Abstract
Background
Age‐related loss of strength is disproportionally greater than the loss of mass, suggesting maladaptations in the neuro‐myo‐tendinous system. Myofibers are often misshaped in aged and diseased muscle, but systematic analyses of large sample sets are lacking. Our aim was to investigate myofiber shape in relation to age, exercise, myofiber type, species and sex.
Methods
Vastus lateralis muscle biopsies (n = 265) from 197 males and females, covering an age span of 20–97 years, were examined. The gastrocnemius and soleus muscles of 11 + 22‐month‐old male C57BL/6 mice were also examined. Immunofluorescence and ATPase stainings of muscle cross‐sections were used to measure myofiber cross‐sectional area (CSA) and perimeter. From these, a shape factor index (SFI) was calculated in a fibre‐type‐specific manner (type I/II in humans; type I/IIa/IIx/IIb in mice), with higher values indicating increased deformity. Heavy resistance training (RT) was performed three times per week for 3–4 months by a subgroup (n = 59). Correlation analyses were performed comparing SFI and CSA with age, muscle mass, maximal voluntary contraction (MVC), rate of force development and specific force (MVC/muscle mass).
Results
In human muscle, SFI was positively correlated with age for both type I (R 2 = 0.20) and II (R 2 = 0.38) myofibers. When subjects were separated into age cohorts, SFI was lower for type I (4%, P < 0.001) and II (6%, P < 0.001) myofibers in young (20–36) compared with old (60–80) and higher for type I (5%, P < 0.05) and II (14%, P < 0.001) myofibers in the oldest old (>80) compared with old. The increased SFI in old muscle was observed in myofibers of all sizes. Within all three age cohorts, type II myofiber SFI was higher than that for type I myofiber (4–13%, P < 0.001), which was also the case in mice muscles (8–9%, P < 0.001). Across age cohorts, there was no difference between males and females in SFI for either type I (P = 0.496/0.734) or II (P = 0.176/0.585) myofibers. Multiple linear regression revealed that SFI, after adjusting for age and myofiber CSA, has independent explanatory power for 8/10 indices of muscle mass and function. RT reduced SFI of type II myofibers in both young and old (3–4%, P < 0.001).
Conclusions
Here, we identify type I and II myofiber shape in humans as a hallmark of muscle ageing that independently predicts volumetric and functional assessments of muscle health. RT reverts the shape of type II myofibers, suggesting that a lack of myofiber recruitment might lead to myofiber deformity.
Keywords: myofiber morphology, physiological function, sarcopenia, shape factor, skeletal muscle
Introduction
It has been known for at least 50 years that myofibers atrophy with ageing, 1 and today, type II myofiber atrophy is a recognized hallmark of muscle ageing. In addition to atrophy, the number of myofibers irreversibly declines with age, 2 due to loss of innervation. 3 Consequently, muscle mass and function, which is critical for independent living, diminish with increasing age. 4 Sarcopenia, the clinical diagnosis for severe loss of muscle mass, strength and function, is observed in 5–10% of individuals >65 years of age and in >25% of individuals >80 years of age, 5 which highlights the importance of preserving muscle mass and function with ageing. An interesting aspect of these age‐related changes is that the loss of muscle function is two‐ to four‐fold greater than the loss of muscle mass, 4 meaning that for a given amount of muscle, less force is produced in older compared with younger adults (i.e., lower functional muscle quality). This is not explained by changes in intrinsic contractile mechanics of myofibers, 6 , 7 indicating the presence of maladaptations in the neuro‐myo‐tendinous system.
One potential such maladaptation is how myofibers are shaped. Geometric analyses of tissue architecture have shown that physical constraint restricts some tissues, including skeletal muscle, into specific organizations. 8 , 9 Myofibers are collectively organized in tessellations of pentagons or hexagons. 8 Crucially, if the physical constraint that maintains organization of packed tissues (such as skeletal muscle) is lifted, deviations from the normal shape will occur. One example of this is seen when neuromuscular transmission is lost, which initiates atrophy and ultimately loss of the myofiber. 10 Consequently, it is common in many neurogenic and myogenic genetic disorders with denervation, to observe atrophic and deformed myofibers amidst myofibers with normal morphology. 11 Furthermore, we have observed that such fibres express denervation markers and their prevalence increases with ageing in healthy cohorts. 3 , 12 Interestingly, a potential explanation for the presence of misshaped fibres is that the healthy innervated myofibers compress denervated myofibers. 9
Four studies have investigated myofiber shape in humans. 13 , 14 , 15 , 16 Type II myofibers are more deformed in older compared with younger adults, while the shape of type I myofibers appears to be less affected by ageing. 13 , 14 , 15 Additionally, there does not seem to be differences in myofiber shape between males and females. 14 , 15 Shape might however be influenced by an individual's level of physical activity, 15 although it has earlier been reported that a 9‐week resistance exercise training intervention did not alter myofiber shape. 16 Furthermore, several earlier studies have provided qualitative descriptions of increased myofiber deformity in both rodents and humans, relating to ageing, 17 , 18 cachexia, 1 denervation 19 and muscular dystrophies. 20 In general, for these studies, it is mostly—if not solely—the shape of type II myofibers that seems to be affected, but systematic analyses of myofiber shape in large human and rodent datasets are lacking.
Currently, it is unknown how myofiber shape is related to physical function and thus whether it represents an undescribed hallmark of ageing. Similarly, the number of studies investigating age‐related changes in myofiber shape is low, and it is completely unknown whether hypertrophy‐inducing resistance training (RT) affects myofiber shape. As such, the aim of this study was to investigate how myofiber shape is influenced by ageing and fibre type in both young, old and very old males and females, with varying levels of physical function, and in young and aged mice. Additionally, hypertrophy‐inducing RT was used as a modality that might positively impact myofiber shape. We hypothesized that the shape of type I and II myofibers would be similar in young, healthy individuals and that ageing would lead to deformation of myofibers, especially in type II myofibers. Furthermore, we expected that hypertrophy‐inducing RT could reverse the deformation of myofiber shape.
Methods
Ethical approval and participants
For this study, 265 muscle biopsies collected from 197 individuals that were part of 7 prior studies previously conducted at Bispebjerg and Frederiksberg hospitals 12 , 21 , 22 , 23 , 24 , 25 , 26 were reanalysed. All studies were approved by appropriate ethics committees (Ref: (KF) 01‐224/94, H‐4‐2013‐068, H‐15005016, H‐15005761, H‐15017223, H‐16014268 and H‐19000881) and were conducted in accordance with the standards set by the Declaration of Helsinki. All subjects signed an informed consent agreement prior to enrolment.
The inclusion and exclusion criteria varied between studies, but in general, young 12 , 21 , 23 and most older adults 12 , 21 , 22 , 23 were healthy, non‐smoking, medication‐free and largely physically inactive males and females aged 20–36 or >60 years. They did not have any knee or hip pain that might prohibit physical testing, use anticoagulant medication, have a high alcohol consumption or have a body mass index (BMI) outside the normal range (<18.5 or >34.0 kg/m2). Some older adults lived in nursing homes and had varying levels of assistance with everyday activities and food preparation. 24 , 26 These participants were medically examined prior to enrolment, to make sure that they did not have surgical or medical diseases that prevented participation. Finally, some of the older adults were patients admitted to the geriatric ward, who were included in the study primarily based on whether they could safely and capably complete the study. 25
Heavy resistance training
Young (n = 7) and older (n = 52) adults underwent 3–4 months of heavy RT, three times per week. 21 , 22 Following 5 min of low‐ to moderate‐intensity cycling, the participants performed 3–5 sets of 8–15 repetitions at 8–15 repetition maximum in leg press, leg extension, leg curl and two optional upper body exercises. The training was supervised, and loads were continuously increased when a target number of repetitions at a given load was achieved.
