Abstract
The non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA) is a neurodegenerative syndrome primarily defined by the presence of apraxia of speech (AoS) and/or expressive agrammatism. In addition, many patients exhibit dysarthria and/or receptive agrammatism. This leads to substantial phenotypic variation within the speech-language domain across individuals and time, in terms of both the specific combination of symptoms as well as their severity. How to resolve such phenotypic heterogeneity in nfvPPA is a matter of debate. ‘Splitting’ views propose separate clinical entities: ‘primary progressive apraxia of speech’ when AoS occurs in the absence of expressive agrammatism, ‘progressive agrammatic aphasia’ (PAA) in the opposite case, and ‘AOS + PAA’ when mixed motor speech and language symptoms are clearly present. While therapeutic interventions typically vary depending on the predominant symptom (e.g. AoS versus expressive agrammatism), the existence of behavioural, anatomical and pathological overlap across these phenotypes argues against drawing such clear-cut boundaries. In the current study, we contribute to this debate by mapping behaviour to brain in a large, prospective cohort of well characterized patients with nfvPPA (n = 104). We sought to advance scientific understanding of nfvPPA and the neural basis of speech-language by uncovering where in the brain the degree of MRI-based atrophy is associated with inter-patient variability in the presence and severity of AoS, dysarthria, expressive agrammatism or receptive agrammatism.
Our cross-sectional examination of brain-behaviour relationships revealed three main observations. First, we found that the neural correlates of AoS and expressive agrammatism in nfvPPA lie side by side in the left posterior inferior frontal lobe, explaining their behavioural dissociation/association in previous reports. Second, we identified a ‘left-right’ and ‘ventral-dorsal’ neuroanatomical distinction between AoS versus dysarthria, highlighting (i) that dysarthria, but not AoS, is significantly influenced by tissue loss in right-hemisphere motor-speech regions; and (ii) that, within the left hemisphere, dysarthria and AoS map onto dorsally versus ventrally located motor-speech regions, respectively. Third, we confirmed that, within the large-scale grammar network, left frontal tissue loss is preferentially involved in expressive agrammatism and left temporal tissue loss in receptive agrammatism.
Our findings thus contribute to define the function and location of the epicentres within the large-scale neural networks vulnerable to neurodegenerative changes in nfvPPA. We propose that nfvPPA be redefined as an umbrella term subsuming a spectrum of speech and/or language phenotypes that are closely linked by the underlying neuroanatomy and neuropathology.
Keywords: articulation, syntactic, sentence production, sentence comprehension, agrammatic, lesion-symptom
Lorca-Puls et al. dissect the neuroanatomy of the most characteristic clinical features of nfvPPA: impaired motor speech and agrammatism. They propose that nfvPPA be reinterpreted as a spectrum disorder subsuming multiple speech-language phenotypes that are closely linked by the underlying neuroanatomy and neuropathology.
Introduction
The non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA) is a neurodegenerative syndrome characterized by effortful, non-fluent speech production, which usually begins in the early sixties.1 Most typically, it is caused by the deposition of abnormal forms of microtubule-associated protein tau in the context of frontotemporal lobar degeneration (FTLD-tau)2-6 that predominantly affects a network of left fronto-insular and subcortical regions.7-9 According to current consensus criteria,10 at least one of two core speech-language features must be present for the clinical diagnosis of nfvPPA: (i) effortful, halting speech with articulatory errors (i.e. apraxia of speech, AoS); and/or (ii) agrammatism in language production (i.e. expressive agrammatism), in the setting of relatively spared single word comprehension and object knowledge. AoS is the most common cause of effortful speech in nfvPPA and is considered a disorder of the motor planning/programming of speech that commonly manifests as distorted speech sound errors, speech sound sequencing errors, articulatory groping, trial-and-error articulation, slow speech rate, difficulty initiating speech and prosodic alterations.11,12 The term ‘expressive agrammatism’ is, on the other hand, traditionally used to refer to morphosyntactically impoverished utterances (i.e. agrammatic speech and writing), which generally result from the omission of function words/morphemes, production of morphosyntactic errors and simplification of sentence structure.13,14
Phenotypic variation within the speech-language domain in nfvPPA, however, extends beyond the question of whether AoS and expressive agrammatism co-occur or not. For example, dysarthria, which is thought to reflect an impairment in the motor control/execution of speech due to neurodegeneration at the level of neural structures or pathways that command the muscles of the pneumo-phono-articulatory system, has frequently been reported to coexist with AoS, particularly in its spastic, hypokinetic or mixed spastic-hypokinetic types.15-17 Although less common, dysarthria has also been described as the first and predominant manifestation of a neurodegenerative condition (i.e. progressive dysarthria), most often in relation to various frontotemporal dementia spectrum clinical and pathological entities.18-20 Finally, many patients with nfvPPA exhibit receptive agrammatism, defined as agrammatic sentence comprehension (especially of morphosyntactically complex structures).21-25
The current cross-sectional study aims to map the neural sites where neurodegeneration is likely to drive phenotypic variation in the expression of these four characteristic speech-language features using a large, prospective cohort of nfvPPA patients. Although the neural correlates of AoS, expressive agrammatism, dysarthria and receptive agrammatism have already been studied individually,26-29 this has never been done (i) in tandem; (ii) within a single cohort of nfvPPA patients; or (iii) with the specific aim of shedding new light on the underlying neural causes of inter-subject variability in behavioural phenotype. Critically, the research question we pose is motivated by prior research findings that, as explained later, point to substantial phenotypic heterogeneity in the speech-language profile of patients with nfvPPA,30,31 both in terms of the specific combination of symptoms as well as their severity, largely reflecting the varying contribution of AoS, dysarthria, expressive agrammatism and receptive agrammatism to the clinical presentation of this syndrome across individuals and time. Moreover, unlike previous reports that segregated patients based on their specific speech-language profile (e.g. AoS with or without co-occurring expressive agrammatism) to then investigate between-group differences in the patterns of brain atrophy,31 our examination of brain-behaviour relationships attempts to advance scientific understanding of why and how motor speech and grammar skills are differentially affected in nfvPPA by directly relating inter-patient variability in the severity of individual speech-language symptoms (e.g. AoS severity) to inter-patient variability in the degree of regional atrophy.
Previous studies have documented the existence of distinguishable speech-language profiles arising from the differential expression of AoS versus expressive agrammatism in patients with nfvPPA.30-34 For example, the term ‘progressive agrammatic aphasia’ (PAA)34 has been coined to refer to patients with expressive agrammatism as the most prominent presenting symptom in the absence of AoS. In contrast, patients exhibiting the opposite speech-language phenotype (AoS without concomitant expressive agrammatism, or aphasia more generally) have been ascribed the term ‘primary progressive apraxia of speech’ (PPAOS).32 Nevertheless, while some authors suggest that these speech-language profiles—linked traditionally to ‘non-fluency’ in the context of left frontal lobe dysfunction—might be associated with specific neuropathological correlates, others contend that considerable overlap exists cross-sectionally and longitudinally. Furthermore, not all patients with impaired motor speech as the most salient clinical feature have AoS only, but rather a combination of AoS and dysarthria, in which the latter might be an equal or even dominant contributor.15,35 In this sense, differences in the definition of AoS and dysarthria, such as using the presence of inconsistent or consistent articulatory errors as a strict diagnostic marker of AoS,36 might contribute to nosological uncertainties. Importantly, evidence showing that even patients with a relatively isolated motor speech disorder initially will often go on to develop agrammatism (and vice versa) as the disease progresses37,38 seems to suggest a continuum of clinical endophenotypes within the larger umbrella of nfvPPA.
Indeed, neighbouring regions of the left posterior fronto-insular cortex have been implicated in articulatory and grammatical aspects of speech production by lesion-symptom mapping studies of stroke patients.26-28 This raises the possibility that two or more neuroanatomically distinct but closely located left frontal loci within the broader ‘speech production’ network might underlie impaired motor speech and expressive agrammatism in nfvPPA. Such spatial arrangement of the neural correlates of impaired motor speech and expressive agrammatism in the left frontal lobe would explain why these nfvPPA symptoms sometimes do initially occur in isolation but tend to merge34,37,38 as the neurodegenerative disease spreads through the vulnerable large-scale neural networks.9,39-42 Put differently, phenotypic variation within the speech-language domain in nfvPPA would be primarily accounted for by the position each patient occupies at any given time along a continuum composed of at least two independent but correlated (due to the neuroanatomy involved) dimensions: motor speech and grammar.
In summary, by mapping each of the four characteristic speech-language features mentioned earlier (i.e. AoS, dysarthria, expressive agrammatism and receptive agrammatism) to their neural substrates in a single, large cohort of patients with nfvPPA, this study thus intends to illuminate the set of brain regions that are likely to give rise to the nfvPPA phenotypic spectrum in the context of network-based neurodegeneration.
Materials and methods
Patient selection criteria
The database of the Memory and Aging Center at University of California, San Francisco (UCSF) was searched for patients who received a clinical diagnosis of nfvPPA based on current diagnostic criteria.10 Patients with nfvPPA were included in the study if they had: (i) a high-resolution T1-weighted MRI scan of sufficient quality for analysis; (ii) a Mini-Mental State Examination43 total score >10 or, alternatively, a Clinical Dementia Rating44 total score <3; and (iii) completed a structured motor speech evaluation, picture description task and/or sentence comprehension task. Of the 114 patients screened for inclusion into the study, seven were excluded on the basis of the first inclusion criterion, two on the basis of the second, and one on the basis of the third, leaving a total of 104 cases, aged between 51 and 81 years [mean age ± standard deviation (SD) = 68.55 ± 7.12; 71 females], who met all three inclusion criteria. Genetic testing for major FTLD gene mutations was performed in 95% (99/104) of the selected patients, four of whom tested positive (see Supplementary Table 1 for more details). Summary demographic, clinical and neuropsychological information for our patient sample can be found in Table 1. Supplementary Fig. 1 shows a histogram of the time elapsed between first symptom onset (as reported by the patient/companion) and scan acquisition. Of note, for each patient, only data-points (including the T1-weighted MRI scan) collected within a maximum period of 6 months were considered in the analyses reported later; other data-points (if any) were treated as missing values.
Table 1.
Demographic, clinical and neuropsychological characteristics of patients
| nfvPPA (n = 104) | |||
|---|---|---|---|
| Mean ± SD | Range | Missing | |
| Demographic | |||
| Age at scan | 68.55 ± 7.12 | 51–82 | 0 |
| Sex (male/female) | 33/71 | N/A | 0 |
| Handedness (right/left/ambidextrous) | 91/11/2 | N/A | 0 |
| Years of education | 16.13 ± 2.88 | 12–28 | 0 |
| Clinical | |||
| Estimated age at first symptom onset | 64.07 ± 7.18 | 44–79 | 1 |
| Estimated years since first symptom onset | 4.39 ± 1.93 | 1–10 | 1 |
| MMSE (30) | 25.24 ± 4.47 | 12–30 | 6 |
| CDR (3) | 0.41 ± 0.40 | 0–2 | 6 |
| Visuospatial function | |||
| Benson figure copy (17) | 14.47 ± 2.10 | 4–17 | 10 |
| VOSP number location (10) | 8.67 ± 1.56 | 3–10 | 16 |
| Visual memory | |||
| Benson figure 10 min free recall (17) | 10.30 ± 3.25 | 0–16 | 10 |
| Verbal memory | |||
| CVLT-MS trials 1–4 (36) | 22.54 ± 6.64 | 9–35 | 14 |
| CVLT-MS 30 s free recall (9) | 6.21 ± 2.31 | 0–9 | 14 |
| CVLT-MS 10 min free recall (9) | 5.82 ± 2.49 | 0–9 | 14 |
| CVLT-MS recognition (9) | 8.19 ± 1.10 | 4–9 | 14 |
| Executive function/working memory | |||
| Backward digit span (8) | 3.49± 1.43 | 0–8 | 10 |
| Modified trails (lines per minute) | 17.83 ± 29.08 | 0–270 | 13 |
| Stroop colour naming (1 min) | 41.98 ± 15.55 | 6–79 | 39 |
| Stroop interference (1 min) | 25.09 ± 11.10 | 1–49 | 35 |
| Design fluency (1 min) | 6.10 ± 2.82 | 0–13 | 12 |
| Letter fluency (D words in 1 min) | 5.90 ± 4.11 | 0–23 | 11 |
| Category fluency (animals in 1 min) | 11.05 ± 6.41 | 0–33 | 10 |
| Language production | |||
| WAB speech fluency rating (10) | 6.19 ± 2.62 | 0–10 | 11 |
| Boston naming test (15) | 12.39 ± 2.95 | 0–15 | 6 |
| WAB repetition (100) | 85.34 ± 15.66 | 15–100 | 15 |
| MSE AoS severity rating (7) | 2.68 ± 1.86 | 0–7 | 1 |
| MSE dysarthria severity rating (7) | 2.09 ± 2.07 | 0–7 | 1 |
| Language comprehension | |||
| Pyramids and palm trees test–pictures (% correct) | 94.25 ± 7.77 | 56–100 | 28 |
| Peabody picture vocabulary test (16) | 14.31 ± 1.96 | 7–16 | 14 |
| Sentence comprehension (% correct) | 88.35 ± 13.27 | 36–100 | 16 |
The numbers in parentheses indicate the maximum possible score (or rating). AoS = apraxia of speech; CDR = Clinical Dementia Rating; CVLT = California Verbal Learning Test; MMSE = Mini-Mental State Examination; MSE = Motor Speech Evaluation; nfvPPA = non-fluent/agrammatic variant of primary progressive aphasia; SD = standard deviation; VOSP = Visual Object and Space Perception Battery; WAB = Western Aphasia Battery.
