Abstract
Extracellular vesicles (EVs) are extremely versatile naturally occurring membrane particles that convey complex signals between cells. EVs of different cellular sources are capable of inducing striking therapeutic responses in neurological disease models. Differently from pharmacological compounds that act by modulating defined signalling pathways, EV-based therapeutics possess multiple abilities via a variety of effectors, thus allowing the modulation of complex disease processes that may have very potent effects on brain tissue recovery. When applied in vivo in experimental models of neurological diseases, EV-based therapeutics have revealed remarkable effects on immune responses, cell metabolism and neuronal plasticity. This multimodal modulation of neuroimmune networks by EVs profoundly influences disease processes in a highly synergistic and context-dependent way. Ultimately, the EV-mediated restoration of cellular functions helps to set the stage for neurological recovery.
With this review we first outline the current understanding of the mechanisms of action of EVs, describing how EVs released from various cellular sources identify their cellular targets and convey signals to recipient cells. Then, mechanisms of action applicable to key neurological conditions such as stroke, multiple sclerosis and neurodegenerative diseases are presented. Pathways that deserve attention in specific disease contexts are discussed. We subsequently showcase considerations about EV biodistribution and delineate genetic engineering strategies aiming at enhancing brain uptake and signalling.
By sketching a broad view of EV-orchestrated brain plasticity and recovery, we finally define possible future clinical EV applications and propose necessary information to be provided ahead of clinical trials. Our goal is to provide a steppingstone that can be used to critically discuss EVs as next generation therapeutics for brain diseases.
Keywords: exosome, immune modulation, cell metabolism, mitochondria, neuronal plasticity
Extracellular vesicles are naturally occurring membrane particles that convey complex signals between cells. Hermann et al. review the recovery-promoting actions of extracellular vesicles in neurological disease models and discuss their potential clinical applications.
Introduction
In CNS diseases, such as stroke, multiple sclerosis or neurodegenerative disorders, damage to neurons, axons or synapses result in the disruption of neuronal networks.1-3 Neuronal damage may be direct or indirect because of demyelination. Independent of the nature of neuronal damage, local and systemic inflammatory responses are activated, which further exacerbate neuronal injury and propagate degenerative processes to distant brain areas.1,4,5 The resulting perpetuated degenerative process inhibits neuronal plasticity, myelin regeneration and rewiring,1,4,5 which in turn lead to the persistent neurological deficits associated with daily life impairments. The complexity of brain damage creates the need of large-scale brain tissue remodelling to compensate for lost functions. However, the endogenous capacity of the brain to cope with injury stressors is extremely limited and integrated into a multiorgan network in which response abilities decline with ageing across life.6
Over the past few years, significant progress has been made towards new therapeutic options in all three disease areas. In ischaemic stroke, the advancement of thrombolysis and mechanical thrombectomy (i.e. strategies to reopen the occluded artery) has markedly reduced ischaemic injuries and improved outcomes.7,8 In multiple sclerosis, immunomodulatory treatments that dampen brain inflammatory responses reduce disease relapses, although modestly affecting disease progression.9,10 In Alzheimer’s disease, a particularly devastating neurodegenerative condition, an immune therapy targeting soluble amyloid-β (Aβ) protofibrils has recently been shown to slow the clinical decline,11 although to moderate extent. Despite the progress made, significant neurological deficits persist in the vast majority of stroke patients,12 while the deficits of most multiple sclerosis and neurodegenerative disease patients still continue to progress in the long run.13,14 Moreover, in none of these three disease areas, neurorestorative treatments have been made available, which could reverse existing injuries or efficiently shift the balance from neurodegeneration to brain repair.
Compared with medicinal chemistry-inspired approaches, advanced therapeutics—including gene delivery methodologies and cellular/acellular therapeutics—hold the promise to provide unprecedented improvement to structural and functional brain plasticity and regeneration. This is achieved by a combination of suppression of neuroinflammation, preservation of host neuronal structures and improvement of motor and cognitive functions.15-18 Among advanced therapeutics, those based on extracellular vesicles (EVs) are particularly versatile therapeutics. EVs are cell-derived, lipid membrane-enclosed vesicles carrying a broad spectrum of biologically active molecules, which play a crucial role in intercellular communication.19 EVs traffic a plethora of signalling molecules, which are dependent on the tissue origin of the producer cells and the molecular determinants of the recipient cells.20 These signalling molecules, including proteins, RNAs and bioactive lipids,21 constrain inflammatory responses that would otherwise result in secondary neuronal injury.22-24 Besides, EVs carry small molecules, critical enzymes, respiratory chain machineries and even entire cell organelles that restore cell metabolism, thus enabling functional neurological recovery.25,26 In contrast to pharmacological compounds, which act by modulating defined signalling pathways, EV therapeutics possess multiple abilities and a variety of effectors allowing the modulation of complex disease processes in a highly synergistic and context-dependent way.21,22
When delivered therapeutically in animal disease models, stem/progenitor cell-derived EVs of different origins may exhibit striking plasticity-promoting restorative effects, leading to functional neurological improvements.27 In the middle cerebral artery occlusion (MCAO) model, intravenously administered mesenchymal stromal cell (MSC)-derived EVs enhance motor-coordination recovery, similarly to parental MSCs, by mechanisms involving long-term neuroprotection, angiogenesis, neurogenesis, axonal sprouting, remyelination and increased synaptic plasticity.28,29 In myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, intracerebroventricularly delivered neural stem cell (NSC)-derived EVs improve clinical outcome in mice almost identically to NSCs, modulating adaptive and innate immune responses, while promoting neuronal survival, remyelination and white matter repair.26,30 In transgenic mouse models of Alzheimer’s disease, intranasally administered MSC-EVs reduce the progression of cognitive deficits via mechanisms involving the polarization of microglia to an anti-inflammatory phenotype and reduction of cerebral Aβ plaque load.31,32 Hence, EVs have very potent effects on brain tissue recovery in multiple disease contexts.
With the demonstration of consistent therapeutic effects of EVs in clinically relevant brain disease models,26,29 including pilot studies in non-human primates,33 the EV field is moving fast towards clinical applications. This review aims to outline our current understanding of the mechanisms of EV action, describing how EVs from various cellular sources interact with brain cells to set the stage for functional recovery. Mechanisms applicable to different neurological diseases will be presented, focusing on pathways that deserve attention in specific disease contexts. By sketching a broader view of EV-orchestrated brain plasticity and recovery, we will further define their possible clinical applications for EVs. Finally, necessary information and quality controls for EV-based therapeutics that should be provided ahead of clinical studies and first human studies will be summarized.
In preparation for this review, we performed a detailed literature search in PubMed combining the keywords (‘extracellular vesicle’ or ‘exosome’) with (‘neuronal plasticity’ or ‘axonal plasticity’ or ‘synaptic plasticity’ or ‘neurological recovery’ or ‘clinical recovery’ or ‘neuronal survival’ or ‘neuroprotection’). Owing to the eminent importance of immune modulation and metabolic recovery in the therapeutic effects of EVs, we also combined (‘extracellular vesicle’ or ‘exosome’) with (‘immunomodulation’ or ‘immunomodulatory’ or ‘anti-inflammation’ or ‘anti-inflammatory’ or ‘immune tolerance’ or ‘metabolic’ or ‘mitochondrial’ or ‘energy metabolism’) and (‘brain’ or ‘central nervous system’ or ‘neuron’). Moreover, literature searches combining the keywords (‘extracellular vesicle’ or ‘exosome’) and (‘ischaemic stroke’ or ‘multiple sclerosis’ or ‘experimental autoimmune encephalomyelitis’ or ‘neurodegeneration’ or ‘neurodegenerative’ or ‘Alzheimer’ or ‘Parkinson’) were performed.
Extracellular vesicle origins, composition and target cell interactions
Cellular origins and heterogeneity
EVs are particles released by virtually all eukaryotic and prokaryotic cells, and they are abundant in all body liquids and tissues including blood, CSF and brain.22 Based on their biogenesis in different cell compartments,21 EVs are classified into various categories, which strongly differ in their size and physiological function (Box 1). Most EVs released from cells are rather small (typically ≤150 nm in diameter). Among these small EVs, exosomes are formed by inward budding of the limiting membrane in the late endosomal compartment,21,22 while nuclear EVs are generated by membrane budding at the inner nuclear membrane.37,38 Conversely, larger EVs (up to 1000 nm or more in diameter) are usually generated as bud-offs from the plasma membrane. Among these, ectosomes are larger than 100 nm,22 while apoptotic bodies, which are typically over 500 nm in size, are released as part of a cellular decomposition process.21 Still, there are considerable size overlaps between the EV categories. Thus, size exclusion strategies can never entirely separate them.
Box 1. Extracellular vesicle categories defined by biogenesis.
Extracellular vesicles (EVs) can be classified into the following categories:
Exosomes are formed by inward budding of the limiting membrane of late endosomes. The resulting intraluminal vesicles are released into the extracellular space by endosomal plasma membrane fusion.21,22 Exosomes are small EVs that typically have diameters of 60–150 nm and have important roles in cellular nutrition and intercellular communication.
Exosomes are also formed by inward limiting membrane budding followed by the extracellular release of autophagosomes/lysosomes.21 The size of autophagosomal/lysosomal exosomes overlaps with late endosomal exosomes, but larger EVs that may include organelles or organelle fragments, including those of mitochondria, can also be secreted.26 The release of autophagosomes/lysosomes and their exosomes represents a cellular waste excretion mechanism when autophagy activity is overchallenged or inhibited.34 Many contents are not involved in intercellular communication.
At the endoplasmic reticulum, EVs are formed by budding at specific membrane contact sites.35 EVs formed at these contact sites are rich in RNAs including microRNAs. Via direct endoplasmic reticulum-endosomal or endoplasmic reticulum-autophagosomal contacts, newly formed proteins are transferred to late endosomes/lysosomes/autophagosomes,36 from where they are further processed or released.
Nuclear EVs are generated by membrane budding at the inner nuclear membrane.37,38 They are passaged across the cytosol and released into the extracellular space. Nuclear EVs are rich in pre-microRNAs. Pre-microRNAs need to be processed to microRNAs to exert biological roles.
Under conditions of inflammation, structurally and functionally intact free and EV-encapsulated mitochondria and mitochondria fractions are released by stem/precursor cells, namely neural stem cells.26 These structures can restore mitochondrial and metabolic dysfunction of inflammatory macrophages.26
Microvesicles are formed by outward budding of the plasma membrane into the extracellular space.21,22 Microvesicles typically have a diameter of 100–1000 nm. They possess important roles in intercellular communication, particularly under conditions of inflammation and injury. Under inflammatory conditions, microvesicles can traffic damage-associated molecular patterns (DAMPs), including IL1α, IL1β and regulated and normal T cell expressed and secreted (RANTES), to adjacent cells, which induces cellular dysfunction and injury.39,40 Under conditions of neurodegenerative diseases, microglia-derived microvesicles can carry misfolded proteins, namely Aβ or α-synuclein, along axonal surfaces, propagating synaptic dysfunction across the brain.41-43
Apoptotic bodies with diameters typically larger than 500 nm are released by the outward budding of larger plasma membrane fractions as part of a cellular decomposition process in apoptotic cell death.21 Apoptotic bodies are typically phosphatidylserine (PS)-decorated on their outer membrane surface, which predisposes them for clearing by phagocytes.44 Despite their large size, size exclusion does not entirely discriminate apoptotic bodies from exosomes. Thus, apoptotic cells may also release EVs in the exosome size that confer pro-inflammatory signals to myeloid leucocytes.45
EV formation can also result from filopodia retraction in migrating cells, during which cellular protrusions condense to vesicles called migrasomes.46 Follicular dendritic cells may release immune complex-loaded vesicles called iccosomes.22
The different origins of EVs imply that they carry diverse cargos and signals. Late endosomes strongly preselect their stocks of proteins determined for cellular secretion, some merging with lysosomes or autophagosomes, which enzymatically process their protein contents.21 Hence, some EVs predominantly release cellular waste products, whereas others contain cargos determined for intercellular communication. Despite these differences, considerable overlaps exist between the protein cargos released by different EV categories. Such overlaps were recently shown in human fibroblasts and HEK293 cells for small EVs released from late endosomes and plasma membrane.47 The overlaps in protein contents suggest that biological signals released by different EV categories may exhibit surprising similarities, despite diverse EV origins and modes of EV release.
