Abstract
A new pulmonary T-cell-like lymphocyte population with the phenotype CD3− CD4+ CD8+ was discovered in mice. CD4+ CD8+ but CD3+ cells among murine intestinal intraepithelial lymphocytes have previously been described. We describe herein a dramatic expansion of the CD3− CD4+ CD8+ cell population in response to experimental respiratory infection. After intranasal Chlamydia pneumoniae infection, CD4+ CD8+ cells became transiently the dominant lymphocyte type (maximum of 87% of all lymphocytes) in the lungs of NIH/S mice but remained virtually undetectable in spleen and blood. The enrichment of these cells was not a C. pneumoniae-specific event, since infection of NIH/S mice with influenza A virus also resulted in an increase in the number of CD4+ CD8+ cells (maximum of 42% of all lymphocytes). In addition to outbred NIH/S mice, two other mouse strains were studied: BALB/c (H-2d) and C57BL/6 (H-2b). C. pneumoniae-infected BALB/c mice responded with an intermediate increase in the number of CD4+ CD8+ cells in lungs, whereas C57BL/6 mice did not respond. The double-positive CD4+ CD8+ cells lacked a major part of the T-cell receptor complex, being both CD3− and TCR αβ−. However, when they were stimulated in vitro with a T-cell mitogen, they responded by proliferation but did not secrete gamma interferon. The dramatic expansion of this cell population at the infection site suggests an active role for them in respiratory infection, but the specification of this requires further study.
Chlamydia pneumoniae is a common gram-negative intracellular pathogen that causes respiratory infections that range from being asymptomatic or mild to severe, such as pneumonia (12). Because of the intracellular nature of chlamydiae, the protective immune reactions are mostly a result of cell-mediated immunity (CMI). However, as with responses to many other intracellular pathogens, CMI also induces pathology associated with the chlamydial disease (4). This makes the understanding of local CMI responses necessary for understanding the pathogenesis of chlamydial and other intracellular infections.
Local mucosal CMI responses to infections may be very different from those detected in the peripheral blood. Many unconventional and extrathymically developed T cells have been described for instance for gut-associated lymphoid tissue. Whereas peripheral T cells express on their surfaces either CD4 or CD8 in association with CD3 as part of the T-cell-receptor (TCR) complex, T cells have been described to express CD3+ CD4− CD8− (16), CD3− CD8+ (13), and CD3+ CD4+ CD8+ (14) in liver and gut. Also, a large proportion of these cells express a γδ TCR instead of an αβ TCR, which is the most abundant of T cells in peripheral blood (9). Although very little is known of the biological function of these unconventional cells, the distinct locations and restricted expression of their TCR genes suggest that these cells have a distinct function in the immune system (reviewed in reference 2). Recent evidence shows that unconventional lymphocytes can be found in the lungs as well as in the liver and gut; pulmonary inflammation induced by mycobacterial cord factor was associated with an appearance of extrathymic T cells in murine lungs (17).
We have studied C. pneumoniae infection using a mouse model (11). The route of infection (intranasal inoculation) and the clinical picture of a self-restricted infection with usually mild inflammation of the lungs resembled the initiation and result of human respiratory infection. In this model we discovered the expansion of a new T-cell-like lymphocyte population (CD3− CD4+ CD8+) in the lungs of infected mice. The appearance of these unusual cells was not strictly a C. pneumoniae-specific event, since we could also demonstrate a similar expansion in influenza A virus-infected mice.
MATERIALS AND METHODS
Mice and experimental infection with C. pneumoniae or influenza A virus.
The NIH/S (outbred; National Public Health Institute, Kuopio, Finland), BALB/c (University of Helsinki, Helsinki, Finland), or C57BL/6 (Bornholtgård Breeding and Research Centre Ltd., Ry, Denmark) mice (6- to 7-week-old females) were infected intranasally with 1 × 106 to 1.5 × 106 inclusion forming units (IFU) of C. pneumoniae Kajaani 6 isolate (8) or 2 × 104 50% egg infectious doses (EID50) of influenza A/PR8/34 (H1N1) virus under light carbon dioxide anesthesia. Reinfection of NIH/S mice with C. pneumoniae was done 6 weeks after primary infection. Certain days postinfection, mice were sacrificed with carbon dioxide and lungs, spleens, and blood were removed.