All participants provided written informed consent in accordance with the Declaration of Helsinki and the study protocols were approved by the UCSF Committee on Human Research.
Speech and language assessment
Our comprehensive speech and language assessment battery allows a wide range of speech-language skills to be evaluated, including speech production, speech comprehension, naming, repetition and reading (for more details, see Gorno-Tempini et al.7). However, in line with the aims of the current study, we focused on three specific component parts described later.
Assessing apraxia of speech and dysarthria through a structured motor speech evaluation
The motor speech evaluation (MSE)45 was designed to detect perceptual features indicative of the presence of AoS and/or dysarthria. To elicit a wide range of motor speech behaviours, the participant was asked, as part of the MSE, to complete a collection of tasks such as vowel prolongation, alternating motion rate, sequential motion rate, multiple repetitions of monosyllabic words, multiple repetitions of multisyllabic words, repetition of words of increasing length (same root word combined with different suffixes) and the reading of a brief, phonetically balanced paragraph (i.e. ‘The Grandfather Passage’).46 Based on the observed motor speech ability of the patient, a certified speech-language pathologist assigned a clinical severity rating for AoS and separately for dysarthria, on a scale from 0 (within normal limits) to 7 (profound). Of the 104 patients included in this study, 103 had an AoS and dysarthria severity rating available. A list of deviant motor speech characteristics used to perceptually judge the presence and severity of AoS and/or dysarthria is provided in Supplementary Table 2. Of note, inter-rater reliability with respect to two independent raters (authors Z.E. and L.D.W., both of whom are certified speech-language pathologists) was established in a subset of 15 patients (14% of the sample) that were quasi-randomly selected according to diagnostic classification and severity. Relative to the first independent rater, this analysis yielded an intraclass correlation coefficient (ICC) of 0.86 for AoS and 0.81 for dysarthria. As for the second independent rater, ICCs were 0.85 for AoS and 0.77 for dysarthria. According to the guidelines for interpretation of ICCs provided by Cicchetti,47 these results indicate excellent inter-rater agreement.
Assessing expressive agrammatism through a picture description task
To assess expressive agrammatism, the participant was prompted to describe a visual scene in as much detail as possible by using sentences. Audio-recorded connected speech samples acquired in this way were available for 91 of the 104 patients included in the study. One patient described the ‘cookie theft’ scene from the Boston Diagnostic Aphasia Examination,48 while the remaining 90 patients described the ‘picnic’ scene from the Western Aphasia Battery.49 Of these 90 patients, 87 described the ‘picnic’ picture orally and the remaining three provided a written picture description. Irrespective of the visual scene employed or response modality, all audio-recorded connected speech samples were sent to www.saltsoftware.com for transcription, coding and analysis. By running the coded transcripts through the Systematic Analysis of Language Transcripts (SALT) software,50 a set of measures was generated, of which we selected exclusively those that permitted us to capture the accuracy and complexity of the sentences produced by the patient (consistent with the definition of expressive agrammatism).
We examined three SALT-derived morphosyntactic variables: (i) % utterances with omission and/or commission errors (%UtWErrors), a measure of morphosyntactic accuracy that captures missing function/content words, omitted bound morphemes, inappropriate word choice and/or incorrect morphosyntactic form; (ii) mean length of utterance (MLU), a measure of morphosyntactic complexity that captures mean sentence length in words; and (iii) subordination index (SI), a measure of morphosyntactic complexity that captures the ratio of the total number of clauses to the total number of utterances. Critically, for oral picture description, only complete (not abandoned or interrupted), intelligible (without any unintelligible segments) and verbal utterances (that contained at least one verbalized word) contributed to the calculation of these three continuous measures. Therefore, two patients who did not produce any such utterances (complete, intelligible and verbal) were excluded, leaving a total of 89 datasets for subsequent analysis. With the goal of creating a single index of expressive grammar ability, the %UtWErrors (after reverse scoring), MLU and SI scores were combined by normalizing each one of them relative to the mean and SD of a group of 18 neurologically intact controls (mean age ± SD = 71.69 ± 5.27 years, range = 56–79 years; 12 females) and then averaging the resulting Z-scores per patient.
As described earlier, here we chose to characterize expressive agrammatism in terms of three SALT-derived morphosyntactic variables following a robust, long-established and widely recognized tradition of quantitative linguistic analysis of the extraordinarily rich data that connected speech samples provide.51-53 However, it is worth noting that there are other alternative approaches to the assessment of grammatical deficits in sentence production, which (due to their relative advantages and disadvantages) may yield partly non-overlapping results. For example, clinician-based auditory-perceptual rating of patients’ connected speech samples28,54 is generally easier to implement (requires less expert knowledge) and might be better suited to detect milder/subtler forms of expressive agrammatism, but usually at the expense of reliability and objective quantification given its inherently qualitative, subjective nature. Alternatively, quantitative assessment of agrammatic sentence production without the need for an overt spoken response is possible using the Northwestern Anagram Test,55 which avoids the potential confound of severely reduced speech fluency/rate and prominent anomia, but at the cost of not being able to capture important aspects of a patient’s grammatical competence, such as impairments of grammatical morphology, thus limiting its ecological validity. Last, written samples could be collected and analysed to assess agrammatism in language production when speech output is significantly compromised, but task demands are not exactly the same as in the spoken modality14 and written samples will likely be sensitive to premorbid differences in reading/writing skills as well as co-occurring deficits, such as hand-motor dysfunction, alexia and agraphia.
Assessing receptive agrammatism through a sentence comprehension task
To assess receptive agrammatism, the participant was prompted to complete either of two auditory sentence-to-picture matching tasks. The first task involved a representative range of sentence types, varying in both length and complexity, taken from the CYCLE-R (www.cycletests.com) as previously reported in Dronkers et al.56 and Amici et al.23 Each patient was instructed to match the meaning of an auditorily-presented sentence with the corresponding line drawing in a three- or four-picture array. The second task was loosely based on the first and has been previously described in Wilson et al.24 The primary difference between these two tasks is that the second included only two pictures (a target and a foil) and explicitly manipulated whether or not it is necessary to attend to syntactic structure to respond correctly. Other than that, both tasks tested the patient’s sentence comprehension skills. Of the 104 patients included in this study, 20 completed the first task and the other 68 performed the second task. These patients’ scores (i.e. % correct) were combined into a single index of receptive grammar ability, yielding a total of 88 datasets for subsequent analysis.
Testing for the presence of expressive and receptive agrammatism
While a MSE clinical severity rating for AoS/dysarthria >0 automatically indicates the presence of AoS/dysarthria as perceptually judged by a certified speech-language pathologist, the performance of an individual patient on the picture description and sentence comprehension tasks cannot be labelled as ‘impaired’ without reference to a normative sample of neurologically intact controls. Therefore, the Bayesian method developed by Crawford et al.,57 paired with a threshold of P < 0.05 one-tailed, was adopted to statistically test if a patient’s score fell within the impaired range (while co-varying out the effects of age and sex). For expressive agrammatism, the three selected morphosyntactic measures (i.e. %UtWErrors, MLU and SI) were considered individually relative to a group of 18 neurologically intact controls (mean age ± SD = 71.73 ± 5.27 years, range = 56–79 years; 12 females). For receptive agrammatism, we used for comparison a group of 10 neurologically intact controls who completed the first sentence-to-picture matching task (mean age ± SD = 61.79 ± 7.69 years, range = 49–75 years; six females) and another group of 26 neurologically intact controls who completed the second sentence-to-picture matching task (mean age ± SD = 69.69 ± 5.77 years, range = 53–79 years; 18 females). The output of these analyses allowed us to obtain a conservative estimate of the frequency of occurrence of expressive and receptive agrammatism across patients.
Neuropathological assessment
Neuropathological diagnoses were ascertained following published consensus criteria58-69 and standard procedures described previously.70-76 The primary neuropathological diagnosis was defined as the entity for which the severity and regional distribution best accounted for the patient’s clinical dementia syndrome.
MRI data acquisition and preprocessing
MRI scans were acquired on either of three Siemens MAGNETOM scanners at the San Francisco Veterans Affairs Medical Center (1.5 T) or UCSF Neurosciences Imaging Center (3 T): 17 patients were imaged on a 1.5 T Vision scanner, 50 on a 3 T Trio scanner and 37 on a 3 T Prisma scanner. In all three cases, a T1-weighted 3D magnetization prepared rapid acquisition gradient echo77 sequence was deployed to collect the whole-brain images. For the 1.5 T Vision scanner, the imaging parameters were: 164 coronal slices; voxel size = 1.0 × 1.5 × 1.0 mm3; field of view = 256 × 256 mm2; matrix size = 256 × 256; repetition time = 10 ms; echo time = 4 ms; inversion time = 300 ms; flip angle = 15°. For the 3 T Trio scanner, these were: 160 sagittal slices; voxel size = 1.0 × 1.0 × 1.0 mm3; field of view = 256 × 256 mm2; matrix size = 256 × 256; repetition time = 2300 ms; echo time = 2.98 ms; flip angle = 9°. For the 3 T Prisma scanner, these were: 160 sagittal slices; voxel size = 1.0 × 1.0 × 1.0 mm3; field of view = 256 × 256 mm2; matrix size = 256 × 256; repetition time = 2300 ms; echo time = 2.90 ms; flip angle = 9°.
All T1-weighted whole-brain images were quality checked by means of visual inspection to rule out the presence of artefacts and/or excessive motion. Next, these T1-weighted images were preprocessed with the Computational Anatomy Toolbox (CAT12; https://neuro-jena.github.io/cat/), using default parameters, in SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) running under MATLAB 2020b (The MathWorks, Inc., Natick, MA, USA). Preprocessing of imaging data in CAT12 for voxel-based statistical analysis comprises two major steps. In the first step, a spatial adaptive non-local means denoising filter78 is applied to the data, followed by internal resampling to properly accommodate low-resolution images and anisotropic spatial resolutions. The data are then bias-corrected, affine-registered and finally submitted to the standard SPM ‘unified segmentation’ routine.79 In the second step, the output images (from the SPM unified segmentation) are skull-stripped and the brain is parcellated into left and right hemispheres, subcortical regions and cerebellum. Subsequently, a local intensity transformation of all tissue classes is performed, before the final adaptive maximum a posteriori (AMAP) segmentation.80 Importantly, the AMAP segmentation is further refined by applying a partial volume estimation.81 Last, the segmented images are (i) spatially normalized into MNI space using an optimized geodesic shooting procedure82; (ii) multiplied by the Jacobian determinants of the deformation field applied during spatial normalization to preserve the absolute amount of a particular tissue; and (iii) written out with an isotropic voxel size of 1.5 × 1.5 × 1.5 mm3.