Muscle mass, strength and specific force
Leg lean mass (LBMleg) was measured by dual‐energy X‐ray absorptiometry (DEXA) in 108 out of 197 subjects. Cross‐sectional area of the quadriceps (CSAquad) was measured by magnetic resonance imaging (MRI) in 104 out of 197 subjects. 21 Maximal voluntary contraction (MVC) force was measured in 149 out of 197 subjects, using a dynamometer (Model 500‐11; Kinetic Communicator) in both isokinetic (60°/s) and isometric (at 70° knee extension, 0° equals straight leg) modes. 12 Peak rate of force development (RFD) was simultaneously derived from the isometric MVC. Specific force (strength per unit mass) was calculated as isokinetic or isometric MVC relative to LBMleg or CSAquad.
Muscle biopsy
Muscle biopsies were obtained from the mid‐belly of the vastus lateralis muscle, under local anaesthesia (lidocaine), using a 5‐ or 6‐mm Bergstrom needle, fitted with manual suction. The biopsied tissue was aligned in Tissue‐Tek (Sakura Finetek) and frozen in isopentane (JT Baker) pre‐cooled by liquid nitrogen. Samples were stored at −80°C.
Animals
Eleven‐ and 22‐month‐old male C57BL/6 mice (Janvier), which were part of a previous study (Danish Animal Inspectorate, Ministry of Justice; #2014‐15‐020100326), 27 were housed individually, under standard conditions (tap water and standard chow) without assess to running wheel in a 12‐h light–dark cycle. The mice were sacrificed by cervical dislocation after which the left gastrocnemius (GAS) and soleus (SOL) muscles were dissected and aligned in Tissue‐Tek (Sakura Finetek) and frozen in pre‐cooled isopentane (JT Baker). Samples were stored at −80°C.
Histochemistry, immunofluorescence and imaging
Muscle samples (human biopsies and mouse muscles) were sectioned (7–10 μm thickness) cross‐sectionally using a cryostat, mounted on SuperFrost® Plus glass slides (Menzel‐Gläser, Thermo Scientific) and stored at −80°C. Human muscle samples were fibre typed using either ATPase histochemistry (97 samples) or immunofluorescence (168 samples).
ATPase—Human tissue
ATPase histochemistry and visualization of the myofiber membrane were performed as previously described. 22 , 26 Briefly, immunohistochemical staining of the myofiber membrane and capillaries were performed using a double staining method combining ulex europaeus lectin 1 (UEA‐1) and collagen type IV staining. 28 ATPase histochemistry for identifying fibre types was performed by preincubating four slides with solutions of pH 4.37, 4.53, 4.57 and 10.30, respectively. 22 Using the immunohistochemical staining for the myofiber membrane, a fibre mask was drawn along the cell borders of the desired number of fibres. Images of the ATPase stainings were then fitted into the fibre mask. A number was assigned by the computer to each fibre and the fibres were then displayed on the screen in multiple images. The individual fibres were therefore easily identified and assigned to a specific fibre type. Myofiber cross‐sectional area (CSA) and perimeter were measured using an image‐analysis software (Tema, Scanbeam, Hadsund, Denmark). Type II isotypes (IIa and IIx) were pooled into type II for the main data but were kept separate in the supporting data. All type I/IIa hybrid fibres, which can be common in the muscle of very old individiuals, 29 , 30 were removed from quantification. A mean ± SD [range] of 73 ± 24 [21–125] type I and 67 ± 23 [18–154] type II fibres per sample were analysed by ATPase.
Immunofluorescence—Human tissue
Immunofluorescence was performed as previously described. 12 , 25 Briefly, sections were removed from the freezer and allowed to dry. Dystrophin (RRID:AB_259245, D8168, Sigma‐Aldrich) or laminin (RRID:AB_2313665, Z0097, Dako) was used as membrane marker together with a myosin heavy chain (MyHC) I antibody (RRID:AB_10540570, A4.951, or RRID:AB_2235587, BA.D5, both DSHB). Primary antibodies were appropriately diluted in a blocking buffer consisting of 1% bovine serum albumin (BSA) and 0.1% sodium azide in Tris‐buffered saline (TBS) and applied overnight at 5°C. The next day, slides were incubated for 45 min at room temperature with corresponding secondary antibodies appropriately diluted in blocking buffer. Finally, sections were mounted with cover glasses using Prolong‐Gold‐Antifade (P36931; Thermo Fisher Scientific). Fixation was performed using either 4% paraformaldehyde (PFA) or Histofix (Histolab) either before primary antibodies or after secondary antibodies were applied. Sections were washed in TBS between all steps. Imaging was performed using either a 0.30 NA/×10 objective and a DP71 Olympus camera on a BX51 Olympus microscope, or 0.80 NA/×20 objective on a confocal laser scanning microscope (LSM710, Carl Zeiss, Oberkochen, Germany). Image analysis was performed using a custom‐build ImageJ macro, S1 as previously described in detail. 31 Type I (MyHC I positive) and type II (MyHC I negative) myofiber CSA and perimeter were measured. A total of 568 (0.96% of all fibres) type I/II hybrid myofibers with weak MyHC I staining were removed from the analyses. A mean ± SD [range] of 231 ± 125 [38–710] type I and 204 ± 127 [31–705] type II myofibers per sample were analysed by immunofluorescence.
Myofiber denervation was assessed in a subset of human samples using immunofluorescence to detect the presence of neural cell adhesion molecule (NCAM) in myofibers, as previously described in detail. 3
Immunofluorescence—Mouse tissue
For mouse muscles, as previously described, 27 two consecutive cross‐sections were stained with laminin (RRID:AB_2313665, Z0097, Dako) and either MyHC1 (RRID:AB_2235587, BA.D5, DSHB) and MyHC2b (RRID:AB_2811120, BF‐F3, DSHB), or MyHC2a (RRID:AB_2147165, SC‐71, DSHB) and MyHC2x (RRID:AB_1157897, 6H1, DSHB). Samples were imaged using a 0.50 NA/×20 objective and a DP71 Olympus camera on a BX51 Olympus microscope. Images were analysed using a custom‐made semi‐automated macro in ImageJ, which aligned images from consecutive cross‐sections, identified fibre type and performed morphometric measurements. Fibres were classified as either type IIa, IIa/IIx, IIx, IIx/IIb or IIb in GAS and I, I/IIa or IIa in SOL. A mean ± SD [range] of 214 ± 99 [65–359] type IIa, 36 ± 24 [5–75] type IIa/IIx, 128 ± 55 [51–233] type IIx, 21 ± 36 [0–133] type IIx/IIb and 734 ± 301 [205–1228] type IIb myofibers per GAS sample and 260 ± 70 [177–441] type I, 7 ± 7 [0–22] type I/IIa and 351 ± 86 [192–485] type IIa myofibers per SOL samples were analysed.
Shape factor index
Myofiber shape was evaluated using the shape factor index (SFI) as previously performed. 13 , 14 , 15 , 16 SFI is a derivative of the ‘Formfactor’ and, thus, represents a dimensionless expression of object shape, with values above 1.00 showing deviation from a circle. S2 Myofibers cut in the cross‐sectional plane have polyhedral shapes rather than circular and have values above 1.00, with greater values indicating further shape deviation. An increased SFI thus means that a myofiber has a greater perimeter relative to its area. SFI was calculated for each myofiber using the measured CSA and perimeter values, with the following formula:
where the denominator represents the squared perimeter of a perfect circle with the given CSA (the smallest possible perimeter). Being a dimensionless expression of shape, objects of the same shape but different sizes will have the same SFI (Figure 1 A ). But two myofibers of equal size can have greatly different shapes (Figure 1 B ). To exemplify further, Figure 1 C shows the SFI of a circle (1.00), pentagon (1.15), hexagon (1.11) and myofibers with gradually increasing SFI (1.14–1.87).
Figure 1.