Differences in cargos for EVs of different origins were also recently shown for RNA contents. Accordingly, RNAs might be more abundant in large EVs than small EVs within the exosome size, at least when stringent isolation techniques are used.48 Bead-capturing experiments using MSC-EVs revealed that EVs recovered by cholera toxin subunit b, a GM1 ganglioside ligand and membrane microdomain marker, contained many exosome markers but hardly any RNAs.49 Conversely, EVs captured by the globotriaosylceramide ligand shiga toxin subunit b were abundant in nuclear markers and contained large amounts of RNA.49 These findings question the relevance of miRNAs as crucial signals mediating functional activities of late endosome-derived EVs.
Membrane organization and target cell tropism
The EV membrane consists of highly organized assemblies of lipids (including cholesterol and sphingolipids) and proteins, which constitute membrane microdomains (see ‘Glossary’). Different membrane microdomains are organized by different proteins, such as tetraspanins and flotillins50,51 (Box 2). Membrane microdomains enrich many signalling proteins, among them several ligands and receptors, forming ligand and receptor platforms that have unique mobility features and signalling properties.60,61 The temporarily restricted interaction of membrane microdomains represents a key principle underlying intercellular communication, and the combination of surface molecules defines the membrane microdomain tropism towards selected cells.60,62 This pattern of organization also relates to the interaction of EVs with target cells. Thus, EVs represent ‘mobile ligand platform carriers’ able to convey complex signals via mutually adjusted platforms. Signalling platforms are markedly altered in response to injury and inflammatory stimuli, when integrins and adhesion molecules are recruited into them.63 This explains the clear tropism of EVs towards injured cells.
Box 2. Composition and signalling properties of extracellular vesicles.
The composition of extracellular vesicles (EVs) closely defines their biological roles:
EVs abundantly contain membrane-organising proteins including tetraspanins and flotillins. Tetraspanins are a family of 34 transmembrane proteins in mammals which contain four transmembrane domains and two extracellular loops, among which are the classical exosomal markers CD9, CD63 and CD81.50 Although each tetraspanin exhibits different tissue and subcellular distributions, they are detected in nearly all cell-types as components of plasma membranes, endosomes and exosomes. Forming homodimers or heterodimers, tetraspanins are able to assemble to tetraspanin-enriched microdomains (TEMs) or ‘tetraspanin webs’. Unlike lipid-rafts organised by the inner membrane proteins flotillins-1 and −2, which are constituents of caveolae and have been described to be insoluble in the non-ionic detergent Triton X-100,52 TEMs are Triton X-100-soluble.51 Tetraspanins arrange the spatial juxtaposition of associated transmembrane proteins and receptors. Clustering with transmembrane integrins, selectins, cell adhesion molecules, cadherins and receptor proteins, tetraspanins regulate biological processes including cell adhesion, motility, proliferation and immune cell activation.
Associated with glycosylphosphatidylinositol (GPI)-anchored proteins and binding proteins on the outer membrane leaflet, EVs carry various protein cargos. These proteins include cytokines, cytokine receptors, enzymes, enzyme inhibitors, ephrins, ephrin receptors, death receptor ligands and major histocompatibility complex (MHC) proteins/complexes.53-55 These cargos have immunomodulatory properties and control cell proliferation, migration and guidance, as well as axonal growth.
EVs may contain RNAs, namely microRNAs, pre-microRNAs, long non-coding RNAs and mRNAs,56 as well as DNA, including mitochondrial DNA26 in their lumen and on their surface. According to recent findings, RNAs might be more abundant in large EVs than small EVs,48 and they might also be more abundant in EVs expressing nuclear markers than EVs expressing late endosomal (i.e. exosome) markers on their surface.49
Important functions of EVs have been attributed to lipids, namely phosphatidic acid, phosphatidylserine (PS), and sphingolipids.21 PS is highly abundant in the inner membrane leaflet, but serves as signal for phagocyte removal when exposed on outer membrane leaflets derived from apoptotic cells.57 The sphingolipids sphingomyelin, ceramide and sphingosine-1 phosphate (S1P) crucially control EV budding and release and modulate cell migration and differentiation upon target cell binding.58,59
Cellular interactions, uptake and signalling
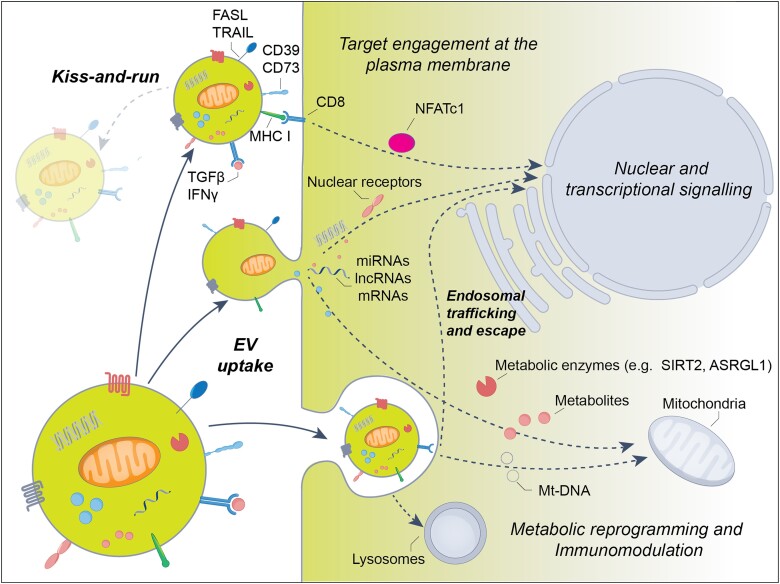
Via mutual platform interactions, EVs may form transient contacts and activate receptors on target cells, while retaining their integrity and shape.64,65 After protease-triggered resolution of cell contacts, the activated receptors are endocytosed to transmit their signals into the cytosol.66,67 Endocytosis is critical to allowing activated receptor platforms to exert their signalling responses.68 For this type of EV-cellular interaction, the term ‘kiss-and-run signalling’ has been coined (Fig. 1). In an example of kiss-and-run signalling, phosphatidylserine (PS)-decorated EVs form contacts with T cells via MHC class I binding to CD8, inducing T cell receptor (TCR) activation associated with the nuclear translocation of the transcription factor nuclear factor (NFATC1).69 Besides the activation of receptors on interacting cells, kiss-and-run contacts have been suggested to enable the formation of transient nanometre-sized fusion pores, via which luminal vesicular cargos might be transferred.64 The release of vesicular cargos across fusion pores is well established for the exocytosis and ultrafast recycling of presynaptic vesicle contents.64,70 The role of kiss-and-run signalling for EVs is far from being fully established. Future studies will need to determine whether fusion pore formation enables the cellular transfer of luminal EV cargos.
Figure 1.
Mechanisms of extracellular vesicle interaction with brain cells. Extracellular vesicles (EVs) interact with brain cells as mobile ligand carriers binding corresponding receptors on the plasma membrane. As receptor ligands, immunomodulatory cytokines/chemokines (e.g. TGFβ, IFNγ) play important roles. According to the ‘kiss-and-run’ hypothesis, EVs conferring a signal get separated from their target cell and fade off before activated receptor platforms are endocytosed. Following target engagement, receptor activation is transmitted to the cytosol and nucleus via a variety of signals that include activated receptor platforms (e.g. nuclear EGFR) and signalling proteins (e.g. SMAD2-4, STAT1). In addition to cytokines/chemokines, endonucleotidases (namely CD73), death receptor ligands such as FAS ligand (FASL) and TNF-related apoptosis-inducing ligand (TRAIL) and major histocompatibility complex (MHC) class-I/II molecules transmit immunomodulatory signals to brain cells. Importantly, kiss-and-run signalling does not enable the cellular uptake of luminal EV cargos. The latter process requires plasma membrane fusion or endocytotic EV uptake. Luminal EV contents transferred to brain cells include metabolic enzymes, metabolites, RNA (including mRNAs, microRNAs and long non-coding RNAs), DNA (specifically mtDNA), mitochondrial membrane fragments and intact mitochondria. Importantly, not all contents transmitted between cells via EVs are involved in intercellular communication. Some contents are transferred for cellular degradation in the lysosome.
Kiss-and-run signalling must be discriminated from ‘cellular EV uptake’ via large-scale plasma membrane fusion or endosomal endocytosis, which results in the transfer of cargo (Fig. 1). Indeed, seminal studies on Cre mRNA and CRE protein transfer studies implied that luminal cargos can functionally be delivered into the cytosol of target cells, which also include brain cells.71 While membrane fusion enables the passage of luminal EV cargos into the cytosol, endosomal endocytosis still maintains a barrier for luminal EV cargos, which need to escape the endosomal compartment and enter the cytosol to exert their function. Previous studies on engineered EVs loaded with luminal cargo proteins suggest that luminal cargos are effectively taken up into the cytosol only in the presence of endosomal escape-facilitating mediators.72 Interestingly, endosomal escape proteins have recently been identified in EVs under select conditions.73 This mechanism appears to be similar to that of viruses that have evolved endosomal escape strategies to deliver nucleic acids into their host cytoplasm (either via fusogenic proteins or dissolution of the endosomal membranes).74 More research is required to identify how EVs transmit signals and overcome membrane barriers to deliver luminal cargos. In the evaluation of endosomal escape mechanisms, it should also be considered that the mechanisms of uptake might differ between in vitro and in vivo conditions.
Physiological and pathogenic roles
Naturally, EVs are abundantly released in brain microenvironments exhibiting vivid intercellular communication, such as cerebral endothelial cells, pericytes, astrocytic end feet at the neurovascular unit,58,75 neurons at synaptic and astrocytic contact sites,76,77 oligodendrocytes,78,79 astrocytes along axonal surfaces80,81 and NSCs within stem cell niches.25,26 In these environments, brain cells are arranged in tight proximity with each other. Here, under physiological conditions, EVs mediate interneuronal and glial-neuronal crosstalk, modulate neuronal survival and synaptic plasticity, regulate myelination and control immune and stress responses.82 The underlying mechanisms will be outlined later, as they are the basis for current efforts to establish EVs as therapeutics.
When considering the brain-protective effects of EVs, it should be considered that under pathophysiological conditions, EVs can also transfer pro-inflammatory damage-associated molecular patterns (DAMPs) to surrounding cells, as shown in a variety of disease models. Moreover, EV trafficking at the blood–brain barrier (BBB) markedly differs from the brain parenchyma. The BBB forms an efficient barrier, which impedes the passage of brain-derived EVs into the blood and of blood-derived EVs into the brain.83 The release of brain-derived EVs into the blood occurs mainly under pathophysiological conditions associated with neuronal injury and BBB breakdown. In response to intracerebral interleukin (IL)-1β injection, astrocytic EVs were shown to accumulate the blood and promote the transmigration of leucocytes into inflammatory brain lesions via mechanisms involving modulation of peripheral cytokine responses through inhibition of peroxisome proliferator-activated receptor-α (PPARα).84 In stroke, macrophage-derived EVs were shown to transfer the DAMPs IL1α, IL1β and regulated and normal T cell expressed and secreted (RANTES) to peri-infarct cells, inducing cellular dysfunction and injury.39 In the multiple sclerosis-like lesion model of lysolecithin-induced axonal demyelination, the local injection of EVs collected from pro-inflammatory microglial cells, which were enriched in IL1α, IL1β and tumour necrosis factor-α (TNFα), prevented the remyelination of corpus callosum axons, whereas that of EVs produced by microglia co-cultured with immunosuppressive MSCs promoted oligodendrocyte precursor cell recruitment and myelin regeneration.40
In neurodegenerative diseases, EVs play a role in the propagation of misfolded proteins. EVs obtained from brain tissue of patients with Alzheimer’s disease exhibited elevated levels of Aβ oligomers85 and hyperphosphorylated tau protein,86 which were shown to act as vehicles for the neuron-to-neuron transfer of those toxic species in vitro and in vivo, respectively. Microglia-derived EVs were found to carry Aβ anterogradely along axonal surfaces, propagating long-term potentiation dysfunction from the entorhinal cortex to the dentate gyrus in the hitherto unaffected brain.41 When obtained from patients with Parkinson’s disease, microglia-derived EVs containing α-synuclein were shown to spread α-synuclein aggregates along axonal connections from the striatum to the substantia nigra.42,43 EV α-synuclein internalization was initiated by α-synuclein binding to toll-like receptor (TLR)-2 of microglia.43 These results indicate that EVs may act as seeds of protein aggregation in remote brain areas. The mechanisms of EV transport on axons are currently being examined.41 By propagating protein folding pathologies, EVs can contribute to neurological disease development. The careful selection of cellular sources is key to the implementation of successful EV-based therapeutics, and signalling mechanisms thoroughly need to be considered in clinically relevant settings.