For each patient, the ensuing modulated-normalized grey matter (GM) and white matter (WM) images from CAT12 were combined into a single whole-brain image (similar to Wilson et al.83) by applying, on a voxel-by-voxel basis, the ‘max’ operator in MATLAB. These combined ‘GM&WM’ images were then spatially smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel to compensate for residual anatomical variability and to permit application of random field theory for statistical inference in SPM12.84
Single-subject atrophy maps
To estimate the frequency with which each voxel in the brain was atrophic across patients, voxel-wise W-maps of the combined GM&WM images were created as described previously.85,86 In brief, W-maps quantify the degree to which voxel-wise brain tissue volumes in each patient deviate from those in neurologically intact controls considering the influence of a set of covariates. Following Ossenkoppele et al.,85 we accounted for the influence of age, sex, total intracranial volume (TIV) and scanner on brain tissue volume by fitting a voxel-wise multiple regression model to the preprocessed imaging data from a sample of 133 neurologically intact controls (mean age ± SD = 67.18 ± 6.89 years, range = 49–80 years; 84 females). The voxel-wise beta coefficients from the regression in neurologically intact controls were then applied to the preprocessed imaging data from each patient (i.e. combined GM&WM images) to derive covariate-adjusted brain tissue volumes (i.e. W-scores) with the following formula: (observed brain tissue volume − expected brain tissue volume) / SD of the residuals for that voxel in neurologically intact controls. Since the distribution of W-scores is analogous to that of Z-scores, the W-map for each patient was binarized using an uncorrected voxel-level threshold of P < 0.05 one-tailed (i.e. W-score < −1.64, as in Iaccarino et al.86) and a cluster extent threshold of at least 100 contiguous voxels, yielding a binary map of the presence or absence of atrophy at each voxel across the brain.
Brain-behaviour relationships
Statistical analysis of brain-behaviour relationships was performed using the general linear model for voxel-based morphometry (VBM)87,88 in SPM12. As described later, we carried out a total of three voxel-based multiple regression analyses. In each of these three analyses, we entered the combined GM&WM images and the following set of nuisance covariates: age, sex, scanner, TIV and % GM + WM {= [(total GM volume + total WM volume) / TIV] × 100}. This latter metric (i.e. % GM + WM) was included to further refine our ability to associate specific behavioural symptoms with the disruption of specific brain regions, after accounting for global effects of whole-brain atrophy beyond the statistical control already provided by TIV (correlation between % GM + WM and TIV = −0.15, P = 0.128). Other confounding factors were expected to have an analysis-specific effect and are therefore detailed later.
VBM Analysis 1: apraxia of speech and dysarthria
This analysis consisted of two regressors of interest: the AoS severity rating and the dysarthria severity rating from our MSE. Based on these regressors, we computed two contrasts. The first T-contrast [(−1 0)] investigated the brain regions where the degree of tissue loss is preferentially associated with the severity of AoS. The second T-contrast [(0 −1)] investigated the brain regions where the degree of tissue loss is preferentially associated with the severity of dysarthria. Controlling for the influence of dysarthria when attempting to isolate the neural correlates of AoS (and vice versa) is critical because these two motor speech disorders are known to share a subset of deviant motor speech characteristics.89 In line with this, there was a significant correlation between the severity of AoS and the severity of dysarthria (r = 0.33, P < 0.001), but not to the point of inducing multicollinearity. A total of 103 patients contributed data to VBM Analysis 1.
VBM Analysis 2: expressive agrammatism
There were two iterations of this analysis, both of which involved the same single regressor of interest: our expressive grammar score (i.e. average of %UtWErrors, MLU and SI). The first iteration (i.e. VBM Analysis 2a) investigated the brain regions where the degree of tissue loss is associated with the severity of expressive agrammatism. A total of 89 patients contributed data to VBM Analysis 2a. The second iteration (i.e. VBM Analysis 2b) included two additional regressors of no interest: (i) words per minute to control for overall speech fluency/rate; and (ii) scores from a 15-item picture naming task (i.e. Boston Naming Test)90 to control for visual-perceptual, object recognition and word retrieval abilities. By adding these two regressors of no interest into the regression, we sought to partial out variance in our expressive grammar score that was not specific to the morphosyntactic encoding of sentences, thereby focusing the inference on brain regions that support expressive grammar skills specifically (as opposed to, for example, word retrieval skills). A total of 85 patients contributed data to VBM Analysis 2b.
VBM Analysis 3: receptive agrammatism
There also were two iterations of this analysis, both of which involved the same single regressor of interest: our receptive grammar score. In the first iteration (i.e. VBM Analysis 3a), we investigated the brain regions where the degree of tissue loss is associated with the severity of receptive agrammatism. A total of 88 patients contributed data to VBM Analysis 3a. In the second iteration (i.e. VBM Analysis 3b), we included two additional regressors of no interest: (i) backward digit span to control for auditory-verbal working memory ability; and (ii) scores from a 16-item auditory word-to-picture matching task (i.e. Peabody Picture Vocabulary Test)91 to control for auditory-perceptual, visual-perceptual, object recognition and word recognition abilities. By adding these two regressors of no interest into the regression, we aimed to partial out variance in our receptive grammar score that was not specific to the morphosyntactic decoding of sentences, thereby focusing the inference on brain regions that support receptive grammar skills specifically (as opposed to, for example, word recognition skills). A total of 78 patients contributed data to VBM Analysis 3b.
Search volume and statistical threshold
For each of the three VBM analyses described earlier, the search volume was defined by an explicit mask, which comprised the union of the grey matter and white matter tissue probability maps provided with SPM12, after being thresholded at a voxel-wise value ≥ 0.2. For each T-contrast computed, the corresponding statistical map was evaluated at a voxel-level threshold of P < 0.005 uncorrected and a cluster-level threshold of P < 0.05 family-wise error (FWE) corrected.
Results
Frequency of occurrence of impaired motor speech and agrammatism
It is beyond the scope of this study to establish how impaired motor speech and agrammatism develop longitudinally in nfvPPA. Nonetheless, with the available cross-sectional data we were able to obtain a general estimate of the frequency of occurrence of these symptoms.
Across the subset of patients with complete datasets (n = 74), impaired motor speech was observed in 99% of the cases: 58% (43/74) had both AoS and dysarthria, 32% (24/74) had AoS only, and 8% (6/74) had dysarthria only, as perceptually judged by certified speech-language pathologists. On the other hand, quantitative evidence of agrammatism (expressive and/or receptive) was identified in 76% of these patients: 46% (34/74) had both expressive and receptive agrammatism, 16% (12/74) had expressive agrammatism only, and 14% (10/74) had receptive agrammatism only. When considered in combination, impaired motor speech (AoS and/or dysarthria) and agrammatism (expressive and/or receptive) were present in 74% (55/74) of the analysed cases. See Supplementary Fig. 2 for a more detailed breakdown of these features.
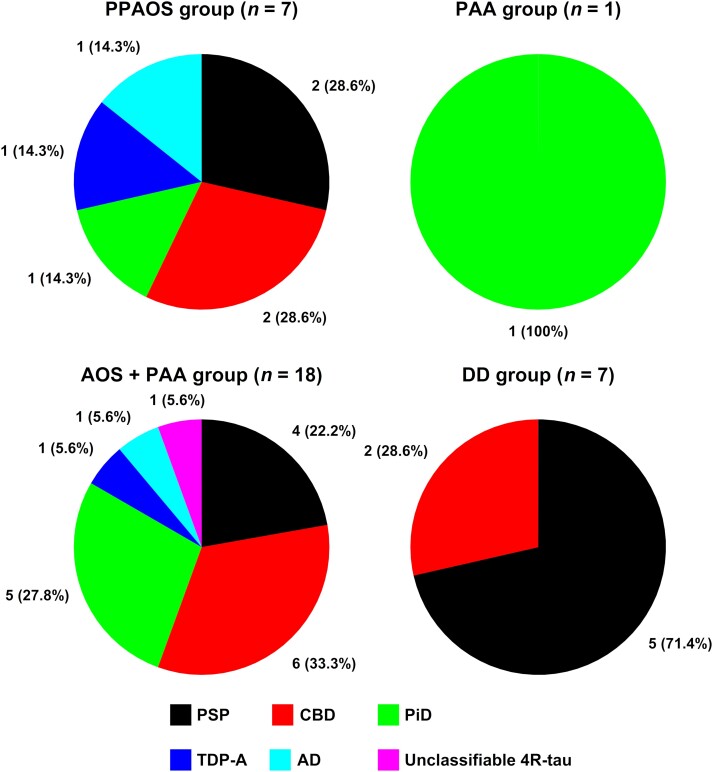
A definitive, autopsy-confirmed neuropathological diagnosis was available for 33 of the 74 (45%) patients with complete datasets. By looking at the incidence of AoS, dysarthria and expressive agrammatism, these 33 cases were further subclassified as follows: seven (21%) met criteria for PPAOS (i.e. AoS in the absence of expressive agrammatism), one (3%) met criteria for PAA (i.e. expressive agrammatism in the absence of AoS), 18 (55%) presented with mixed features (AOS + PAA) and the remaining seven (21%) patients were characterized primarily by a progressive motor speech impairment in which dysarthria rather than AoS was the most salient clinical feature (dominant dysarthria). Beyond the known association between these distinct clinical presentations of nfvPPA and FTLD-tau subtypes, and that between dysarthria and progressive supranuclear palsy (PSP) pathology, no other clear clinico-pathological correlations emerged from our examination. See Fig. 1 for more details.
Figure 1.
Distribution of neuropathological subtypes across distinct clinical presentations of nfvPPA. The figure illustrates the overlap, in terms of underlying neuropathology, between different speech-language phenotypes subsumed under the umbrella term ‘nfvPPA’. Apart from an increased frequency of PSP pathology in patients with dominant dysarthria (DD group), no other clinico-pathological correlations were observed [disregarding the progressive agrammatic aphasia (PAA) group which comprised one case only]. The vast majority of patients (28/33 = 85%) exhibited FTLD-tau (PSP, CBD, PiD, and unclassifiable 4R-tau) as primary neuropathology. AD = Alzheimer’s disease; AOS = apraxia of speech; CBD = corticobasal degeneration; FTLD = frontotemporal lobar degeneration; nfvPPA = non-fluent/agrammatic variant of primary progressive aphasia; PiD = Pick’s disease; PSP = progressive supranuclear palsy; PPAOS = primary progressive apraxia of speech; TDP-A = transactive response DNA-binding protein 43 kD type A.
Distribution of atrophy in nfvPPA
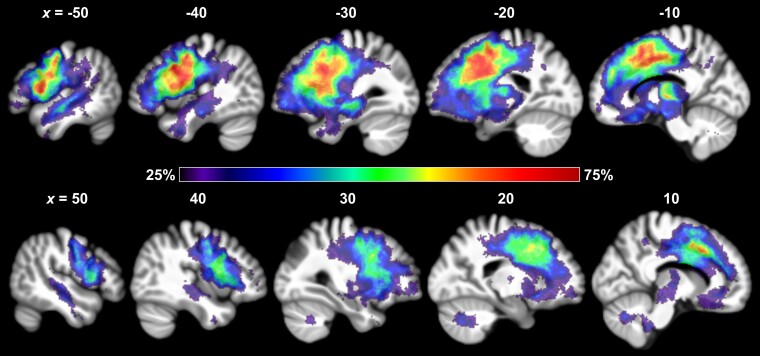
Across patients, we observed the expected pattern of atrophy in nfvPPA, including greater involvement of the left than the right hemisphere. In particular, the most frequently atrophic brain regions were, bilaterally, the primary motor cortex, premotor cortex, pars opercularis, deep frontal operculum, insula, putamen, supplementary motor area (SMA), pre-SMA and neighbouring white matter (Fig. 2).
Figure 2.
Atrophy frequency map of 104 patients with nfvPPA. The figure shows the distribution of tissue loss across the brain, with the colour scale depicting the percentage of patients with atrophy at each given voxel in sagittal slices. nfvPPA = non-fluent/agrammatic variant of primary progressive aphasia.
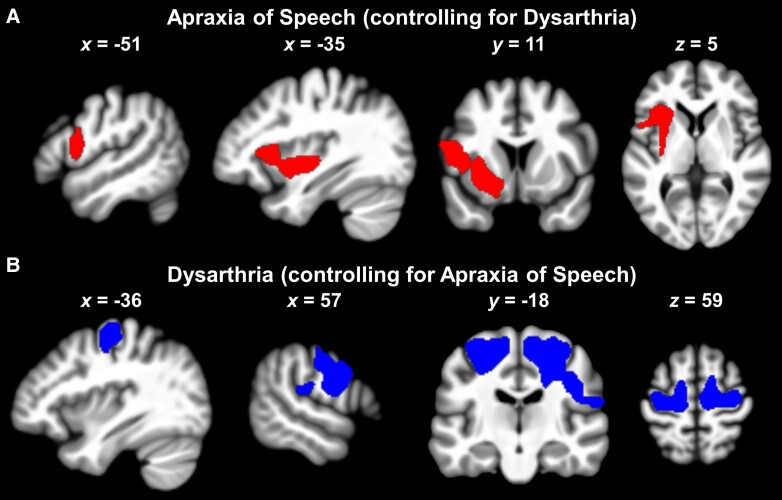
Neural correlates of impaired motor speech
Within the motor speech network, AoS (controlling for dysarthria) was preferentially associated with tissue loss in a cluster of left-hemisphere regions spanning ventral premotor cortex, posterior-most aspect of pars opercularis, deep frontal operculum, anterior insula, putamen and neighbouring white matter (Table 2 and Figs 3A and 4). In contrast, dysarthria (controlling for AoS) was preferentially related to tissue loss in two separate clusters of regions: one in the right hemisphere and one in the left hemisphere (Table 2 and Figs 3B and 4). The right-hemisphere cluster (i.e. the most prominent effect) comprised the primary somatosensory cortex, primary motor cortex, premotor cortex, SMA (posterior-most part only), mid corpus callosum and surrounding white matter. The left-hemisphere cluster encompassed the dorsal primary motor cortex, dorsal premotor cortex and neighbouring white matter (extending medially towards but without reaching SMA).