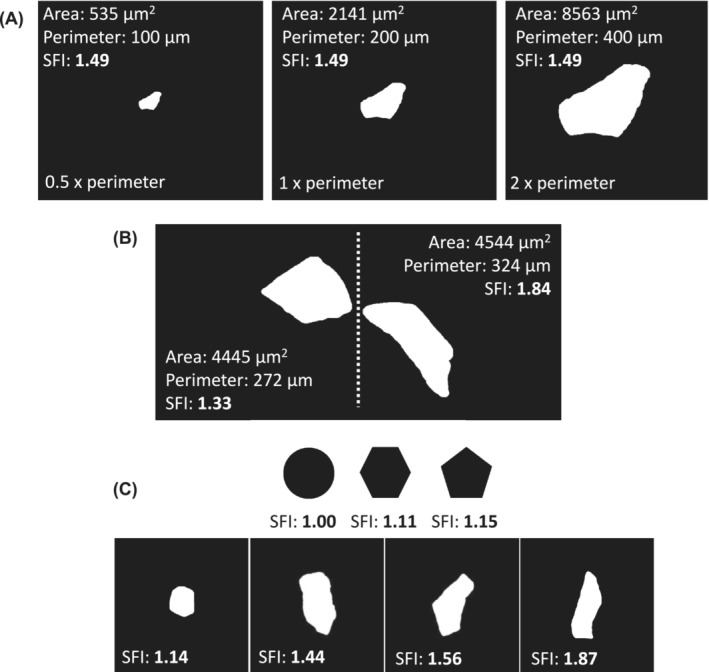
Shape factor index (SFI) overview. (A) SFI is a dimensionless descriptor of shape, as shown with the same myofiber in three different sizes. (B) High SFI values indicate increased deviation from a circle, as illustrated by two fibres with similar cross‐sectional area but different shapes. (C) SFI values for a circle and four myofibers of different shapes. The area and perimeter values are from actual measurements of myofibers on cryosections.
For each tissue sample, the mean SFI of all myofibers analysed was calculated to represent that sample. Furthermore, SFI distribution was expressed in 0.10 increments from <1.10 to >2.00, as well as in relation to myofiber CSA in 1000 μm2 increments from 0 to >6000 μm2. Finally, coefficient of variation (CV) for fibre‐type‐specific SFI and CSA was calculated within each subject to explore heterogeneity. S3
Statistics
Data in graphs are presented as means ± SEM or means with individual values. Data were analysed using t‐tests (paired or unpaired), one‐way analyses of variance (ANOVAs), two‐way repeated measures ANOVAs and mixed‐effects models. The statistical test used for a given dataset is specified in the corresponding figure legend. Relationship between SFI, or CSA, and muscle mass and function was evaluated using simple regressions (Pearson), whereas the association between SFI and age was evaluated using second‐order polynomial regression analyses. Multiple linear regressions (backward elimination) were performed to explore the explanatory power of five model parameters (age, type I SFI, type II SFI, type I CSA and type II CSA) on muscle mass and function. Type II semi‐partial correlation coefficients, which represent the additional explanatory power of the given parameter after accounting for the other parameters in the model, are reported for significant predictors. Graphs were prepared in Prism (v.8, GraphPad Software). Statistical analyses of the histograms (two‐way repeated measures ANOVAs and mixed‐effects models) were performed in Prism, multiple linear regressions were performed in SAS (v.9.2, SAS, Cary, NC, USA) and all other statistical analyses were performed in SigmaPlot (v. 13.0, Systat Software). Statistical significance was set at 0.05.
Results
Subject characteristics
In the present study, muscle biopsies from 197 subjects, which were part of 7 prior studies, were analysed for SFI. Key subject characteristics are summarized in Table 1 . The number of female (F) and male (M) subjects represented in each age cohort was 12 F and 22 M in young, 13 F and 98 M in old and 22 F and 30 M in oldest old. Sex‐specific subject characteristics are presented in Supporting Information S1. The old and oldest old groups were phenotypically aged; compared with young, old demonstrated lower LBMleg (11%, P < 0.05), CSAquad (16%, P < 0.05) and isometric MVC (21%, P < 0.001). Compared with old, oldest old demonstrated lower LBMleg (21%, P < 0.001), CSAquad (26%, P < 0.001) and isometric MVC (33%, P < 0.001). Subject characteristics from each of the seven studies are provided separately in Supporting Information S2. The percentage type I fibres were 51.2 ± 13.8%, 54.8 ± 15.8% and 50.5 ± 15.9% for young, old and oldest old, respectively, with no significant differences between groups.
Table 1.
Subject characteristics for young, old and oldest old
| Young (12 F/22 M) | Old (13 F/98 M) | Oldest old (22 F/30 M) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Average ± SD | Min–max | N | Average ± SD | Min–max | N | Average ± SD | Min–max | N | |
| Age (years) | 25 ± 4 | 20–36 | 34 | 71 ± 4 | 60–79 | 111 | 86 ± 3 | 81–97 | 52 |
| Height (m) | 1.77 ± 0.10 | 1.57–1.93 | 34 | 1.77 ± 0.08 | 1.55–1.95 | 111 | 1.70 ± 0.11 | 1.42–1.88 | 40 |
| Weight (kg) | 75 ± 15 | 53–111 | 34 | 81 ± 13 | 56–109 | 111 | 72 ± 14 | 45–98 | 40 |
| BMI (kg/m2) | 23.9 ± 3.1 | 19.0–30.4 | 34 | 25.7 ± 3.3 | 18.9–32.5 | 111 | 24.6 ± 3.3 | 18.7–33.2 | 40 |
| LBMleg (kg)*, # | 22 ± 4 | 17–30 | 22 | 20 ± 3 | 12–26 | 53 | 16 ± 3 | 9–21 | 33 |
| CSAquad (cm2)*, # | 73 ± 15 | 55–99 | 7 | 62 ± 12 | 36–91 | 65 | 45 ± 11 | 26–69 | 32 |
| Isometric MVC (Nm)*, # | 272 ± 62 | 186–364 | 22 | 188 ± 38 | 85–278 | 93 | 126 ± 42 | 22–205 | 34 |
| Isokinetic MVC (Nm)*, # | 307 ± 67 | 200–425 | 22 | 207 ± 46 | 75–313 | 94 | 109 ± 45 | 18–219 | 35 |
| Isometric RFD (Nm/s)* | 2322 ± 839 | 1135–4048 | 22 | 1259 ± 472 | 251–2746 | 93 | 1126 ± 531 | 692–2295 | 8 |
Note: Data are shown as means ± SD with range and number of subjects represented in each measurement. LBMleg, CSAquad, MVCs and RFD were compared using one‐way analyses of variance. Subject characteristics separated by gender and from each study can be found in Supporting Information S1 and S2, respectively. Abbreviations: BMI, body mass index; CSAquad, quadriceps cross‐sectional area; F, female; LBMleg, leg lean mass; M, male; MVC, maximal voluntary contraction; RFD, rate of force development.
P < 0.05 between young and old.
P < 0.05 between old and oldest old.
Shape factor index is fibre type dependent and increases with ageing
SFI for both type I and II myofibers demonstrated positive correlations with age (Figure 2 A ; R 2 = 0.20 and 0.38, P < 0.001), with myofibers becoming increasingly misshapen with age. There was no difference in SFI between males and females in young, old and oldest old (Supporting Information S3). To further explore the different trajectories between fibre types, subjects were divided into three age cohorts (Figure 2 B ), where it became clear that the fibre type difference in SFI was present even in young (P < 0.001). Additionally, old had higher SFI values for both fibre types than young (P < 0.001), while the oldest old had higher values than old (P < 0.05–0.001). The age‐related increase in SFI was smaller for type I compared with type II myofibers. Specifically, compared with young, type I SFI was 3.9% and 5.2% higher in old and oldest old, respectively. For type II fibres, the equivalent values were 6.2% and 13.6%. SFI CV was similar between age groups for type I fibres but higher in oldest old compared with old (P < 0.01) and young (P < 0.05) (Supporting Information S4). Examples of fibres with the full range of SFI values are shown in Figure 2 C–E and Supporting Information S5. Type I/IIa and IIa/IIx hybrid fibres were analysed using ATPase in 52 subjects from old (Supporting Information S6), with IIa/IIx fibres having an SFI similar to IIa fibres but different from IIx fibres. Only 50% of the subjects had one or more type I/IIa hybrid fibres, so these could not be reliably evaluated (not shown).
Figure 2.