Recovery of C. pneumoniae or influenza A virus from lung samples.
Infection by C. pneumoniae was demonstrated, as previously described (11), by culturing samples obtained from supernatants of homogenized lungs on Vero cell monolayers by centrifugation (500 × g for 1 h) and cycloheximide (0.5 μg/ml) for enhancement of in vitro infection of cells. Influenza A virus culturing was done according to the principles presented in the work of Dowdle and Schild (7) by using allantoic incubation of 11-day-old embryonated eggs and EID50 end-point-infectivity titration.
Isolation of mononuclear cells and flow cytometry.
Mononuclear cells were enriched from pooled (2–7), mechanically homogenized lungs or spleens by filtering (filter pore size, 70 μm) the tissue debris and by lysing erythrocytes with a short hypothonic shock with H2O. For blood, Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden) was used to isolate lymphocytes. In the flow cytometry analysis, enriched mononuclear cells (0.4 × 106 for each test) were stained with 5 μl (each) of the following antibodies: phycoerythrin (PE)-conjugated anti-rat immunoglobulin G2b (as a control for nonspecific binding) or PE-conjugated anti-CD4 (YTS 191.1) and fluorescein isothiocyanate-conjugated anti-CD8 (α-chain specific; CT-CD8a), all purchased from Caltag (South San Francisco, Calif.). Unstained cells were used for adjustment of a FACScan (Becton Dickinson, San Jose, Calif.) and gating of lymphocytes by size. Data were typically collected from 10,000 gated events. For a more detailed analysis of CD4+ CD8+ cells, the cell suspension of lung homogenates was stained also with PE- or fluorescein isothiocyanate-conjugated antibodies specific to CD3-ɛ (145-2C11; PharMingen, San Diego, Calif.) and TCR Vαβ chain (H57-597; PharMingen). Correlations were assessed by linear-regression analysis.
Lymphoproliferation assay.
Freshly isolated pulmonary mononuclear cells in complete RPMI 1640 medium (Sigma, St. Louis, Mo.) containing 10% fetal calf serum (Integro b.v., Zaandam, The Netherlands), 10 mM HEPES (Sigma), 0.3 mg of l-glutamine (Gibco BRL, Life Technologies Ltd., Paisley, Scotland) per ml, 10 U of penicillin (Sigma) per ml, 10 μg of streptomycin (Sigma) per ml, 50 μM 2-mercaptoethanol (Sigma), and 5 μg of concanavalin A (ConA) (Sigma) per ml were plated into round-bottomed 96-well plates at 0.2 × 106 cells per well. The proliferative response was measured by incorporation of 1 μCi of 3H-labeled thymidine (Amersham, Aylesbury, United Kingdom) per well over the last 16 to 20 h of a 2-day culture period at 37°C in a 5% CO2 atmosphere. The proliferation index was calculated as (ConA-induced proliferation − background proliferation)/background proliferation.
Analysis of IFN-γ, IL-10, and IL-4 secretion.
Cells isolated from infected mice as described above were cultured in complete RPMI 1640 medium containing 5 μg of ConA per ml at 37°C in a 5%-CO2-saturated, humidified incubator for 72 h. The supernatants were collected, frozen, and analyzed later by enzyme immunoassay. For the enzyme immunoassay, on 96-well plates (Labsystems, Helsinki, Finland) 5 μg of anti-mouse interleukin-10 (IL-10) (JES-5A2; kindly given by K. Varkila, Orion Pharma, Espoo, Finland) per ml, 2 μg of anti-mouse IL-4 (BVD4-1D11; PharMingen) per ml, or 1 μg of anti-mouse gamma interferon (IFN-γ) (R4-6A2; PharMingen) per ml was used as a first antibody and biotinylated anti-mouse IL-10 (SXC-1; PharMingen), anti-mouse IL-4 (BVD6-24G2; PharMingen), or anti-mouse IFN-γ (XMG1.2; PharMingen) was used as a second antibody. Recombinant mouse IL-10 (PharMingen), recombinant mouse IL-4 (kindly given by K. Varkila), and ConA-induced cell culture supernatant of a Th1-type T-cell line (kindly given by K. Varkila) that had been previously standardized against recombinant IFN-γ (a kind gift from DNAX Research Institute, Palo Alto, Calif.) were used as standards. The intensity of the color reaction after incubation with streptavidin peroxidase (Zymed Laboratories Inc., South San Francisco, Calif.) was measured with a Multiskan MCC/340 (Labsystems). The correlations were assessed by linear-regression analysis.
