Summary
Background
Older adults were more likely to be socially isolated during the COVID-19 pandemic, with increased risk of depression and loneliness. We aimed to investigate whether a behavioural activation intervention delivered via telephone could mitigate depression and loneliness in at-risk older people during the COVID-19 pandemic.
Methods
BASIL+ (Behavioural Activation in Social Isolation) was a pragmatic randomised controlled trial conducted among patients recruited from general practices in England and Wales, and was designed to assess the effectiveness of behavioural activation in mitigating depression and loneliness among older people during the COVID-19 pandemic. Eligible participants were aged 65 years and older, socially isolated, with a score of 5 or higher on the Patient Health Questionnaire-9 (PHQ-9), and had multiple long-term conditions. Participants were allocated in a 1:1 ratio to the intervention (behavioural activation) or control groups by use of simple randomisation without stratification. Behavioural activation was delivered by telephone; participants were offered up to eight weekly sessions with trained BASIL+ Support Workers. Behavioural activation was adapted to maintain social connections and encourage socially reinforcing activities. Participants in the control group received usual care with existing COVID-19 wellbeing resources. The primary clinical outcome was self-reported depression severity, assessed by the PHQ-9, at 3 months. Outcomes were assessed masked to allocation and analysis was by treatment allocation. This trial is registered with the ISRCTN registry (ISRCTN63034289).
Findings
Between Feb 8, 2021, and Feb 28, 2022, 449 eligible participants were identified and 435 from 26 general practices were recruited and randomly assigned (1:1) to the behavioural activation intervention (n=218) or to the control group (usual care with signposting; n=217). The mean age of participants was 75·7 years (SD 6·7); 270 (62·1%) of 435 participants were female, and 418 (96·1%) were White. Participants in the intervention group attended an average of 5·2 (SD 2·9) of eight remote behavioural activation sessions. The adjusted mean difference in PHQ-9 scores between the control and intervention groups at 3 months was –1·65 (95% CI –2·54 to –0·75, p=0·0003). No adverse events were reported that were attributable to the behavioural activation intervention.
Interpretation
Behavioural activation is an effective and potentially scalable intervention that can reduce symptoms of depression and emotional loneliness in at-risk groups in the short term. The findings of this trial add to the range of strategies to improve the mental health of older adults with multiple long-term conditions. These results can be helpful to policy makers beyond the pandemic in reducing the global burden of depression and addressing the health impacts of loneliness, particularly in at-risk groups.
Funding
UK National Institute for Health and Care Research.
Introduction
The mental health of the population deteriorated during the COVID-19 pandemic.1 People reported greater social isolation, and the incidence of depression and anxiety increased for older people and those with medical vulnerabilities.2 A plausible reason for this deterioration was that COVID-19 restrictions led to disruption of daily routines, loss of social contact, and heightened isolation and increased loneliness. Social isolation, social disconnectedness, perceived isolation, and loneliness are known to be linked to common mental health problems, such as depression in older people.3 Loneliness is a risk factor for depression and seems detrimental to physical health and life expectancy.4 It is recognised that strategies that maintain social connectedness could be important in ensuring the mental health of older people.5
Behavioural activation is an evidence-based psychological treatment that explores how physical inactivity and low mood are linked to and result in a reduction of valued activities.6 Within behavioural activation, the therapist and patient work together to develop a collaborative treatment plan to reinstate (or replace, if former activities are no longer possible) behaviours that connect people to sources of positive reinforcement (ie, meaningful activities), including social connectedness.
Research in context.
Evidence before this study
Before designing the BASIL+ trial we updated reviews of behavioural activation. We searched key databases (MEDLINE, Embase, CINAHL, PsycINFO, HMIC, CENTRAL, and DARE) for English language publications from database inception to June 1, 2020, for collaborative care studies and trials of behavioural activation, and independently extracted data. We used search terms relating to loneliness and social isolation (“[Loneliness” OR “Lonel* OR social isolat*]”), combined with terms for behavioural activation (“[exp behavior therapy/ OR exp behavior modification/ OR behavior change/ OR behavior contracting/behavioral activation system/]”) and collaborative care (“[exp Collaborative Care OR exp Shared Care OR exp Integrative Care]”). We included randomised controlled trials conducted in any country or care setting in adults and older adults with depression. We found no large-scale UK trials of collaborative care and no large-scale trials of behavioural activation (and no meta-analyses of trials) addressing loneliness in socially isolated older people in any setting.
Added value of this study
To the best of our knowledge, the BASIL+ trial is the first large-scale, fully powered trial of a brief psychological intervention to mitigate loneliness, alongside other common mental health problems such as depression and anxiety. The findings of this trial demonstrate that it is feasible to deliver a behavioural activation intervention for older people with long-term conditions who were socially isolated during the COVID-19 pandemic. Older people readily engaged with behavioural activation as a remotely delivered (via telephone) psychological intervention. The results of the BASIL+ trial contribute to the evidence base for behavioural or cognitive interventions.
Implications of all the available evidence
Behavioural activation was a plausible intervention to mitigate depression and loneliness in at-risk older populations during the COVID-19 pandemic; this evidence will be useful to practitioners and policy makers beyond the pandemic in preventing loneliness in vulnerable populations.
Behavioural activation is known to be effective in treating depression in older adults,7 and small-scale trials of behavioural activation delivered to socially isolated older people have produced encouraging preliminary results,8 making this a credible candidate approach. In March–April, 2020, we adapted an ongoing programme of work into the role of behavioural activation in multiple long-term conditions (multimorbidities) to answer the following overarching question: can a brief behavioural intervention delivered via telephone mitigate depression and loneliness in at-risk older people during COVID-19 isolation?
The results of an external pilot trial of the Behavioural Activation in Social Isolation (BASIL+) intervention showed a significant effect in reducing loneliness at 3 months in the behavioural activation group compared with the control group (De Jong Gierveld Loneliness Scale: adjusted mean difference –0·87; 95% CI –1·56 to –0·18).9 Evidence from a living systematic review (PROSPERO CRD42021298788)9 shows a growing evidence base of the clinical effectiveness of cognitive or behavioural approaches, or both, in mitigating the effects of loneliness (standardised mean difference [SMD] –0·48, 95% CI –0·70 to –0·27) and depression (SMD –0·31, 95% CI –0·51 to –0·11).
The BASIL+ trial was a fully powered multicentre randomised controlled trial of manualised behavioural activation, adapted specifically to be delivered at scale and remotely (via telephone) to older adults who were at risk of social isolation as a consequence of COVID-19 restrictions (including in the post-pandemic period); we aimed to investigate whether this behavioural activation intervention could mitigate depression and loneliness in this population.
Methods
Study design and participants
BASIL+ was a parallel-group randomised controlled trial conducted among patients recruited from general practices in England and Wales. The study design was informed by an external developmental phase. BASIL+ study recruitment and follow-up procedures were first tested in an external pilot randomised controlled trial (BASIL-C19)10, 11 with a concurrent qualitative study.12
The COVID-19 responsive BASIL trials programme was supported by the UK National Institute for Health and Care Research (NIHR) under grant RP-PG-0217-20006, and was adopted by the NIHR Urgent Public Health programme on May 28, 2020. The protocol and design for the BASIL+ trial was registered on ISRCTN (ISRCTN63034289) on Feb 8, 2021, and is publicly available.13 Recruitment took place between Feb 8, 2021, and Feb 28, 2022.