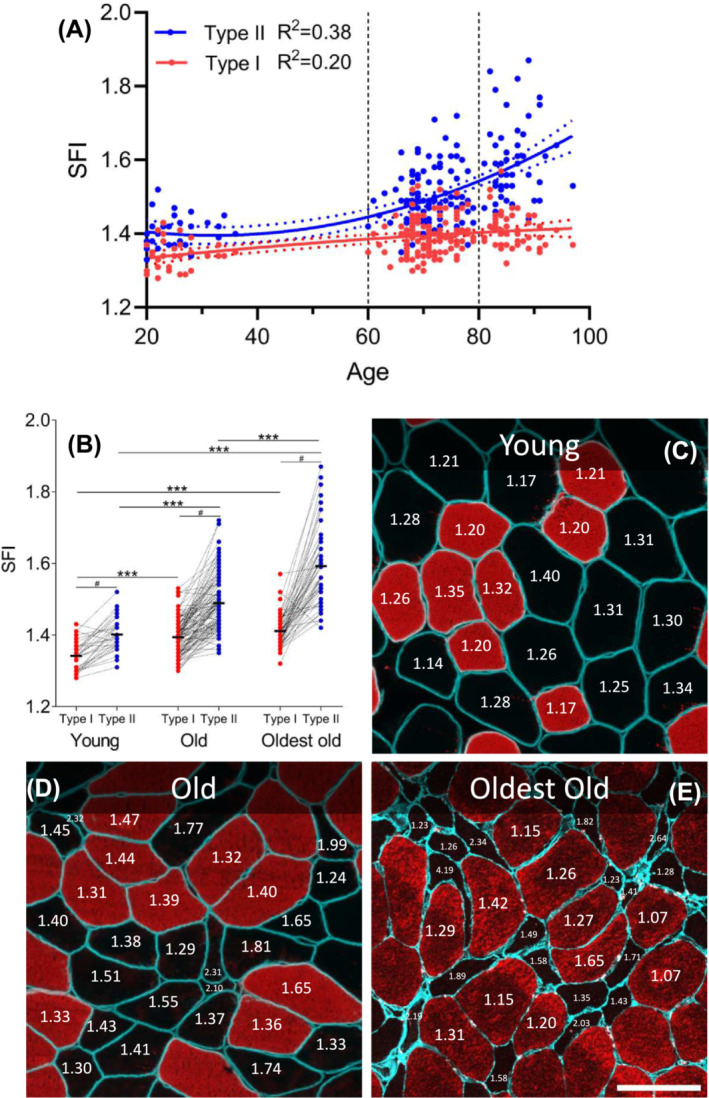
Myofiber shape factor index (SFI) increases with ageing and in type II fibres. (A) Association between age and type I (red, P < 0.001) and II (blue, P < 0.001) myofiber SFI was determined by second‐order polynomial regression (solid line). Horizontal dashed lines indicate arbitrary separation into three age cohorts: young (n = 34), old (n = 111) and oldest old (n = 52). (B) Type I and II myofiber SFI for young, old and oldest old displayed as means, with connected individual values. Each data point represents one individual. Data were analysed using two‐way repeated measures analysis of variance (age group × fibre type), with significant main effects (P < 0.001) and interactions (P < 0.001). *** indicates effect of age group, P < 0.001. # indicates effect of fibre type, P < 0.001. (C–E) Representative examples of muscle biopsy cross‐sections from young (C), old (D) and oldest old (E), stained for dystrophin (C, D, cyan), laminin (E, cyan) and myosin heavy chain type I (C–E, red). SFI values are provided for selected fibres. Scale bar = 100 μm.
Shape factor index is higher in older individuals for fibres of all sizes
With increasing age, fibres with high SFI values make up a larger proportion of fibres (Figure 3 A ). For type I fibres, the young group has a higher proportion than the two older groups in the SFI increments below 1.3. From the increments starting at 1.4 and higher, this pattern is reversed. A similar picture is clear for type II fibres, with the shift occurring at SFI of 1.5. Interestingly, only one of the type I fibre SFI increments demonstrates a difference between the old and the oldest old, while seven of the type II fibre SFI increments display this difference. To probe whether the age‐related change in SFI was driven by a higher abundance of atrophic fibres, SFI was expressed according to myofiber CSA, in 1000 μm2 increments. As seen in Figure 3 B , the greater SFI in old compared with young, and oldest old compared with old, was present in the majority of CSA increments, confirming that high SFI is not driven by a higher prevalence of atrophic fibres alone. The number of fibres and subjects represented in each category is provided in Supporting Information S7.
Figure 3.
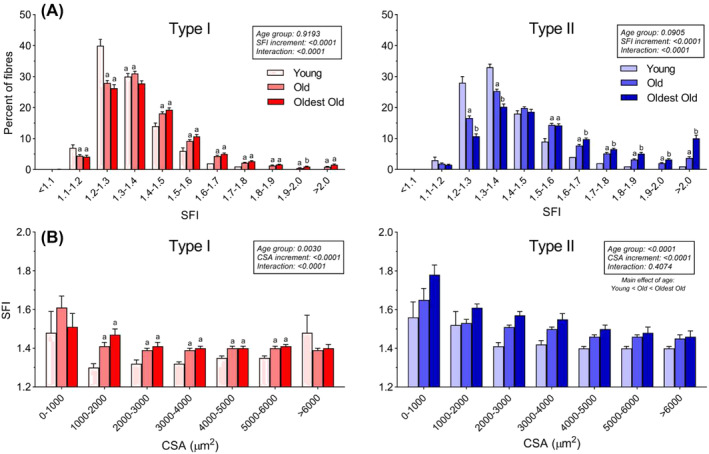
Shape factor index (SFI) distribution. (A) Percentage of type I and II myofibers in 0.1 increments of SFI for young (n = 34), old (n = 111) and oldest old (n = 52). Data were analysed using two‐way repeated measures analysis of variance (age group × cross‐sectional area [CSA] increment), with main effects and interactions indicated in the figure. Results of the post hoc test are indicated by letters; bars that do not have the same letter are significantly different within the respective SFI increment (P < 0.05). (B) SFI of type I and II myofibers binned in 1000 μm2 CSA increments. Data are averages of all subjects within each age group and presented as means ± SEM. Data were analysed using mixed‐effects model (age group × CSA increment), with main effects and interactions indicated in the figure. Results of the post hoc test are indicated by letters; bars that do not have the same letter are significantly different within the respective CSA increment (P < 0.05). See Supporting Information S7 for details on the numbers of participants represented in each increment.
We also assessed SFI for denervated myofibers (determined by NCAM expression) in a subset of samples (Supporting Information S8). Most denervated myofibers are atrophic and present with high SFI values (>1.60), while a smaller portion of myofibers had a shape corresponding to that found in young (SFI 1.20–1.50).
Myofiber shape factor index shows similar patterns in mice
To address whether this was conserved across species, we sampled GAS and SOL muscles from C57BL/6 mice (Supporting Information S9). In confirmation of the observations in humans, SFI values were higher in type II compared with type I myofibers (P < 0.001). Additionally, in the fast‐twitch GAS, this seemed to vary with type II isotypes, with increasing values from IIa to IIx and IIb (P < 0.05). However, SFI was not higher in old compared with young mice for any fibre type in any muscle.
The importance of shape factor index for physical function
To better understand how an increased SFI influences muscle function in humans, SFI and CSA were correlated with in vivo measurements of muscle mass (DEXA and MRI), muscle function (MVC and RFD) and specific force (strength per unit mass). As shown in Figure 4 and in Supporting Information S10, in general, SFI demonstrated strong, significant negative correlations with in vivo muscle mass and function measures, with higher SFI values associated with lower mass and function. CSA demonstrated strong, significant positive correlations with the same outcomes, with lower CSA values associated with lower mass and function. Multiple linear regression with backward elimination was used to probe whether SFI and CSA had independent explanatory power when adjusting for confounding variables, including age. As shown in Figure 4 E and in Supporting Information S11, SFI was an independent predictor of 8 of the 10 variables tested. Specifically, type II fibre SFI (and not type I fibre SFI) was an independent predictor of CSAquad, isometric MVC, isokinetic MVC and three types of specific force. Type I fibre SFI was an independent predictor of LBMleg, and SFI for both type I and II fibres were independent predictors of one measure of specific force. For comparison, type II fibre CSA only came out as an independent predictor of 4 out of the 10 variables tested.