RESULTS
Effect of experimental C. pneumoniae infection on the distribution of lymphocyte subtypes in the lungs.
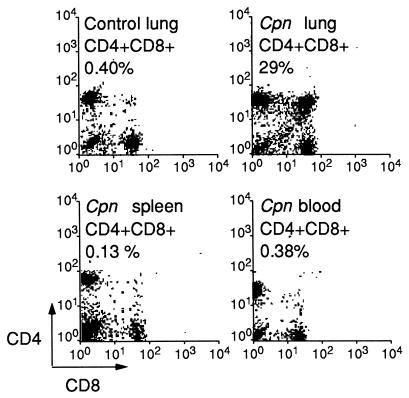
Intranasal inoculation of C. pneumoniae leads to a self-restricted pulmonary infection which reaches its infection peak in 1 week and is cleared in about 4 weeks in both NIH/S and BALB/c mice (reference 14a and our unpublished data). The T-lymphocyte composition of the lung homogenate of healthy NIH/S mice was typically as follows: 50 to 60% (of all lymphocytes) were CD4+ cells, 10 to 20% were CD8+ cells, and <1 to 5% were CD4+ CD8+ cells. During the C. pneumoniae infection, the number of isolated mononuclear cells in infected lungs (mean, 13 × 106 cells per mouse) was approximately threefold above that in uninfected lungs. The cell composition in the lungs changed, and the proportion of double-positive (CD4+ CD8+) cells expanded substantially in the NIH/S mice (up to 70% in one set of experiments) (Table 1). The proportion of these cells stayed elevated until the last time point determined, which was over 2 weeks after clearance of infection, as judged by culture (Table 1). The appearance of lymphocytes expressing a CD4+ CD8+ phenotype was tissue specific: CD4+ CD8+ cells were not detected in other tissues, such as spleen or peripheral blood, of the infected mice (Fig. 1) and also no bacteria could be cultured from these tissues. Expansion of the CD4+ CD8+ cell population appeared also during reinfection, but at a somewhat lower level than during primary infection (data not shown). Infection of BALB/c (H-2d) mice with C. pneumoniae also resulted in an increase of CD4+ CD8+ cells, but the proportion of these cells remained lower (maximally 10% of lymphocytes) than in outbred NIH/S mice (Mann-Whitney U test, P = 0.048) (Table 1). In contrast, only diminutive numbers of double-positive cells (<1%) (Table 1) were seen after infection of C57BL/6 (H-2b) mice with C. pneumoniae.
TABLE 1.
Examples of the appearance of CD4+ CD8+ cells in the lungs during intranasal infection of mice with C. pneumoniae or influenza A virus
| Mouse strain | No. of mice | Infectious agenta | No. of days after infection | IFU/sampleb | EID50/mlc | % CD4+ CD8+ cellsd |
|---|---|---|---|---|---|---|
| NIH/S | 56 | C. pneumoniae | 0 | <4 | 2 | |
| 7 | 9 | 1,714 | 10 | |||
| 7 | 17 | <4 | 70 | |||
| 7 | 24 | <4 | 21 | |||
| 7 | 42 | <4 | 67 | |||
| BALB/c | 6 | C. pneumoniae | 0 | <4 | 0 | |
| 4 | 4 | 29,534 | 0 | |||
| 4 | 8 | 28,818 | 10 | |||
| 4 | 18 | 208 | 0 | |||
| C57BL/6 | 5 | C. pneumoniae | 2 | 865 | 1 | |
| 5 | 6 | 31,197 | 0 | |||
| 5 | 12 | 41,162 | 0 | |||
| 5 | 27 | 7 | 0 | |||
| NIH/S | 6 | Influenza A virus | 2 | 130,000 | 42 | |
| 6 | 4 | 3,200 | 33 | |||
| 5 | 7 | 1,000 | <1 |
The infectious doses were 1.5 × 106 IFU of C. pneumoniae for NIH/S and BALB/c mice, 1 × 106 IFU of C. pneumoniae for C57BL/6 mice, and 2 × 104 EID50 of influenza A virus for NIH/S mice.