Based on the definition of multimorbidity used by the UK Academy of Medical Sciences14 we recruited older adults (aged ≥65 years) with two or more physical long-term conditions from primary care registers in 26 general practices in England and Wales. Eligible participants had to have a score of 5 or greater on the Patient Health Questionnaire (PHQ-9), putting them at risk of clinical depression or indicating already established minor depressive symptoms. Eligible participants included those who were subject to UK Government guidelines about COVID-19 self-isolation, physical distancing, and shielding as relevant to their health conditions and age.
We excluded older adults who had cognitive impairment, bipolar disorder, psychosis, or psychotic symptoms; alcohol or drug dependence; were in the palliative phase of illness; had active suicidal ideation; were receiving psychological therapy; or were unable to speak or understand English. Older adults living in residential or care homes were not excluded.
Potentially eligible patients were identified and contacted via telephone by staff working at general practices. Interested patients could also complete an online consent form or contact the study team directly. Once eligibility had been ascertained, additional informed consent was obtained (where online consent had not already been provided). In response to the need to deliver the trial remotely, the informed consent process involved taking consent verbally via telephone.
The BASIL+ trial received ethical approval from the Yorkshire and the Humber–Leeds West Research Ethics Committee on Dec 11, 2021 (Ref: 20/YH/0347). The sponsor for BASIL+ was Tees, Esk and Wear Valleys NHS Foundation Trust.
Randomisation and masking
After providing consent, eligible participants completed a baseline questionnaire by telephone. Participants were then randomly assigned and informed of their group allocation (the BASIL+ intervention or usual care with signposting). Participants were allocated in a 1:1 ratio by use of simple randomisation without stratification. The allocation schedule was generated in Stata version 16 by York Trials Unit staff not otherwise involved in recruitment. Treatment allocation was concealed from study researchers at the point of recruitment by use of an automated computer data entry system, administered remotely by the York Trials Unit, UK. Owing to the nature of the intervention, participants, general practices, study clinicians, and BASIL+ Support Workers could not be masked to treatment allocation. Researchers facilitating the telephone-based outcome assessments were masked to treatment allocation.
Procedures
The behavioural activation intervention (within a collaborative care framework) was adapted for the purposes of the BASIL+ trial; a description of the adaptation for social isolation due to COVID-19 has been published previously.9, 10 The overall content and delivery of the intervention was informed by detailed qualitative preparatory work.12 Briefly, behavioural activation is a structured, brief, simple psychotherapeutic approach designed to increase engagement in rewarding and adaptive activities, decrease engagement in activities that maintain depression, and solve problems that limit access to rewards or that maintain avoidance.15
Within the BASIL behavioural activation intervention, the therapist (the BASIL+ Support Worker) and participant worked together to develop a collaborative treatment plan to reinstate (or replace, if former activities were no longer possible because of social isolation or long-term conditions, or both) behaviours that connect participants to sources of positive reinforcement (ie, valued activities). Behavioural activation has the potential to address depression and loneliness in the presence of social isolation in this way and the simplicity of behavioural activation made it suitable for delivery in the context of the COVID-19 pandemic.
In the intervention group, participants were offered up to eight weekly sessions by trained BASIL+ Support Workers, accompanied by participant materials: a BASIL+ behavioural activation booklet that was modified to take account of UK Government guidance about the need for social isolation or physical distancing and enforced isolation for those people most at risk (defined as clinically extremely vulnerable people). For example, the BASIL+ booklet discussed ways to replace activities that are no longer possible with ones that preserve physical distancing while helping participants stay connected with the activities and people important to them; illustrative patient stories included in the booklet were modified to take account of COVID-19 restrictions. Behavioural activation acknowledged the disruption to people's lives and usual routines, and encouraged the establishment of a balanced daily routine. Intervention sessions were delivered remotely via telephone. An additional offer of a video call was taken up by only three participants. The first session was scheduled as soon as convenient after randomisation, and was scheduled to last approximately 1 h, with subsequent sessions lasting approximately 30 min. The intervention could be extended to include involvement of a participant's informal caregiver or partner.
Participants in the control group received usual care as provided by the UK National Health Service (NHS) or third sector providers, or both. Additionally, participants in the control group were signposted to reputable sources of self-help and information, including advice on how to keep mentally and physically well. Examples of such sources were Public Health England's guidance for the public on the mental health and wellbeing aspects of coronavirus (COVID-19)16 and Age UK.17
This was a pragmatic trial and no treatment was withheld by reason of participation in the BASIL+ trial in either the intervention or control groups.
Outcomes
Outcome measures were collected at baseline and 1, 3, and 12 months after randomisation. The primary clinical outcome and endpoint was self-reported symptoms of depression, assessed by the PHQ-9,18 at 3 months.
Other secondary outcomes were perceived loneliness (measured by the De Jong Gierveld Scale: the 11-item loneliness scale, and its subscales for Social Loneliness [5 items] and Emotional Loneliness [6 items]),19 anxiety (measured by the General Anxiety Disorder-7 [GAD-7] scale),20 health-related quality of life (measured by the Short Form 12-item [SF-12v2] Mental Health Component Score and Physical Health Component Score,21 and the European Quality of Life 5 Dimensions 3 Level Version [EQ-5D-3L]),22 social networks (measured by the 6-item Lubben Social Network Scale [LSNS6]),23 and questions relating to COVID-19 circumstances. For each of the clinical outcomes, missing item-level data were scored and handled in accordance with the user guides; full details are provided in the statistical analysis plan (appendix pp 1–14). We recorded details of any serious adverse events experienced by study participants. All potential serious adverse events were reviewed by a clinician independently of the BASIL study team and serious adverse events were reported to the Yorkshire and the Humber–Leeds West Research Ethics Committee within 15 days.
Here, we only report outcomes at 1 month and 3 months since these timepoints include our primary outcome, and the 12-month outcomes are not yet available for analysis. Future analyses will include a quantitative and economic evaluation.
Statistical analysis
In an older population with subthreshold depression, a difference of 1·3 in the PHQ-9 has been found to be clinically effective and cost-effective24 with an SD of 4. The final design of the BASIL+ trial aimed to detect a standardised effect size of 0·3 in the primary outcome, with 90% power. We drew on data from our pilot trial9, 10 to estimate 10% attrition (from mortality and loss to follow-up). Additionally, within the pilot trial, we calculated the correlation between the PHQ-9 score at baseline and the primary outcome timepoint to be 0·58 among participants who scored 5 or higher at baseline (which would be the sample eligible for the main trial). Assuming a correlation of at least 0·5,25 90% power, two-sided 5% alpha, 0·3 effect size, and 10% attrition, we estimated that the BASIL+ trial needed to recruit and randomly assign 392 participants (power calculations were done in Stata version 15). In the original trial design, we did not incorporate a correlation between baseline and the primary outcome and we were able to revise our study sample size to incorporate these data as the results of the BASIL pilot emerged (appendix p 8). The final statistical analysis plan was approved by the independent BASIL+ Trial Steering and Data Monitoring & Ethics Committee on June 15, 2022.
All analyses were conducted in Stata version 17 on a pseudo intention-to-treat basis (using available data, analysing participants in the groups to which they were randomly assigned) by use of two-sided tests at the 5% significance level. The trial is reported in accordance with CONSORT guidelines. Baseline data are summarised descriptively by trial group both as randomised and as included in the primary analysis.