Figure 4.
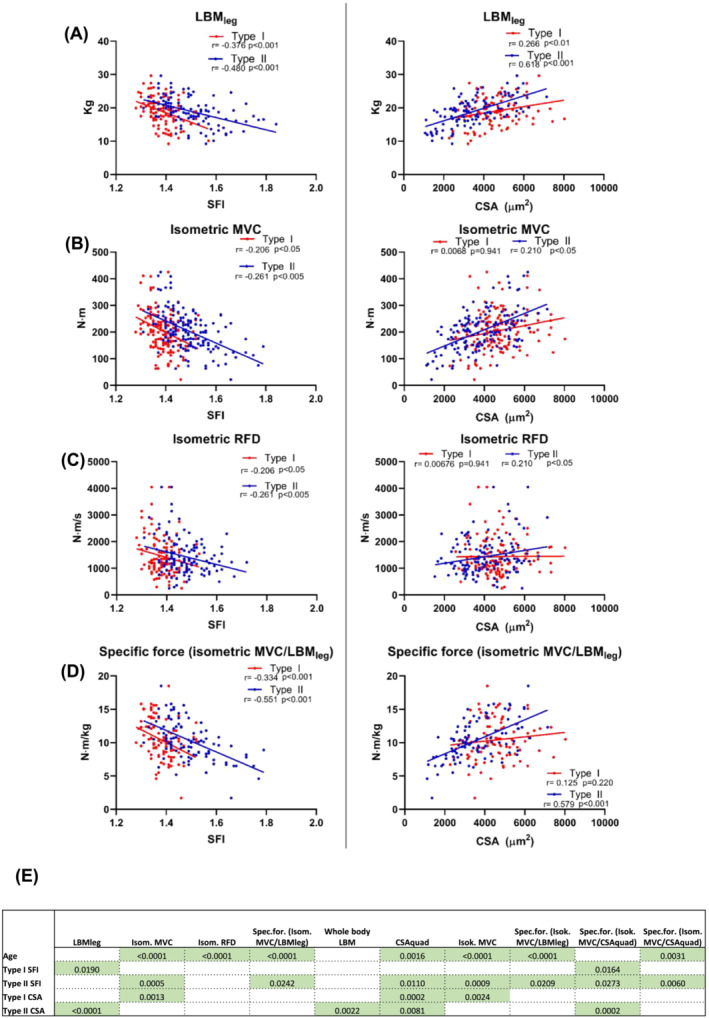
Linear correlation analyses between shape factor index (SFI) (left) and cross‐sectional area (CSA) (right) for type I and II myofibers with in vivo measures of muscle mass and function. Each data point represents one individual, ranging in age from 20 to 94 years, and all in the untrained state. Strength of association is indicated by r and P‐values. Correlations are displayed for (A) leg lean mass (LBMleg) (n = 108), (B) isometric (Isom.) maximal voluntary contraction (MVC) (n = 150), (C) isometric rate of force development (RFD) (n = 123) and (D) specific force (Spec.for.) (MVC/LBMleg) (n = 98). (E) P‐values of the model parameters that were significant independent predictors of 10 indices of muscle mass and function. Refer to Supporting Information S11 for full outline of the multiple linear regression analyses. Additional correlations can be found in Supporting Information S10. Isok., isokinetic.
Heavy resistance training reverses the higher shape factor index of type II myofibers
To test whether RT‐induced hypertrophy could alter myofiber shape, SFI was evaluated before and after 3–4 months of RT. In both young and old, RT reduced type II SFI significantly (P < 0.001), while type I SFI remained unchanged (Figure 5 A ). Individual delta values are shown in Supporting Information S12. As shown in Figure 5 B , there were significant negative correlations between baseline SFI and change in SFI with RT, for both type I (R 2 = 0.266, P < 0.001) and II (R 2 = 0.233, P < 0.001) myofibers.
Figure 5.
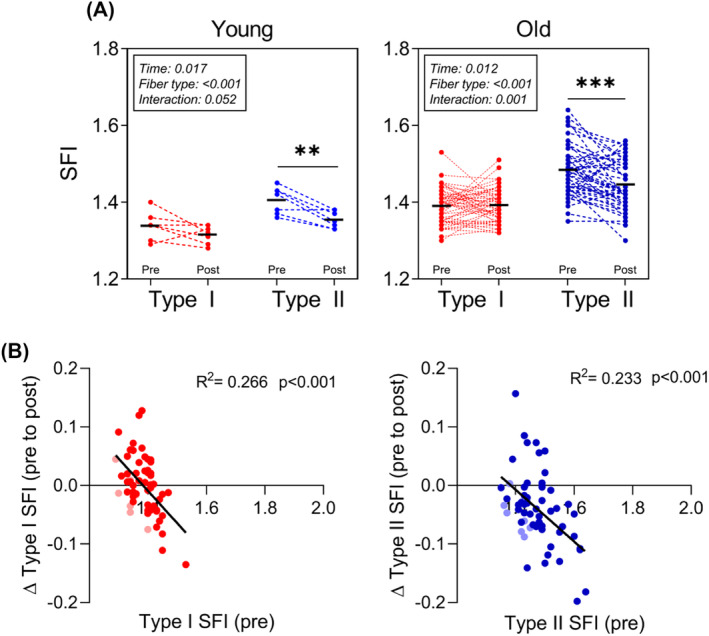
Shape factor index (SFI) modification with resistance training (RT). (A) SFI for type I (red) and II (blue) myofibers before and after 3 or 4 months of RT. Data are presented for young (n = 7) and old (n = 52) and were analysed using two‐way repeated measures analysis of variance (time × fibre type) within each age group, with main effects and interactions indicated in the figure. **P < 0.01 post versus pre. ***P < 0.001 post versus pre. (B) Linear correlation analysis between SFI at baseline and changes following RT for type I (red) and II (blue) myofibers. Data are pooled young and old participants from A, and young is shown in faded colours. R 2 and P‐values are provided.
Discussion
Myofiber size is traditionally reported as the most important morphological feature of skeletal muscle biopsy specimens collected from healthy and diseased individuals of all ages and in response to physical activity and disuse. In this study, with 265 human muscle tissue samples, we focus instead on the shape of the myofiber and find that age, fibre type and exercise alter myofiber shape. Furthermore, myofiber shape, investigated using the SFI, was a significant independent predictor of several in vivo indices of muscle mass and function, even after adjusting for age and CSA. Importantly, hypertrophy‐inducing RT partially reversed the deformity of type II myofibers. These findings demonstrate that myofiber shape is a hallmark of muscle ageing. We argue that SFI should therefore be included alongside CSA in histological investigations of aged and diseased muscle, to enhance understanding of the full extent of myofiber maladaptation and the degree of its reversibility with mechanical loading.
Age‐related myofiber deformation is linked to lack of myofiber recruitment
It is well documented that particularly type II myofibers undergo atrophy with ageing and physical inactivity, 32 but the potential significance of myofiber shape in aged muscle has received limited attention. 13 , 14 , 15 , 16 In this study, we comprehensively investigated myofiber shape in relation to ageing, physical function, species and sex. In contrast to our hypothesis and previous findings, 13 , 14 , 15 we found that type II myofibers were deformed compared with type I myofibers, even in young healthy individuals. However, in agreement with our hypothesis but in contrast to previous findings, 13 , 14 , 15 we found that both type I and II myofibers became increasingly misshaped with ageing. This finding was not replicated in mice, suggesting that mouse muscle is affected differently than human muscle, at least for mice at 22 months of age. Our immunofluorescent approach in humans did not allow for a comprehensive evaluation of hybrid fibres; however, samples from 52 subjects in old were analysed by ATPase, providing insight into the type I/IIa and IIa/IIx hybrid fibres. Interestingly, type IIa/IIx hybrid fibres had SFI values similar to pure type IIa fibres but different from IIx fibres. Type I/IIa fibres, which in rodents have been reported to be in some sort of disarray, 19 were rare and could not be reliably evaluated.
