Abstract
Background/Aims
In stereomicroscopic sample isolation processing, the cutoff value (≥4 mm) of stereomicroscopically visible white cores indicates high diagnostic sensitivity. We aimed to evaluate endoscopic ultrasound-guided tissue acquisition (EUS-TA) using a simplified stereomicroscopic on-site evaluation of upper gastrointestinal subepithelial lesions (SELs).
Methods
In this multicenter prospective trial, we performed EUS-TA using a 22-gauge Franseen needle in 34 participants with SELs derived from the upper gastrointestinal muscularis propria, requiring pathological diagnosis. The presence of stereomicroscopically visible white core (SVWC) in each specimen was assessed using stereomicroscopic on-site evaluation. The primary outcome was EUS-TA’s diagnostic sensitivity with stereomicroscopic on-site evaluation based on the SVWC cutoff value (≥4 mm) for malignant upper gastrointestinal SELs.
Results
The total number of punctures was 68; 61 specimens (89.7%) contained stereomicroscopically visible white cores ≥4 mm in size. The final diagnoses were gastrointestinal stromal tumor, leiomyoma, and schwannoma in 76.5%, 14.7%, and 8.8% of the cases, respectively. The sensitivity of EUS-TA with stereomicroscopic on-site evaluation based on the SVWC cutoff value for malignant SELs was 100%. The per-lesion accuracy of histological diagnosis reached the highest level (100%) at the second puncture.
Conclusions
Stereomicroscopic on-site evaluation showed high diagnostic sensitivity and could be a new method for diagnosing upper gastrointestinal SELs using EUS-TA.
Keywords: Endoscopic ultrasound-guided fine-needle aspiration, Interventional ultrasonography, Upper gastrointestinal tract
Graphic abstract
INTRODUCTION
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is a safe procedure with relatively low risks of morbidity (0.98%) and mortality (0.02%).1 EUS-TA is useful for diagnosing upper gastrointestinal subepithelial lesions (SELs). Real-time on-site cytopathological (ROSE) interpretation improves sample adequacy and diagnostic yield of EUS-TA.2 During ROSE, cytopathologists can evaluate whether additional sampling is required for auxiliary diagnosis.3-6 Only half the centers in Asia and Europe have adopted ROSE.7 Sample isolation processing by stereomicroscopy (SIPS) was developed as an alternative to ROSE. SIPS increases the accuracy of EUS-TA by determining the presence of stereomicroscopically visible white cores (SVWCs) in specimens collected using a 22-gauge needle. SIPS has a high sensitivity of 98.8% for diagnosing malignancy in upper gastrointestinal SELs when an SVWC cutoff value of ≥3.5 mm is obtained using a 22-gauge fine-needle aspiration (FNA) needle and 98.7% when an SVWC cutoff value of ≥4 mm is attained using a 22-gauge fine-needle biopsy (FNB) needle.8,9 However, SIPS is complicated and time-consuming.10 A previous study comparing isolated and non-isolated samples revealed that the degree of blood contamination was significantly lower with the SIPS method (p<0.01). However, the accuracy (95% vs. 95%) and histological adequacy (4 vs. 4) were comparable, suggesting that the isolation steps in SIPS may not be necessary for pathological diagnosis.10 A previous study on pancreatic cancer reported that isolation is unnecessary.11 The present multicenter prospective trial focused on upper gastrointestinal SELs and aimed to verify the usefulness of a new, simplified assessment method (stereomicroscopic on-site evaluation [SOSE]) that omits the SIPS processes and simply confirms whether the SVWC cutoff value has been reached.
METHODS
Study design
This multicenter prospective study was conducted at three Japanese institutions (Kitasato University Hospital, Isehara Kyodo Hospital, and the Japan Community Health Care Organization Sagamino Hospital). The primary outcome was the sensitivity of EUS-TA with SOSE for diagnosing malignant upper gastrointestinal SELs, based on the SVWC cutoff value (≥4 mm). Secondary outcomes included the following: (1) accuracy (using predefined criteria for the final malignancy diagnosis) and (2) adverse events.