The primary analysis compared the severity of depression as measured by the PHQ-9 between the two groups by use of a covariance pattern mixed-effects linear regression model, incorporating data from the 1-month and 3-month follow-up timepoints. Treatment group, timepoint, treatment-by-time interaction, and baseline PHQ-9 score were fixed effects, with participant nested within site as random effects. An exchangeable covariance pattern for the correlation between the observations for a participant over time was specified (based on minimising the Akaike's information criterion).26 Estimates of the difference in total PHQ-9 score were extracted for each timepoint as an adjusted mean difference, with 95% CI, and p value.
Model assumptions were checked as follows: the normality of the standardised residuals was checked with a QQ plot, and homoscedasticity was assessed by means of a scatter plot of the standardised residuals against fitted values.
Intervention adherence, including the total number of behavioural activation sessions completed per participant and the average duration of sessions, is summarised descriptively. A complier average causal effect (CACE) analysis27 was done to assess the impact of compliance on the primary estimate, with a two-stage instrumental variable regression approach with randomised group as the instrumental variable, adjusting for baseline score and with robust standard errors to account for clustering within site. Compliance was defined in two ways: as a continuous measure of the number of behavioural activation sessions attended, and as a dichotomous measure to indicate that at least five sessions were attended. A pre-specified subgroup analysis exploring the effects of differential intervention depending on the baseline level of depressive symptoms (PHQ-9 score 5–9 [mild or subthreshold], or ≥10 [moderate to severe]) was conducted by repeating the primary analysis but including an indicator variable for whether the participant scored 5–9 or 10 or higher at baseline as a covariate (rather than the continuous score) plus an interaction term between treatment allocation and baseline PHQ-9 threshold.
In light of an observed differential dropout between the trial groups, post-hoc analyses were conducted to investigate missing data. To investigate the effect of missing data on the treatment effect, any baseline variables associated with non-response at the 3-month follow-up (ie, no valid PHQ-9 score) were identified and included as covariates in the primary analysis model. Multiple imputation of the primary analysis was also conducted with chained equations28 (20 imputations, burn-in=10) based on predictors: allocation, gender, age, ethnicity, number of comorbidities, academic degree, marital status, and baseline scores for PHQ-9 and all secondary outcomes.
The secondary outcomes of the De Jong Gierveld Loneliness Scale (the two subscales separately, and the total score), GAD-7, LSNS6, SF-12v2 (mental and physical health component scores separately), and EQ-5D-3L (visual analogue scale [VAS] score and index value score based on the UK Tariff29) were analysed in a similar way to the primary outcome, swapping the baseline PHQ-9 score with the baseline value of the outcome as a covariate.
Serious and non-serious adverse events are summarised by trial group and overall.
Patient and public involvement
The BASIL trial was informed by a Patient and Public Involvement Advisory Group (PPI AG) that was working with the research collective on the existing NIHR-funded research programme. This PPI AG included older adults with a lived experience of mental health or physical health conditions, or both, as well as their caregivers.
Role of the funding source
This project was funded by the NIHR Programme Grants for Applied Research (PGfAR) programme (RP-PG-0217-20006). The scope of our pre-existing research into multimorbidity in older people was extended at the outset of the COVID-19 pandemic with the agreement of the funder to consider depression and loneliness in this vulnerable group. The NIHR PGfAR programme had no role in the writing of this manuscript or the decision to submit it for publication.
Results
Approximately 11 900 study information packs were mailed out to potentially eligible participants registered at 27 general practices within England and Wales from Feb 8, 2021, to Dec 17, 2021. Randomisation took place between Feb 25, 2021, and Feb 28, 2022; participants were recruited from 26 general practices (one of the original 27 practices did not recruit any participants).
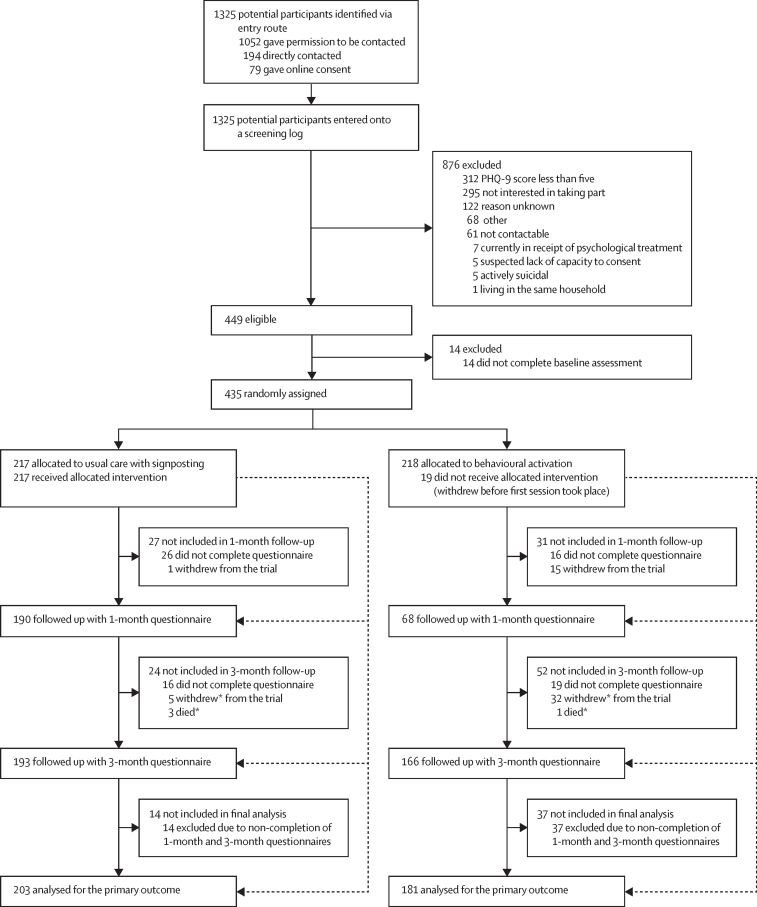
Following receipt of a postal study pack, 1325 patients across 26 general practices expressed an interest in the study (1052 [79·4%] gave permission to be contacted, 194 [14·6%] were directly contacted, and 79 [6·0%] gave online consent), of whom 449 (33·9%) were identified as eligible (figure 1). Of those who were eligible, 14 did not go on to complete a baseline assessment and were not recruited; therefore, 435 (96·9%) participants were recruited and randomly assigned (218 to the behavioural activation intervention and 217 to usual care, with signposting).
Figure 1.
Trial profile
PHQ-9=Patient Health Questionnaire-9. *Withdrawals and deaths are cumulative.
The mean age of participants was 75·7 years (SD 6·7); 270 (62·1%) of 435 participants were female, and 418 (96·1%) were White (table 1). Cardiovascular conditions (288 [66·2%] of 435) and arthritis (186 [42·8%]) were the most frequently reported long-term health conditions. Baseline characteristics were similar between the two groups. Most participants (296 [68·0% of 435) were engaging in physical distancing and reported adhering to the UK Government's guidance in relation to COVID-19 restrictions. 200 (46·0%) of 435 participants reported living alone, and 364 (83·7%) had received one or both doses of a COVID-19 vaccine at the point of trial entry. A slightly lower proportion of participants in the intervention group had subthreshold depression (a PHQ-9 score of 5–9, as opposed to ≥10, which would indicate more severe depressive symptoms) at baseline than in the control group (115 [52·8%] of 218 vs 136 [62·7%] of 217). The total De Jong Gierveld loneliness score can be categorised as follows: 0–2=not lonely, 3–8=moderately lonely, and 9–11=severely lonely.30 Loneliness was balanced between the two groups, with a mean loneliness score across the two groups of 5·5 (SD 3·1).
