiquitous metabolite of various vitamins, lipids, and steroids in both prokaryotic and eukaryotic cells (8, 49, 56). Therefore, it is of interest to elucidate whether the γδ T cells induced by E. coli recognize such unique antigens in a manner different from that of αβ T cells.
Another notable finding is that IL-15 was involved in protection against E. coli infection. LPS-hyporesponsive C3H/HeJ mice carry the lpsd mutation on chromosome 4, and macrophages and B cells in these mice respond poorly to LPS (11, 36). Consistently, the macrophages induced by E. coli infection in C3H/HeJ mice showed an impaired expression of monokine genes such as those for TNF-α and IL-6 compared with that in C3H/HeN mice. In correlation to the sensitivity of macrophages to LPS, γδ T-cell numbers in the peritoneal cavity were remarkably increased after E. coli infection in LPS-responsive C3H/HeN mice but not in LPS-hyporesponsive C3H/HeJ mice. IL-15 promoter regions contained binding elements for LPS-inducible transcription factors such as NF-IL-6 and NF-κB (60). Consistent with this finding, C3H/HeN mice infected with E. coli expressed higher levels of IL-15 mRNA, especially the longer transcript, than did C3H/HeJ mice infected with E. coli. We have recently found that IL-15 mRNA containing a longer alternative exon 5 is translated most efficiently among IL-15 mRNA isoforms (40). Administration of anti-IL-15 MAb inhibited, albeit partially, the increase in γδ T cells after E. coli infection and impaired the host defense against E. coli. These results suggest that IL-15 is at least partly responsible for the increase in γδ T cells in the peritoneal cavity after E. coli infection. γδ T cells induced by E. coli infection did not produce IL-2, nor did IL-2-producing αβ T cells appear in the peritoneal cavity after infection (data not shown). Thus, we speculate that IL-15 released from LPS-stimulated macrophages is responsible for the increase in γδ T cells and consequently for the defense against E. coli infection in mice. We have previously reported that γδ T cells proliferate to produce IFN-γ in response to IL-15 in vitro (18, 39). Therefore, IL-15 derived from LPS-stimulated macrophages may be responsible for local expansion of γδ T cells preexisting in the peritoneal cavity in vivo. However, the Vγ6/Vδ1 T-cell subset is rare in the peritoneal cavity. γδ T cells are stimulated with LPS in the absence of accessory cells. Our preliminary experiments revealed that addition of anti-IL-15 MAb in in vitro culture did not inhibit the γδ T-cell proliferation in the presence of LPS. Taken together, IL-15 may not play a very important role in LPS-induced proliferation of γδ T cells in situ. IL-15 is reported to have a strong chemotactic activity for T cells (32, 37). Therefore, we speculate that IL-15 released from LPS-stimulated macrophages may be more important in accumulation of γδ T cells in the peritoneal cavity than in the expansion in vivo after E. coli infection. Anti-IL-15 administration only partially inhibited the appearance of the γδ T cells. Skeen and Ziegler reported that peritoneal γδ T cells proliferated in response to IL-1 and IL-7 (52). It has been reported that TNF-α and IL-12 synergistically stimulate human γδ T-cell proliferation (59). Although it cannot be ruled out that the amount of anti-IL-15 MAb was insufficient to cause inhibition in our experiments, it appears that the increase in γδ T cells may be attributable in part to cytokines other than IL-15.
In conclusion, LPS-stimulated γδ T cells play important roles in the host defense against E. coli infection. IL-15 released from LPS-stimulated macrophages may be involved in the accumulation of γδ T cells during E. coli infection.
ACKNOWLEDGMENTS
This work was supported in part by grants to Y.Y. from the Ministry of Education, Science and Culture and JSPS-RFTF (97L00703) and by a Searle Scientific Research Fellowship to H.N.
We thank A. Kato, Y. Kitagawa, and K. Itano for preparing the manuscript, Daniel Murozek for reading the manuscript, and Y. Yamakawa for technical assistance with the EPICS sorting.