Patient eligibility
We enrolled consecutive patients who underwent EUS-TA for upper gastrointestinal SELs derived from the muscularis propria at three institutions between August 2020 and August 2021. The inclusion criteria were as follows: (1) age ≥20 years, (2) upper gastrointestinal SELs derived from the muscularis propria that required EUS-TA, and (3) pathological diagnosis to determine the treatment strategy. The exclusion criteria were as follows: (1) patients with serious mental disorders and (2) patients with abnormal coagulation parameters.
Procedure
EUS-TA was performed using a GF-UCT 260 or TGF-260J echoendoscope (Olympus Medical Systems Corp.) and a 22-gauge Franseen needle (Acquire; Boston Scientific Corp.), with the patient under conscious sedation in all cases. After withdrawing the stylet, 20 to-and-fro movements within the lesion were made with negative pressure applied using a 20 mL syringe. The procedure was performed by board-certified trainers from the Japan Gastroenterological Endoscopy Society or trainees; the latter performed the procedure under the supervision of board-certified trainers. Two punctures were made in each case and SOSE was performed separately after each puncture to evaluate the adequacy of the specimens. SOSE was performed according to a previously reported methodology.11 SOSE omits the processes of SIPS and simply confirms whether the SVWC cutoff value (≥4 mm) has been reached. In step 1, the sample in the puncture needle was extruded into a Petri dish by compressing the air in the syringe using a stylet. In step 2, the sample was immersed in a buffer solution, and the liquid component was sent for cytology. In step 3, the SVWC length fragments were measured under a stereomicroscope (×20) to estimate whether the SVWC lengths in the sample met the cutoff value (Fig. 1). The time required for step 3 was measured using a stopwatch. In step 4, the sample was placed in close contact with a filter paper and placed in a formalin container. SOSE was performed on-site at each puncture by one of the three evaluators designated in advance. Each evaluator had performed at least 10 SOSE procedures at the start of the study and had mastered the technique. EUS-TA was terminated if the SVWC cutoff value was reached at least once after the first or second puncture. The 30-day incidence of adverse events after EUS-TA was evaluated based on a previous report.12
Fig. 1.
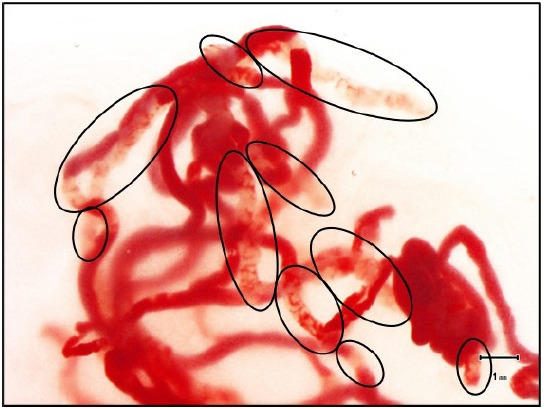
The stereomicroscopic on-site evaluation procedure. A stereomicroscope (×20) was used to measure each fragment of the observed stereomicroscopically visible white core (SVWC) length. The scale on the screen of the microscope monitor was used to determine whether the sum of SVWC lengths in a sample met the cutoff value (≥4 mm). The areas encircled in black were defined as SVWC, and the total length exceeded 4 mm.
Histological assessment
Hematoxylin and eosin (H&E)-stained specimens were prepared for histopathological diagnosis. The required immunohistochemical staining for diagnosis was performed using formalin-fixed paraffin-embedded samples containing the largest amount of tissue from repeated EUS-TA for each lesion or all samples at the discretion of a specialized pathologist. Immunohistochemical staining included CD34, CD117, gastrointestinal stromal tumor (GIST)-1, DOG1 in GISTs, α-smooth muscle actin in leiomyomas, and S-100 and vimentin in schwannomas. Other samples used to prepare H&E-stained specimens alone were considered to have been examined correctly if the H&E findings showed the same pathological signs as the samples that underwent immunohistochemical staining. Among the SELs derived from the upper gastrointestinal muscularis propria, only GISTs were considered malignant. Histological evaluation was performed by two or more pathologists. For patients whose lesions were surgically resected, EUS-TA and surgical histopathology results were consistent. Subsequent clinical patients with unresected lesions were followed-up for at least six months, and imaging was performed as appropriate. The final diagnosis was considered correct if it was consistent with EUS-TA results.