Restorative mechanisms supporting brain tissue recovery
Immunomodulation
When therapeutically administered into the blood, EVs interact with adhesion molecules on inflamed endothelial cells, enabling their passage into the injured tissue parenchyma.23,87 This process involves EV interactions with extracellular matrix (ECM) proteoglycans in the corona of EVs, which expose signals and mediate their binding to cell membranes (Box 2). Among the signals mediating therapeutic responses, cytokines such as transforming growth factor-β (TGFβ) play a decisive role (Fig. 1). Bound to EVs via the proteoglycan betaglycan, TGFβ interacts with TGFβ receptors (TGFβR) on cell membranes.53 TGFβ signalling involves the endosomal uptake of activated TGFβ-TGFβR complexes.68 Following MCAO, TGFβ localized on intravenously administered microglia-derived EVs was found to promote neuronal survival, angiogenesis and M2 microglia polarization by activating the small body size-mothers-against-decapentaplegic (SMAD)-2/3 pathway in ischaemic brain tissue.88
Besides cytokines, EVs can also directly transfer active cytokine receptors to target cells and modulate their biological responses in the nervous system. For example, under pro-inflammatory conditions, NSC-EVs were found to transfer functional interferon-γ receptor-1 (IFNγR1) to recipient cells, in which EV IFNγ-IFNγR1 complexes promoted signal transducer and activator of transcription-1 (STAT1) signalling.89 The latter processes likely involved cytokines and cytokine receptors decorated on the EV surface. Whether cytokines, cytokine receptors or associated signalling proteins encapsulated in the EV lumen can transmit signals to recipient cells, as proposed by some studies,90,91 needs further assessment. To the best of our knowledge, there still is no unequivocal evidence indicating functionally significant delivery of luminal cytokine or cytokine receptor cargos from EVs to target cells in the brain.
Chemokines, which are able to induce directional cell movements along concentration gradients, are also present on EVs and can attract cells to modulate their biological responses to cell injury.92 Among these, CC-chemokine ligand-2 (CCL2) is a chemokine, which in the brain is produced by astrocytes and decorates glycosaminoglycan sidechains of proteoglycans on the EV surface.93,94 In models of breast, lung and prostate cancer, EV-bound CCL2 was found to induce cancer cell migration across a 3D BBB in vitro and promote brain metastasis in vivo via its receptor C-C-chemokine receptor-2 (CCR2).93,94 Upon brain injury, chemokines play a crucial role in the homing of inflammatory cells to the site of brain damage.92 In spinal cord trauma, CCL2 on astrocytic EVs increased microglial activation and neuronal death via CCR2 in the acute injury phase,95 whereas CCR2 activation induced spinal motor circuit synapse pruning in the recovery phase.96
In addition to cytokines and chemokines, EV can carry death receptor ligands, such as FAS ligand (FASL) or TNF-related apoptosis-inducing ligand (TRAIL) (Fig. 1), and checkpoint proteins, namely cytotoxic T lymphocyte antigen-4 (CTLA4) or programmed death-ligand-1 (PD-L1), on their surface, which can induce immune tolerance via corresponding receptor binding on T and NK cells.53 Receptor binding of these ligands was shown to induce immune cell death, providing protection against autoimmune pathologies, e.g. under conditions of EAE.53 When released by oligodendroglioma cells, EV-bound FASL and TRAIL cooperatively promoted cell death of astrocytes and neurons and prevented neurite growth.97
Further, ECM proteoglycans and proteins on EVs can directly modify cellular signalling responses. For example, the laminin-binding protein fibulin-2, which is enriched on astrocyte-derived EVs, was shown to activate the TGFβR/SMAD2 pathway in primary cortical neurons, enhancing spine and synapse formation.98 Fibulin-2 knockdown abolished SMAD2-dependent spine and synapse growth. On the surface of EVs, several ECM proteases and glycosidases including membrane-type 1 matrix metalloproteinase (MT1-MMP), insulin-degrading enzyme, sialidase and heparanase, among others, have furthermore been localized.99 These surface enzymes were shown to retain their activity and degrade their natural substrates present in the extracellular space. To date, ECM enzymes on EVs have been associated with the mobilization of growth factors, degradation of ECM macromolecules and destruction of Aβ plaques.99 Their role in brain remodelling and plasticity still requires assessment. ECM proteins and proteoglycans play a decisive role in regulating neuronal survival and plasticity.100,101
EV can also carry ectonucleotidases like CD39 and CD73 on their surface (Fig. 1), which restrain brain inflammatory responses by cleaving damage-associated adenosine triphosphate (ATP) to anti-inflammatory adenosine.102 Via adenosine A2 receptor binding on target cells, adenosine was found to suppress CD4+/FoxP3+ regulatory T cell103 and CD8+ effector T cell102 activities. In glioblastoma, tumour EV-bound CD73 inhibited aerobic T cell glycolysis, reduced T cell proliferation and promoted tumour growth.104 In EAE, CD39 and CD73 activation mediated activing-A-induced neurological improvements and axonal remyelination by inhibiting pro-inflammatory Th17 cells.105 In Parkinson’s disease, conversely, CD73-mediated adenosine formation sustained adenosine A2A receptor overactivation, resulting in the promotion of neuronal degeneration, motor and cognitive impairments.106 In MCAO, CD73−/− did not influence ischaemic injury or neurological outcome.107 Possibly, the role of CD39 and CD73 depends on pathophysiological contexts and cellular targets.
Some EVs display functional major histocompatibility (MHC) class-I and II complexes on their surfaces (Fig. 1), which present endogenous or exogenous antigens to T cells.108 Dendritic cells may reveal antigens to T cells via EV-bound MHC complexes. This process, termed ‘crossdressing’, circumvents cellular antigen uptake and processing that is otherwise required for antigen presentation.109 EV-mediated antigen presentation may contribute to autoimmune brain pathology.22 Upregulation of MHC complexes and integrins on EVs of IL1β-preconditioned astrocytes was made responsible for the inhibition of neurite outgrowth under neuroinflammatory conditions.110 EV transfer is particularly intense in areas of immune cell contacts, where the transmitted signals, MHC complexes and costimulatory molecules coordinate interactions between cells.22 By taking up MHC complexes, recipient cells can achieve new immunological features which fundamentally reprogram injury responses.
Nuclear signalling, transcriptional and post-transcriptional regulation
EVs can transport nuclear constituents and signals, among other proteins and RNAs, to the nucleus of target cells (Fig. 1).21 Nuclear receptors carried via EVs bind DNA and modulate gene transcription. Using mutant receptor constructs, cancer cells were found to transport EV-bound epidermal growth factor receptor (EGFR, also called ErbB1) and androgen receptor (AR, also called nuclear receptor-3C4) to recipient cell nuclei, where they activated transcriptional responses.111 EGFR is a tyrosine kinase which upon activation and dimerization phosphorylates a variety of transcription factors, whereas activated AR directly acts a DNA-binding transcription factor. Nuclear EGFR delivery was shown to confer chemotherapy resistance in cancer,112 while EGFR activation by EGF reduced neurological deficits and histopathological damage in EAE.113
EVs can also deliver mRNAs to target cells, where they are translated into proteins (Box 2 and Fig. 1). For example, EV-encapsulated mRNAs from human endothelial progenitor cells were found to promote endothelial survival, proliferation and tube formation.114 The successful transfer and translation of mRNA in endothelial cells was shown by EV-encapsulated Gfp mRNA transduction and the biological relevance by the angiogenic effect of EV-mRNA extract delivered by lipofectamine.114 In MCAO mice, mRNAs enriched in brain-derived EVs were most often of microglial and oligodendroglial origin.115 They were involved in immune signalling, cell differentiation, adhesion and motility, indicating brain-reparative roles.
Several studies reported EV-encapsulated non-coding RNA (ncRNA) transfer to target cells under conditions of cerebral hypoxia-ischaemia. The current literature on EV-associated ncRNAs has been reviewed recently.56 We therefore focus the following paragraphs on implications for neuronal survival, neuroplasticity and neurological recovery. MicroRNAs (miRNAs) are short single-strand non-coding RNAs, which typically are 21–23 nucleotides in size. Released from the nucleus as pre-miRNA hairpins, they are processed in the cytosol to mature miRNAs.116 As part of the RNA-induced silencing complex (RISC),117 miRNAs interact with complementary gene sequences of target mRNA, repressing gene expression by mRNA cleavage or interference with mRNA-ribosome interactions.118-120 The human genome contains >600 genes with robust evidence of miRNA functions,121 which target >60% of all genes.122 Thus, miRNAs have potent biological effects when transferred via EVs, which modify disease recovery.
Following MCAO, miR-133b has been found to mediate effects of MSC-EVs on axonal plasticity and neurological recovery in rats via mechanisms involving downregulation of the miR-133b targets connective tissue growth factor and Ras-homolog gene-family member-A.123 Besides, miR-17–92, which was enriched in MSC-EVs, stimulated oligodendrogenesis, neurogenesis and axon-myelin remodelling following MCAO by downregulating the miR-17–92 target phosphatase-and-tensin homolog (PTEN).124 Also following MCAO, MSC-EV miR-25–3p decreased neuronal autophagic flux and injury and enhanced neurological recovery in mice by downregulating the miR-25–3p target p53 and B cell lymphoma protein-2 (BCL2)-interacting protein-3 (BNIP3).125 In type-2 diabetic mice exposed to cortical photothrombotic stroke, endothelial cell-derived EV miR-126 promoted axonal plasticity, myelin remodelling and neurological recovery by mechanisms involving M2 macrophage polarization and enhanced angiogenesis.126
In contrast to miRNAs, long ncRNAs (lncRNAs) are transcripts with more than 200 nucleotides,127 which control gene expression in multiple ways, acting as transcription regulators, regulators of epigenetic modifications, assistants of DNA repair and regulators of mRNA processing.128,129 Owing to their circular structure, circRNAs have high exonuclease resistance.130 They act as miRNA sponges and scaffolds for chromatin-modification, transcription regulation and mRNA splicing.131,132
Primary astrocyte EV-associated circSHOC2 has been shown to increase neuronal survival and inhibited autophagy in mice exposed to MCAO via miR-7670-3p sponging that resulted in the elevation of the miR-7670-3p target sirtuin (SIRT)-1.133 Under conditions of oxidative stress, MSC-EV lncRNA metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) increased HT22 neuronal survival and proliferation via mechanisms involving serine and arginine rich splicing factor (SRSF)-2 recruitment, alternative protein kinase (PK)-CδII splicing and BCL2 elevation.134 Following photothrombotic stroke, circSCMH1 enriched in EVs of genetically engineered HEK293T cells increased dendritic and synaptic plasticity, reduced microglial activation, reduced pro-inflammatory cytokine (IL1β, TNFα and IL6) formation and improved neurological recovery in mice and rhesus monkeys through repression of transcription factor methyl-CpG binding protein (MeCP)-2, a nuclear transcription factor directly binding methylated DNA.135 Through MeCP2 binding, MeCP2 target gene transcription repression was released.
A prerequisite for biological actions is that EV-encapsulated RNAs reach their targets in the cell, specifically in the nucleus and endoplasmic reticulum. Importantly, not all EV-contained RNAs are involved in intercellular communication. Several RNAs are released for cellular waste disposal.56 Some RNAs may also represent artefacts, since RNAs tend to precipitate with EVs.56 Further research is needed on the mechanisms responsible for RNA packaging into EVs and the mechanisms enabling the delivery of EV-loaded RNAs to their subcellular targets in recipient cells.