Table 2.
VBM statistical details: neural correlates of impaired motor speech
| Brain region | Peak MNI coordinates | Voxel-level inference | Cluster-level inference | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | t-value | P-value (unc) | Extent | P-value (FWE-corr) | |
| VBM Analysis 1: apraxia of speech (controlling for dysarthria) | |||||||
| LPut | −32 | −14 | 0 | 4.91 | 0.000 | 5462a | 0.003 |
| LWM/LPut | −27 | 18 | 3 | 4.32 | 0.000 | ||
| LPut | −27 | 3 | −9 | 4.18 | 0.000 | ||
| LWM/LPut | −18 | 12 | −14 | 3.97 | 0.000 | ||
| LFO/LadIns | −38 | 20 | 3 | 3.45 | 0.000 | ||
| LpOp/LvPMC | −48 | 9 | 16 | 3.37 | 0.001 | ||
| LWM/LPut | −30 | −3 | 9 | 3.33 | 0.001 | ||
| LadIns/LFO | −44 | 8 | −2 | 2.78 | 0.003 | ||
| VBM Analysis 1: dysarthria (controlling for apraxia of speech) | |||||||
| RvPreCG | 57 | 2 | 26 | 5.89 | 0.000 | 13578a | 0.000 |
| RWM/RdPreCG | 36 | −12 | 57 | 4.83 | 0.000 | ||
| RWM/RdPreCG | 12 | −14 | 60 | 4.44 | 0.000 | ||
| RWM/RdPreCG | 16 | −12 | 60 | 4.43 | 0.000 | ||
| RvPreCG | 42 | −8 | 36 | 4.43 | 0.000 | ||
| RWM/RvPreCG | 44 | −8 | 40 | 4.42 | 0.000 | ||
| RvPostCG | 56 | −8 | 42 | 4.32 | 0.000 | ||
| RWM/RmCS | 15 | −9 | 46 | 3.82 | 0.000 | ||
| RavSMG/RPO | 54 | −26 | 16 | 3.72 | 0.000 | ||
| CC | 8 | −12 | 24 | 3.61 | 0.000 | ||
| RWM | 18 | −8 | 34 | 3.45 | 0.000 | ||
| RmCS/RmCG | 9 | 4 | 40 | 3.18 | 0.001 | ||
| LWM/LdPreCG | −28 | −15 | 52 | 3.91 | 0.000 | 3452a | 0.025 |
| LWM/LdPreCG | −15 | −20 | 62 | 3.89 | 0.000 | ||
| LWM/LdPreCG | −34 | −12 | 56 | 3.84 | 0.000 | ||
| LWM/LdPreCG | −14 | −3 | 58 | 3.45 | 0.000 | ||
The table lists representative peak voxels. ad = anterodorsal; CC = corpus callosum; CG = cingulate gyrus; CS = cingulate sulcus; d = dorsal; FO = frontal operculum; FWE-corr = family-wise error corrected; Ins = insula; L = left; m = mid; MNI = Montreal Neurological Institute space; PMC = premotor cortex; PO = parietal operculum; pOp = pars opercularis; PostCG = postcentral gyrus; PreCG = precentral gyrus; Put = putamen; R = right; SMG = supramarginal gyrus; unc = uncorrected; v = ventral; VBM = voxel-based morphometry; WM = white matter.
aUsing a cluster-forming voxelwise threshold of P < 0.005 uncorrected.
Figure 3.
Patterns of brain tissue loss associated with impaired motor speech in nfvPPA. (A) Apraxia of speech effect from VBM Analysis 1. (B) Dysarthria effect from VBM Analysis 1. Images are shown in neurological orientation. nfvPPA = non-fluent/agrammatic variant of primary progressive aphasia; VBM = voxel-based morphometry.
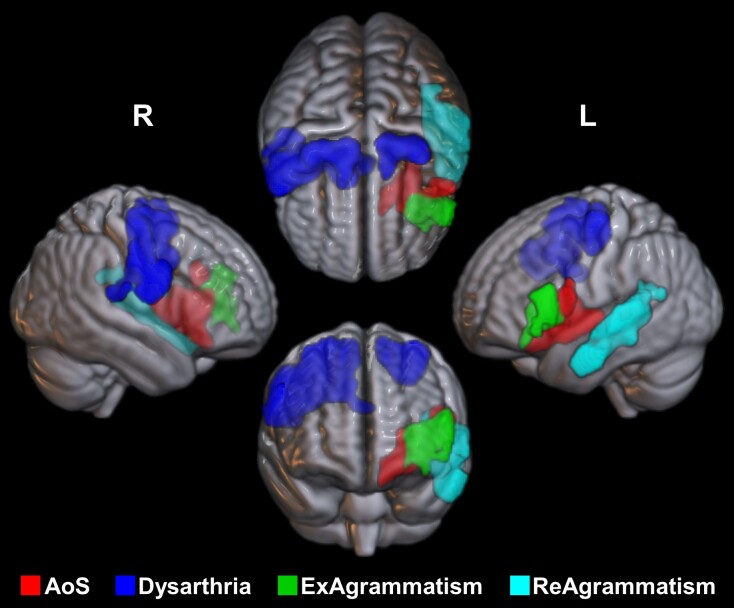
Figure 4.
3D volume rendering of thresholded statistical maps. The figure illustrates the relative location of the neural substrates of apraxia of speech (AoS) and dysarthria (both from VBM Analysis 1), as well as expressive (Ex) and receptive (Re) agrammatism (from VBM Analyses 2b and 3b, respectively). There was a confined area of overlap between the effects of AoS and expressive agrammatism within the deep left frontal operculum. L = left hemisphere; R = right hemisphere; VBM = voxel-based morphometry.
Neural correlates of agrammatism
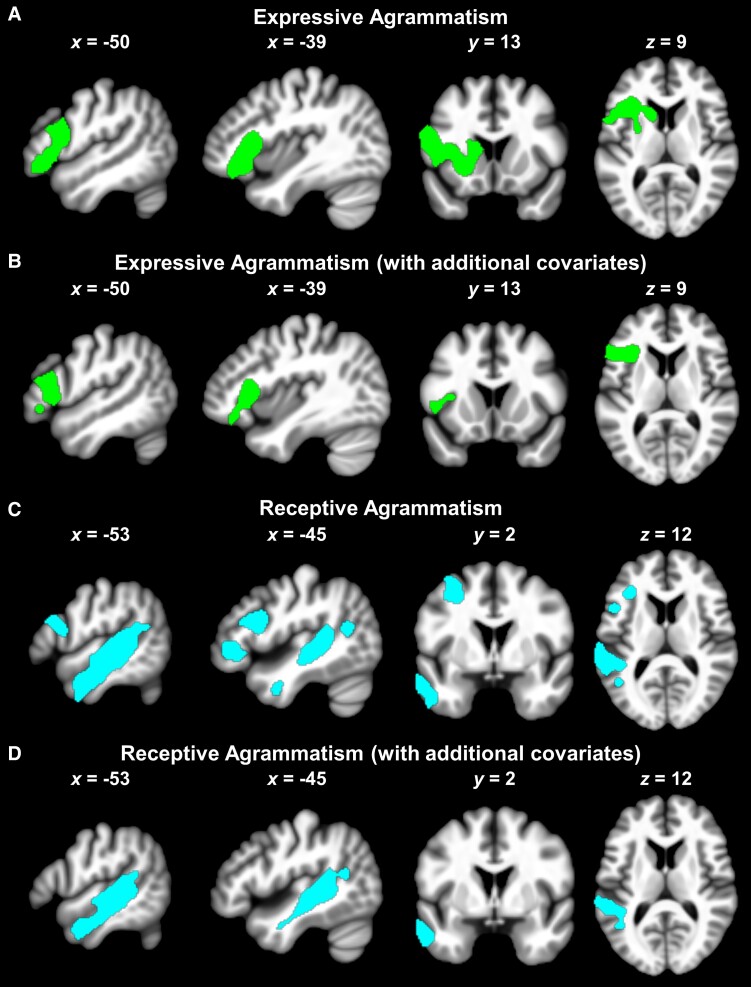
Within the grammar network, expressive agrammatism was linked to tissue loss in a cluster of left-hemisphere regions involving the pars opercularis (extending posteriorly into ventral premotor cortex), pars triangularis (extending anteriorly into pars orbitalis), deep frontal operculum, putamen, caudate and surrounding white matter (Table 3 and Fig. 5A). Critically, when the analysis co-varied out variance unrelated to morphosyntactic encoding (e.g. reflecting inter-patient differences in object recognition, word retrieval and/or speech fluency), expressive agrammatism was uniquely associated with tissue loss in the posterior half of the left pars triangularis (extending into its anterior half and pars orbitalis), anterior-most portion of pars opercularis, deep frontal operculum and neighbouring white matter (Table 3 and Figs 4 and 5B).
Table 3.
VBM statistical details: neural correlates of agrammatism
| Brain region | Peak MNI coordinates | Voxel-level inference | Cluster-level inference | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | t-value | P-value (unc) | Extent | P-value (FWE-corr) | |
| VBM Analysis 2a: expressive agrammatism | |||||||
| LFO/LpTri | −39 | 22 | 4 | 5.12 | 0.000 | 7961a | 0.000 |
| LCau | −12 | 4 | 20 | 4.62 | 0.000 | ||
| LpOrb/LFP | −39 | 33 | −12 | 4.01 | 0.000 | ||
| LpOrb | −45 | 30 | −8 | 3.81 | 0.000 | ||
| LpOp | −52 | 10 | 18 | 3.64 | 0.000 | ||
| LPut | −28 | 9 | −2 | 3.40 | 0.001 | ||
| LOFC | −24 | 20 | −20 | 3.02 | 0.002 | ||
| VBM Analysis 2b: expressive agrammatism (with additional covariates) | |||||||
| LFO/LpTri | −40 | 22 | 6 | 4.83 | 0.000 | 3487a | 0.019 |
| LIFS/LpTri | −51 | 30 | 18 | 3.62 | 0.000 | ||
| LpTri | −56 | 26 | 12 | 3.41 | 0.001 | ||
| LpOrb/LFP | −39 | 34 | −14 | 3.22 | 0.001 | ||
| LpTri/LpOrb | −52 | 30 | 8 | 3.05 | 0.002 | ||
| LpTri | −56 | 30 | −2 | 3.04 | 0.002 | ||
| VBM Analysis 3a: receptive agrammatism | |||||||
| LaMTG | −56 | −9 | −24 | 5.41 | 0.000 | 9637a | 0.000 |
| LmSTS | −63 | −28 | −3 | 5.22 | 0.000 | ||
| LmSTS | −60 | −22 | −6 | 5.01 | 0.000 | ||
| LpSTG/LpSTS | −52 | −36 | 4 | 4.55 | 0.000 | ||
| LpMTG/LavAG | −46 | −57 | 16 | 3.46 | 0.000 | ||
| LWM | −39 | −28 | −9 | 3.07 | 0.001 | ||
| LWM/LpOp | −48 | 16 | 20 | 4.89 | 0.000 | 5300a | 0.003 |
| LWM/LpMFG | −32 | 6 | 51 | 4.51 | 0.000 | ||
| LFO | −34 | 32 | 3 | 4.14 | 0.000 | ||
| LWM/LpMFG | −34 | 14 | 34 | 3.98 | 0.000 | ||
| LpMFG/LpSFS | −26 | 18 | 45 | 3.37 | 0.001 | ||
| VBM Analysis 3b: receptive agrammatism (with additional covariates) | |||||||
| LaMTG | −57 | −2 | −27 | 4.96 | 0.000 | 7753a | 0.000 |
| LpSTS/LpSTG | −46 | −38 | 3 | 4.27 | 0.000 | ||
| LpSTS/LpSTG | −48 | −33 | 2 | 4.26 | 0.000 | ||
| LmSTS/LmSTG | −60 | −32 | 0 | 4.11 | 0.000 | ||
| LmSTS/LmSTG | −62 | −20 | −4 | 3.47 | 0.000 | ||
| LaSTS/LaMTG | −60 | −8 | −9 | 3.40 | 0.001 | ||
| LpMTG/LavAG | −45 | −56 | 15 | 3.25 | 0.001 | ||
| LWM/LpMTG | −40 | −51 | 16 | 3.14 | 0.001 | ||
The table lists representative peak voxels. a = anterior; AG = angular gyrus; av = anteroventral; Cau = caudate; FO = frontal operculum; FP = frontal pole; FWE-corr = family-wise error corrected; IFS = inferior frontal sulcus; L = left; m = mid; MFG = middle frontal gyrus; MNI = Montreal Neurological Institute space; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; P = posterior; pOp = pars opercularis; pOrb = pars orbitalis; pTri = pars triangularis; Put = putamen; R = right; SFS = superior frontal sulcus; STG = superior temporal gyrus; STS = superior temporal sulcus; VBM = voxel-based morphometry; WM = white matter.
aUsing a cluster-forming voxelwise threshold of P < 0.005 uncorrected.