Statistical analyses
In a previous study,9 the sensitivity for histological diagnosis when SVWC cutoff values were obtained was 98.7%. Assuming that the frequency of obtaining SVWC cutoff values was 86.7% and the tissue diagnosis success rate per puncture was 93.3%,9 the number of required specimens was calculated to be 63, with α and power values of 0.025 and 80%, respectively, and an equivalence tolerance margin of 10%. Assuming a dropout rate of approximately 5%, the planned sample size was set at 35 patients (70 specimens). The presence of SVWC and tissue sampling were classified as positive if white samples were visible under a stereomicroscope and tissue lesions were visible using optical microscopy. Categorical variables were compared using Fisher's exact probability test. Two-tailed p-values <0.05 were considered significant. Statistical analyses were performed using R statistical package ver. 3.2.4 (R Foundation for Statistical Computing).
Ethical statements
The individual review boards of all three participating institutions approved the study (No: C20-124, 111, and 202010). The study was conducted in accordance with the guidelines of the Declaration of Helsinki as revised in in 2013 (clinical trial registration number: UMIN000041270). All patients provided written informed consent before undergoing EUS-TA.
RESULTS
Thirty-five patients were initially enrolled. One patient with a suspected lesion derived from the muscularis propria of the stomach during a prior EUS was excluded from the analysis; a subsequent EUS-TA revealed gastric wall invasion due to lymph node metastasis of an unknown primary cancer (adenocarcinoma).
Among the 34 included participants, 15 (44.1%) were men and 19 (55.9%) were women, with a median age of 66 years (24–87 years). The median maximum diameter of the lesions, calculated using EUS imaging, was 20 mm (14–150 mm). The lesions were located in the esophagus in four (11.8%) participants and in the stomach in 30 (88.2%).
The technical success rate was 100%. Twelve endoscopists, five of whom were trainees, participated as operators. Punctures were performed twice for all SELs; the SVWC cutoff value was achieved after the first or second puncture, and was considered completed. The puncture sites were the esophagus in eight (12%) passes and the stomach in 60 (88%) passes. Mild hemorrhage from the puncture site during EUS-TA occurred in one patient (3%) diagnosed with GIST.
Table 1 shows the results of pathological examination and tissue diagnosis of EUS-TA with SOSE. Insufficient microscopic tissue was obtained from one specimen for diagnosis. The tissue sampling rates per-pass and per-lesion analyses were 99% and 100%, respectively. The per-lesion accuracy of histological diagnosis reached the highest score (100%) at the second puncture. When the SVWC cutoff value was achieved, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS-TA for the diagnosis of malignancy were all 100%. The sensitivity was significantly higher in specimens that met the SVWC cutoff value than in those that did not meet this criterion (p<0.01) (Table 2). The 95% confidence interval was -1.06 to 5.46 when calculated using the difference in the population ratio (2.2%) test based on the previously reported sensitivity (98.7%)9; the calculated value was within the 10% equivalence tolerance margin.
Table 1.