Table 1.
Baseline characteristics
| Intervention (n=218) | Usual care (n=217) | Total (n=435) | ||
|---|---|---|---|---|
| Age, years | 75·2 (6·4) | 76·2 (6·9) | 75·7 (6·7) | |
| Gender | ||||
| Female | 137 (63%) | 133 (61%) | 270 (62%) | |
| Male | 81 (37%) | 84 (39%) | 165 (38%) | |
| Long-term health conditions* | ||||
| Cardiovascular conditions | 144 (66%) | 144 (66%) | 288 (66%) | |
| Arthritis | 98 (45%) | 88 (41%) | 186 (43%) | |
| Diabetes | 77 (35%) | 60 (28%) | 137 (31%) | |
| Respiratory conditions | 60 (28%) | 61 (28%) | 121 (28%) | |
| Chronic pain | 44 (20%) | 37 (17%) | 81 (19%) | |
| Cancer | 26 (12%) | 26 (12%) | 52 (12%) | |
| Neurological conditions | 23 (11%) | 16 (7%) | 39 (9%) | |
| Osteoporosis | 19 (9%) | 14 (6%) | 33 (8%) | |
| Stroke | 12 (6%) | 16 (7%) | 28 (6%) | |
| Other† | 46 (21%) | 51 (24%) | 97 (22%) | |
| Ethnicity | ||||
| White (English, Welsh, Scottish, Northern Irish, or British) | 201 (92%) | 201 (93%) | 402 (92%) | |
| White (Irish) | 3 (1%) | 2 (1%) | 5 (1%) | |
| White (Other White background) | 5 (2%) | 6 (3%) | 11 (3%) | |
| Asian (Indian) | 1 (<1%) | 1 (<1%) | 2 (<1%) | |
| Asian (Pakistani) | 0 | 1 (<1%) | 1 (<1%) | |
| Asian (Chinese) | 1 (<1%) | 0 | 1 (<1%) | |
| Asian (Other) | 2 (1%) | 1 (<1%) | 3 (1%) | |
| Black (African) | 2 (1%) | 4 (2%) | 6 (1%) | |
| Black (Caribbean) | 1 (<1%) | 1 (<1%) | 2 (<1%) | |
| Prefer not to say | 1 (<1%) | 0 | 1 (<1%) | |
| Mixed or multiple ethnic groups (Other) | 1 (<1%) | 0 | 1 (<1%) | |
| Smoking status | ||||
| I have never smoked | 83 (38%) | 93 (43%) | 176 (40%) | |
| I currently smoke | 20 (9%) | 16 (7%) | 36 (8%) | |
| I am an ex-smoker | 115 (53%) | 108 (50%) | 223 (51%) | |
| Alcohol intake (>2 units daily) | ||||
| Yes | 36 (17%) | 39 (18%) | 75 (17%) | |
| No | 180 (83%) | 176 (81%) | 356 (82%) | |
| Don't know | 2 (1%) | 2 (1%) | 4 (1%) | |
| Education after compulsory school leaving age | ||||
| Yes | 136 (62%) | 122 (56%) | 258 (59%) | |
| Marital status | ||||
| Married | 95 (44%) | 104 (48%) | 199 (46) | |
| Widowed | 57 (26%) | 64 (29%) | 121 (28%) | |
| Divorced or separated | 37 (17%) | 32 (15%) | 69 (16%) | |
| Single | 16 (7%) | 14 (6%) | 30 (7%) | |
| Cohabiting | 8 (4%) | 2 (1%) | 10 (2%) | |
| Civil partnership | 4 (2%) | 1 (<1%) | 5 (1%) | |
| Not reported | 1 (<1%) | 0 | 1 (<1%) | |
Data are mean (SD) or n (%).
Long-term health conditions were participant self-reported and were not mutually exclusive.
Other long-term health conditions included: hypothyroidism (n=35), gastrointestinal conditions (n=19), renal conditions (n=13), osteoarthritis (n=6), mental health conditions (n=5), inflammatory conditions (n=5), dermatological conditions (n=5), sleep apnoea (n=4), sensory conditions (n=4), haematological conditions (n=4), pain-related conditions (n=3), unknown (n=3), low immunity conditions (n=2), B12 deficiency (n=2), gynaecological conditions (n=1), hyperthyroidism (n=1), post-COVID-19 condition (also known as long COVID; n=1), lymphoedema (n=1), and autoimmune conditions (n=1). Participants could have reported multiple other conditions.
Participants in the intervention group attended an average of 5·2 (SD 2·9) of eight remote sessions. 19 (8·7%) of 218 participants attended 0 sessions, 139 (63·8%) attended at least five sessions, and 80 (36·7%) attended all eight. The first behavioural activation session took place an average of 15·4 days after randomisation (SD 10·9, median 13·0), and subsequent sessions took place over an average of 7·9 weeks (SD 3·7, range 1–18). 155 (71·1%) participants in the intervention group attended their last session before their 3-month follow-up was due. Based on data collected across 1114 sessions, sessions lasted on average 36·2 min (SD 13·2).
Overall, 358 (82·3%) of 435 participants (168 [77·1%] of 218 assigned to the intervention and 190 [87·6%] of 217 assigned to usual care) completed the 1-month follow-up questionnaire and 359 (82·5%) of 435 participants (166 [76·1%] assigned to the intervention and 193 [88·9%] assigned to usual care) completed the 3-month follow-up questionnaire; the response rate was therefore higher in the control group at both timepoints (figure 1). There were no adverse events reported that were attributable to the behavioural activation intervention. Where returned, 1-month questionnaires were completed a median of 5 weeks after randomisation, and 3-month questionnaires were completed a median of 14 weeks after randomisation.
Of the 435 participants randomly assigned, 384 (88·3%) were included in the primary analysis; 51 (11·7%) were omitted due to non-completion of both the 1-month and 3-month follow-up questionnaires. The primary analysis included some participants who completed 3-month follow-up data but had not completed 1-month follow-up data, and vice versa. No participants were excluded from the primary analysis on the basis of missing baseline covariates (PHQ-9 score at baseline), as this was provided for all randomly assigned participants.