Metabolic and mitochondrial reprogramming
Most evidence supporting a role for EVs in regulating cell metabolism comes from non-neural cells. Upon glucose deprivation, cardiomyocytes increase the synthesis and secretion of EVs, which are loaded with functional glucose transporters and glycolytic enzymes that increase glucose uptake, glycolysis and pyruvate production in recipient endothelial cells.136 Similarly, EVs produced by prostate cells (exosome-like prostasomes) contain glycolytic enzymes and enzymes involved in ATP turnover (e.g. adenylate kinase, ATPase, 5'-nucleotidase), which contribute to ATP formation when supplied with substrates.137
This intrinsic metabolic activity of EVs plays an important role in cancer, where energy metabolism is targeted to block tumour progression.138 Indeed, up to one-quarter of proteins enriched in cancer derived large EVs (i.e. oncosomes) are enzymes involved in glucose, glutamine and amino acid metabolism,139 processes relevant to cancer progression. Via EV-bound amino acids and tricarboxylic cycle intermediates, tumours induce a metabolic switch of their microenvironment from oxidative phosphorylation to glycolysis.140 The resulting lactate is used by cancer cells to promote tumour growth. Oxidative phosphorylation/glycolysis balance decisively controls neuronal survival and synaptic plasticity in the injured CNS through astrocytes.141
Recent data obtained from CNS cells have shown that NSC-EVs harbour a specific L-asparaginase activity due to the presence of the asparaginase-like protein-1 (ASRGL1) (Fig. 1), a key enzyme specific for asparagine that is devoid of glutaminase activity.25 Thereby, EVs act as independent, metabolically active units capable of perturbing the extracellular milieu by influencing metabolic substrate levels.
In the brain, axons are critical sites at which energy metabolism is stabilized by oligodendrocyte-derived78,79 and astrocyte-derived81,142 EVs. An important mechanism is the transcellular delivery of the NAD-dependent deacetylase SIRT2 (Figs 1 and 2), which is produced in oligodendrocytes and transferred to neurons via EVs.79 EVs obtained from wild-type, but not Sirt2−/− oligodendrocytes induced mitochondrial adenine nucleotide translocases-1/2 (ANT1/2) deacetylation, elevated ATP level and rescued mitochondrial integrity in Sirt2−/− mouse spinal cords.79
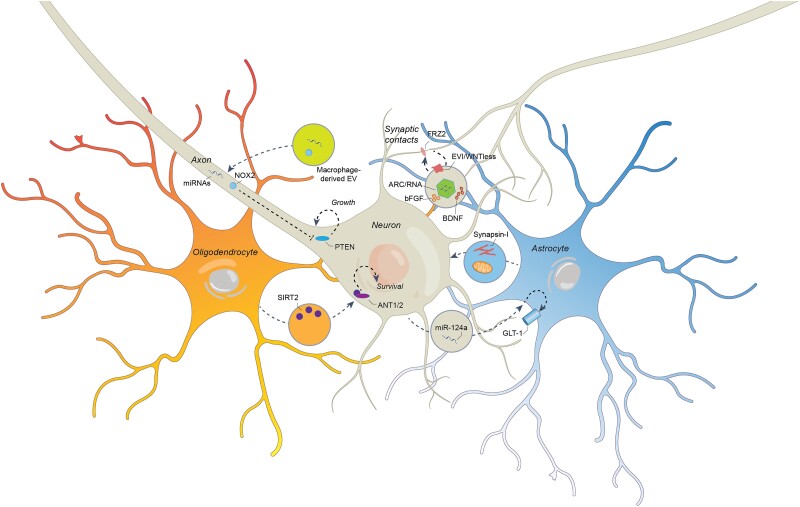
Figure 2.
Molecular mechanisms and signals via which extracellular vesicles induce neuronal plasticity and functional recovery. In the injured brain, extracellular vesicles (EVs) carrying a large variety of proregenerative signals are released by neurons, oligodendrocytes and astrocytes. EVs derived from oligodendrocytes (in orange) can transfer NAD-dependent deacetylase SIRT2 to neurons (in grey), which helps to stabilize cellular energy state and prevents axonal degeneration via ANT1/2 deacetylation. Under conditions of ischaemia, astrocytes (in blue) can shuttle synapsin-I and functional mitochondria to neurons via EVs, promoting cell survival and neurite growth. In amyotrophic lateral sclerosis, neurons can traffic EV-encapsulated miR-124a to astrocytes, elevating the glutamate transporter GLT1 by transcriptional regulation, which reduces extracellular glutamate levels and reverses synaptic over-activation that otherwise threatens neuronal survival. In the inflamed brain, EVs are furthermore released by macrophages, which can transport functional NADPH oxidase NOX2 and miRNAs to neuronal axons, from which they are retrogradely carried to the perikaryon, inducing axonal regeneration via PTEN deactivation. A unique, recently discovered mechanism is the activity-dependent EV release at the presynaptic membrane at synaptic contact sites. By trafficking the FRZ2 ligand EVI/WNTless and RNA-loaded capsid-like structures formed by the retrotransposon ARC to the postsynaptic membrane, these EVs can coordinate pre- and postsynaptic growth. The growth factors bFGF and BDNF are major modulators of EV release at synapses.
Deficient oligodendrocytic metabolic support was made responsible for progressive axonal degeneration in proteolipid protein (Plp)−/− and 2′,3′-cyclic-nucleotide-3′-phosphodiesterase (Cnp)−/− mice characterized by deficient retrograde and anterograde axonal transport and axonal swelling.78 EV release of oligodendrocytes was reduced in both mice, indicating roles of PLP and CNP in EV biogenesis. Notably, EVs of Plp−/− and Cnp−/− oligodendrocytes revealed reduced SIRT2 and heat shock protein-72 contents compared with wild-type oligodendrocyte EVs.78 Progressive axonal degeneration and transport in both mice were reversed by wild-type oligodendrocyte EVs. The mechanisms via which oligodendroglial EVs sustain axonal structure and function have recently been thoroughly reviewed.143 The latter review specifically pointed out the cooperation between exosome-dependent and metabolic support mechanisms in the maintenance of axonal integrity.
Oxidative stress in mitochondria closely accompanies delayed neuronal loss, brain atrophy and cognitive impairment in rodent traumatic brain injury models.142 Astrocytic EVs can reduce neuronal loss, brain atrophy and mitochondrial oxidative stress, as shown in post traumatic brain injury by activating nuclear factor erythroid-2-related factor-2 (NRF2)/heme oxygenase-1 signalling and increasing antioxidant superoxide dismutase (SOD) and catalase activity.142 The neuroprotective effects of astrocytic EVs were abrogated in brain-specific Nrf2−/− mice.
EVs also contain mitochondrial proteins, mitochondrial DNA and even entire mitochondria.144,145 EVs may help to unload injured mitochondria from stressed cells in a process termed ‘transmitophagy’ as demonstrated for retinal ganglion cell axons releasing acidified mitochondria associated with lysosomes, which were taken up by neighbouring astrocytes for degradation.146 Lysosomal uptake protects the cells against inflammatory responses elicited by oxidized mitochondrial proteins.147 The Parkinson’s disease-associated protein parkin recognizes damaged mitochondrial proteins and membrane fractions and directs them to the lysosomes.147 Less severely injured mitochondria may be reused by recipient cells. Thus, depolarized mitochondria released from MSCs via EVs were engulfed and restored by macrophages and regained bioenergetic function.144
Upon ischaemia, astrocytes can release functionally intact mitochondria, through a calcium-dependent mechanism involving CD38/cyclic ADP-ribose signalling, which are transferred to adjacent neurons.145 When administered to MCAO mice, the mitochondrial transfer increased cellular ATP level, neuronal survival and dendritic growth.145 CD38 knockdown reduced cellular mitochondrial transfer and worsened neurological outcome. Endothelial precursor cells similarly can release viable mitochondria, which are taken up by brain endothelial cells, promoting intracellular ATP level, microvascular integrity and angiogenesis.148
Structurally and functionally intact (free and EV-encapsulated) mitochondria can finally be released by NSCs.26 These MitoEVs can rescue the mitochondrial dysfunction of mitochondrial DNA-deficient L929 Rho0 cells and integrate into mitochondria of inflammatory macrophages, modifying their metabolic profile and pro-inflammatory gene expression in vitro and in vivo in rodents with chronic EAE.26 These effects are relevant for persistent neuroinflammation.149 Further research is required on the mechanisms underlying mitochondrial packaging, release and cellular uptake.
Promotion of neuronal plasticity
Via their immunomodulatory, transcriptional/post-transcriptional and metabolic effects, therapeutically administered EVs help to create a CNS microenvironment favourable for neuronal plasticity and neurological recovery. Axons and dendrites in the vicinity of and at a distance from brain lesions sprout, accompanied by myelin remodelling, enabling functional neuronal network rewiring in rodents.123,124 This plasticity-promoting action was recently also demonstrated in the perilesional cortex of rhesus monkeys exposed to motor cortical cold injury, in which intravenously delivered MSC-EVs increased dendritic branching and synaptic spine density.33 In this rhesus monkey study, the plasticity-promoting structural effects went along with functional fine motor improvements.33 The authors of this study found that microglial immunomodulatory responses were crucially involved in the plasticity-promoting actions of MSC-EVs.150 Mechanistically, a number of genuine nervous system-inherent actions of EVs also exist that specifically contribute to axonal growth, axon-myelin interaction, astrocytic function and synaptic plasticity. These effects are outlined in the following.
EV delivery profoundly regulates axonal signalling. This process involves communication with the perikaryon and nucleus. For example, macrophage EVs were found to transfer functional NADPH oxidase-2 (NOX2) to injured mouse axons, in which NOX2 was taken up by endocytosis.151 In axonal endosomes, active NOX2 was retrogradely transported to the soma through an importin-β1-dynein-dependent mechanism (Fig. 2). Endosomal NOX2 oxidized PTEN, leading to its inactivation, stimulating phosphatidylinositol-3 kinase (PI3K)/Akt signalling and regenerative axon growth.151 Furthermore, internalized EVs obtained from ischaemic cerebral endothelial cells can specifically transfer miRNAs to the nucleus via retrograde transport, and these have the ability to downregulate axonal growth inhibitors in distal axons via gene expression repression.152 Blockade of axonal transport suppressed cerebral endothelial EV miRNA and protein responses in neuronal somata but not in distal axons.
Neurites and astrocytes mutually support each other following brain injury via EV-bound signals. Astrocytes play decisive roles in the maintenance of neuronal energy metabolism, most notably via lactate shuttling.141 They also have important trophic functions, controlling neuronal survival and plasticity.141 The oligomannose-mimicking peptide synapsin-I is a neurite growth stimulant released from mouse astrocytes via EVs (Fig. 2). When transferred to neurons, astrocytic synapsin-I increased neurite outgrowth and promoted neuronal survival after hydrogen peroxide treatment or oxygen-glucose deprivation.80 Coculture experiments using wild-type neurons and wild-type or synapsin-I-deficient glial cells showed enhanced neurite outgrowth when synapsin-I was expressed by glial cells. Synapsin-I-induced neurite outgrowth was dependent on oligomannose on synapsin-I and on neural cell adhesion molecule (NCAM) at the neuronal cell surface.80
Perisynaptic astrocytes express glutamate transporters, namely glutamate transporter-1 (GLT1), which control extracellular glutamate levels at tripartite synapses and modulate synaptic activation and plasticity. EVs released from mouse neurons were found to contain abundant microRNAs and other small RNAs.77 When internalized into astrocytes, these EVs increased GLT1 protein levels via mechanisms involving miR124a transfer (Fig. 2).77 Intrastriatal injection of antisense RNA against miR-124a into adult mice dramatically reduced GLT1 protein expression and glutamate uptake in the striatum, yet without reducing Glt1 mRNA levels.77 miR-124a was reduced in spinal cords of endstage SOD1 G93A mice, an amyotrophic lateral sclerosis model. Exogenous miR-124a delivery prevented the loss of GLT1 protein in spinal cord astrocytes of SOD1 G93A mice.77
Synaptic contacts are sites of activity-dependent plasticity.153 In the regulation of activity-dependent plasticity, EVs possess a central role. EVs are constantly released at the presynaptic membrane in an activity-dependent way.76 Activity-dependent EV release involves syntaxin-1A (SYX1A), a protein otherwise involved in synaptic vesicle secretion, as shown in Drosophila.76 EVs released via SYX1A were found to contain the Wingless-binding protein Evenness-interrupted (EVI)/WNTless that binds to Frizzled-2 (FRZ2) at the pre- and postsynaptic membrane (Fig. 2), inducing coordinated synaptic growth at both membranes occurring in a glycogen synthase kinase-3β (GSK3β)/β-catenin-dependent way.76
The cytoskeleton-associated protein ARC regulates activity-dependent synaptic plasticity. ARC protein was demonstrated to self-assemble into capsid-like structures with a size of 20–60 nm that encapsulate RNA.73 In mouse hippocampal neurons, ARC protein capsids released via EVs were shown to transfer mRNA into recipient neurons, in which this mRNA was successfully translated (Fig. 2).73 Structurally, ARC resembles retroviral GAG retrotransposons which may have been repurposed phylogenetically for synaptic communication.154 The retrotransposon ARC might provide an endosomal packaging and escape mechanism, via which mRNA and miRNA can be exchanged between cells.