Pathological examination and tissue diagnosis of samples obtained by endoscopic ultrasound-guided tissue acquisition and evaluated with stereomicroscopic
| Characteristic | Value |
|---|---|
| Presence of spindle cells in cytology (n=34) | |
| First pass | 18 (52.9) |
| Second pass | 15 (44.1) |
| Cumulative rate for up to two passes (per lesion) | 23 (67.6) |
| Tissue sampling rate | |
| All passes (n=68) | 67 (98.5) |
| Per lesion (n=34) | 34 (100) |
| Histological diagnosis rate (n=34) | |
| First pass | 33 (97.1) |
| Second pass | 33 (97.1) |
| Cumulative rate for up to two passes (per lesion) | 34 (100) |
| Malignant diagnosis based on the SVWC cutoff value (per-pass analysis) | |
| Sensitivity (n=47) | 47 (100) |
| Specificity (n=14) | 14 (100) |
| Positive predictive value (n=47) | 47 (100) |
| Negative predictive value (n=14) | 14 (100) |
| Accuracy (n=61) | 61 (100) |
Values are presented as number (%).
SVWC, stereomicroscopically visible white core.
Table 2.
Results of endoscopic ultrasound-guided tissue acquisition according to the obtained SVWC cutoff value
| Positive (n) | Negative (n) | Sensitivity (%) | p-value | |
|---|---|---|---|---|
| SVWC ≥4 mm | 61 | 0 | 100 | <0.01 |
| SVWC <4 mm | 5 | 2 | 71 |
SVWC, stereomicroscopically visible white core.
The results of the stereomicroscopic and microscopic evaluations and the final diagnoses are presented in Table 3. SOSE was performed for all 68 samples, and the SVWC cutoff value collection rate was 100% for each lesion, with 85.3%, 94.1%, and 89.7% in the first, second, and all passes, respectively. The median time spent in step 3 of SOSE was 53 seconds (23–120 seconds). The final diagnoses were GIST in 26 patients (76.5%; 25 in the stomach and one in the esophagus), leiomyoma in five (14.7%; three in the esophagus and two in the stomach), and schwannoma in three (8.8%; all in the stomach). During the study period, 18 patients with GIST underwent surgical resection. Postoperative pathological diagnoses were similar to those of EUS-TA. For serious concomitant disease or other reasons, five patients with GIST did not undergo surgical resection within the study period. Of the remaining three patients with GIST, two were awaiting surgery and one with an unresectable lesion was treated with chemotherapy. The final diagnoses in other patients with benign diseases (five leiomyomas and three schwannomas) were made during the monitored clinical courses of the patients over 6 months.
Table 3.
Stereomicroscopic and microscopic assessments and final diagnoses
| Assessment | Value |
|---|---|
| Presence of the SVWC cutoff value | |
| First pass (n=34) | 29 (85.3) |
| Second pass (n=34) | 32 (94.1) |
| All passes (n=68) | 61 (89.7) |
| Per lesion (n=34) | 34 (100) |
| SOSE step 3 duration (sec) | 53 (23–120) |
| Final diagnosis (n=34) | |
| Gastrointestinal stromal tumor | 26 (76.5) |
| Stomach | 25 (73.5) |
| Esophagus | 1 (2.9) |
| Leiomyoma | 5 (14.7) |
| Stomach | 3 (8.8) |
| Esophagus | 2 (5.9) |
| Schwannoma of the stomach | 3 (8.8) |
Values are presented as number (%) and median (range).
SVWC, stereomicroscopically visible white core; SOSE, stereomicroscopic on-site evaluation.
DISCUSSION
In the present study, EUS-TA was performed, and specimens were collected for definitive diagnosis of upper gastrointestinal SELs. By implementing SOSE, we achieved high sensitivity (100%) for diagnosing malignancy in samples that satisfied the SVWC cutoff value; the sensitivity was comparable to that of one of a previous study9 that used the SIPS method.
Upper gastrointestinal SELs originating from the muscularis propria include GISTs, schwannomas, leiomyomas, ectopic pancreatic lesions, glomus tumors, and some neuroendocrine neoplasms.13 Their characteristics vary; some have malignant potential, whereas others follow a benign course that does not require surgical resection.14 Thus, obtaining a confirmatory pathological diagnosis is significant in distinguishing the different types of SELs and determining a proper treatment strategy. GISTs are the most common type of SELs, accounting for up to 3% of all gastrointestinal tumors.15 EUS-TA and specific immunohistochemical staining can be used to distinguish GISTs from other SELs.