Figure 5.
Patterns of brain tissue loss associated with agrammatism in nfvPPA. (A) Expressive agrammatism effect from VBM Analysis 2a. (B) Expressive agrammatism effect from VBM Analysis 2b (i.e. co-varying out variance unrelated to morphosyntactic encoding). (C) Receptive agrammatism effect from VBM Analysis 3a. (D) Receptive agrammatism effect from VBM Analysis 3b (i.e. co-varying out variance unrelated to morphosyntactic decoding). Images are shown in neurological orientation. nfvPPA = non-fluent/agrammatic variant of primary progressive aphasia; VBM = voxel-based morphometry.
Receptive agrammatism was related to tissue loss in two separate clusters of left temporal and frontal areas (Table 3 and Fig. 5C). The left temporal lobe cluster spanned the mid-to-posterior superior temporal gyrus, mid-to-posterior superior temporal sulcus, middle temporal gyrus (extending ventrally into inferior temporal gyrus) and underlying white matter. The left frontal lobe cluster comprised the dorsal pars opercularis, anterior half of pars triangularis (extending rostrally into the pars orbitalis and medially into the deep frontal operculum), inferior frontal junction/sulcus, posterior middle frontal gyrus (extending dorsally into superior frontal sulcus) and surrounding white matter. Notably, when the analysis regressed out variance not attributable to morphosyntactic decoding (e.g. due to inter-patient differences in object recognition, word recognition and/or auditory-verbal working memory), expressive agrammatism was uniquely associated with tissue loss in the left temporal, but not frontal lobe (Table 3 and Figs 4 and 5D). Adding a binary regressor to VBM Analysis 3b to partial out effects specific to one or the other of the two sentence comprehension tasks used to create our index of receptive grammar ability weakened the results (e.g. peak voxel t-value: 4.96 without ‘task’ regressor versus 4.75 with ‘task’ regressor) but, crucially, did not alter their overall pattern.
Finally, a post hoc analysis (n = 73) designed to examine the neural substrates of expressive and receptive agrammatism simultaneously (while co-varying out variance unrelated to morphosyntactic abilities: i.e. VBM Analyses 2b and 3b combined) confirmed the preferential involvement of (i) left frontal lobe tissue loss in expressive agrammatism; and (ii) left temporal lobe tissue loss in receptive agrammatism.
Discussion
Using a single, large patient cohort, we mapped the structural anatomy of four characteristic speech-language symptoms of nfvPPA: AoS, dysarthria, expressive agrammatism and receptive agrammatism. We found that the neural substrates of AoS and expressive agrammatism (the two core clinical features of nfvPPA) lie side by side in the left posterior inferior frontal lobe, explaining why they co-occur more often than not (cross-sectionally and longitudinally) in the context of network-based neurodegeneration. For impaired motor speech, we identified a distributed set of areas arranged primarily along the central sulcus bilaterally, with dysarthria mapping within the left hemisphere onto dorsally located motor-speech regions compared to ventrally located ones in the case of AoS. For agrammatism, we detected a broader left-lateralized fronto-temporal network, with evidence suggesting that left frontal sites may play a more prominent role in morphosyntactic processing for sentence production, while left temporal sites may play a more prominent role in morphosyntactic processing for sentence comprehension. All four of these symptoms were most frequently associated with FTLD-tau, although patients with dominant dysarthria showed an increased prevalence of PSP pathology specifically (consistent with Santos-Santos et al.35). Based on these findings, we propose that nfvPPA could be conceptualized as a spectrum disorder encompassing multiple (and often overlapping) speech-language phenotypes, all of which are closely linked by the underlying neuroanatomy and neuropathology.
Next, we discuss the scientific and clinical implications of our findings in relation to prior literature.
Neural correlates of impaired motor speech
Impaired motor speech is a hallmark clinical feature of nfvPPA, very frequently taking the form of AoS with or without concomitant dysarthria. For example, nearly 100% of the patients in our cohort exhibited AoS and/or dysarthria. Interestingly, our investigation into brain-behaviour relationships revealed a neuroanatomical distinction between AoS versus dysarthria, that it is possible to recapitulate in two main axes: left-right and ventral-dorsal. The first left-right axis appears to suggest that, in contrast to the preponderance of the left hemisphere for the motor planning/programming of speech (i.e. the presumed locus of impairment in AoS), the right hemisphere plays a relevant role in the motor control/execution of speech (i.e. the presumed locus of impairment in dysarthria), since we found that the severity of dysarthria is influenced by the degree of tissue loss in right-hemisphere motor-speech regions including the white matter in the territory of, for instance, the corticobulbar tract. Indeed, prior studies have shown (i) that speech articulation is normally subserved by a bilaterally distributed neural system arranged primarily along the lateral and medial cortical surface surrounding the central sulcus92-94; and (ii) that progressive (spastic/hypokinetic) dysarthria is correlated with tissue loss in the right sensorimotor cortex as well as underlying white matter.20,95 In stroke survivors, although its severity is usually worse (but still within the mild-to-moderate range) after unilateral infarction of the left than the right hemisphere, substantial or even complete recovery from dysarthria has been consistently observed following both left- and right-sided lesions.29 This, coupled with our own findings, raises an interesting hypothesis for future studies: right-hemisphere motor-speech regions may be able to compensate for the loss of those in the left hemisphere (and vice versa), with more severe forms of dysarthria emerging as a function of the degree of involvement of the contralateral (typically the right) sensorimotor cortex and underlying white matter.
Regarding the second ventral-dorsal axis, it captures our finding that, within the left hemisphere, dysarthria and AoS are preferentially associated with tissue loss in dorsally versus ventrally located motor-speech regions, respectively. Notably, the same (or a very similar) part of the left dorsal precentral gyrus, where tissue loss is linked to dysarthria according to our results, has previously been implicated in either progressive AoS (irrespective of subtype)96 or progressive dysarthria.20 Such inconsistent brain-behaviour mapping across studies might be in part a consequence of the existence of a subset of altered motor speech behaviours that can be explained equally well by AoS and dysarthria.89 Here, we were able to tease their effects apart by statistically controlling for the influence of one or the other of these two motor speech disorders in a whole-brain voxel-based analysis that yielded evidence of a unique relationship between an impairment in the motor control/execution of speech (i.e. dysarthria) and the structural integrity of this left dorsal precentral region. In favour of our interpretation, prior work in neurologically intact controls has indicated that a seemingly overlapping dorsal area within the left precentral gyrus subserves major aspects of motor speech function, such as pitch and voicing,97 due to its role in laryngeal and respiratory motor control.98,99 For example, direct electrical stimulation over this left dorsal precentral region (called dorsal laryngeal motor cortex) has been reported to induce laryngeal movements in participants under general anaesthesia and involuntary vocalizations in awake participants, confirming its causal involvement in the feed-forward motor control of laryngeal muscles.97 In contrast, focal surgical resection of a region located more anteriorly in the left posterior middle frontal gyrus resulted in AoS in a single patient following surgery for astrocytoma removal,100 which may (or may not) be explained by atypical functional anatomy or indirect effects of white matter disconnection. Future studies are now needed to specifically investigate the potential existence of a rostral-to-caudal functional gradient extending from the left posterior middle frontal gyrus to the left dorsal precentral gyrus,101,102 employing methods capable of resolving fine-grained functional and syndromic distinctions in nfvPPA.
With respect to the neural correlates of AoS, we identified a collection of ventrally located left posterior frontal regions that are likely to contribute to the motor planning/programming of speech, including a swath of tissue centred around the ventral precentral sulcus, anterior insula, putamen and neighbouring white matter. Despite it being possible that some of the AoS effects in the left posterior inferior frontal gyrus may have been partly driven by the presence of agrammatism (given how frequently these two symptoms co-occurred across patients), these brain-behaviour associations are broadly in agreement with the existing body of knowledge on the neural basis of AoS.11,26,27,103,104 For example, Rohrer et al.105 reported in 16 patients with nfvPPA that reduced diadochokinetic rate (used as a surrogate measure of AoS severity) correlated with greater tissue loss in the left posterior inferior frontal cortex. In addition, Ogar et al.106 noted that left-hemisphere stroke patients with more extensive lesions involving the anterior insula as well as posterior inferior frontal cortex and basal ganglia tended to have more severe AoS than those with more focal lesions affecting the anterior insula. We have extended these and other previous observations in a much larger sample of nfvPPA patients by showing that, above and beyond the effect of dysarthria, there is a linear relationship between the severity of AoS (as clinically rated) and the degree of tissue loss in these regions, each of which might play a unique role in the motor planning/programming of speech. According to one of the most influential neurocomputational models of the motor control of speech (i.e. DIVA/GODIVA),107 AoS-like behaviours would primarily result from damage to the left posterior inferior frontal sulcus, left ventral premotor cortex, the connections between these two, or any combination thereof. Our results partially confirm this prediction, highlighting the functional relevance of the neural site at the intersection of the left ventral premotor cortex and the left pars opercularis. Critically, Mugler et al.108 revealed in patients undergoing awake craniotomy for glioma removal that neural activity in the left ventral premotor cortex encodes articulatory gestures (i.e. context-dependent speech sound representations) to a greater extent than phonemes (i.e. context-independent speech sound representations), while the left pars opercularis encodes both articulatory gestures and phonemes. Given how frequently atrophy in patients with nfvPPA impinges on the left pars opercularis,7 those new to the field are thus advised to devote special attention to differentiating speech production errors that reflect an impairment at the level of phonological (language-based) versus phonetic encoding (articulatory-based).109,110
Neural correlates of agrammatism
In addition to impaired motor speech, patients with nfvPPA often have expressive agrammatism with or without co-occurring receptive agrammatism. For example, quantitative evidence of either or both forms of agrammatism was detected in 76% of the cases included in our large patient cohort. Crucially, in keeping with previous studies of sentence processing,111-114 we were able to dissect a distributed network of fronto-temporal sites in which left frontal versus temporal lobe structures might differentially contribute to expressive versus receptive grammar abilities, respectively. Specifically, worse symptom severity was preferentially associated with greater tissue loss in a cluster of regions centred on (i) the left posterior pars triangularis/anterior pars opercularis for expressive agrammatism; and (ii) the left middle temporal gyrus for receptive agrammatism. These empirical findings align well with the neuroanatomical model of morphosyntactic processing proposed by Matchin and Hickok,115 which assigns a critical role to the left pars triangularis and mid-to-posterior middle temporal gyrus in carrying out fundamental morphosyntactic computations. Moreover, by providing an unbiased description of the extent of these effects and appreciating the importance of the underlying white matter, our whole-brain results complement the Region of interest × Condition interactions reported in Matchin et al.,116 who primarily associated agrammatic sentence production with damage to the left posterior pars triangularis/anterior pars opercularis and agrammatic sentence comprehension with damage to the left posterior middle temporal gyrus/superior temporal sulcus. Likewise, Matchin et al.28 documented a significant relationship between damage to Broca’s area and the presence of expressive agrammatism (as clinically rated) after adjusting for overall speech fluency (words per minute) in the context of a region of interest analysis. We have replicated and refined this finding by highlighting which parts of Broca’s area were found to be most important in a whole-brain analysis that controlled for overall speech fluency and other components (e.g. word retrieval skills) that could have influenced the performance of the patients on the picture description task that we used to derive our quantitative index of expressive grammar ability. Importantly, converging evidence in support of our brain-behaviour findings for expressive agrammatism comes from a study of patients undergoing awake craniotomy where direct electrical stimulation over the left posterior inferior frontal cortex neighbouring the ascending ramus of the lateral sylvian fissure (i.e. posterior pars triangularis and anterior pars opercularis) selectively interfered with the morphosyntactic encoding of sentence structure during production,117 albeit only in half of the subjects, implying substantial variability in functional anatomy.