Activity-dependent EV release at synapses is controlled by neurotrophic growth factors. In a model of electrophysiological stimulus-induced EV release in primary rat hippocampal neurons, basic fibroblast growth factor (bFGF) was found to increase the activity-dependent release of EVs by late endosomes (Fig. 2).155 Proteome analysis showed that EVs released by bFGF were rich in vesicle-associated membrane protein-3 (VAMP3).155 VAMP3 was indispensable for bFGF-induced EV secretion. VAMP3 knockdown attenuated the bFGF-induced EV release.
Brain-derived neurotrophic factor (BDNF) coordinates the sorting and release of miRNAs via neuronal EVs, which promote synaptic plasticity (Fig. 2). In mouse cortical neurons, miR-132-5p, miR-218-5p and miR-690 were packaged into small EVs upon BDNF-induced TrkB activation.156 EV formation occurred in a neutral sphingomyelinase and ceramide-dependent way. When added to mouse hippocampal neurons, BDNF-induced EVs increased excitatory synapse formation by elevating a broad set of developmental and synaptogenesis-related genes (such as Sema4a, -6c and -7a, Wnt7a/b, NeuroD2), which depended on EV-associated miRNA transfer.156 BDNF-induced EVs furthermore amplified synaptic vesicle clustering, thereby increasing synaptic transmission and synchronous neuronal activity.156
The presynaptic endosomal system maintains a stock of release-competent EVs and EV cargos, which supports activity-dependent plasticity. The formation of this stock relies on the functionality of endocytic proteins, namely nervous wreck (NWK), shibire/dynamin and AP2 adaptor complex.157 In Drosophila, the deficiency of these proteins locally depleted EV cargos from presynaptic terminals. As such, Nwk mutants exhibited synaptic plasticity defects phenocopying those associated with deficiency of synaptotagmin-4 (SYT4), a known EV cargo.157 Mechanistically, NWK assisted in the loading of cargos into EVs. Activity-dependent synaptic EV signalling has not been modulated therapeutically in the injured brain. Stimulating or mimicking synaptic EV responses might enhance use-dependent plasticity, e.g. under conditions of neurorehabilitation.
Pharmacokinetics and tissue targeting
Pharmacokinetics and tissue targets of genetically unmodified extracellular vesicles
To reach potential targets in the CNS, systemically administered EVs need to pass the BBB, a tight barrier preventing the diffusion of macromolecules. In direction to the brain, the penetration of blood-injected unmodified EVs is scarce under physiological conditions in rodents71,158-160 and macaque monkeys.161 Pharmacokinetics is disappointingly rapid, and circulation time is short.71,158-161 In macaques, EV concentrations in the brain after intravenous administration were 100–1000 times lower than concentrations in the liver and spleen and 10–50 times lower than concentrations in the lungs and heart.161 EV uptake in the brain was markedly increased under inflammatory conditions, e.g. upon peripheral lipopolysaccharide administration or in cancer, as shown by CRE recombinase reporter expression analysis.71,162 EV uptake by neurons was augmented by neuronal activity.162 In rodents, intranasal EV administration allowed significantly more efficient EV uptake into the ischaemic brain than intravenous delivery, as shown by gold particle labelling.163 Unfortunately, the enhanced brain uptake following intranasal delivery could not be replicated in macaque monkeys. In macaques, brain EV uptake was even lower after intranasal than after intravenous delivery.161 EV biodistribution carefully needs to be considered in the preparation of human proof-of-concept studies. It remains to be determined, in which disease settings systemically administered EVs achieve sufficient concentrations in the brain that allow to modify disease processes.
In view to the limited brain uptake, the systemic intravenous delivery of EVs in neurological diseases remains a matter of concerns. Yet, the therapeutic effects of EVs may not only be dependent on tissue concentrations, and the brain uptake of EVs may even not be required for EVs to exert their recovery-promoting effects. Following intravenous delivery, EVs are rapidly taken up by peripheral blood leucocytes, specifically in monocytes, granulocytes and B cells, both in rodents or macaque monkeys within minutes.161 Leucocytes invade the injured brain parenchyma in all major neurological diseases, although to different extents.23,164 Hence, leucocytes might mediate the recovery-promoting effects of EVs even in the absence of EV BBB passage. In line with this notion, the protective effects of MSC-EVs on neurological deficits and brain injury following ischaemic stroke induced by MCAO critically depended on their anti-inflammatory actions, namely the prevention of polymorphonuclear neutrophil, monocyte and lymphocyte entry in the ischaemic brain tissue.23,24 Neutrophil depletion by delivery of an antibody against the neutrophil-specific antigen Ly6G mimicked the effects of intravenously administered MSC-EVs on neurological deficits, brain injury and brain monocyte/macrophage and lymphocyte infiltrates.23 In neutrophil-depleted mice, MSC-EVs did not have any further effect on neurological deficits and brain injury, and brain monocyte/macrophage and lymphocyte infiltrates were not reduced by MSC-EVs.23 Notably, the role of peripheral blood leucocytes in mediating post-ischaemic actions of MSC-EVs was not limited to the acute stroke phase. When administered post-acutely, from 24 h to 5 days post MCAO, EVs obtained from hypoxic MSCs were found to promote peri-infarct angiogenesis.165 The angiogenic effects of the MSC-EVs were abolished in neutrophil-depleted mice.165 Apparently, polymorphonuclear neutrophils are early brain invaders after MCAO, which promote brain monocyte/macrophage and lymphocyte entry and exacerbate ischaemic damage in the early injury phase,166,167 but support brain tissue remodelling and recovery in response to MSC-EV treatment in the recovery phase. The modulation of peripheral immune responses might represent a potent mode of action via which disease processes can be modified even under conditions in which brain EV uptake is low.
Besides blood leucocytes, the inflamed cardiovascular system may represent a target that mediates the therapeutic actions of systemically administered EVs. In response to MCAO, ischaemic brain endothelial cells exhibit pro-inflammatory responses, indicated by the upregulation of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM1), on the luminal endothelial cell surface,23 which facilitate brain leucocyte entry.168,169 Intravenous delivery of MSC-EVs reduced ICAM1 abundance on ischaemic brain endothelial cells and reduced brain leucocyte invasion.23 These findings suggest that recovery-promoting actions of systemically administered EVs may be transferred to the brain via anti-inflammatory responses of endothelial cells. Such anti-inflammatory responses involve cardiovascular structures remote of the brain lesion: Indeed, blood-derived M1-like (i.e. classically-activated) macrophage infiltrates have been shown post MCAO in rodent hearts closely associated with stroke-induced myocardial hypertrophy, interstitial fibrosis and left ventricular dysfunction.170,171 Splenectomy significantly reduced cardiac macrophage infiltrates and decreased myocardial hypertrophy, fibrosis, and dysfunction.171 In type-2 diabetic MCAO mice exhibiting exacerbated post-stroke myocardial inflammation, hypertrophy, fibrosis and dysfunction, the intravenous delivery of CD133+ MSC-EVs reduced cardiac M1-like macrophage infiltrates, myocardial hypertrophy and left ventricular dysfunction.172 The authors concluded that pro-inflammatory communication axes exist between the brain and heart upon ischaemic injury, which are reregulated by MSC-EVs.173 Remote organ interactions of the injured brain may go beyond the cardiovascular system, as recently suggested. Stroke-induced inflammatory responses, i.e. serum amyloid-A (SAA) and IL6, have been observed in the liver and kidneys of type-2 diabetic mice post MCAO.174 Interestingly, intravenously administered MSC-EVs reduced hepatic steatosis, fibrosis and hepatocyte ballooning, and they also reduced elevated serum alanine aminotransferase (ALT) levels.174 The liver plays a central role in the removal of EVs, including PS-decorated EVs meant for degradation, from the bloodstream.56 These EVs are taken up by Kupffer cells and hepatocytes and metabolized,44 and their remnants are excreted into the intestine via the biliary system. Since many of these EVs have pro-inflammatory functions, the restoration of hepatocyte function may potentially contribute to the recovery-promoting effects of MSC-EVs.
Target optimization and functionalization of genetically modified extracellular vesicles
Although extremely versatile in nature, unmodified EV therapeutics suffer from their very limited uptake and fast clearance in target tissues. Therefore, genetic engineering methods are employed to allow the modification of EVs to prolong their circulation in the blood, enhance brain uptake and enhance their signalling action (Fig. 3). In drug pharmacokinetics, the characteristics of drug absorption, biodistribution, metabolism and excretion (widely abbreviated using the acronym ADME) are systematically evaluated for new drug candidates.175 Like pharmacological drugs, these aspects are also relevant for the development of biological therapeutics, and they can be manipulated genetically to enhance the brain tissue targeting and efficacy of therapeutically administered EVs.
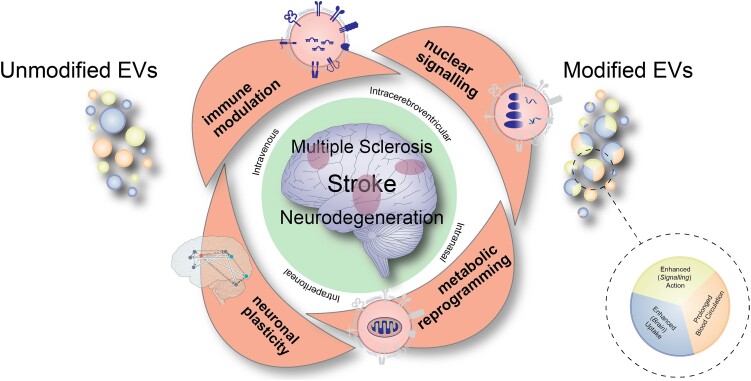
Figure 3.
Cartoon summarizing major modes of actions of extracellular vesicles that are therapeutically administered via different routes in diverse disease conditions including stroke, multiple sclerosis or neurodegenerative diseases. The different modes of action, which comprise immune modulation, nuclear signalling, metabolic reprogramming and promotion of neuronal plasticity, synergistically contribute to the recovery-promoting effects of extracellular vesicles (EVs). For therapeutic purposes, unmodified EVs are currently evaluated, as well as EVs that have genetically been modified enabling prolonged EV circulation in the blood, enhanced brain uptake or enhanced signalling action, respectively.
To increase circulation time in the blood and improve their delivery to target tissues, one promising approach is the decoration of EVs with the polyether polyethylene glycol (PEG), known as PEGylation. PEGylation is expected to delay EV degradation and increase circulation time, as described for lipid nanoparticles showing 10–15-fold increased blood half-life compared with unmodified lipid nanoparticles.176 The prevention of EV phagocytosis by host innate immune cells using ‘do-not-eat’ signalling molecules is another means to prolong the circulation time of EVs in the blood. As such, EV decoration with the do-not-eat signalling protein CD47 was found to reduce EV endocytosis by macrophages, augment circulation time and increase EV uptake in tumours following systemic injection in rat cancer models.177 The strategy induced a more than 2-fold concentration increase in tumours compared with conventional EVs.