Previously devised SIPS is an on-site evaluation method which does not require a cytopathologist as in ROSE.8 The diagnostic performance of SIPS is greatly improved when the SVWC cutoff value is met.8,9 However, SIPS is a complicated procedure and requires time for separation from the red component and full-length SVWC measurement. Another prospective exploratory study suggested that the SIPS isolation process may be unnecessary for pathological diagnosis only.10 Therefore, this prospective study was conducted to assess whether SOSE could achieve the same sensitivity as that observed for SIPS in previous studies. The study results confirmed that when the SVWC cutoff value was reached with SOSE, the sensitivity was as high as that previously reported for SIPS.9
The correct diagnosis rate reaches a maximum after two punctures with an FNB needle in EUS-TA for solid pancreatic masses, such as pancreatic cancer.11,16 In this study, we obtained one or more SVWC cutoff values in all cases using up to two punctures (one puncture for seven cases and two for 27 cases). The maximum per-lesion accuracy for histological diagnosis was 97% for the first puncture and 100% for the second puncture. Given the favorable results of FNB needles, some researchers have suggested that rapid evaluation methods, such as ROSE, are no longer necessary when using FNB needles in EUS-TA for pancreatic tumors17-19; a similar conclusion may be drawn for SELs. However, several meta-analyses have found that ROSE improved sample adequacy and diagnostic yield in pancreatic masses.20,21 Nevertheless, some meta-analyses do not support these advantages,22,23 and the current evidence is inconsistent. Therefore, the European Society of Gastrointestinal Endoscopy recommends the use and non-use of ROSE (moderate-quality evidence, strong recommendation).24 Yang et al.25 concluded that ROSE has distinct clinical benefits and should be applied, especially by endosonographers in the learning stage of EUS-FNA and at centers where specimen adequacy rates are insufficient. However, only a few facilities can implement ROSE. Factors associated with this include limited cytopathologist staffing, cost-effective performance, longer procedural duration, and a lack of belief in its added value.7 Therefore, we propose that the advantage of SOSE is its high sensitivity when the cutoff value is attained. SOSE does not directly evaluate the validity of collected samples (cells) by cytopathologists as in ROSE, but indirectly predicts whether high sensitivity can be attained by determining the presence or absence of the SVWC cutoff value. SOSE could be a useful and rapid evaluation method for facilities that cannot implement ROSE. A head-to-head comparison between SOSE and ROSE is needed to prove its superiority in future research.
This study had some limitations. First, the sample size (and thereby, the number of specimens) was small and was calculated from the results of previous prospective exploratory studies. Although revalidation with a larger-scale study is needed, we achieved a predicted sensitivity of 98.7%. Second, SVWC assessment in SOSE was subjective, and the evaluators were limited to three individuals trained in advance. However, only SVWC length measurement in an enlarged image using a stereomicroscope was required; nearly identical evaluations were possible after training with approximately 10 cases. Third, SOSE omits most of the processes in SIPS, and direct comparisons could not be made because previous SIPS process times were unknown. In current clinical practice, the processing time for SIPS is approximately 5–7 minutes per sample. However, the time required for SOSE is short (median value of 53 seconds), as shown in this study, which is an acceptable in clinical practice.
In conclusion, we attained an extremely good sensitivity with SOSE in this multicenter study, equivalent to that with SIPS in previous studies. SOSE could be a new, standardized diagnostic method that is simpler than SIPS for EUS-TA of upper gastrointestinal SELs originating from the muscularis propria. Therefore, SOSE can become a standard procedure in facilities where ROSE cannot be performed. Randomized controlled trials with larger sample sizes should be considered in the future to analyze the applicability of SOSE for diagnosing upper gastrointestinal SELs.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: SN, KO, HI; Data curation: SN, KO, MW, TI, TM, RH, HM, TK, AT, JI, AI; Formal analysis: KO; Investigation: SN, MW; Supervision: MK, CK; Writing–original draft: SN, KO; Writing–review & editing: all authors.