REFERENCES
- 1.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P F, Grisso C L, Abrams J S, Band H, Rea T H, Modlin R L. γδ T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone J A, Cron R Q, Cotterman M, Houlden B A, Matis L A. Structure and specificity of T cell receptor γ/δ on major histocompatibility complex antigen-specific CD3+, CD4−, CD8− T lymphocytes. J Exp Med. 1988;168:1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, Brennan P, O’Brien R. Recognition of a peptide antigen by heat shock-reactive γδ T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- 5.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson W E, Giri J G, Lindemann M J, Linett M L, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri M A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo V K, Weiner H L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 8.Constant P, Davodeau F, Peyrat M-A, Poquet Y, Puzo G, Bonneville M, Fournié J-J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 9.Emoto M, Danbara H, Yoshikai Y. Induction of γ/δ T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992;176:363–372. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans T J, Strivens E, Carpenter A, Cohen J. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous gram-negative infection. J Immunol. 1993;150:5033–5040. [PubMed] [Google Scholar]
- 11.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 12.Follows G A, Munk M E, Gatrill A J, Conradt P, Kaufmann S H E. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in γ/δ T-cell cultures after activation with bacteria. Infect Immun. 1992;60:1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujihashi K, McGhee J R, Kweon M-N, Cooper M D, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, Kiyono H. γ/δ T cell-deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med. 1996;183:1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabstein K H, Eisenman J, Shaneback K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, Johnson L, Alderson M R, Watson J D, Anderson D M, Giri J G. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 16.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 17.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone J A, Nomoto K. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inagaki-Ohara K, Nishimura H, Mitani A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing γδ T cell receptor in mice. Eur J Immunol. 1997;27:2885–2891. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Yoshikai Y, Matsuzaki G, Nomoto K. Early appearing γ/δ-bearing T cells during infection with Calmette Guérin bacillus. J Immunol. 1991;146:2754–2762. [PubMed] [Google Scholar]
- 20.Ito K, Bonneville M, Takagaki Y, Nakanishi N, Kanagawa O, Krecko E G, Tonegawa S. Different γδ T cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci USA. 1989;86:631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy D B, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse γδ T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 22.Itohara S, Farr A G, Lafaille J J, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 23.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke A R, Hooper M L, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 24.Janis E M, Kaufmann S H E, Schwartz R H, Pardoll D M. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 25.Johnson R M, Lancki D W, Sperling A I, Dick R F, Spear P G, Fitch F W, Bluestone J A. A murine CD4−, CD8− T cell receptor-γδ T lymphocyte clone specific for herpes simplex virus glycoprotein I. J Immunol. 1992;148:983–988. [PubMed] [Google Scholar]
- 26.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann S H E. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozbor D, Trinchieri G, Monos D S, Isobe M, Russo G, Haney J A, Zmijewski C, Croce C M. Human TCR-γ+/δ+ CD8+T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J Exp Med. 1989;169:1847–1851. doi: 10.1084/jem.169.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladel C H, Blum C, Dreher A, Reifenberg K, Kaufmann S H E. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 30.Leclercq G, Plum J. Stimulation of TCR Vγ3 cells by gram-negative bacteria. J Immunol. 1995;154:5313–5319. [PubMed] [Google Scholar]
- 31.Matis L A, Fry A M, Cron R Q, Cotterman M M, Dick R F, Bluestone J A. Structure and specificity of a class II MHC alloreactive γδ T cell receptor heterodimer. Science. 1989;245:746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- 32.McInnes I B, Mughales J, Field M, Leung B P, Huang F P, Dixon R, Sturrock R D, Wilkinson P C, Liew F Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 33.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H E. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:353. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 34.Morita C T, Beckman E M, Bukowski J F, Tanaka Y, Band H, Bloom B R, Golan D E, Brenner M B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 35.Mukasa A, Lahn M, Pflum E K, Born W, O’Brien R L. Evidence that the same γδ T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- 36.Nakano M, Shinomiya H. The Lps mutational defect in C3H/HeJ mice. In: Morrison D C, Ryan J L, editors. Bacterial endotoxin lipopolysaccharides. 1. Molecular biochemistry and cellular biology. Boca Raton, Fla: CRC Press; 1992. p. 311. [Google Scholar]