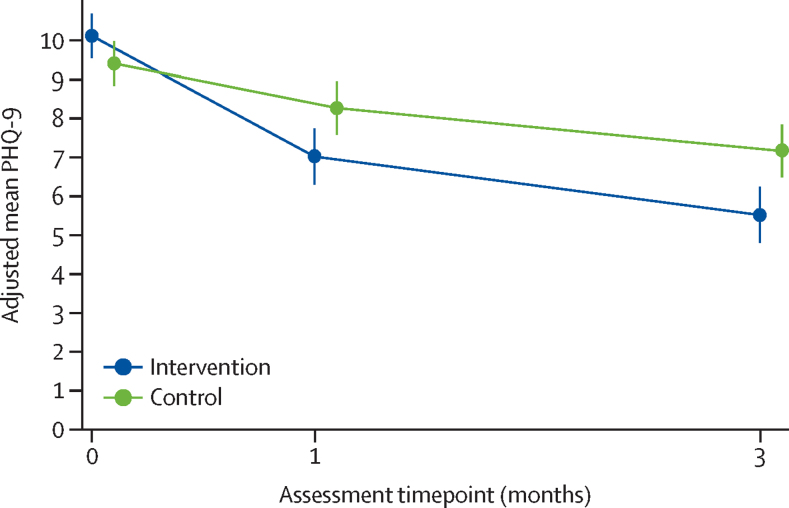
On average, unadjusted and adjusted mean PHQ-9 scores decreased over time in both groups (Table 2, Table 3; figure 2). Overall the mean PHQ-9 score was 9·8 [SD 4·3] at baseline, 7·7 [SD 5·0] at 1 month, and 6·4 [SD 4·8] at 3 months. The observed correlation between baseline and 3-month PHQ-9 scores was 0·48 (95% CI 0·40 to 0·56) at 1 month and 0·42 (95% CI 0·33 to 0·50) at 3 months. There was evidence of a difference in depression severity favouring the intervention group across the follow-up period, at both 1 month (PHQ-9 score adjusted mean difference –1·25, 95% CI –2·15 to –0·35, p=0·0064) and 3 months (primary endpoint of primary outcome, adjusted mean difference –1·65, 95% CI –2·54 to –0·75, p=0·0003; figure 2; table 3). The corresponding SMD score (Cohen's d) for the primary outcome was –0·39 (95% CI –0·60 to –0·18). Assessment of the model assumptions showed no major violations. Findings were robust to sensitivity analyses.
Table 2.
Raw summaries of patient-reported outcome measures by trial group and timepoint
|
Intervention group |
Usual care group |
|||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | |
| PHQ-9 | ||||||
| Baseline | 218 | 10·1 (4·3) | 9·0 (7·0–12·0) | 217 | 9·4 (4·2) | 8·0 (6·0–11·0) |
| 1 month | 167 | 7·2 (4·5) | 6·0 (4·0–10·0) | 190 | 8·1 (5·5) | 7·0 (4·0–11·0) |
| 3 months | 165 | 5·7 (4·0) | 5·0 (3·0–8·0) | 192 | 6·9 (5·3) | 6·0 (3·0–10·0) |
| GAD-7 | ||||||
| Baseline | 218 | 5·7 (4·4) | 4·5 (2·0–8·0) | 216 | 6·2 (4·9) | 5·0 (2·0–9·5) |
| 1 month | 164 | 4·7 (4·2) | 4·0 (1·0–7·0) | 189 | 5·7 (4·7) | 5·0 (2·0–8·0) |
| 3 months | 164 | 3·8 (3·8) | 3·0 (1·0–5·5) | 192 | 4·6 (4·6) | 3·0 (1·0–7·0) |
| De Jong Gierveld Loneliness Scale | ||||||
| Baseline | 218 | 5·5 (3·1) | 6·0 (3·0–8·0) | 217 | 5·7 (3·1) | 6·0 (3·0–8·0) |
| 1 month | 164 | 5·0 (3·3) | 5·0 (2·0–8·0) | 189 | 5·1 (3·2) | 5·0 (3·0–8·0) |
| 3 months | 162 | 4·6 (3·1) | 4·0 (2·0–7·0) | 190 | 4·9 (3·3) | 4·0 (2·0–8·0) |
| De Jong Gierveld Emotional Loneliness subscale | ||||||
| Baseline | 218 | 3·3 (1·8) | 3·0 (2·0–5·0) | 217 | 3·5 (1·8) | 4·0 (2·0–5·0) |
| 1 month | 164 | 2·9 (1·9) | 3·0 (1·0–4·5) | 189 | 3·0 (1·8) | 3·0 (2·0–4·0) |
| 3 months | 162 | 2·6 (1·9) | 3·0 (1·0–4·0) | 190 | 3·0 (1·9) | 3·0 (1·0–5·0) |
| De Jong Gierveld Social Loneliness subscale | ||||||
| Baseline | 218 | 2·2 (1·8) | 2·0 (0·0–4·0) | 217 | 2·2 (1·8) | 2·0 (0·0–4·0) |
| 1 month | 164 | 2·0 (1·8) | 2·0 (0·0–4·0) | 189 | 2·1 (1·9) | 2·0 (0·0–4·0) |
| 3 months | 162 | 2·0 (1·8) | 2·0 (0·0–4·0) | 190 | 1·9 (1·9) | 1·0 (0·0–4·0) |
| LSNS6 | ||||||
| Baseline | 218 | 13·8 (5·6) | 14·0 (10·0–18·0) | 217 | 13·2 (5·8) | 14·0 (9·0–17·0) |
| 1 month | 164 | 13·9 (6·1) | 14·0 (9·0–19·0) | 189 | 13·7 (6·1) | 13·0 (9·0–18·0) |
| 3 months | 161 | 14·7 (6·0) | 14·0 (11·0–19·0) | 190 | 14·3 (6·4) | 14·0 (10·0–19·0) |
| SF-12v2 (Physical Health Component Score) | ||||||
| Baseline | 218 | 37·3 (11·3) | 36·8 (28·5–45·3) | 217 | 37·6 (10·8) | 37·5 (28·9–44·4) |
| 1 month | 164 | 37·0 (11·1) | 35·2 (28·2–44·9) | 189 | 37·7 (11·6) | 38·2 (28·1–45·2) |
| 3 months | 161 | 37·6 (11·8) | 36·7 (28·2–45·8) | 190 | 38·1 (11·0) | 38·4 (28·9–46·5) |
| SF-12v2 (Mental Health Component Score) | ||||||
| Baseline | 218 | 44·4 (10·0) | 44·4 (38·1–51·7) | 217 | 44·0 (10·2) | 43·0 (37·6–50·7) |
| 1 month | 164 | 46·1 (9·8) | 47·2 (39·9–53·5) | 189 | 45·8 (10·6) | 46·3 (38·5–53·7) |
| 3 months | 161 | 48·3 (9·3) | 49·4 (42·9–55·0) | 190 | 46·4 (10·8) | 47·7 (40·4–54·8) |
| EQ-5D-3L index value score | ||||||
| Baseline | 217 | 0·608 (0·267) | 0·656 (0·587–0·760) | 217 | 0·619 (0·279) | 0·691 (0·587–0·796) |
| 1 month | 164 | 0·631 (0·277) | 0·691 (0·620–0·796) | 187 | 0·622 (0·284) | 0·691 (0·587–0·796) |
| 3 months | 161 | 0·642 (0·275) | 0·725 (0·620–0·796) | 190 | 0·642 (0·276) | 0·691 (0·620–0·796) |
| EQ-5D-3L VAS | ||||||
| Baseline | 218 | 58·8 (20·2) | 60·0 (45·0–75·0) | 216 | 59·3 (19·2) | 60·0 (50·0–75·0) |
| 1 month | 164 | 61·9 (19·4) | 65·0 (50·0–75·0) | 187 | 59·7 (20·9) | 62·0 (50·0–75·0) |
| 3 months | 160 | 61·3 (20·1) | 65·0 (50·0–80·0) | 190 | 60·2 (22·4) | 65·0 (50·0–80·0) |
Data are mean (SD) or median (IQR). Range of possible scores: PHQ-9, 0–27; GAD-7, 0–21; De Jong Gierveld total, 0–11, emotional subscale, 0–6, social subscale, 0–5 (higher score indicates worse outcome); LSNS6, 0–30; SF-12v2 subscales, 0–100; EQ-5D-3L index value score −0·285 (worse than death) to −1 (full health); EQ-5D-3L VAS, 0–100 (higher score indicates better outcome). PHQ-9=Patient Health Questionnaire-9. GAD-7=Generalised Anxiety Disorder-7. LSNS6=6-item Lubben Social Network Scale. SF-12v2=Short Form 12-item. EQ-5D-3L=European Quality of Life 5 Dimensions 3 Level Version. VAS=visual analogue scale.