REFERENCES
- 1.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 2.Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 3.Hocke M, Topalidis T, Braden B, et al. "Clinical" cytology for endoscopists: a practical guide. Endosc Ultrasound. 2017;6:83–89. doi: 10.4103/eus.eus_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes CV, Dedavid E, Silva TL, et al. The value of endoscopic ultrasound-fine needle aspiration in the suspicion of pancreatic hydatid cyst in endemic areas with negative serology (with video) Endosc Ultrasound. 2017;6:350–351. doi: 10.4103/eus.eus_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Chai N, Feng J, et al. A prospective study of endoscopic ultrasonography features, cyst fluid carcinoembryonic antigen, and fluid cytology for the differentiation of small pancreatic cystic neoplasms. Endosc Ultrasound. 2018;7:335–342. doi: 10.4103/eus.eus_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biermann K, Lozano Escario MD, Hébert-Magee S, et al. How to prepare, handle, read, and improve EUS-FNA and fine-needle biopsy for solid pancreatic lesions: the pathologist's role. Endosc Ultrasound. 2017;6(Suppl 3):S95–S98. doi: 10.4103/eus.eus_71_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: outcome of a global survey. Endosc Int Open. 2016;4:E360–E370. doi: 10.1055/s-0042-101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masutani H, Okuwaki K, Kida M, et al. On-site stereomicroscope quality evaluations to estimate white core cutoff lengths using EUS-FNA biopsy sampling with 22-gauge needles. Gastrointest Endosc. 2019;90:947–956. doi: 10.1016/j.gie.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Okuwaki K, Masutani H, Kida M, et al. Diagnostic efficacy of white core cutoff lengths obtained by EUS-guided fine-needle biopsy using a novel 22G Franseen biopsy needle and sample isolation processing by stereomicroscopy for subepithelial lesions. Endosc Ultrasound. 2020;9:187–192. doi: 10.4103/eus.eus_18_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuwaki K, Imaizumi H, Kida M, et al. Usefulness of the automated multiband imaging system for EUS-FNA biopsy specimen evaluation in patients with upper gastrointestinal subepithelial lesions. Endosc Ultrasound. 2022;11:283–290. doi: 10.4103/EUS-D-21-00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Okuwaki K, Kida M, et al. Multicenter prospective study of the efficacy of stereomicroscopic on-site evaluation in endoscopic ultrasound-guided tissue acquisition in patients with pancreatic cancer. Pancreatology. 2022;22:311–316. doi: 10.1016/j.pan.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Kida M, Kawaguchi Y, Miyata E, et al. Endoscopic ultrasonography diagnosis of subepithelial lesions. Dig Endosc. 2017;29:431–443. doi: 10.1111/den.12854. [DOI] [PubMed] [Google Scholar]
- 14.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–645. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 15.Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer. 2005;41:2868–2872. doi: 10.1016/j.ejca.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Ishigaki K, Nakai Y, Oyama H, et al. Endoscopic ultrasound-guided tissue acquisition by 22-gauge Franseen and standard needles for solid pancreatic lesions. Gut Liver. 2020;14:817–825. doi: 10.5009/gnl19171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedenström P, Marschall HU, Nilsson B, et al. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions: a prospective, comparative study. Surg Endosc. 2018;32:1304–1313. doi: 10.1007/s00464-017-5808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–1438. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc. 2019;11:454–471. doi: 10.4253/wjge.v11.i8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159–171. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 23.Kong F, Zhu J, Kong X, et al. Rapid on-site evaluation does not improve endoscopic ultrasound-guided fine needle aspiration adequacy in pancreatic masses: a meta-analysis and systematic review. PLoS One. 2016;11:e0163056. doi: 10.1371/journal.pone.0163056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline: March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Liu E, Sun S. Rapid on-site evaluation (ROSE) with EUS-FNA: the ROSE looks beautiful. Endosc Ultrasound. 2019;8:283–287. doi: 10.4103/eus.eus_65_19. [DOI] [PMC free article] [PubMed] [Google Scholar]