The age‐related increase in SFI in humans was seen across all fibre size increments, albeit most predominantly in the smallest type II fibres, which are more abundant in old muscle. Age‐related changes in myofiber shape are thus cumulatively driven by a general deformation of all myofibers alongside an increased proportion of small type II fibres. The age‐related change in shape was less in type I fibres compared with type II. In fact, the SFI of type I myofibers in aged muscle is comparable with the SFI of type II myofibers in young. For both type I and II SFI, there was a strong correlation to in vivo volumetric and functional assessments of muscle health. In many cases, the correlations were as strong or stronger than those of the classic hallmark of muscle ageing, myofiber CSA. Consequently, it can be argued that myofiber shape is indicative of muscle health in an ageing context. Additionally, these data clearly demonstrate why it is not appropriate to use minimum Feret diameter in ageing muscle tissue samples, as the age‐related change in myofiber shape will give artificially low diameters.
The increased abundance of highly deformed (SFI > 1.6) and often very atrophic fibres (CSA < 2000 μm2) was observed predominantly in the oldest old and is particularly interesting. The CV for type II myofiber SFI was also found increased in this group compared with old and young, indicating increased shape heterogeneity within individuals. We speculate that the source of these fibres is myofiber denervation and failed reinnervation. When a myofiber becomes denervated, through motoneuron decay or NMJ destabilization, its gene and protein expression is altered, 33 fibre type uniformity is lost 34 and the fibre atrophies 35 and loses core structural features. 36 It has been shown that temporary loss of neuromuscular transmission exerts similar negative effects on myofiber size and structure as actual denervation. 37 Given that recruitment of fast‐twitch motor units only occurs during high speed and high force movements, it is therefore a reasonable assumption that older adults not engaging in such activities will experience denervation‐like ramifications to their type II myofibers. To explore whether the increased SFI observed in older adults was related to neuromuscular disturbances, we assessed SFI in denervated myofibers by immunofluorescence staining for NCAM. 38 NCAM gene expression is upregulated in myonuclei following sciatic nerve transection 39 and is one of the several tentative markers of denervation. 38 While highly variable, a large proportion of the denervated myofibers were both atrophic and deformed (SFI > 1.60 values), whereas some NCAM+ fibres retained a normal shape and size and are thus presumably caught early in the denervation process. We speculate that the very deformed and atrophic myofibers observed are long‐term denervated and therefore no longer able to become reinnervated, whereas the NCAM+ myofibers that present with a normal histological profile suffer from a lack of recruitment or are recently denervated.
Inherent fibre type difference in myofiber shape
Surprisingly, we found a significant, albeit minor, fibre type difference in SFI in the young individuals, potentially due to the large number of myofibers included in the analyses (>200 per fibre type). The SFI increment with the highest proportion of type I fibres was 1.20–1.30, while for type II, it was 1.30–1.40. This finding is surprising, as the myofibers in the young and healthy would be expected to have a favourable myofiber shape that is unperturbed by the effects of ageing. We decided to investigate this further in mice and found that this inherent fibre type difference was conserved across species. Additionally, we also observed that type IIx and IIb fibres had a higher SFI than type IIa fibres. It is unclear whether this reflects the divergent physiology of type I and II fibre types, with SFI relating to a fibre's intrinsic force‐generating capacity. 40 Alternatively, it may be acquired due to a predominately sedentary lifestyle, where fast‐twitch motor units would only rarely be recruited, and this lack of fibre recruitment could negatively influence the shape of the myofibers. Importantly, type II myofiber SFI was reduced in the seven young subjects that underwent RT, implying that new‐found recruitment and subsequent hypertrophy of these myofibers also alters their shape. It should also be pointed out that while the subjects were generally considered to be sedentary, they did bike and walk for transportation purposes, which may also influence myofiber shape.
Heavy resistance training reverses myofiber deformation
Given that SFI of both fibre types was so closely correlated with indices of physical function, we wanted to explore whether hypertrophy‐inducing RT would normalize myofiber shape. To do that, we examined muscle biopsies from young and old individuals who had completed 3 or 4 months of RT that lead to significant hypertrophy at both the whole muscle and myofiber levels. 21 , 22 In agreement with our hypothesis, we found that SFI declined in type II myofibers following the intervention, demonstrating that increased neuromuscular activity not only results in increased size of the myofibers but also restores a favourable shape. It is also clear that the individuals with the highest SFI values had the largest decrements in SFI. This suggests that myofiber shape remains amendable to change even when we reach our 70s. As we did not have any subjects from the oldest old taking part in the training intervention, we do not know if this muscle plasticity stretches into the last decades of life.
Conclusions and perspectives
We provide evidence in males and females to support myofiber shape—investigated using the SFI—as a prominent hallmark of skeletal muscle ageing. Furthermore, myofiber shape is a strong, independent predictor of indices of muscle health measured in vivo, and the degree of myofiber deformity is improved by several months of RT. We argue that increased myofiber deformity is related to neuromuscular disturbances and myofiber denervation, although this remains speculative. Due to the ease of assessing SFI (when muscle biopsies are collected), the strong relationship between SFI and several indices of muscle health and the substantial adaptability of SFI in response to increased myofiber recruitment, we believe that SFI should be assessed in conjunction with other morphological muscle parameters, such as CSA. This is especially warranted when mapping the effects of interventions that seek to improve muscle quality in elderly or diseased muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Supporting information
Supporting Information S1. Subject characteristics for males and females separately, shown as means ± SD with range and number of subjects represented in each measurement. BMI, body mass index. LBMleg, leg lean mass. CSAquad, quadriceps cross‐sectional area. MVC, maximal voluntary contraction.
Supporting Information S2. Subject characteristics for each of the original studies separately, shown as means ± SD with minimum and maximum values. These subjects characteristics have previously been reported in the original publications13,24–29. BMI, body mass index. LBMleg, leg lean mass. CSAquad, quadriceps cross‐sectional area. MVC, maximal voluntary contraction.
Supporting Information S3. SFI in males and females separately. Type I (red) and II (blue) myofiber SFI for Young (a, n = 22 males and 12 females), Old (b, n = 98 males and 13 females), and Oldest Old (c, n = 30 males and 22 females). Data were analyzed using Two‐Way Repeated Measures ANOVA (sex x fiber type) within each group, with main effects of fiber type indicated in the figure. SFI, shape factor index.
Supporting Information S4. Coefficient of variation (CV) of SFI (A) and CSA (B) for type I and II myofibers in Young (n = 34), Old (n = 111) and Oldest Old (n = 52). Data were analyzed using Two‐Way Repeated Measures ANOVA (age group x fiber type). *p < 0.05, **p < 0.01, ****p < 0.0001. CV, coefficient of variation. SFI, shape factor index. CSA, cross‐sectional area.
Supporting Information S5. Representative split channel images of muscle biopsy cross‐sections from Young, Old, and Oldest Old, stained for dystrophin (Young, Old) or laminin (Oldest Old), and MyHC I. Scale bar = 100 μm.
Supporting Information S6. SFI in hybrid fibers. The SFI of IIa/IIx hybrid fibers were evaluated in a subset of samples (n = 52). Data were analyzed using One‐Way Repeated Measures ANOVA (fiber type). *p < 0.05, **p < 0.005, ***p < 0.005, ****p < 0.0001. SFI, shape factor index.
Supporting Information S7. a) Number of subjects and myofibers represented in each type I and II myofiber SFI increment for Young, Old, and Oldest Old. These data supplement figure 3.a. b) Number of subjects and myofibers represented in each type I and II myofiber CSA increment for Young, Old, and Oldest Old. These data supplement figure 3.b.
Supporting Information S8. Split channel view of myofibers in healthy older adults stained with dystrophin (cyan), NCAM (magenta) and nuclei (white). Denervated myofiber is identified by arrow, with the SFI provided below. Scale bar = 100 μm. NCAM, neural cell adhesion molecule. SFI, shape factor index.