While the critical contribution of left temporal regions to the morphosyntactic decoding of auditorily-presented sentences is widely recognized,24,56,118-123 the link between receptive agrammatism and left posterior inferior frontal regions remains controversial. For example, conflicting evidence has led some authors to conclude that the structural integrity of the left posterior inferior frontal cortex is essential for successful sentence comprehension,24,118,120 whereas others have challenged its causal involvement in agrammatic sentence comprehension.119,122,123 In the current study, we did initially find a significant association between poorer receptive grammar ability and greater tissue loss in several left frontal as well as temporal regions. However, only the left temporal, but not frontal, effects of receptive agrammatism held after regressing out variance attributable to several task components (e.g. word recognition) other than morphosyntactic decoding. Importantly, these left temporal effects are unlikely to be explained by the peripheral and central auditory processing deficits previously documented in nfvPPA,124-126 because any influence of auditory impairments would have been partialled out after the inclusion of the ‘auditory word recognition’ and ‘auditory-verbal working memory’ nuisance covariates in the analysis. Consequently, we hypothesize that, within the grammar network, a partial division of labour between left frontal and temporal sites in morphosyntactic processing for sentence production (frontal > temporal) versus comprehension (temporal > frontal) could explain previous as well as our own findings. In this context, we argue that beyond their predominant involvement in morphosyntactic processing for sentence production, specific parts of the left posterior inferior frontal cortex are likely to play a complementary role in sentence comprehension under certain circumstances (e.g. when sentences are more complex and/or ambiguous),127 possibly through generating top-down predictions of morphosyntactic structure that might facilitate parsing and interpretation in the relevant left temporal regions.128 It therefore follows that relatively focal neurodegeneration of the left posterior inferior frontal lobe in patients with nfvPPA should generally result in mild (or no) receptive agrammatism, with the more severe forms of agrammatic sentence comprehension arising as atrophy spreads to critical regions of the left temporal lobe, especially in advanced cases (e.g. 25% of the patients in our cohort were >5 years since first symptom onset) with underlying Pick’s disease (PiD) pathology.129,130
Reinterpreting nfvPPA as a spectrum disorder
The term ‘nfvPPA’, as a broad diagnostic label, has been challenged on three main grounds: (i) AoS occurs in some patients as the most salient clinical feature in the absence of expressive agrammatism and vice versa; (ii) since these two symptoms dissociate in these patients, the resulting behavioural phenotypes (PPAOS versus PAA) may be better thought of as two completely separate syndromic entities; and (iii) ascribing a ‘PPA’ diagnosis in the former scenario (PPAOS) would be incorrect because of the absence of expressive agrammatism (and aphasia more generally). In this context, our examination of brain-behaviour relationships revealed that the neural correlates of the two defining features of nfvPPA, namely AoS and expressive agrammatism, lie next to each other in the left posterior inferior frontal lobe, explaining why these two symptoms do not always co-occur. But, considering the spatial proximity of their neural substrates, the most natural prediction would be that patients who initially present with AoS but not expressive agrammatism (PPAOS) or expressive agrammatism but not AoS (PAA) will represent the exception rather than the rule. Indeed, across the subset of patients with complete datasets in our sample (n = 74), 16 (22%) met criteria for PPAOS, one (1%) met criteria for PAA and 43 (58%) presented with mixed features (AOS + PAA). Furthermore, phenotypic overlap between PPAOS and PAA is only expected to increase as the disease spreads through the ‘speech production’ network, thereby blurring diagnostic boundaries.
Consistent with these predictions, in a related study, we demonstrate statistically that patients with nfvPPA cannot be robustly clustered into separate syndromic entities (e.g. PPAOS versus PPA) but rather fall along a clinical continuum/spectrum with substantial overlap behaviourally, anatomically and pathologically.131 However, this does not negate the presence of phenotypic variation within nfvPPA; on the contrary, we embrace it, while at the same time highlighting the fact that substantial overlap also exists. To reconcile both phenomena, here we propose that nfvPPA is best conceptualized as a spectrum disorder comprising several speech-language phenotypes that exhibit graded distinctions but not sharp boundaries (due to the neuroanatomy involved and the progressive nature of the underlying disease). Importantly, given that in clinical decision-making, the primary symptomatology may serve to (i) guide the choice of treatment approaches to remediate speech-language deficits; and (ii) inform prognosis, we suggest that a two-level diagnostic scheme is most appropriate, where the first level establishes whether the behavioural phenotype falls within the nfvPPA spectrum and then the second level records the presenting or most salient clinical feature (e.g. nfvPPA predominantly apraxic, nfvPPA predominantly agrammatic, nfvPPA predominantly dysarthric, etc.). This diagnostic scheme effectively conveys that these speech-language profiles belong to the same clinical spectrum (i.e. nfvPPA), without neglecting their graded distinctions and unnecessarily adding an extra layer of complexity by introducing other diagnostic labels.
Although the term ‘nfvPPA’ has been widely adopted by the frontotemporal dementia (FTD) community since its inception, a major point of criticism is that it does not readily accommodate patients with a relatively isolated motor speech impairment (i.e. AoS and/or dysarthria) in the absence of expressive agrammatism (and aphasia more generally). Therefore, we anticipate that a new, more inclusive umbrella term for this spectrum will eventually be agreed upon. To spark such a debate, we tentatively offer the following alternative designation: ‘progressive non-fluent speech and aphasia spectrum’, thereby better accounting for the existence of a relatively isolated (at least initially) apraxic/dysarthric presentation. Crucially, this new umbrella term is primarily intended for research purposes rather than being introduced as a new diagnostic category for clinical use. However, we fully endorse that, prior to implementing non-trivial changes to established nomenclature, further discussion and refinement, guided by the latest advances in the field of biomarkers for neurodegenerative diseases, are still required.
Limitations
The current study is not without limitations, such as the inclusion of varying numbers of patients in each analysis depending on available data. Nevertheless, our study comprises the largest nfvPPA cohort reported to date. In what follows, we consider four specific aspects of our work that could be improved in future studies.
First, the presence and severity of AoS and dysarthria were determined here, as in the vast majority of previous studies, based on auditory-perceptual judgements of deviant motor speech characteristic made by expert speech-language pathologists. Given their perceptual nature, such clinical ratings may be susceptible to different sources of error and bias.132 To alleviate these concerns, we have historically (for over 20 years) adopted a rigorous and systematic approach to the assessment of motor speech disorders, including extensive clinical training, discussion and review of the videotaped MSE for difficult cases, and consensus diagnosis of the presence and severity of AoS and/or dysarthria whenever deemed necessary, as reflected in the results of our inter-rater reliability analysis. Moreover, inspired by work from Richardson et al.27 and Basilakos et al.,133 we treated our clinical ratings of AoS and dysarthria severity as continuous variables during the examination of brain-behaviour relationships, which yielded a collection of brain regions that the prior literature has robustly associated with motor speech function. Critically, this speaks to the face validity of our findings and strongly suggests that our auditory-perceptual ratings of apraxic and dysarthric speech were, in fact, able to capture the behavioural phenomena they were intended to capture. The lack of involvement of the entire left SMA and most of the right SMA may, on the other hand, be due to the potential existence of a fine-grained neuroanatomical distinction between AoS versus dysarthria within the supplementary motor cortex, which we could not tease apart with the available data. To complement the insights afforded by auditory-perceptual clinical ratings, future studies could aim to develop and examine new objective, quantitative measures of motor speech function, an area of research that is rapidly gaining attention.134,135 For example, in a recent study,136 we showed that acoustic features automatically extracted from audio-recorded speech samples, such as articulation rate, might help to differentiate between patients with nfvPPA due to corticobasal degeneration (CBD) versus PSP. While these results are promising, it should be noted that the auditory-perceptual assessment of motor speech disorders is still regarded as the ‘gold standard’ in clinical decision-making.
Second, at least six major types of dysarthria have been documented,137 each potentially mapping onto partially distinct neural correlates.138 In nfvPPA, the most prevalent dysarthria types are spastic, hypokinetic or a combination of these two.15-17 Owing to competing time pressures on our team of certified speech-language pathologists, this ‘dysarthria type’ information has historically been assigned a lower priority compared to the recording of other clinical variables like dysarthria severity, which explains why it was only available for a subset of patients (55% = 57/104). Reassuringly, however, among these patients, 26% (15/57) presented with spastic dysarthria, 18% (10/57) with hypokinetic dysarthria and 39% (22/57) with mixed forms, consistent with prior reports on this topic. Since our analysis of brain-behaviour relationships was blind to dysarthria type, it is then sensible to assume that the patterns of atrophy we have associated with dysarthria are likely (i) those that are common to various types of dysarthria; and (ii) driven primarily by those patients with spastic/hypokinetic speech features in the context of FTLD-tau (especially PSP and CBD). Plausibly, our VBM analysis did not implicate the more ventrally located portions of the left primary motor cortex, such as the orofacial region, because of methodological challenges in dissociating the neural correlates of dysarthria and AoS within the left posterior inferior frontal cortex, particularly considering that in our patient sample, dysarthria almost always co-occurred with AoS but, critically, not the other way around. Although largely based on inconclusive evidence lacking the support of modern voxel-based brain-behaviour mapping methods, neuroimaging findings from studies of post-stroke dysarthria have also involved other lesion sites in the striatocapsular area and brainstem (roughly located along the course of the corticobulbar tract) as well as in the cerebellum.138-140 Whether these discrepancies represent false positives in the prior literature or false negatives in our study remains to be established. We note, however, that differences in the distribution of brain damage (Fig. 2) and, therefore, statistical power between cortical and subcortical structures (including the cerebellum) might have played a role here.
Third, Utianski et al.96 recently proposed that at least two subtypes of progressive AoS exist: a phonetic subtype in which distorted speech sound errors dominate, and a prosodic subtype in which slow, segmented speech dominate. Such an AoS subclassification scheme highlights the relative predominance of one collection of speech characteristics compared to the other, given that the vast majority of patients exhibit both phonetic and prosodic features. As this distinction between phonetic and prosodic AoS is relatively new, it has not yet been widely adopted by the FTD community or replicated by an independent research team. Therefore, we have opted to remain neutral as to the potential advantages or disadvantages of AoS subtyping, until the auditory-perceptual distinction between phonetic and prosodic AoS put forward by Utianski et al.96 receives support from an unbiased whole-brain voxel-based analysis that unequivocally demonstrates differential patterns of atrophy associated with each subtype upon direct statistical comparison of two well defined and well matched patient groups. In the meantime, it is worth pointing out that our neuroanatomical findings for dysarthria partially overlap with the areas of atrophy reported for progressive AoS (irrespective of subtype) in some studies.2,31,32,37,96 However, the same areas of atrophy have also been linked to progressive spastic dysarthria by the prior literature,20,141 perhaps with opposed left-right asymmetry. It is unclear what factors might explain these inter-study inconsistencies. One possibility is methodological differences. For example, we directly searched for brain regions where there was evidence of a unique linear relationship between AoS or dysarthria severity (controlling for the other) and the degree of tissue loss, rather than adopting the more indirect approach of inferring brain-behaviour relationships by investigating the patterns of atrophy in a group of patients with AoS or dysarthria relative to neurologically intact controls. Another possibility is that disparate clinical diagnostic criteria may have been used across studies blurring the boundaries between dysarthria and prosodic AoS (i.e. the subtype that departs most markedly from the traditional definition of AoS). To conduct an evidence- and consensus-based revision of the clinical diagnostic criteria for AoS (including potential subtypes) and dysarthria, assembling an international panel of expert clinicians and scientists would be the first step. In addition, future studies using new objective, quantitative measures of dysarthria and AoS have the potential of solving these discrepancies.