Geometric means calculated from individual mice in each experiment.
Geometric means calculated from individual mice in two to three experiments.
Analysis of cells isolated from a pool of lungs; lymphocytes were gated by forward- and side-scatter in fluorescence-activated cell sorter analysis.
FIG. 1.
Expression of CD4 and CD8 surface markers on lymphocytes in lung, spleen, and peripheral blood samples of intranasally C. pneumoniae (Cpn)-infected (1.5 × 106 IFU/mouse) and uninfected (control) NIH/S mice.
Lung-derived CD4+ CD8+ cells lack a major part of the TCR complex.
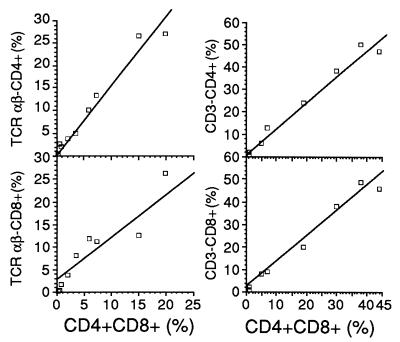
For further characterization of the CD4+ CD8+ cells, additional mice were infected and their isolated pulmonary cells were simultaneously double stained with either CD3- or TCR Vαβ-chain-specific antibody and with CD4- or CD8-specific antibody. The increase in the number of CD4+ CD8+ cells correlated with the appearance of both CD4+ CD3− and CD8+ CD3− cells (r = 0.988 with P = 0.0001 and r = 0.987 with P = 0.0001, respectively), as well as with CD4+ TCR Vαβ− and CD8+ TCR Vαβ− cells (r = 0.983 with P = 0.0001 and r = 0.936 with P = 0.0002, respectively) (Fig. 2). From these results, we conclude that the CD4+ CD8+ cells obtained from the infected murine lungs were both CD3− and TCR Vαβ− and that they differ in this respect from previously described double-positive cells in the murine gut (14).
FIG. 2.
Correlation, assessed by linear-regression analysis, between proportions (percentages of lymphocytes) of CD4+ CD8+ cells and TCR Vαβ− or CD3− cells (stained also with antibody against CD4 or CD8) in cells isolated from pools of lungs from C. pneumoniae-infected NIH/S mice.
Lung-derived CD4+ CD8+ cells respond to ConA by proliferation but not by producing IFN-γ.
Function of the double-positive cells was studied by analyzing their in vitro responses to the T-cell mitogen ConA. We measured the proliferation and the production of IFN-γ as well as IL-10 and IL-4, typical hallmarks of Th1- and Th2-type cytokines, respectively. A sample of pooled pulmonary mononuclear cells from two C. pneumoniae-infected mice which contained an unusually high proportion of CD4+ CD8+ cells, 87%, was chosen and compared with pulmonary mononuclear cells isolated from uninfected mice (with ∼2% CD4+ CD8+ cells). Stimulation of the sample led to a proliferative response (proliferation index, 113) comparable to that of uninfected mice (mean index of seven pools, 6 to 10 mice in each pool, 60 ± 34). However, IFN-γ production in the ConA-stimulated sample was low (5.3 ng/ml) in comparison to that in the uninfected mice (mean of eight pools, 39.4 ± 17 ng/ml) and IL-10 production could not be detected (detection limit, 5.6 U/ml). The sample that was 87% double-positive cells produced 0.16 ng of IL-4 per ml, whereas the samples from uninfected mice produced no detectable IL-4 (detection limit, 0.07 ng/ml).