Table 3.
Adjusted mean differences between the behavioural activation and control groups at various timepoints
| Intervention group (95% CI) | Usual care group (95% CI) | Mean difference (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| PHQ-9 (3 months) | 5·5 (4·8 to 6·2) | 7·2 (6·5 to 7·8) | −1·65 (−2·54 to −0·75) | 0·0003 |
| Sensitivity analyses of PHQ-9 | ||||
| Adjusting for predictors of missing data* | ||||
| 1 month | 7·1 (6·3 to 7·8) | 8·2 (7·5 to 8·9) | −1·18 (−2·06 to −0·29) | 0·0094 |
| 3 months | 5·5 (4·8 to 6·2) | 7·1 (6·4 to 7·8) | −1·58 (−2·47 to −0·69) | 0·0005 |
| Analysis using multiply imputed data† | ||||
| 1 month | 7·1 (6·4 to 7·8) | 8·3 (7·7 to 9·0) | −1·19 (−2·10 to −0·29) | 0·0097 |
| 3 months | 5·6 (4·9 to 6·3) | 7·2 (6·6 to 7·8) | −1·63 (−2·50 to −0·77) | 0·0002 |
| Secondary outcomes at 1 month | ||||
| PHQ-9 | 7·0 (6·3 to 7·7) | 8·3 (7·6 to 8·9) | −1·25 (−2·15 to −0·35) | 0·0064 |
| GAD-7 | 4·9 (4·3 to 5·4) | 5·5 (5·0 to 6·0) | −0·65 (−1·41 to 0·11) | 0·092 |
| De Jong Gierveld Loneliness Scale | 5·1 (4·7 to 5·5) | 5·1 (4·7 to 5·5) | −0·03 (−0·49 to 0·43) | 0·89 |
| De Jong Gierveld Emotional Loneliness subscale | 3·0 (2·8 to 3·3) | 3·0 (2·8 to 3·2) | 0·05 (−0·24 to 0·33) | 0·75 |
| De Jong Gierveld Social Loneliness subscale | 2·0 (1·8 to 2·3) | 2·14 (1·9 to 2·4) | −0·09 (−0·37 to 0·19) | 0·53 |
| Lubben Social Network Scale (6-item) | 13·5 (12·7 to 14·2) | 14·2 (13·5 to 14·9) | −0·72 (−1·57 to 0·14) | 0·10 |
| SF-12v2 (Physical Health Component Score) | 37·1 (36·1 to 38·2) | 37·9 (36·9 to 38·9) | −0·80 (−2·24 to 0·68) | 0·30 |
| SF-12v2 (Mental Health Component Score) | 46·0 (44·7 to 47·3) | 45·9 (44·7 to 47·1) | 0·10 (−1·66 to 1·87) | 0·90 |
| EQ-5D-3L index value score | 0·639 (0·599 to 0·679) | 0·623 (0·585 to 0·661) | 0·016 (−0·031 to 0·063) | 0·51 |
| EQ-5D-3L VAS score | 61·7 (59·0 to 64·4) | 60·1 (57·6 to 62·6) | 1·64 (−2·03 to 5·30) | 0·38 |
| Secondary outcomes at 3 months | ||||
| GAD-7 | 3·9 (3·3 to 4·5) | 4·6 (4·0 to 5·1) | −0·67 (−1·43 to 0·09) | 0·084 |
| De Jong Gierveld Loneliness Scale | 4·6 (4·2 to 5·0) | 5·0 (4·6 to 5·4) | −0·42 (−0·88 to 0·04) | 0·076 |
| De Jong Gierveld Emotional Loneliness subscale | 2·6 (2·4 to 2·9) | 3·0 (2·8 to 3·2) | −0·37 (−0·68 to −0·06) | 0·018 |
| De Jong Gierveld Social Loneliness subscale | 1·9 (1·7 to 2·2) | 1·99 (1·7 to 2·3) | −0·05 (−0·33 to 0·23) | 0·72 |
| Lubben Social Network Scale (6-item) | 14·3 (13·5 to 15·0) | 14·6 (13·9 to 15·3) | −0·36 (−1·21 to 0·50) | 0·41 |
| SF-12v2 (Physical Health Component Score) | 37·4 (36·2 to 38·6) | 37·9 (36·8 to 39·0) | −0·50 (−2·14 to 1·10) | 0·53 |
| SF-12v2 (Mental Health Component Score) | 48·4 (47·1 to 49·7) | 46·4 (45·2 to 47·6) | 1·99 (0·22 to 3·76) | 0·028 |
| EQ-5D-3L index value score | 0·633 (0·593 to 0·673) | 0·641 (0·604 to 0·679) | −0·008 (−0·055 to 0·038) | 0·73 |
| EQ-5D-3L VAS score | 60·8 (58·1 to 63·5) | 60·0 (57·5 to 62·4) | 0·84 (−2·83 to 4·52) | 0·65 |
Data are mean (95% CI). Adjusted for baseline measure of the outcome as a covariate. PHQ-9=Patient Health Questionnaire-9. GAD-7=Generalised Anxiety Disorder-7. SF-12v2=Short Form 12-item. EQ-5D-3L=European Quality of Life 5 Dimensions 3 Level Version. VAS=visual analogue scale.
Additionally adjusted for sex, educated to degree level or equivalent, baseline total De Jong Gierveld Loneliness Scale score, and baseline Lubben Social Network Scale score.
With data derived by multiple imputation based on the following predictors: allocation, sex, age, ethnicity, number of comorbidities, academic degree, marital status, and baseline scores for PHQ-9 and all secondary outcomes.
Figure 2.
Depression severity, measured by adjusted mean PHQ-9, across the follow-up period (1 month and 3 months)
Error bars depict 95% CIs. PHQ-9=Patient Health Questionnaire-9.
For the measure of loneliness (measured on the De Jong Gierveld Loneliness Scale), there was evidence of a difference in emotional loneliness, favouring the intervention group, at 3 months (adjusted mean difference –0·37, 95% CI –0·68 to –0·06, p=0·018), but there was no evidence of a difference for social loneliness (–0·05, 95% CI –0·33 to 0·23, p=0·72). For the total loneliness score, the adjusted mean difference did not show a significant reduction in the severity of loneliness at 3 months in the intervention group (–0·42, 95% CI –0·88 to 0·04, p=0·076). At 1 month there was no evidence of a significant benefit in any aspect of measured loneliness (table 2).