The functionalization of PEG derivatives on EVs with nanobodies aims to increase the target tissue specificity of EVs. In a proof-of-concept study, nanobodies directed against EGFR were conjugated to phospholipid-PEG derivatives.178 This process did not affect EV morphology, size distribution or composition. After introduction of PEG-conjugated anti-EGFR nanobodies to EVs, cellular binding to EGFR-expressing cancer cells was increased compared with PEG-conjugated control antibody.178 Whereas unmodified EVs were rapidly cleared from the circulation within 10 min after intravenous injection in mice, EVs modified with nanobody-PEG-phospholipids were detectable in plasma for more than 60 min.178
To increase brain parenchymal targeting, click chemistry is a particularly versatile method for conjugating ligands to the EV surface.179 Specifically, click chemistry-based expression of the neuropilin-1 receptor peptide RGERPPR has been shown to increase BBB passage and promote the therapeutic efficacy of systemically administered EVs in a rodent glioma model.180 Combined with hyperthermic therapy, RGERPPR-engineered EVs revealed a synergistic anti-tumour effect.180
For the functionalization of EVs, two alternative modalities approaches have become available, each of which has its own pros and cons. These approaches entail the loading of the EV producer cell line with defined cargoes or the direct loading of EVs with cargoes using a variety of loading strategies. One of the most exciting strategies is EV functionalization using key components of the genome editing machinery through EV-Cas9 ribonucleoprotein (RNP) complexes.181 While EV-Cas9 RNP therapeutics have been validated in acute liver injury, chronic liver fibrosis and hepatocellular carcinoma mouse models, the applicability of these new principles of tissue specific therapeutic gene editing for brain disease is yet to be established. Modified EVs are not the main focus of this work. Technologies used for EV engineering have been reviewed in depth recently.182 This earlier review addresses both target tissue delivery and functionality aspects of EVs.
Therapeutic potential and clinical translation
As pointed out in this review, when obtained from the right cell sources, EVs have multimodal actions that promote neurological recovery by modulating gene expression, immune responses and cell metabolism, while stimulating neuron-glia interactions, neuronal survival and plasticity (Fig. 3). Whereas each of these actions may be beneficial in defined disease states, we have to assume that most EVs have a plethora of signalling mechanisms, which act in synergy to set the stage for functional neurological recovery. The combination of actions explains the potent effects of EVs. Having a clear therapeutic potential in a variety of disease contexts, supported by a large number of experimental studies, clinical translation is promising. First clinical proof-of-concept studies are on the way. We need to rule out that critical mistakes are made at this stage.
Therapeutic effects of EVs, besides cell sources, critically depend on culturing conditions and EV isolation protocols. MSC-EVs, for example, may have immune tolerance-promoting or cytotoxic actions depending on the MSC culturing conditions even when a defined MSC donor is used.23,24 Preconditioning in the right setting by physiological or chemical stimuli may augment the restorative effects of EVs, whereas inappropriate preconditioning and loading with pro-inflammatory signals (e.g. DAMPs) or pathogenic proteins (e.g. Aβ) may abolish brain protective effects or even confer detrimental activities. When applied in ischaemic stroke models, for example, hypoxic preconditioning enhanced the neurovascular, angiogenic and long-term neuroprotective effects of MSC-EVs by modifying a large number of EV proteins.23,165 When administered in brain tumours, the same hypoxic stimulus was found to increase tumour malignancy and growth.183-185 Solid pathophysiological concepts are needed, along with in-depth knowledge about cell sources and culturing conditions, to ensure that EV preparations are used that successfully stimulate neurological recovery. In order to retain restorative properties, cell sources, culturing conditions and EV isolation protocols should be standardized in clinical studies and precisely mirror those in experimental studies (Box 3). Since the biological activity of EVs differs from preparation to preparation even when the same cell source is used, the biological activity of each EV preparation should be evaluated with potency assays before being administered to human patients (Box 3).
Box 3. Tasks for the successful clinical implementation of extracellular vesicles.
The following steps, procedures and principles will have to enable the successful clinical implementation of extracellular vesicles (EVs):
Cell sources, culturing conditions and EV isolation protocols should be standardized and precisely mimic those in experimental studies. Protocols should not be modified for large scale production of EVs without again confirming therapeutic actions in experimental model systems.
The biological activity of each EV preparation should be evaluated with well-selected potency assays before EVs are administered to human patients. The biological activity of EVs differs from preparation to preparation, even when the same cell source is used. Accordingly, the biological activity should be monitored in subsequent EV preparations. The activities evaluated should measure biological responses relevant for the presumed modes of action. Depending on the disease context, sets of assays may have to be screened.
Clinical study protocols should closely mirror experimental conditions in animal studies, including disease severities, temporal disease progression, age profile and comorbidities. EV delivery routes should be identical to those in experimental studies. Treatment dosing and timing should match.
Early clinical (phase 1/2a) studies should vividly examine biological actions of EVs by surrogate markers. In case of systemic EV delivery, surrogate markers in the blood or CSF may prove that a given mode of action (e.g. anti-inflammation) can successfully be targeted in human patients. The surrogate markers ideally reflect read-outs of experimental studies and potency assays.
Subsequent clinical implementation will require randomized, double-blind, placebo-controlled phase 2b/3 studies. These studies will have to evaluate therapeutic responses with endpoints able to detect clinical improvements relevant for daily life.
In the preparation of clinical studies, an important question relates to the selection of cellular EV sources. Certain mechanisms of action, namely mitochondrial stabilization and neuronal plasticity promotion, have genuinely been linked to EVs derived from neural cell sources, namely NSCs, oligodendrocytes or astrocytes.26,79,80 In contrast, potent immunomodulatory actions have been reported following the delivery of MSC-EVs.23,24 Hence, the selection of optimal cell sources will depend on disease contexts. Studies targeting inflammatory responses may prefer MSC-EVs,23 while studies primarily modulating neuronal plasticity might prefer those that are brain-derived, namely NSC-EVs.26 Another key question is the mode of EV delivery. Potent immune modulation can be achieved by systemic (namely intravenous) EV delivery,23,24 whereas mitochondrial stabilization may require more local, i.e. intracerebroventricular, EV administration.26 In the choice of EV delivery strategies, potential benefits of a certain mode of administration need to be weighed carefully against associated efforts and risks. Owing to the risk of peri-procedural bleeding and infection, the intracerebral delivery of EVs, for example, via a trephination of the skull is not feasible in a large number of disease contexts. Repeated EV doses will be needed in several disease settings.
Mitochondrial disturbances are a joint hallmark of various neurodegenerative and neuroinflammatory conditions. Thus, EVs with mitochondria-stabilizing action may have broad application not only in contexts in which they have hitherto been evaluated (e.g. stroke, multiple sclerosis, Parkinson’s disease) but also beyond, e.g. in rare hereditary neurodegenerative diseases, in which they should be able to restore the cellular energy state. Gene therapies are currently making great progress in the treatment of metabolic disturbances in rare hereditary neurodegenerative diseases.186,187 To enhance their biological properties, EVs may genetically be loaded with defined genes or proteins (Fig. 3). As outlined earlier, genetic engineering strategies may also be used to increase EV circulation time in the blood or enhance EV brain tissue targeting (Fig. 3). Interestingly, compelling evidence exists in support of a multi-cargo biological anti-ageing signature of genetically non-modified small EVs, which can be used therapeutically to delay the degenerative processes associated with ageing and frailty.188
An important requirement for clinical studies is that the proof-of-concept for a given mode of action has unequivocally been documented in experimental disease models. This implies that the assumed mediator (i.e. a protein or RNA) reaches its target on the surface or inside recipient cells. Considering that cargos encapsulated in the EV lumen must escape endosomal confinements, proofs-of-concept built on luminal EV signals may pose greater challenges than those built on membrane-bound signals. We urgently need to learn more about the target cell uptake of EVs, specifically about how EV cargos reach their site of action in recipient cells.
Concluding remarks and outlook
In envisaging the clinical translation of EV therapeutics, several tasks currently remain to be resolved. To ensure therapeutic efficacy, EV production should be standardized, and EV activity should be evaluated in well-selected potency assays. Potency testing raises important challenges (Box 3). Depending on the disease context, sets of assays may have to be screened. Clinical implementation will require stringent proof-of-concept studies that closely mimic experimental studies regarding cell sources, EV isolation strategies and delivery protocols. Often, information about EV sources and isolation strategies is critically missing in ongoing interventional clinical trials (Table 1). Future clinical phase 1/2a studies should rigorously examine surrogate markers (e.g. immune responses in blood or CSF), which ideally should match read-outs in experimental studies and potency assays. These surrogate markers may provide the proof-of-concept that a presumed mode of action (e.g. anti-inflammation) can successfully be modified in human patients ahead of phase 2b/3 efficacy studies. These principles are pivotal for the success of clinical trials; the scientific community should not risk the clinical implementation of EVs by neglecting them in premature studies.
Table 1.
List of clinical trials on clinicaltrials.gov investigating the delivery of extracellular vesicles for the treatment of brain diseases
| NCT Number | Source | Conditions | Phase | Country | Status |
|---|---|---|---|---|---|
| NCT03384433 | Allogenic MSC-derived EVs transfected with miR-124 | Ischaemic stroke | 1/2 | Iran | Passed completion date |
| NCT04202770 | Amniotic fluid EVs | Dementia, depression, anxiety | 1 | USA | Suspended (pending COVID-19 pandemic) |
| NCT04202783 | EVs (not further specified) | Craniofacial neuralgia | 1 | USA | Suspended (pending COVID-19 pandemic) |
| NCT04388982 | Allogenic adipose MSC-derived EVs | Alzheimer's disease | 1/2 | China | Passed completion date |
| NCT05490173 | MSC-derived EVs | Neurodevelopmental disorders of prematurity | 1 | Russia | Not yet recruiting |
COVID-19 = coronavirus disease 2019; EV = extracellular vesicle; MSC = mesenchymal stromal cell; NCT = National Clinical Trial.
Contributor Information
Dirk M Hermann, Department of Neurology, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany.
Luca Peruzzotti-Jametti, Department of Clinical Neurosciences and National Institute for Health Research (NIHR) Biomedical Research Centre, University of Cambridge, Cambridge CB2 0AH, UK; Department of Metabolism, Digestion and Reproduction, Imperial College London, London W12 0NN, UK.
Bernd Giebel, Institute of Transfusion Medicine, University Hospital Essen, University of Duisburg-Essen, D-45147 Essen, Germany.
Stefano Pluchino, Department of Clinical Neurosciences and National Institute for Health Research (NIHR) Biomedical Research Centre, University of Cambridge, Cambridge CB2 0AH, UK.
Funding
Supported by the German Research Foundation [grants 389030878, 405358801 (within FOR2879), 428817542 (within FOR2879), 449437943 (within TRR332) and 514990328], by the German Federal Ministry of Education and Science (3DOS; grant 161L0278B), by Fondazione Italiana Sclerosi Multipla FIMS (grants 2018/R/14 and 2022/R-Single/011) and through an Italian Multiple Sclerosis Association AISM senior research fellowship. Co-financed by ‘5 per mille’ public funding cod. 2017/B/5, an Isaac Newton Trust research grant RG 97440, a Ferblanc Foundation grant RRAG_267, a National MS Society grant RG 1802-30200 and a Bascule Charitable Trust grant RG98181. L.P.J. is the recipient of a Wellcome Trust Clinical Research Career Development Fellowship (G105713).
Competing interests
D.M.H. and B.G. hold patents for the application of extracellular vesicles for the treatment of inflammatory conditions (EP2687219A1; US9877989B2). B.G. is founding director of Exosla Ltd., scientific advisory board member of Innovex Therapeutics SL, Mursla Ltd., PL Bioscience GmbH and ReNeuron Plc., and a consultant for Fujifilm. S.P. is founder, chief scientific officer and shareholder (>5%) of CITC Ltd. and chair of the scientific advisory board of ReNeuron Plc.
Glossary
Activity-regulated cytoskeleton-associated protein (ARC): Master-regulator controlling synaptic plasticity, which was suggested to act as phylogenetically repurposed retrotransposon packaging/unpackaging RNA in EVs. ARC might represent an endosomal escape mechanism allowing EV-encapsulated RNA transfer into the cytosol.
Axonal demyelination: Loss of axonal myelin-sheaths associated with oligodendrocyte death during brain injury/inflammation.
Axonal remyelination: Reconstruction of myelin-sheaths by surviving or new-formed oligodendrocytes during brain repair.
Endosome: Organelle involved cellular nutrition, sorting, transport and waste disposal.
Endosomal escape: Ability of luminal endosomal contents (including EVs) to pass the endosomal limiting membrane to accumulate in the cytosol.
Exosome: Small EV (diameter typically 60–150 nm) formed by inward budding of late endosomes or autophagosomes/lysosomes. The vesicle is released into the extracellular space by endosomal plasma membrane fusion.
Experimental autoimmune encephalomyelitis (EAE): Model of multiple sclerosis induced by CNS antigen immunization.
Extracellular vesicle: Heterogeneous class of vesicles released from different cell compartments, which strongly differ in their function and size.