Fourth, we combined scores (% correct) from two auditory sentence-to-picture matching tasks to create our quantitative index of receptive grammar ability. Even though both these tasks were very similar, each measuring the patient’s sentence comprehension skills, they were not identical. Furthermore, while one subset of patients completed the first task, the other completed the second task. It is therefore expected that some noise might have been added when combining their sentence comprehension scores. This, paired with our hypothesis of a partial division of labour within the fronto-temporal grammar network for sentence production (frontal > temporal) versus comprehension (temporal > frontal), might explain why only the most robust effects in the left temporal lobe (but not those in the left frontal lobe) were found to be significant after attempting to focus the inference on true grammatical processing impairments during sentence comprehension (i.e. receptive agrammatism). Co-varying out alternative sources of variance is fundamental (as we have done here), especially because working memory and/or executive control deficits could influence the sentence comprehension performance of patients with nfvPPA,23 as domain-general and language-selective regions have been shown to coexist in close anatomical proximity within the left posterior inferior frontal cortex.142 Moreover, by including a ‘task’ regressor in the analysis, we attempted to adjust for effects that were specific to one or the other of the two sentence comprehension tasks making up our index of receptive grammar ability. However, this is not a perfect strategy as it could potentially remove not only some of the noise but also some of the signal of interest (i.e. not all task-specific effects are necessarily noise), ultimately weakening the results. Therefore, future studies should aim to replicate our results using a representative, large cohort of nfvPPA patients who have been administered the same sentence comprehension task.
Future directions
Collectively, our findings open up new avenues of inquiry for future research. For instance, the nfvPPA is known to be caused by a heterogeneous group of neuropathological changes that, in the majority of cases, fall under the rubric of one of three tauopathies2-6: PSP, CBD or PiD. This raises the question of whether the three neural sites that we have identified in the left frontal lobe (one for dysarthria, one for AoS and one for expressive agrammatism) signal distinct sub-networks within the larger speech production network, each of which might be differentially targeted by either PSP, CBD or PiD in the early stages of the disease.9,143 Similarly, the possibility that the neurodegenerative process may start in brain regions that we have associated with dysarthria prompts debate as to whether a relatively isolated progressive impairment of the motor control/execution of speech (i.e. dysarthria)18-20 should be regarded as one of the speech-language phenotypes that comprise the nfvPPA phenotypic spectrum. Finally, the brain-behaviour relationships that we have delineated in this study allow testable predictions about atrophy progression in the brains of patients with nfvPPA to be derived from the clinical expression of certain speech-language symptoms over the course of the disease.
Conclusion
Taken together, our clinical, pathological and neuroanatomical findings provide evidence in favour of considering nfvPPA as a multidimensional, spectrum disorder with several possible clinical presentations, including relatively isolated motor speech impairments (i.e. without accompanying expressive agrammatism, or aphasia more generally). While recognizing that nfvPPA as an umbrella term is imperfect from both behavioural (some patients present with PPAOS or progressive dysarthria) and phenomenological (fluency is a complex construct) perspectives, we also accept that it has been widely adopted in the FTD community to refer to motor speech and/or grammatical impairments caused by left frontal damage. Future developments in basic and translational science of neurodegenerative diseases will likely indicate new, refined terminology.144
Supplementary Material
Acknowledgements
We are indebted to the patients and their relatives for their generous assistance with our research.
Contributor Information
Diego L Lorca-Puls, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Sección de Neurología, Departamento de Especialidades, Facultad de Medicina, Universidad de Concepción, Concepción, 4070105, Chile.
Andrea Gajardo-Vidal, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Centro de Investigación en Complejidad Social (CICS), Facultad de Gobierno, Universidad del Desarrollo, Santiago, 7590943, Chile; Dirección de Investigación y Doctorados, Vicerrectoría de Investigación y Doctorados, Universidad del Desarrollo, Concepción, 4070001, Chile.
Maria Luisa Mandelli, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Ignacio Illán-Gala, Sant Pau Memory Unit, Department of Neurology, Biomedical Research Institute Sant Pau, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Madrid, 28029, Spain; Global Brain Health Institute, University of California, San Francisco, CA 94143, USA.
Zoe Ezzes, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Lisa D Wauters, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Department of Speech, Language and Hearing Sciences, University of Texas, Austin, TX 78712-0114, USA.
Giovanni Battistella, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Department of Otolaryngology, Head and Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School, Boston, MA 02114, USA.
Rian Bogley, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Buddhika Ratnasiri, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Abigail E Licata, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Petronilla Battista, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Global Brain Health Institute, University of California, San Francisco, CA 94143, USA; Laboratory of Neuropsychology, Istituti Clinici Scientifici Maugeri IRCCS, Bari, 70124, Italy.
Adolfo M García, Global Brain Health Institute, University of California, San Francisco, CA 94143, USA; Centro de Neurociencias Cognitivas, Universidad de San Andrés, Buenos Aires, B1644BID, Argentina; Departamento de Lingüística y Literatura, Facultad de Humanidades, Universidad de Santiago de Chile, Santiago, 9160000, Chile.
Boon Lead Tee, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Global Brain Health Institute, University of California, San Francisco, CA 94143, USA.
Sladjana Lukic, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Department of Communication Sciences and Disorders, Ruth S. Ammon College of Education and Health Sciences, Adelphi University, Garden City, NY 11530-0701, USA.
Adam L Boxer, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Howard J Rosen, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
William W Seeley, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Department of Pathology, University of California San Francisco, San Francisco, CA 94143, USA.
Lea T Grinberg, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Global Brain Health Institute, University of California, San Francisco, CA 94143, USA; Department of Pathology, University of California San Francisco, San Francisco, CA 94143, USA.
Salvatore Spina, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Bruce L Miller, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA; Global Brain Health Institute, University of California, San Francisco, CA 94143, USA.
Zachary A Miller, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Maya L Henry, Department of Speech, Language and Hearing Sciences, University of Texas, Austin, TX 78712-0114, USA; Department of Neurology, Dell Medical School, University of Texas, Austin, TX 78712, USA.
Nina F Dronkers, Department of Psychology, University of California, Berkeley, CA 94720, USA; Department of Neurology, University of California, Davis, CA 95817, USA.
Maria Luisa Gorno-Tempini, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, SanFrancisco, CA 94158, USA.
Data availability
The conditions of our ethics approval do not permit public archiving of anonymized study data. Data requests can be submitted here: https://memory.ucsf.edu/research-trials/professional/open-science. Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement. Commercial use will not be approved.
Funding
This work was supported by the National Institutes of Health (M.L.G.-T., NINDS R01 NS050915, NIDCD K24 DC015544, NIA U01 AG052943; B.L.M., NIA P50 AG023501, NIA P01 AG019724; N.D., NIDCD R01 DC016345; L.T.G., NIA K24 AG053435). The UCSF Neurodegenerative Disease Brain Bank receives funding support from NIH grants P30 AG062422, P01 AG019724, U01 AG057195, and U19 AG063911, as well as the Rainwater Charitable Foundation and the Bluefield Project to Cure FTD. A.M.G. is an Atlantic Fellow at the Global Brain Health Institute (GBHI) and is partially supported by the NIH National Institute on Aging (R01AG075775); ANID (FONDECYT Regular 1210176, 1210195); GBHI, Alzheimer’s Association, and Alzheimer’s Society (Alzheimer’s Association GBHI ALZ UK-22-865742); and the Multi-partner Consortium to Expand Dementia Research in Latin America (ReDLat), which is supported by the Fogarty International Center, the National Institutes of Health, the National Institute on Aging (R01AG057234, R01AG075775, R01AG21051, and CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable Foundation’s Tau Consortium, the Bluefield Project to Cure FTD and the Global Brain Health Institute. B.L.T. is supported by the Alzheimer’s Association (AACSFD-22-972143), University of California, San Francisco, and National Institutes of Health (NIA R21AG068757, R01AG080469, R01AG083840). D.L.L.-P. and A.G.-V. were each supported by a postdoctoral fellowship from the Chilean National Agency for Research and Development (ANID BECAS-CHILE 74200073 and ANID BECAS-CHILE 74200065, respectively). D.L.L.-P. is supported with funding from the Chilean National Agency for Research and Development (ANID SUBVENCIÓN A LA INSTALACIÓN EN LA ACADEMIA 85220006).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grossman M, Powers J, Ash S, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol. 2017;81:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandelli ML, Vilaplana E, Brown JA, et al. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain. 2016;139:2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. [DOI] [PubMed] [Google Scholar]
- 12. Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- 13. Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11(4-5):297–321. [Google Scholar]
- 14. Tetzloff KA, Utianski RL, Duffy JR, et al. Quantitative analysis of agrammatism in agrammatic primary progressive aphasia and dominant apraxia of speech. J Speech Lang Hear Res. 2018;61:2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21:S23–S30. [DOI] [PubMed] [Google Scholar]
- 16. Duffy JR, Strand EA, Josephs KA. Motor speech disorders associated with primary progressive aphasia. Aphasiology. 2014;28(8-9):1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poole ML, Brodtmann A, Darby D, Vogel AP. Motor speech phenotypes of frontotemporal dementia, primary progressive aphasia, and progressive apraxia of speech. J Speech Lang Hear Res. 2017;60:897–911. [DOI] [PubMed] [Google Scholar]
- 18. Santens P, van Borsel J, Foncke E, et al. Progressive dysarthria. Dement Geriatr Cogn Disord. 1999;10:231–236. [DOI] [PubMed] [Google Scholar]
- 19. Soliveri P, Piacentini S, Carella F, Testa D, Ciano C, Girotti F. Progressive dysarthria: Definition and clinical follow-up. Neurol Sci. 2003;24:211–212. [DOI] [PubMed] [Google Scholar]
- 20. Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson EJ, Josephs KA. Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol. 2014;21:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grossman M, Mickanin J, Onishi K, et al. Progressive nonfluent aphasia: Language, cognitive, and pet measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135–154. [DOI] [PubMed] [Google Scholar]
- 22. Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: A comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–524. [DOI] [PubMed] [Google Scholar]
- 23. Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci. 2010;30:16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charles D, Olm C, Powers J, et al. Grammatical comprehension deficits in non-fluent/agrammatic primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2014;85:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldo J V, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex. 2011;47:800–807. [DOI] [PubMed] [Google Scholar]
- 27. Richardson JD, Fillmore P, Rorden C, LaPointe LL, Fridriksson J. Re-establishing broca’s initial findings. Brain Lang. 2012;123:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matchin W, Basilakos A, Stark BC, den Ouden DB, Fridriksson J, Hickok G. Agrammatism and paragrammatism: A cortical double dissociation revealed by lesion-symptom mapping. Neurobiol Lang (Camb). 2020;1:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urban PP, Rolke R, Wicht S, et al. Left-hemispheric dominance for articulation: A prospective study on acute ischaemic dysarthria at different localizations. Brain. 2006;129:767–777. [DOI] [PubMed] [Google Scholar]
- 30. Rohrer JD, Rossor MN, Warren JD. Syndromes of nonfluent primary progressive aphasia. Neurology. 2010;75:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain. 2012;135:1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tetzloff KA, Duffy JR, Clark HM, et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain. 2018;141:302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tetzloff KA, Duffy JR, Clark HM, et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain. 2019;142:2466–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol. 2016;73:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staiger A, Finger-Berg W, Aichert I, Ziegler W. Error variability in apraxia of speech: A matter of controversy. J Speech Lang Hear Res. 2012;55:S1544–S1561. [DOI] [PubMed] [Google Scholar]
- 37. Josephs KA, Duffy JR, Strand EA, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137:2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Utianski RL, Duffy JR, Clark HM, et al. Clinical progression in four cases of primary progressive apraxia of speech. Am J Speech Lang Pathol. 2018;27:1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. [DOI] [PubMed] [Google Scholar]
- 42. Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci. 2018;21:1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 44. Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 45. Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: The disorder and its management. Grune & Stratton; 1984. [Google Scholar]
- 46. Reilly J, Fisher JL. Sherlock holmes and the strange case of the missing attribution: A historical note on “the grandfather passage”. J Speech Lang Hear Res. 2012;55:84–88. [DOI] [PubMed] [Google Scholar]
- 47. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284-290. [Google Scholar]
- 48. Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea & Febiger; 1983. [Google Scholar]
- 49. Kertesz A. The western aphasia battery. Grune & Stratton; 1982. [Google Scholar]