There was no significant reduction in anxiety (GAD-7) in the intervention group at 1 month or 3 months (table 2). Similarly, in the LSNS6 measure of social networks, there was no evidence of a significant difference at 1 month or at 3 months (table 2).
There was also no evidence of a significant difference in health-related quality of life as measured by the EQ-5D-3L index or VAS scores between treatment groups at 1 month or 3 months (table 2).
In the CACE analysis defining compliance as a continuous measure, there was an indication that, for every behavioural activation session attended, there was a reduction in depression severity of 0·26 points on the PHQ-9 at 3 months (95% CI –0·42 to –0·10; p=0·0019). Where compliance was treated as a dichotomous measure, there was an indication of a reduction in depression severity of two points on the PHQ-9 at 3 months when at least five behavioural activation sessions were completed (2·02 [95% CI –3·29 to –0·76], p=0·0017).
Of the 435 randomly assigned participants, 251 (57·7%) scored 5–9 on the PHQ-9 at baseline (115 [52·8%] of 218 assigned to the intervention and 136 [62·7%] of 217 assigned to usual care), and 184 (42·3%) scored ten or more (103 [47·2%] assigned to the intervention and 81 [37·3%] assigned to usual care). There was weak evidence of an interaction between PHQ-9 score at baseline and trial group (interaction effect p=0·10).
The adjusted mean difference between the intervention and usual care groups was estimated to be –1·13 (95% CI –2·26 to 0·01, p=0·051) among those scoring 5–9 on the PHQ-9 at baseline, but a larger effect was seen among those scoring 10 or higher at baseline (–2·48 [95% CI –3·81 to 1·16], p=0·0002).
Discussion
We conducted a randomised controlled trial within the primary care setting of a psychosocial intervention for older people with long-term conditions during the COVID-19 pandemic. This population was vulnerable to the psychological impacts of COVID-19 restrictions. Our main finding is that behavioural activation, a brief telephone-delivered psychological intervention adapted to mitigate depression and loneliness, showed short-term positive psychological impact. There was evidence of an immediate benefit in terms of depression severity at 1 month, and this was evident at the primary trial endpoint of 3 months. The results of the BASIL+ trial were also in line with the effect size that is observed in meta-analyses of depression and loneliness outcomes for brief psychological interventions.10 The results of the BASIL+ trial will be added to future updates of a prospectively registered living systematic review (PROSPERO CRD42021298788).
For important secondary outcome measures, behavioural activation reduced levels of emotional loneliness (but not social loneliness) and also improved quality of life relating to mental health (but not physical health). There was some evidence that behavioural activation reduced levels of anxiety, although the findings were not significant. The psychological benefit was greatest for those with more severe depression and among people who engaged with five or more behavioural activation sessions.
The BASIL+ trial followed a developmental phase where behavioural activation was first adapted to meet the needs of socially isolated older adults at the beginning of the COVID-19 pandemic, and was tested in an external pilot trial.9 The BASIL+ trial was co-produced and supported by a specially convened COVID-19 research prioritisation and delivery mechanism that focused mainly (although not exclusively) on the roll-out of randomised trials in response to the COVID-19 pandemic. The BASIL+ trial was one of only two trials of psychosocial interventions designed to address unmet psychological needs during this unprecedented time.31 The BASIL+ trial was also designed to mitigate levels of loneliness in a vulnerable population, and a novel finding from BASIL+ is that a scalable behavioural intervention reduces levels of emotional loneliness. Trials of psychosocial interventions targeting loneliness, to date, have been small-scale studies and the BASIL+ trial is, by some margin, the largest trial of a behavioural intervention conducted to mitigate loneliness. The need for scalable psychological solutions to social isolation and loneliness was identified as an important research priority at the start of the pandemic,32 and the findings of the BASIL+ trial help to fill this evidence gap.
The BASIL+ trial had various limitations. The first limitation is that there was differential attrition. Although follow-up rates were high (and in line with predicted rates of retention based on our pilot), we noted some differential rates of attrition between the intervention and control groups. However, findings for the primary outcome were robust to post-hoc sensitivity analyses investigating the effect of missing data. The second limitation is that we were unable to mask participants to trial allocation and we sought to mitigate this potential bias by ensuring that, where possible, the outcome was assessed by researchers who were masked to treatment allocation. A further limitation is that our original proposed sample size was not feasible during the trial recruitment period. However, we were able to recruit slightly above the revised sample size target, which was calculated to account for correlation between baseline and follow-up measurements. Finally, we designed a pragmatic trial to assess the effectiveness of a behavioural intervention, but the choice of comparator of usual care does not allow the specific effects of behavioural approaches to be assumed. BASIL+ was designed as a two-arm trial with a usual care plus signposting comparator, and an alternative design might have been to add a third group with an attention control condition. We note that our control condition included signposting to ensure that participants were also offered a credible self-help option in line with best practice and policy recommendations during the pandemic. We also noted a dose–response effect from our CACE analysis, which supports the principle that engagement with the intervention maximised the effectiveness of behavioural activation.
COVID-19 highlighted the importance of loneliness as a threat to population health, and it remains a clinically, socially, and economically important issue that is now increasingly recognised as a post-pandemic priority.33 There are several approaches that can be helpful to policy makers, but there are also important evidence gaps in terms of what works in preventing or mitigating loneliness.34 This is a rapidly advancing area and successful delivery of the BASIL+ trial during pandemic conditions contributes to an evolving evidence base in response to social isolation and the risk of psychological deterioration in at-risk groups. Looking to the future, behavioural activation could be used to mitigate depression and the risk of loneliness in the presence of shocks to health systems and populations, such as future pandemics or other shocks that could increase anxiety and depression among vulnerable groups, such as the climate emergency.35
Data sharing
Anonymised data will be made available upon reasonable request, which must include a protocol and statistical analysis plan and not be in conflict with our prespecified publication plan, consistent with our data sharing policy (available on request via email from SG). The BASIL trials collective is especially keen that the BASIL data contribute to prospective meta-analyses and individual patient data meta-analyses. Requests for data sharing will be considered by SG and the independent trial steering and data monitoring committee.
Declaration of interests
SG and DE are members of the NICE Depression Guideline (update) Development Group. CAC-G declares royalties from Cambridge University Press Primary Care Mental Health, honoraria as Editor-in-Chief of Health Expectations and for organisation of the Royal College of General Practitioners (RCGP) One Day Essential learning event, and is Chair of the Society for Academic Primary Care awards panel and the RCGP Research Paper of the Year. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to thank the participants for taking part in the trial, general practices and North East and North Cumbria Local Clinical Research Network staff for identifying and facilitating recruitment of participants, participating NHS Trusts and Age UK organisations for recruitment, intervention delivery, and follow-ups, independent members of the Joint Programme/Trial Steering and Data Monitoring and Ethics Committee for overseeing the study, and our PPI AG members for their insightful contributions and collaboration.