EV isolation: EV enrichment in supernatants/fluids using physicochemical (e.g. differential ultracentrifugation, precipitation, affinity-selection) techniques.
Immune modulation: Shifting immune balance towards cytotoxicity or immune tolerance.
Kiss-and-run signalling: Temporally restricted EV-cell interaction associated with receptor activation followed by protease-triggered EV-cell contact resolution, upon which activated receptors are endocytosed to transmit their signals to the cell. This mechanism does not require cytosolic ligand uptake.
Long non-coding RNA: RNA containing >200 nucleotides which is not translated into proteins with roles in transcriptional/post-transcriptional regulation.
Membrane microdomain: Assembly of lipids (including cholesterol, sphingolipids) and proteins within membranes that forms the basis for ligand/receptor platforms.
Mesenchymal stromal cell: Multipotent cell with capacity to differentiate into osteogenic, chrondrogenic, myogenic and adipogenic cell lineages
MicroRNA: Non-coding RNA containing 21–23 nucleotides with roles in transcriptional/post-transcriptional regulation.
Microvesicle: EV formed by outward budding of the plasma membrane (diameter 100–1000 nm).
Middle cerebral artery occlusion: Blockage of a major artery that supplies large parts of the striatum and overlying cerebral cortex.
Mitochondrial transfer: Cell-to-cell exchange of injured or healthy mitochondria.
Neural stem/precursor cell: Multipotent cell with capacity to differentiate into neurons, astrocytes and oligodendrocytes.
Neurological recovery: Recovery of sensorimotor, cognitive, or language impairments following brain injury/inflammation.
Neuronal plasticity: Capacity of neurons, axons, dendrites and synapses to change through growth and reorganization.
Phase 1/2a study: Early clinical study with focus on surrogate markers and side-effects.
Phase 2b/3 study: Pivotal clinical study evaluating therapeutic efficacy using predefined endpoints.
Potency assay: Assay able to predict therapeutic effects of EVs.
Retrotransposon: Gene product copying and pasting RNA into different genomic locations, which includes RNA transport in capsid-like complexes. Within synapses, ARC was found to package/unpackage RNA-loaded capsid-like structures in EVs presumably as a phylogenetically repurposed retrotransposon. ARC might represent an endosomal escape mechanism for EV-RNA.
Transmitophagy: Cell-to-cell exchange of injured mitochondria for remote degradation.
References
- 1. Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10:225–238. [DOI] [PubMed] [Google Scholar]
- 3. Tzioras M, McGeachan RI, Durrant CS, Spires-Jones TL. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol. 2023;19:19–38. [DOI] [PubMed] [Google Scholar]
- 4. Kuhlmann T, Moccia M, Coetzee T, et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023;22:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bettcher BM, Tansey MG, Dorothée G, Heneka MT. Peripheral and central immune system crosstalk in Alzheimer disease—A research prospectus. Nat Rev Neurol. 2021;17:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 2023;29:1221–1231. [DOI] [PubMed] [Google Scholar]
- 7. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 8. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 9. Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen JA, Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020;19:336–347. [DOI] [PubMed] [Google Scholar]
- 10. Pluchino S, Smith JA, Peruzzotti-Jametti L. Promises and limitations of neural stem cell therapies for progressive multiple sclerosis. Trends Mol Med. 2020;26:898–912. [DOI] [PubMed] [Google Scholar]
- 11. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. [DOI] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 13. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;397:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17:676–688. [DOI] [PubMed] [Google Scholar]
- 15. Somia N, Verma IM. Gene therapy: Trials and tribulations. Nat Rev Genet. 2000;1:91–99. [DOI] [PubMed] [Google Scholar]
- 16. Temple S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell. 2023;30:512–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. [DOI] [PubMed] [Google Scholar]
- 18. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. [DOI] [PubMed] [Google Scholar]
- 19. Roudi S, Rädler JA, El Andaloussi S. Therapeutic potential of extracellular vesicles in neurodegenerative disorders. Handb Clin Neurol. 2023;193:243–266. [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Martin R, Brandao BB, Thomou T, Altindis E, Kahn CR. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep. 2022;38:110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dixson AC, Dawson TR, Di Vizio D, Weaver AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 2023;24:454–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23:236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C, Borger V, Sardari M, et al. Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke. 2020;51:1825–1834. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Börger V, Mohamud Yusuf A, et al. Postischemic neuroprotection associated with anti-inflammatory effects by mesenchymal stromal cell-derived small extracellular vesicles in aged mice. Stroke. 2022;53:e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iraci N, Gaude E, Leonardi T, et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peruzzotti-Jametti L, Bernstock JD, Willis CM, et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021;19:e3001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang ZG, Buller B, Chopp M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193–203. [DOI] [PubMed] [Google Scholar]
- 28. Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doeppner TR, Herz J, Gorgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Buller BA, Zhang ZG, et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol. 2022;347:113895. [DOI] [PubMed] [Google Scholar]
- 31. Cone AS, Yuan X, Sun L, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Losurdo M, Pedrazzoli M, D'Agostino C, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medalla M, Chang W, Calderazzo SM, et al. Treatment with mesenchymal-derived extracellular vesicles reduces injury-related pathology in pyramidal neurons of monkey perilesional ventral premotor Cortex. J Neurosci. 2020;40:3385–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minakaki G, Menges S, Kittel A, et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy. 2018;14:98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barman B, Sung BH, Krystofiak E, et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev Cell. 2022;57:974–994.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yorimitsu T, Klionsky DJ. Eating the endoplasmic reticulum: Quality control by autophagy. Trends Cell Biol. 2007;17:279–285. [DOI] [PubMed] [Google Scholar]
- 37. Hagen C, Dent KC, Zeev-Ben-Mordehai T, et al. Structural basis of vesicle formation at the inner nuclear membrane. Cell. 2015;163:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arya SB, Chen S, Jordan-Javed F, Parent CA. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol. 2022;24:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biemmi V, Milano G, Ciullo A, et al. Inflammatory extracellular vesicles prompt heart dysfunction via TRL4-dependent NF-κB activation. Theranostics. 2020;10:2773–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lombardi M, Parolisi R, Scaroni F, et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathol. 2019;138:987–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gabrielli M, Prada I, Joshi P, et al. Microglial large extracellular vesicles propagate early synaptic dysfunction in Alzheimer's disease. Brain. 2022;145:2849–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo M, Wang J, Zhao Y, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020;143:1476–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xia Y, Zhang G, Kou L, et al. Reactive microglia enhance the transmission of exosomal α-synuclein via toll-like receptor 2. Brain. 2021;144:2024–2037. [DOI] [PubMed] [Google Scholar]
- 44. Kamps JA, Morselt HW, Scherphof GL. Uptake of liposomes containing phosphatidylserine by liver cells in vivo and by sinusoidal liver cells in primary culture: In vivo-in vitro differences. Biochem Biophys Res Commun. 1999;256:57–62. [DOI] [PubMed] [Google Scholar]
- 45. Park SJ, Kim JM, Kim J, et al. Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci U S A. 2018;115:E11721–E11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu S, Yu L. Migrasome biogenesis and functions. FEBS J. 2022;289:7246–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fordjour FK, Guo C, Ai Y, Daaboul GG, Gould SJ. A shared, stochastic pathway mediates exosome protein budding along plasma and endosome membranes. J Biol Chem. 2022;298:102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lai RC, Tan SS, Yeo RW, et al. MSC Secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. [DOI] [PubMed] [Google Scholar]
- 51. Claas C, Stipp CS, Hemler ME. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–7984. [DOI] [PubMed] [Google Scholar]
- 52. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. [DOI] [PubMed] [Google Scholar]
- 53. Buzás EI, Tóth E, Sódar BW, Szabó-Taylor K. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rai A, Fang H, Claridge B, Simpson RJ, Greening DW. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J Extracell Vesicles. 2021;10:e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gong J, Körner R, Gaitanos L, Klein R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J Cell Biol. 2016;214:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hermann DM, Xin W, Bähr M, Giebel B, Doeppner TR. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics. 2022;12:5776–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. [DOI] [PubMed] [Google Scholar]
- 58. Mohamud Yusuf A, Hagemann N, Zhang X, et al. Acid sphingomyelinase deactivation post-ischemia promotes brain angiogenesis and remodeling by small extracellular vesicles. Basic Res Cardiol. 2022;117:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. [DOI] [PubMed] [Google Scholar]
- 60. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. [DOI] [PubMed] [Google Scholar]
- 61. Jacobson K, Liu P, Lagerholm BC. The lateral organization and mobility of plasma membrane components. Cell. 2019;177:806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833:2526–2541. [DOI] [PubMed] [Google Scholar]
- 63. Jaudon F, Thalhammer A, Cingolani LA. Integrin adhesion in brain assembly: From molecular structure to neuropsychiatric disorders. Eur J Neurosci. 2021;53:3831–3850. [DOI] [PubMed] [Google Scholar]
- 64. Alabi AA, Tsien RW. Perspectives on kiss-and-run: Role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. [DOI] [PubMed] [Google Scholar]
- 65. Watanabe S, Boucrot E. Fast and ultrafast endocytosis. Curr Opin Cell Biol. 2017;47:64–71. [DOI] [PubMed] [Google Scholar]
- 66. Gáborik Z, Hunyady L. Intracellular trafficking of hormone receptors. Trends Endocrinol Metab. 2004;15:286–293. [DOI] [PubMed] [Google Scholar]
- 67. Sorkin A, Von Zastrow M. Signal transduction and endocytosis: Close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. [DOI] [PubMed] [Google Scholar]
- 68. Shelke GV, Yin Y, Jang SC, et al. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. 2019;8:1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rausch L, Flaskamp L, Ashokkumar A, et al. Phosphatidylserine-positive extracellular vesicles boost effector CD8(+) T cell responses during viral infection. Proc Natl Acad Sci U S A. 2023;120:e2210047120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valtorta F, Meldolesi J, Fesce R. Synaptic vesicles: Is kissing a matter of competence? Trends Cell Biol. 2001;11:324–328. [DOI] [PubMed] [Google Scholar]
- 71. Ridder K, Keller S, Dams M, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Heath N, Osteikoetxea X, de Oliveria TM, et al. Endosomal escape enhancing compounds facilitate functional delivery of extracellular vesicle cargo. Nanomedicine (Lond). 2019;14:2799–2814. [DOI] [PubMed] [Google Scholar]
- 73. Pastuzyn ED, Day CE, Kearns RB, et al. The neuronal gene arc encodes a repurposed retrotransposon gag protein that mediates intercellular RNA transfer. Cell. 2018;172:275–288.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liao Z, Tu L, Li X, Liang XJ, Huo S. Virus-inspired nanosystems for drug delivery. Nanoscale. 2021;13:18912–18924. [DOI] [PubMed] [Google Scholar]
- 75. Brahmer A, Neuberger E, Esch-Heisser L, et al. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J Extracell Vesicles. 2019;8:1615820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koles K, Nunnari J, Korkut C, et al. Mechanism of evenness interrupted (evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morel L, Regan M, Higashimori H, et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frühbeis C, Kuo-Elsner WP, Müller C, et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020;18:e3000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chamberlain KA, Huang N, Xie Y, et al. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron. 2021;109:3456–3472.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang S, Cesca F, Loers G, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leggio L, L'Episcopo F, Magri A, et al. Small extracellular vesicles secreted by nigrostriatal astrocytes rescue cell death and preserve mitochondrial function in Parkinson's disease. Adv Healthc Mater. 2022;11:e2201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ranganathan M, Rahman M, Ganesh S, et al. Analysis of circulating exosomes reveals a peripheral signature of astrocytic pathology in schizophrenia. World J Biol Psychiatry. 2022;23:33–45. [DOI] [PubMed] [Google Scholar]
- 83. Krämer-Albers EM. Extracellular vesicles at CNS barriers: Mode of action. Curr Opin Neurobiol. 2022;75:102569. [DOI] [PubMed] [Google Scholar]
- 84. Dickens AM, Tovar YRLB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10:eaai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinha M S, Ansell-Schultz A, Civitelli L, et al. Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ruan Z, Pathak D, Venkatesan Kalavai S, et al. Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. 2021;144:288–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jerabkova-Roda K, Dupas A, Osmani N, Hyenne V, Goetz JG. Circulating extracellular vesicles and tumor cells: Sticky partners in metastasis. Trends Cancer. 2022;8:799–805. [DOI] [PubMed] [Google Scholar]