Supporting Information S9. SFI in muscles of 11 and 22 month old mice. a) Split channel and merged images of a mouse GAS muscle stained with MyHC I (cyan), MyHC IIb (magenta), MyHC IIa (yellow), MyHC IIx (green) and laminin (grey) across two consecutive cross‐section. b) Type I and type II myofiber SFI in gastrocnemius (GAS) and soleus (SOL) combined. C) SFI in GAS, for type I, Iia, IIa/IIx, IIx, IIx/IIb and IIb. d) SFI in SOL, for type I, I/IIa and IIa. Data are shown as means ± SEM and were analyzed using a Two‐Way Repeated Measures ANOVA (muscle x fiber type), with a significant main effect of fiber type (p < 0.001). *p < 0.05, **p < 0.005, ***p < 0.005, ****p < 0.0001. N = 7 and 6 for 11 and 22 month old, respectively. SFI, shape factor index.
Supporting Information S10. Linear correlation analyses between SFI (left) and CSA (right) for type I and II myofibers with in vivo measures of muscle mass and function. Each data point represents one individual, ranging in age from 20–94y, and all in the untrained state. Strength of association is indicated by r and p values. Correlations are displayed for whole body LBM, CSAQuad, isokinetic MVC, specific force (isokinetic MVC/CSAQuad), specific force (isokinetic MVC/LBMleg), and specific force (isometric MVC/CSAQuad). LBM, lean body mass. CSAQuad, quadriceps cross‐sectional area. LBMleg, leg lean mass. SFI, shape factor index. CSA, cross‐sectional area. RFD, rate of force development. MVC, maximal voluntary contraction.
Supporting Information S11. Result of backward elimination multiple linear regression. Ten indices of muscle mass and function were evaluated (1–4 in figure 4 and 5–10 in Supporting Information S10). Model parameters included: Age, type I SFI, type II SFI, type I CSA and type II CSA. SE, standard error. SFI, shape factor index. CSA, cross‐sectional area. R squared for the individual parameters represent type II semi‐partial correlation coefficients.
Supporting Information S12. SFI delta values from pre to post RT, for type I (red) and II (blue) myofibers (n = 59). Young and Old subjects are pooled, but Young is shown in faded colors. Delta values were compared using paired t‐tests. ***p < 0.001. RT, heavy resistance training. SFI, shape factor index.
Data S1. Supporting Information.
Acknowledgements
Funding from the Lundbeck Foundation (R344‐2020‐254, R402‐2022‐1387) and the Nordea Foundation (Centre for Healthy Aging) is gratefully acknowledged. The authors are thankful for the technical assistance that was provided by lab technicians Camilla Brink Sørensen, Ann‐Christina Ronnie Reimann and Anja Jokipii‐Utzon. The monoclonal antibodies A4.951 and BA.D5 (type I MyHC), developed by Blau, H.M., and Schiaffino, S., respectively, were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. S4
Soendenbroe C, Karlsen A, Svensson RB, Kjaer M, Andersen JL, Mackey AL. Marked irregular myofiber shape is a hallmark of human skeletal muscle ageing and is reversed by heavy resistance training. Journal of Cachexia, Sarcopenia and Muscle 2023; 10.1002/jcsm.13405
Jesper L. Andersen and Abigail L. Mackey have contributed equally to this study.
References
- 1. Tomlinson BE, Walton JN, Rebeiz JJ. The effects of ageing and of cachexia upon skeletal muscle. A histopathological study. J Neurol Sci 1969;9:321–346. [DOI] [PubMed] [Google Scholar]
- 2. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 3. Soendenbroe C, Heisterberg MF, Schjerling P, Karlsen A, Kjaer M, Andersen JL, et al. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve 2019;60:453–463. [DOI] [PubMed] [Google Scholar]
- 4. Suetta C, Haddock B, Alcazar J, Noerst T, Hansen OM, Ludvig H, et al. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle 2019;10:1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology—update 2014. J Cachexia Sarcopenia Muscle 2014;5:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, et al. Improved single muscle fiber quality in the oldest‐old. J Appl Physiol 1985;2016:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grosicki GJ, Zepeda CS, Sundberg CW. Single muscle fibre contractile function with ageing. J Physiol 2022;600:5005–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sánchez‐Gutiérrez D, Sáez A, Gómez‐Gálvez P, Paradas C, Escudero LM. Rules of tissue packing involving different cell types: human muscle organization. Sci Rep 2017;7:40444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sánchez‐Gutiérrez D, Tozluoglu M, Barry JD, Pascual A, Mao Y, Escudero LM. Fundamental physical cellular constraints drive self‐organization of tissues. EMBO J 2016;35:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol (Lond) 2016;594:1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 2013;45:2191–2199. [DOI] [PubMed] [Google Scholar]
- 12. Soendenbroe C, Dahl CL, Meulengracht C, Tamáš M, Svensson RB, Schjerling P, et al. Preserved stem cell content and innervation profile of elderly human skeletal muscle with lifelong recreational exercise. J Physiol 2022;600:1969–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkeby S, Garbarsch C. Aging affects different human muscles in various ways. An image analysis of the histomorphometric characteristics of fiber types in human masseter and vastus lateralis muscles from young adults and the very old. Histol Histopathol 2000;15:61–71. [DOI] [PubMed] [Google Scholar]
- 14. Barnouin Y, McPhee JS, Butler‐Browne G, Bosutti A, De Vito G, Jones DA, et al. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J Cachexia Sarcopenia Muscle 2017;8:647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Messa GAM, Piasecki M, Rittweger J, McPhee JS, Koltai E, Radak Z, et al. Absence of an aging‐related increase in fiber type grouping in athletes and non‐athletes. Scand J Med Sci Sports 2020;30:2057–2069. [DOI] [PubMed] [Google Scholar]
- 16. Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch 1997;433:238–244. [DOI] [PubMed] [Google Scholar]
- 17. Scelsi R, Marchetti C, Poggi P. Histochemical and ultrastructural aspects of m. vastus lateralis in sedentary old people (age 65–89 years). Acta Neuropathol 1980;51:99–105. [DOI] [PubMed] [Google Scholar]
- 18. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 2003;13:40–47. [DOI] [PubMed] [Google Scholar]
- 19. Purves‐Smith FM, Solbak NM, Rowan SL, Hepple RT. Severe atrophy of slow myofibers in aging muscle is concealed by myosin heavy chain co‐expression. Exp Gerontol 2012;47:913–918. [DOI] [PubMed] [Google Scholar]
- 20. Kaido M, Arahata K, Hoffman EP, Nonaka I, Sugita H. Muscle histology in Becker muscular dystrophy. Muscle Nerve 1991;14:1067–1073. [DOI] [PubMed] [Google Scholar]
- 21. Karlsen A, Soendenbroe C, Malmgaard‐Clausen NM, Wagener F, Moeller CE, Senhaji Z, et al. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J 2020;34:6418–6436. [DOI] [PubMed] [Google Scholar]
- 22. Heisterberg MF, Andersen JL, Schjerling P, Lund A, Dalskov S, Jønsson AO, et al. Losartan has no additive effect on the response to heavy‐resistance exercise in human elderly skeletal muscle. J Appl Physiol (1985) 2018;125:1536–1554. [DOI] [PubMed] [Google Scholar]
- 23. Bechshøft CJL, Jensen SM, Schjerling P, Andersen JL, Svensson RB, Eriksen CS, et al. Age and prior exercise in vivo determine the subsequent in vitro molecular profile of myoblasts and nonmyogenic cells derived from human skeletal muscle. Am J Physiol Cell Physiol 2019;316:C898–C912. [DOI] [PubMed] [Google Scholar]
- 24. Bechshøft RL, Malmgaard‐Clausen NM, Gliese B, Beyer N, Mackey AL, Andersen JL, et al. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp Gerontol 2017;92:96–105. [DOI] [PubMed] [Google Scholar]
- 25. Karlsen A, Cullum CK, Norheim KL, Scheel FU, Zinglersen AH, Vahlgren J, et al. Neuromuscular electrical stimulation preserves leg lean mass in geriatric patients. Med Sci Sports Exerc 2020;52:773–784. [DOI] [PubMed] [Google Scholar]