- 37.Nieto M, del Pozo M A, Sanchez-Madrid F. Interleukin-15 induces adhesion receptor redistribution in T lymphocytes. Eur J Immunol. 1996;26:1302–1307. doi: 10.1002/eji.1830260619. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura H, Emoto M, Sakai T, Katsuno M, Hiromatsu K, Gomi H, Ikeda T, Itohara S, Yoshikai Y. The role of γδ T cells in priming macrophages to produce tumor necrosis factor-α. Eur J Immunol. 1995;25:1465–1468. doi: 10.1002/eji.1830250551. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura H, Hiromatsu K, Kobayashi N, Grabstein K H, Paxton R, Sugamura K, Bluestone J A, Yoshikai Y. IL-15 is novel growth factor for murine γδ T cells induced by Salmonella infection. J Immunol. 1996;156:663–669. [PubMed] [Google Scholar]
- 40.Nishimura H, Washizu J, Nakamura N, Enomoto A, Yoshikai Y. Translation efficiency is upregulated by alternative exon in murine interleukin-15 mRNA. J Immunol. 1998;160:936–942. [PubMed] [Google Scholar]
- 41.Nitta T, Imai H, Ogasawara Y, Nakano M. Mitogenicity of bacterial lipopolysaccharide on the T lymphocyte population bearing the γδ T cell receptor. J Endotoxin Res. 1994;1:101–107. [Google Scholar]
- 42.O’Brien R L, Fu Y-X, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding S R, Kubo R, Born W K. Heat shock protein Hsp60-reactive γδ T cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohga S, Yoshikai Y, Takeda Y, Hiromatsu K, Nomoto K. Sequential appearance of γ/δ- and α/β-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990;20:533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- 44.Roak E C, Vollmer M K, Campbell P A, Born W K, O’Brien R L. Response of a γδ+ T cell receptor invariant subset during bacterial infection. J Immunol. 1996;156:2214–2220. [PubMed] [Google Scholar]
- 45.Roberts S J, Smith A L, West A B, Wen L, Findly R C, Owen M J, Hayday A C. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rust C J, Verreck F, Vietor H, Koning F. Specific recognition of staphylococcal enterotoxin A by human T cells bearing receptors with the Vγ9 region. Nature. 1990;346:572–574. doi: 10.1038/346572a0. [DOI] [PubMed] [Google Scholar]
- 47.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 48.Schild H, Mavaddat N, Litzenberger C, Ehrich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y H. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 49.Schoel B, Sprenger S, Kaufmann S H. Phosphate is essential for stimulation of Vγ9/Vδ2 T lymphocytes by mycobacterial low molecular weight ligand. Eur J Immunol. 1994;24:1886–1892. doi: 10.1002/eji.1830240826. [DOI] [PubMed] [Google Scholar]
- 50.Sciammas R, Johnson R M, Sperling A I, Brady W, Linsley P S, Spear P G, Fitch F W, Bluestone J A. Unique antigen recognition by a herpesvirus-specific TCR-γδ cell. J Immunol. 1994;152:5392–5397. [PubMed] [Google Scholar]
- 51.Skeen M J, Ziegler H K. Induction of murine peritoneal γ/δ T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skeen M J, Ziegler H K. Intercellular interactions and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on γ/δ T cells. J Exp Med. 1993;178:985–996. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T, Hiromatsu K, Ando Y, Okamoto T, Tomoda Y, Yoshikai Y. Regulatory role of γδ T cells in uterine intraepithelial lymphocytes in maternal anti-fetal immune responses. J Immunol. 1995;154:4476–4484. [PubMed] [Google Scholar]
- 54.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 55.Takada H, Hiromatsu K, Matsuzaki G, Muramori K, Nomoto K. Peritoneal γδ T cells induced by Escherichia coli infection in mice. Correlation between Thy-1 phenotype and host minor lymphocyte-stimulating phenotype. J Immunol. 1993;151:2062–2069. [PubMed] [Google Scholar]
- 56.Tanaka Y, Morita C T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 57.Tsukaguchi K, Balaji K N, Boom W H. CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 58.Tsuru S, Nomoto K, Mitsuyama M, Zinnaka Y, Takeya K. Importance of polymorphonuclear leukocytes in protection of mice against Escherichia coli. J Gen Microbiol. 1981;122:335–338. doi: 10.1099/00221287-122-2-335. [DOI] [PubMed] [Google Scholar]
- 59.Ueta C, Kawasumi H, Fujiwara H, Miyagawa T, Kida H, Ohmoto Y, Kishimoto S, Tsuyuguchi I. Interleukin-12 activates human γδ T cells: synergistic effect of tumor necrosis factor α. Eur J Immunol. 1996;26:3066–3073. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]
- 60.Washizu, J., H. Nishimura, N. Nakamura, Y. Nimura, and Y. Yoshikai. NF-κB binding site is essential for transcriptional activation of IL-15. Immunogenetics, in press. [DOI] [PubMed]