- 88. Zhang L, Wei W, Ai X, et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/smad2/3 pathway. Cell Death Dis. 2021;12:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cossetti C, Iraci N, Mercer TR, et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via ifngr1 to activate stat1 signaling in target cells. Mol Cell. 2014;56:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A system of cytokines encapsulated in ExtraCellular vesicles. Sci Rep. 2018;8:8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xia Y, Hu G, Chen Y, et al. Embryonic stem cell derived small extracellular vesicles modulate regulatory T cells to protect against ischaemic stroke. ACS Nano. 2021;15:7370–7385. [DOI] [PubMed] [Google Scholar]
- 92. Gyoneva S, Ransohoff RM. Inflammatory reaction after traumatic brain injury: Therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol Sci. 2015;36:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hajal C, Shin Y, Li L, Serrano JC, Jacks T, Kamm RD. The CCL2-CCR2 astrocyte-cancer cell axis in tumor extravasation at the brain. Sci Adv. 2021;7:eabg8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lima LG, Ham S, Shin H, et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat Commun. 2021;12:3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rong Y, Ji C, Wang Z, et al. Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J Neuroinflammation. 2021;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rotterman TM, Akhter ET, Lane AR, et al. Spinal motor circuit synaptic plasticity after peripheral nerve injury Depends on microglia activation and a CCR2 mechanism. J Neurosci. 2019;39:3412–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cicero A L, Schiera G, Proia P, et al. Oligodendroglioma cells shed microvesicles which contain TRAIL as well as molecular chaperones and induce cell death in astrocytes. Int J Oncol. 2011;39:1353–1357. [DOI] [PubMed] [Google Scholar]
- 98. Patel MR, Weaver AM. Astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-β signaling. Cell Rep. 2021;34:108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sanderson RD, Bandari SK, Vlodavsky I. Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biol. 2019;75-76:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dzyubenko E, Fleischer M, Manrique-Castano D, et al. Inhibitory control in neuronal networks relies on the extracellular matrix integrity. Cell Mol Life Sci. 2021;78:5647–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dzyubenko E, Hermann DM. Role of glia and extracellular matrix in controlling neuroplasticity in the central nervous system. Semin Immunopathol. 2023;45:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schneider E, Winzer R, Rissiek A, et al. CD73-mediated Adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat Commun. 2021;12:5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smyth LA, Ratnasothy K, Tsang JY, et al. CD73 Expression on extracellular vesicles derived from CD4+ CD25+ foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. [DOI] [PubMed] [Google Scholar]
- 104. Wang M, Jia J, Cui Y, Peng Y, Jiang Y. CD73-positive Extracellular vesicles promote glioblastoma immunosuppression by inhibiting T-cell clonal expansion. Cell Death Dis. 2021;12:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morianos I, Trochoutsou AI, Papadopoulou G, et al. Activin-A limits Th17 pathogenicity and autoimmune neuroinflammation via CD39 and CD73 ectonucleotidases and hif1-α-dependent pathways. Proc Natl Acad Sci U S A. 2020;117:12269–12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Carmo M, Gonçalves FQ, Canas PM, et al. Enhanced ATP release and CD73-mediated adenosine formation sustain adenosine A(2A) receptor over-activation in a rat model of Parkinson's disease. Br J Pharmacol. 2019;176:3666–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schädlich IS, Schnapauff O, Pöls L, et al. Nt5e deficiency does not affect post-stroke inflammation and lesion size in a murine ischemia/reperfusion stroke model. iScience. 2022;25:104470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. [DOI] [PubMed] [Google Scholar]
- 110. You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles. 2020;9:1706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Read J, Ingram A, Al Saleh HA, et al. Nuclear transportation of exogenous epidermal growth factor receptor and androgen receptor via extracellular vesicles. Eur J Cancer. 2017;70:62–74. [DOI] [PubMed] [Google Scholar]
- 112. Wu S, Luo M, To KKW, et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer. 2021;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nicoletti F, Mazzon E, Fagone P, et al. Prevention of clinical and histological signs of MOG-induced experimental allergic encephalomyelitis by prolonged treatment with recombinant human EGF. J Neuroimmunol. 2019;332:224–232. [DOI] [PubMed] [Google Scholar]
- 114. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 115. Bub A, Brenna S, Alawi M, et al. Multiplexed mRNA analysis of brain-derived extracellular vesicles upon experimental stroke in mice reveals increased mRNA content with potential relevance to inflammation and recovery processes. Cell Mol Life Sci. 2022;79:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lund E, Dahlberg JE. Substrate selectivity of exportin 5 and dicer in the biogenesis of microRNAs. Cold Spring Harb Symp Quant Biol. 2006;71:59–66. [DOI] [PubMed] [Google Scholar]
- 117. Rana TM. Illuminating the silence: Understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. [DOI] [PubMed] [Google Scholar]
- 118. Guo H, Ingolia N, Weissman J, Bartel D. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. [DOI] [PubMed] [Google Scholar]
- 121. Fromm B, Billipp T, Peck LE, et al. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the human microRNAome. Annu Rev Genet. 2015;49:213–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–-2746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xin H, Katakowski M, Wang F, et al. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kuang Y, Zheng X, Zhang L, et al. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles. 2020;10:e12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Venkat P, Cui C, Chopp M, et al. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes Mellitus mice. Stroke. 2019;50:2865–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kapranov P, Cheng J, Dike S, et al. RNA Maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. [DOI] [PubMed] [Google Scholar]
- 128. Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: Physiology and disease. Noncoding RNA. 2019;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wu P, Mo Y, Peng M, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ashwal-Fluss R, Meyer M, Pamudurti N, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. [DOI] [PubMed] [Google Scholar]
- 132. Meng S, Zhou H, Feng Z, et al. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen W, Wang H, Zhu Z, Feng J, Chen L. Exosome-Shuttled circSHOC2 from IPASs regulates neuronal autophagy and ameliorates ischemic brain injury via the miR-7670-3p/SIRT1 axis. Mol Ther Nucleic Acids. 2020;22:657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bassit GE, Patel RS, Carter G, et al. MALAT1 In human adipose stem cells modulates survival and alternative splicing of PKCδII in HT22 cells. Endocrinology. 2017;158:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yang L, Han B, Zhang Z, et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation. 2020;142:556–574. [DOI] [PubMed] [Google Scholar]
- 136. Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408. [DOI] [PubMed] [Google Scholar]
- 137. Ronquist KG, Ek B, Stavreus-Evers A, Larsson A, Ronquist G. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am J Physiol Endocrinol Metab. 2013;304:E576–E582. [DOI] [PubMed] [Google Scholar]
- 138. Bernstock JD, Kang KD, Klinger NV, et al. Targeting oncometabolism to maximize immunotherapy in malignant brain tumors. Oncogene. 2022;41:2663–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Minciacchi VR, You S, Spinelli C, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhao H, Yang L, Baddour J, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Borbor M, Yin D, Brockmeier U, et al. Neurotoxicity of ischemic astrocytes involves STAT3-mediated metabolic switching and depends on glycogen usage. Glia. 2023;71:1553–1569. [DOI] [PubMed] [Google Scholar]
- 142. Zhang W, Hong J, Zhang H, Zheng W, Yang Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY). 2021;13:21642–21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Krämer-Albers EM, Werner HB. Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat Rev Neurosci. 2023;24:474–486. [DOI] [PubMed] [Google Scholar]
- 144. Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Davis CH, Kim KY, Bushong EA, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111:9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Todkar K, Chikhi L, Desjardins V, El-Mortada F, Pépin G, Germain M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat Commun. 2021;12:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hayakawa K, Chan SJ, Mandeville ET, et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36:1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Peruzzotti-Jametti L, Willis CM, Hamel R, Krzak G, Pluchino S. Metabolic control of smoldering neuroinflammation. Front Immunol. 2021;12:705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Go V, Bowley BGE, Pessina MA, et al. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged rhesus monkeys. Geroscience. 2020;42:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hervera A, De Virgiliis F, Palmisano I, et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol. 2018;20:307–319. [DOI] [PubMed] [Google Scholar]
- 152. Zhang Y, Qin Y, Chopp M, et al. Ischemic cerebral endothelial cell-derived exosomes promote axonal growth. Stroke. 2020;51:3701–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Magee JC, Grienberger C. Synaptic plasticity forms and functions. Annu Rev Neurosci. 2020;43:95–117. [DOI] [PubMed] [Google Scholar]
- 154. Hantak MP, Einstein J, Kearns RB, Shepherd JD. Intercellular communication in the nervous system goes viral. Trends Neurosci. 2021;44:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kumar R, Tang Q, Müller SA, et al. Fibroblast growth factor 2-mediated regulation of neuronal exosome release Depends on VAMP3/cellubrevin in hippocampal neurons. Adv Sci (Weinh). 2020;7:1902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Antoniou A, Auderset L, Kaurani L, et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023;42:112063. [DOI] [PubMed] [Google Scholar]
- 157. Blanchette CR, Scalera AL, Harris KP, et al. Local regulation of extracellular vesicle traffic by the synaptic endocytic machinery. J Cell Biol. 2022;221:e202112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wiklander OP, Nordin JZ, O'Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gupta D, Liang X, Pavlova S, et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. 2020;9:1800222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Driedonks T, Jiang L, Carlson B, et al. Pharmacokinetics and biodistribution of extracellular vesicles administered intravenously and intranasally to Macaca nemestrina. J Extracell Biol. 2022;1:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kur IM, Prouvot PH, Fu T, et al. Neuronal activity triggers uptake of hematopoietic extracellular vesicles in vivo. PLoS Biol. 2020;18:e3000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Betzer O, Perets N, Angel A, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. [DOI] [PubMed] [Google Scholar]
- 164. Berriat F, Lobsiger CS, Boillée S. The contribution of the peripheral immune system to neurodegeneration. Nat Neurosci. 2023;26:942–954. [DOI] [PubMed] [Google Scholar]
- 165. Gregorius J, Wang C, Stambouli O, et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res Cardiol. 2021;116:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46:2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129:259–277. [DOI] [PubMed] [Google Scholar]
- 168. Furuya K, Takeda H, Azhar S, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: A bedside-to-bench study. Stroke. 2001;32:2665–2674. [DOI] [PubMed] [Google Scholar]
- 169. Zhang RL, Chopp M, Li Y, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. [DOI] [PubMed] [Google Scholar]
- 170. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-Heart interaction: Cardiac complications after stroke. Circ Res. 2017;121:451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yan T, Chen Z, Chopp M, et al. Inflammatory responses mediate brain-heart interaction after ischemic stroke in adult mice. J Cereb Blood Flow Metab. 2020;40:1213–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Venkat P, Cui C, Chen Z, et al. CD133+Exosome Treatment improves cardiac function after stroke in type 2 diabetic mice. Transl Stroke Res. 2021;12:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Venkat P, Chen J, Chopp M. Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab. 2018;38:2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Venkat P, Gao H, Findeis EL, et al. Therapeutic effects of CD133 + exosomes on liver function after stroke in type 2 diabetic mice. Front Neurosci. 2023;17:1061485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014;87:93–120. [DOI] [PubMed] [Google Scholar]
- 176. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Belhadj Z, He B, Deng H, et al. A combined “eat me/don't eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9:1806444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kooijmans SAA, Fliervoet LAL, van der Meel R, et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. [DOI] [PubMed] [Google Scholar]
- 179. Smyth T, Petrova K, Payton NM, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25:1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. [DOI] [PubMed] [Google Scholar]
- 181. Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X. Exosome-mediated delivery of cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8:eabp9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. [DOI] [PubMed] [Google Scholar]
- 184. Tang T, Yang Z, Zhu Q, et al. Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cells metastasis, proliferation, and self-renewal by targeting E-cadherin. FASEB J. Published online 6 September 2018. doi: 10.1096/fj201801013R [DOI] [PubMed] [Google Scholar]
- 185. Zhang X, Sai B, Wang F, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. [DOI] [PubMed] [Google Scholar]
- 187. Mercuri E, Muntoni F, Baranello G, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:832–841. [DOI] [PubMed] [Google Scholar]
- 188. Sanz-Ros J, Romero-García N, Mas-Bargues C, et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci Adv. 2022;8:eabq2226. [DOI] [PMC free article] [PubMed] [Google Scholar]