Contributors
SG, DE, CAC-G, EL, DMcM, CH, AHi, DB, and SB planned the trial, contributed to the trial design, and drafted the trial protocol. SG and DE led the writing of the manuscript, managed the trial as chief investigators, and critically revised the manuscript. SG, DE, EL, DMcM, CAC-G, CH, PC, GT-T, AC, TG, AHi, KL, SD-S, CS, EN, H-IW, and JW contributed to trial design and trial management meetings. SG, CAC-G, DE, DMcM, DB, and CS designed and contributed to the intervention and training materials for BASIL+ Support Workers, and DB, DMcM, CAC-G, and DE delivered the training of BASIL+ Support Workers. EL led the day-to-day management of the trial. SB, LB, LA, and RW were the trial coordinators. DB, SC, and DMcM provided clinical supervision to BASIL+ Support Workers. SB, LB, AHe, SP, KH, ER, LS, LA, and RW facilitated participant recruitment and follow-up data collection, and participated in trial management meetings. ER and LS assisted with delivery of the behavioural activation intervention. CF, KB, and CH developed the statistical analysis plan and analysed the quantitative data. CF, KB, and CH directly accessed and verified the underlying data reported in the manuscript. All authors contributed to the drafts of manuscripts and read the final manuscript. The York Trials Unit acts as the data custodian.
Supplementary Material
References
- 1.Patel K, Robertson E, Kwong ASF, et al. Psychological distress before and during the COVID-19 pandemic among adults in the United Kingdom based on coordinated analyses of 11 longitudinal studies. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steptoe A, Di Gessa G. Mental health and social interactions of older people with physical disabilities in England during the COVID-19 pandemic: a longitudinal cohort study. Lancet Public Health. 2021;6:e365–e373. doi: 10.1016/S2468-2667(21)00069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SL, Pearce E, Ajnakina O, et al. The association between loneliness and depressive symptoms among adults aged 50 years and older: a 12-year population-based cohort study. Lancet Psychiatry. 2021;8:48–57. doi: 10.1016/S2215-0366(20)30383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valtorta NK, Kanaan M, Gilbody S, Hanratty B. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur J Prev Cardiol. 2018;25:1387–1396. doi: 10.1177/2047487318792696. [DOI] [PubMed] [Google Scholar]
- 5.Newman MG, Zainal NH. The value of maintaining social connections for mental health in older people. Lancet Public Health. 2020;5:e12–e13. doi: 10.1016/S2468-2667(19)30253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samad Z, Brealey S, Gilbody S. The effectiveness of behavioural therapy for the treatment of depression in older adults: a meta-analysis. Int J Geriatr Psychiatry. 2011;26:1211–1220. doi: 10.1002/gps.2680. [DOI] [PubMed] [Google Scholar]
- 7.Orgeta V, Brede J, Livingston G. Behavioural activation for depression in older people: systematic review and meta-analysis. Br J Psychiatry. 2017;211:274–279. doi: 10.1192/bjp.bp.117.205021. [DOI] [PubMed] [Google Scholar]
- 8.Choi NG, Pepin R, Marti CN, Stevens CJ, Bruce ML. Improving social connectedness for homebound older adults: randomized controlled trial of tele-delivered behavioral activation versus tele-delivered friendly visits. Am J Geriatr Psychiatry. 2020;28:698–708. doi: 10.1016/j.jagp.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbody S, Littlewood E, McMillan D, et al. Behavioural activation to prevent depression and loneliness among socially isolated older people with long-term conditions: the BASIL COVID-19 pilot randomised controlled trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Littlewood E, McMillan D, Chew Graham C, et al. Can we mitigate the psychological impacts of social isolation using behavioural activation? Long-term results of the UK BASIL urgent public health COVID-19 pilot randomised controlled trial and living systematic review. Evid Based Ment Health. 2022;25:e49–e57. doi: 10.1136/ebmental-2022-300530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbody S, Brabyn S, Mitchell A, et al. Can we prevent depression in at-risk older adults using self-help? The UK SHARD Trial of Behavioral Activation. Am J Geriatr Psychiatry. 2022;30:197–207. doi: 10.1016/j.jagp.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Shearsmith L, Coventry PA, Sloan C, et al. Acceptability of a behavioural intervention to mitigate the psychological impacts of COVID-19 restrictions in older people with long-term conditions: a qualitative study. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-064694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke L, Littlewood E, Gascoyne S, et al. Behavioural Activation for Social IsoLation (BASIL+) trial (Behavioural activation to mitigate depression and loneliness among older people with long-term conditions): protocol for a fully-powered pragmatic randomised controlled trial. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Academy of Medical Sciences Multimorbidity: a priority for global health research. April, 2018. https://acmedsci.ac.uk/policy/policy-projects/multimorbidity
- 15.Dimidjian S, Barrera M, Jr, Martell C, Muñoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- 16.Public Health England COVID-19: guidance for the public on mental health and wellbeing. Advice and information on how to look after your mental health and wellbeing during the coronavirus (COVID-19) outbreak. March 29, 2020. https://www.gov.uk/government/publications/covid-19-guidance-for-the-public-on-mental-health-and-wellbeing
- 17.Age UK Staying safe and well. Sept 15, 2021. https://www.ageuk.org.uk/information-advice/coronavirus/staying-safe-and-well-at-home/
- 18.Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann. 2002;32:509–521. [Google Scholar]
- 19.De Jong-Gierveld J, Kamphuls F. The development of a Rasch-type loneliness scale. Appl Psychol Meas. 1985;9:289–299. [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Kosinski M, Keller SD. New England Medical Centre; Boston, MA: 1995. SF-12: how to score the SF-12 physical and mental health summary scales. [Google Scholar]
- 22.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 23.Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 24.Gilbody S, Lewis H, Adamson J, et al. Effect of collaborative care vs usual care on depressive symptoms in older adults with subthreshold depression: the CASPER randomized clinical trial. JAMA. 2017;317:728–737. doi: 10.1001/jama.2017.0130. [DOI] [PubMed] [Google Scholar]
- 25.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 27.Dunn G. International Encyclopedia of Statistical Science. Springer; Berlin, Heidelberg: 2011. Complier-average causal effect (CACE) estimation; pp. 273–274. [Google Scholar]
- 28.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 29.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Uysal-Bozkir Ö, Fokkema T, MacNeil-Vroomen JL, van Tilburg TG, de Rooij SE. Translation and validation of the De Jong Gierveld loneliness scale among older migrants living in the Netherlands. J Gerontol B Psychol Sci Soc Sci. 2017;72:109–119. doi: 10.1093/geronb/gbv044. [DOI] [PubMed] [Google Scholar]
- 31.Gilbody S, Littlewood E, Gascoyne S, et al. Mitigating the impacts of COVID-19: where are the mental health trials? Lancet Psychiatry. 2021;8:647–650. doi: 10.1016/S2215-0366(21)00204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes EA, O'Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Lancet Loneliness as a health issue. Lancet. 2023;402:79. doi: 10.1016/S0140-6736(23)01411-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Lloyd-Evans B, Giacco D, et al. Social isolation in mental health: a conceptual and methodological review. Soc Psychiatry Psychiatr Epidemiol. 2017;52:1451–1461. doi: 10.1007/s00127-017-1446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harmer A, Eder B, Gepp S, Leetz A, van de Pas R. WHO should declare climate change a public health emergency. BMJ. 2020;368:m797. doi: 10.1136/bmj.m797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data will be made available upon reasonable request, which must include a protocol and statistical analysis plan and not be in conflict with our prespecified publication plan, consistent with our data sharing policy (available on request via email from SG). The BASIL trials collective is especially keen that the BASIL data contribute to prospective meta-analyses and individual patient data meta-analyses. Requests for data sharing will be considered by SG and the independent trial steering and data monitoring committee.