- 26. Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports 2007;17:422–430. [DOI] [PubMed] [Google Scholar]
- 27. Olesen AT, Malchow‐Møller L, Bendixen RD, Kjær M, Svensson RB, Andersen JL, et al. Age‐related myofiber atrophy in old mice is reversed by ten weeks voluntary high‐resistance wheel running. Exp Gerontol 2021;143:111150. [DOI] [PubMed] [Google Scholar]
- 28. Qu Z, Andersen JL, Zhou S. Visualisation of capillaries in human skeletal muscle. Histochem Cell Biol 1997;107:169–174. [DOI] [PubMed] [Google Scholar]
- 29. Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 1999;22:449–454. [DOI] [PubMed] [Google Scholar]
- 30. Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol (1985) 2000;88:627–633. [DOI] [PubMed] [Google Scholar]
- 31. Karlsen A, Bechshøft RL, Malmgaard‐Clausen NM, Andersen JL, Schjerling P, Kjaer M, et al. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83–94‐year‐old men and women. Acta Physiol (Oxf) 2019;227:e13271. [DOI] [PubMed] [Google Scholar]
- 32. Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 2015;96:183–195. [DOI] [PubMed] [Google Scholar]
- 33. Lang F, Aravamudhan S, Nolte H, Türk C, Hölper S, Müller S, et al. Dynamic changes in the mouse skeletal muscle proteome during denervation‐induced atrophy. Dis Model Mech 2017;10:881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ansved T, Larsson L. Effects of denervation on enzyme‐histochemical and morphometrical properties of the rat soleus muscle in relation to age. Acta Physiol Scand 1990;139:297–304. [DOI] [PubMed] [Google Scholar]
- 35. Viguie CA, Lu D‐X, Huang S‐K, Rengen H, Carlson BM. Quantitative study of the effects of long‐term denervation on the extensor digitorum longus muscle of the rat. Anat Rec 1997;248:346–354. [DOI] [PubMed] [Google Scholar]
- 36. Carraro U. Chronic denervation of rat hemidiaphragm: maintenance of fiber heterogeneity with associated increasing uniformity of myosin isoforms. J Cell Biol 1985;100:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, et al. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol 1988;127:1–11. [DOI] [PubMed] [Google Scholar]
- 38. Soendenbroe C, Andersen JL, Mackey AL. Muscle‐nerve communication and the molecular assessment of human skeletal muscle denervation with aging. Am J Physiol Cell Physiol 2021;321:C317–C329. [DOI] [PubMed] [Google Scholar]
- 39. Lin H, Ma X, Sun Y, Peng H, Wang Y, Thomas SS, et al. Decoding the transcriptome of denervated muscle at single‐nucleus resolution. J Cachexia Sarcopenia Muscle 2022;13:2102–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harridge SDR, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjörnsson M, et al. Whole‐muscle and single‐fibre contractile properties and myosin heavy chain isoforms in humans. Pflügers Archiv ‐ Eur J Physiol 1996;432:913–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1. Subject characteristics for males and females separately, shown as means ± SD with range and number of subjects represented in each measurement. BMI, body mass index. LBMleg, leg lean mass. CSAquad, quadriceps cross‐sectional area. MVC, maximal voluntary contraction.
Supporting Information S2. Subject characteristics for each of the original studies separately, shown as means ± SD with minimum and maximum values. These subjects characteristics have previously been reported in the original publications13,24–29. BMI, body mass index. LBMleg, leg lean mass. CSAquad, quadriceps cross‐sectional area. MVC, maximal voluntary contraction.
Supporting Information S3. SFI in males and females separately. Type I (red) and II (blue) myofiber SFI for Young (a, n = 22 males and 12 females), Old (b, n = 98 males and 13 females), and Oldest Old (c, n = 30 males and 22 females). Data were analyzed using Two‐Way Repeated Measures ANOVA (sex x fiber type) within each group, with main effects of fiber type indicated in the figure. SFI, shape factor index.
Supporting Information S4. Coefficient of variation (CV) of SFI (A) and CSA (B) for type I and II myofibers in Young (n = 34), Old (n = 111) and Oldest Old (n = 52). Data were analyzed using Two‐Way Repeated Measures ANOVA (age group x fiber type). *p < 0.05, **p < 0.01, ****p < 0.0001. CV, coefficient of variation. SFI, shape factor index. CSA, cross‐sectional area.
Supporting Information S5. Representative split channel images of muscle biopsy cross‐sections from Young, Old, and Oldest Old, stained for dystrophin (Young, Old) or laminin (Oldest Old), and MyHC I. Scale bar = 100 μm.
Supporting Information S6. SFI in hybrid fibers. The SFI of IIa/IIx hybrid fibers were evaluated in a subset of samples (n = 52). Data were analyzed using One‐Way Repeated Measures ANOVA (fiber type). *p < 0.05, **p < 0.005, ***p < 0.005, ****p < 0.0001. SFI, shape factor index.
Supporting Information S7. a) Number of subjects and myofibers represented in each type I and II myofiber SFI increment for Young, Old, and Oldest Old. These data supplement figure 3.a. b) Number of subjects and myofibers represented in each type I and II myofiber CSA increment for Young, Old, and Oldest Old. These data supplement figure 3.b.
Supporting Information S8. Split channel view of myofibers in healthy older adults stained with dystrophin (cyan), NCAM (magenta) and nuclei (white). Denervated myofiber is identified by arrow, with the SFI provided below. Scale bar = 100 μm. NCAM, neural cell adhesion molecule. SFI, shape factor index.
Supporting Information S9. SFI in muscles of 11 and 22 month old mice. a) Split channel and merged images of a mouse GAS muscle stained with MyHC I (cyan), MyHC IIb (magenta), MyHC IIa (yellow), MyHC IIx (green) and laminin (grey) across two consecutive cross‐section. b) Type I and type II myofiber SFI in gastrocnemius (GAS) and soleus (SOL) combined. C) SFI in GAS, for type I, Iia, IIa/IIx, IIx, IIx/IIb and IIb. d) SFI in SOL, for type I, I/IIa and IIa. Data are shown as means ± SEM and were analyzed using a Two‐Way Repeated Measures ANOVA (muscle x fiber type), with a significant main effect of fiber type (p < 0.001). *p < 0.05, **p < 0.005, ***p < 0.005, ****p < 0.0001. N = 7 and 6 for 11 and 22 month old, respectively. SFI, shape factor index.
Supporting Information S10. Linear correlation analyses between SFI (left) and CSA (right) for type I and II myofibers with in vivo measures of muscle mass and function. Each data point represents one individual, ranging in age from 20–94y, and all in the untrained state. Strength of association is indicated by r and p values. Correlations are displayed for whole body LBM, CSAQuad, isokinetic MVC, specific force (isokinetic MVC/CSAQuad), specific force (isokinetic MVC/LBMleg), and specific force (isometric MVC/CSAQuad). LBM, lean body mass. CSAQuad, quadriceps cross‐sectional area. LBMleg, leg lean mass. SFI, shape factor index. CSA, cross‐sectional area. RFD, rate of force development. MVC, maximal voluntary contraction.
Supporting Information S11. Result of backward elimination multiple linear regression. Ten indices of muscle mass and function were evaluated (1–4 in figure 4 and 5–10 in Supporting Information S10). Model parameters included: Age, type I SFI, type II SFI, type I CSA and type II CSA. SE, standard error. SFI, shape factor index. CSA, cross‐sectional area. R squared for the individual parameters represent type II semi‐partial correlation coefficients.
Supporting Information S12. SFI delta values from pre to post RT, for type I (red) and II (blue) myofibers (n = 59). Young and Old subjects are pooled, but Young is shown in faded colors. Delta values were compared using paired t‐tests. ***p < 0.001. RT, heavy resistance training. SFI, shape factor index.
Data S1. Supporting Information.