- 50. Miller JF, Andriacchi K, Nockerts A, eds. Assessing language production using SALT software: A clinician’s guide to language sample analysis. SALT Software LLC; 2019. [Google Scholar]
- 51. Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: Procedure and data. Brain Lang. 1989;37:440–479. [DOI] [PubMed] [Google Scholar]
- 52. Thompson C, Shapiro LP, Li L, Schendel L. Analysis of verbs and verb-argument structure: A method for quantification of aphasic language production. Clinical Aphasiology. 1995;23:121–140. [Google Scholar]
- 53. Rochon E, Saffran EM, Berndt RS, Schwartz MF. Quantitative analysis of aphasic sentence production: Further development and new data. Brain Lang. 2000;72:193–218. [DOI] [PubMed] [Google Scholar]
- 54. Casilio M, Rising K, Beeson PM, Bunton K, Wilson SM. Auditory-perceptual rating of connected speech in aphasia. Am J Speech Lang Pathol. 2019;28:550–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: Measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dronkers NF, Wilkins DP, van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. [DOI] [PubMed] [Google Scholar]
- 57. Crawford JR, Garthwaite PH, Ryan K. Comparing a single case to a control sample: Testing for neuropsychological deficits and dissociations in the presence of covariates. Cortex. 2011;47:1166–1178. [DOI] [PubMed] [Google Scholar]
- 58. Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–2019. [DOI] [PubMed] [Google Scholar]
- 59. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology. 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 60. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 61. Hyman BT, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 63. Dickson DW, Bergeron C, Chin SS, et al. Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. [DOI] [PubMed] [Google Scholar]
- 64. Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131:1416–1432. [DOI] [PubMed] [Google Scholar]
- 66. Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2009;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mackenzie IRA, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: Clinical and pathological features. Brain. 2011;134:1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging–Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tartaglia MC, Sidhu M, Laluz V, et al. Sporadic corticobasal syndrome due to FTLD-TDP. Acta Neuropathol. 2010;119:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140:3329–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim EJ, Brown JA, Deng J, et al. Mixed TDP-43 proteinopathy and tauopathy in frontotemporal lobar degeneration: Nine case series. J Neurol. 2018;265:2960–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seo SW, Thibodeau MP, Perry DC, et al. Early vs late age at onset frontotemporal dementia and frontotemporal lobar degeneration. Neurology. 2018;90:e1047–e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spina S, Brown JA, Deng J, et al. Neuropathological correlates of structural and functional imaging biomarkers in 4-repeat tauopathies. Brain. 2019;142:2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Spina S, la Joie R, Petersen C, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain. 2021;144:2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Oh JY, Walsh CM, Ranasinghe K, et al. Subcortical neuronal correlates of sleep in neurodegenerative diseases. JAMA Neurol. 2022;79:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mugler JP III, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15:152–157. [DOI] [PubMed] [Google Scholar]
- 78. Manjón JV, Coupé P, Martí-Bonmatí L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. [DOI] [PubMed] [Google Scholar]
- 79. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 80. Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–186. [DOI] [PubMed] [Google Scholar]
- 81. Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. [DOI] [PubMed] [Google Scholar]
- 82. Ashburner J, Friston KJ. Diffeomorphic registration using geodesic shooting and gauss–newton optimisation. Neuroimage. 2011;55:954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flandin G, Friston KJ. Topological inference. In: Toga AW, ed. Brain mapping. Academic Press; 2015:495–500. [Google Scholar]
- 85. Ossenkoppele R, Cohn-Sheehy BI, la Joie R, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36:4421–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Iaccarino L, la Joie R, Edwards L, et al. Spatial relationships between molecular pathology and neurodegeneration in the Alzheimer’s disease continuum. Cereb Cortex. 2021;31:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 88. Mechelli A, Price JC, Friston JK, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Curr Med Imaging. 2005;1:105–113. [Google Scholar]
- 89. Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014;51:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kaplan EGSW, Goodglass HW, Weintraub S. The Boston naming test. Lea & Febiger; 1983. [Google Scholar]
- 91. Dunn LM, Dunn LM. PPVT-III: Peabody picture vocabulary test. American Guidance Service; 1997. [Google Scholar]
- 92. Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tourville JA, Nieto-Castañón A, Heyne M, Guenther FH. Functional parcellation of the speech production cortex. J Speech Lang Hear Res. 2019;62(8S):3055–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Correia JM, Caballero-Gaudes C, Guediche S, Carreiras M. Phonatory and articulatory representations of speech production in cortical and subcortical fMRI responses. Sci Rep. 2020;10:4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen Y, Zhu G, Liu D, et al. Brain morphological changes in hypokinetic dysarthria of Parkinson’s disease and use of machine learning to predict severity. CNS Neurosci Ther. 2020;26:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018;184:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dichter BK, Breshears JD, Leonard MK, Chang EF. The control of vocal pitch in human laryngeal motor cortex. Cell. 2018;174:21–31.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Eichert N, Papp D, Mars RB, Watkins KE. Mapping human laryngeal motor cortex during vocalization. Cereb Cortex. 2020;30:6254–6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Belyk M, Brown R, Beal DS, et al. Human larynx motor cortices coordinate respiration for vocal-motor control. Neuroimage. 2021;239:118326. [DOI] [PubMed] [Google Scholar]
- 100. Chang EF, Kurteff G, Andrews JP, et al. Pure apraxia of speech after resection based in the posterior middle frontal gyrus. Neurosurgery. 2020;87:E383–E389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Castellucci GA, Kovach CK, Howard MA, Greenlee JDW, Long MA. A speech planning network for interactive language use. Nature. 2022;602:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hickok G, Venezia J, Teghipco A. Beyond broca: Neural architecture and evolution of a dual motor speech coordination system. Brain. 2023;146:1775–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Robles S G, Gatignol P, Capelle L, Mitchell MC, Duffau H. The role of dominant striatum in language: A study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mandelli ML, Caverzasi E, Binney RJ, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. 2014;34:9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rohrer JD, Rossor MN, Warren JD. Apraxia in progressive nonfluent aphasia. J Neurol. 2010;257:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97:343–350. [DOI] [PubMed] [Google Scholar]
- 107. Miller HE, Guenther FH. Modelling speech motor programming and apraxia of speech in the DIVA/GODIVA neurocomputational framework. Aphasiology. 2021;35:424–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mugler EM, Tate MC, Livescu K, Templer JW, Goldrick MA, Slutzky MW. Differential representation of articulatory gestures and phonemes in precentral and Inferior frontal gyri. J Neurosci. 2018;38:9803–9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Buchwald A, Miozzo M. Finding levels of abstraction in speech production: Evidence from sound-production impairment. Psychol Sci. 2011;22:1113–1119. [DOI] [PubMed] [Google Scholar]
- 110. Buchwald A, Miozzo M. Phonological and motor errors in individuals with acquired sound production impairment. J Speech Lang Hear Res. 2012;55:S1573–S1586. [DOI] [PubMed] [Google Scholar]
- 111. Grodzinsky Y, Friederici AD. Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol. 2006;16:240–246. [DOI] [PubMed] [Google Scholar]
- 112. Walenski M, Europa E, Caplan D, Thompson CK. Neural networks for sentence comprehension and production: An ALE-based meta-analysis of neuroimaging studies. Hum Brain Mapp. 2019;40:2275–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lukic S, Thompson CK, Barbieri E, et al. Common and distinct neural substrates of sentence production and comprehension. Neuroimage. 2021;224:117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Giglio L, Ostarek M, Weber K, Hagoort P. Commonalities and asymmetries in the neurobiological infrastructure for language production and comprehension. Cereb Cortex. 2022;32:1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Matchin W, Hickok G. The cortical organization of syntax. Cereb Cortex. 2020;30:1481–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Matchin W, Basilakos A, den Ouden DB, Stark BC, Hickok G, Fridriksson J. Functional differentiation in the language network revealed by lesion-symptom mapping. Neuroimage. 2022;247:118778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chang EF, Kurteff G, Wilson SM. Selective interference with syntactic encoding during sentence production by direct electrocortical stimulation of the inferior frontal gyrus. J Cogn Neurosci. 2018;30:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tyler LK, Marslen-Wilson WD, Randall B, et al. Left inferior frontal cortex and syntax: Function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thothathiri M, Kimberg DY, Schwartz MF. The neural basis of reversible sentence comprehension: Evidence from voxel-based lesion symptom mapping in aphasia. J Cogn Neurosci. 2012;24:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wilson SM, DeMarco AT, Henry ML, et al. Variable disruption of a syntactic processing network in primary progressive aphasia. Brain. 2016;139:2994–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pillay SB, Binder JR, Humphries C, Gross WL, Book DS. Lesion localization of speech comprehension deficits in chronic aphasia. Neurology. 2017;88:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rogalsky C, LaCroix AN, Chen KH, et al. The neurobiology of agrammatic sentence comprehension: A lesion study. J Cogn Neurosci. 2018;30:234–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kristinsson S, Thors H, Yourganov G, et al. Brain damage associated with impaired sentence processing in acute aphasia. J Cogn Neurosci. 2020;32:256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hardy CJD, Agustus JL, Marshall CR, et al. Functional neuroanatomy of speech signal decoding in primary progressive aphasias. Neurobiol Aging. 2017;56:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Grube M, Bruffaerts R, Schaeverbeke J, et al. Core auditory processing deficits in primary progressive aphasia. Brain. 2016;139:1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hardy CJD, Frost C, Sivasathiaseelan H, et al. Findings of impaired hearing in patients with nonfluent/agrammatic variant primary progressive aphasia. JAMA Neurol. 2019;76:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Riva M, Wilson SM, Cai R, et al. Evaluating syntactic comprehension during awake intraoperative cortical stimulation mapping. J Neurosurg. 2022;138:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jakuszeit M, Kotz SA, Hasting AS. Generating predictions: Lesion evidence on the role of left inferior frontal cortex in rapid syntactic analysis. Cortex. 2013;49:2861–2874. [DOI] [PubMed] [Google Scholar]
- 129. Whitwell JL, Tosakulwong N, Schwarz CC, et al. Longitudinal anatomic, functional, and molecular characterization of pick disease phenotypes. Neurology. 2020;95:e3190–e3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mesulam MM, Coventry CA, Bigio EH, et al. Neuropathological fingerprints of survival, atrophy and language in primary progressive aphasia. Brain. 2022;145:2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Illan-Gala I, Lorca-Puls DL, Ezzes Z, et al. Clinical dimensions along the non-fluent variant primary progressive aphasia spectrum. Brain . Published online 21 November 2023. doi: 10.1093/brain/awad396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kent RD. Hearing and believing: Some limits to the auditory-perceptual assessment of speech and voice disorders. Am J Speech Lang Pathol. 1996;5:7–23. [Google Scholar]
- 133. Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke. 2015;46:1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cordella C, Quimby M, Touroutoglou A, Brickhouse M, Dickerson BC, Green JR. Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology. 2019;92:e1992–e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Landin-Romero R, Liang CT, Monroe PA, et al. Brain changes underlying progression of speech motor programming impairment. Brain Commun. 2021;3:fcab205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. García AM, Welch AE, Mandelli ML, et al. Automated detection of speech timing alterations in autopsy-confirmed nonfluent/agrammatic variant primary progressive aphasia. Neurology. 2022;99:e500–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Duffy JR, Kent RD. Darley’s contributions to the understanding, differential diagnosis, and scientific study of the dysarthrias. Aphasiology. 2001;15:275–289. [Google Scholar]
- 138. Kent RD, Duffy JR, Slama A, Kent JF, Clift A. Clinicoanatomic studies in dysarthria: Review, critique, and directions for research. J Speech Lang Hear Res. 2001;44:535–551. [DOI] [PubMed] [Google Scholar]
- 139. Urban PP, Hopf HC, Fleischer S, Zorowka PG, Müller-Forell W. Impaired cortico-bulbar tract function in dysarthria due to hemispheric stroke. Functional testing using transcranial magnetic stimulation. Brain. 1997;120:1077–1084. [DOI] [PubMed] [Google Scholar]
- 140. Urban PP, Wicht S, Vukurevic G, et al. Dysarthria in acute ischemic stroke: Lesion topography, clinicoradiologic correlation, and etiology. Neurology. 2001;56:1021–1027. [DOI] [PubMed] [Google Scholar]
- 141. Whitwell JL, Duffy JR, Strand EA, et al. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol. 2013;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca’s area. Curr Biol. 2012;22:2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mandelli ML, Welch AE, Vilaplana E, et al. Altered topology of the functional speech production network in non-fluent/agrammatic variant of PPA. Cortex. 2018;108:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Murley AG, Coyle-Gilchrist I, Rouse MA, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. 2020;143:1555–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The conditions of our ethics approval do not permit public archiving of anonymized study data. Data requests can be submitted here: https://memory.ucsf.edu/research-trials/professional/open-science. Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement. Commercial use will not be approved.