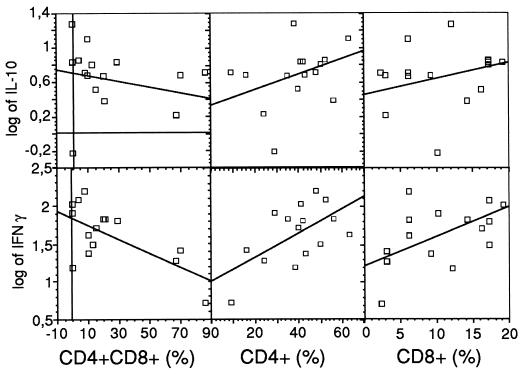
These findings were confirmed by assessing the function of the CD4+ CD8+ cells indirectly from samples of several different experiments containing various proportions of double-positive cells (0 to 87%, with a proliferation index range of 32 to 119). Consistent with the former results, the production of IFN-γ was inversely correlated with the number of CD4+ CD8+ cells (r = −0.663, P = 0.007) whereas the production of IL-10 showed neither a positive nor a negative correlation with the number of the double-positive cells (Fig. 3). In contrast, a positive correlation was demonstrated, as expected, between production of IFN-γ and both CD4+ CD8− (r = 0.588, P = 0.021) and CD4− CD8+ (r = 0.481, P = 0.069) single-positive cells. Based on this data it can be concluded that IFN-γ is not produced by CD4+ CD8+ cells when they are stimulated with ConA. Although some IL-4 secretion was detected in the sample with 87% double-positive cells, no correlation between IL-4 secretion and the number of double-positive cells could be demonstrated (data not shown).
FIG. 3.
Correlation, assessed by linear-regression analysis, between ConA-induced secretion of IFN-γ or IL-10 and the proportion of double-positive (CD4+ CD8+) or single-positive (CD4+ CD8− or CD4− CD8+) cells (percentages of lymphocytes) in cells isolated from pools of lungs from C. pneumoniae-infected NIH/S mice.
The proportion of CD4+ CD8+ cells increases in experimental influenza A virus infection.
To determine whether the appearance of CD4+ CD8+ cells observed was associated with respiratory infection in general or with C. pneumoniae in particular, we studied NIH/S mice infected with influenza A virus. We established an experimental infection model where mice were inoculated intranasally with 2 × 104 EID50 of influenza A/PR8/34 (H1N1) virus. This led to an infection during which the virus could be cultured from days 2 to 7 from the lungs of the mice (Table 1). Two days after inoculation, the proportion of CD4+ CD8+ cells of all lymphocytes in the lung samples was 42% and declined to less than 1% by day 7 (Table 1).
DISCUSSION
In this study we demonstrate the appearance of an unusual type of lymphocyte population (CD3− CD4+ CD8+) at the infection site (lung) during respiratory infection. These cells could be detected only at very low numbers from the lungs of uninfected mice or from other tissues of infected mice, whereas their proportion in all lymphocytes in the lungs during C. pneumoniae infection expanded to the maximum of 87%. The expansion of this CD4+ CD8+ cell population was not a C. pneumoniae-specific event, since intranasal infection with influenza A virus also resulted in the appearance of these double-positive cells. Dose-dependent response to infection was studied in a pilot experiment using three different inoculum doses of C. pneumoniae. The usual inoculum (106 IFU/mouse) yielded at day 9 after infection 7% CD4+ CD8+ cells, whereas 104 or 102 IFU/mouse had no effect on the proportion of double-positive cells (0.4 and 0.9%, respectively). Nonetheless, we cannot completely rule out the possibility of additional inductory mechanisms for expansion of the double-positive cells, such as nonbiological assaults or nonviable organisms.
The double-positive cells appear to be a distinct group of cells with a surface marker composition different from that of CD4+ or CD8+ single-positive cells in the peripheral blood or lymph nodes. The lung-derived CD4+ CD8+ cells described in this report differ also from the previously described double-positive cells of the gut; the lung-derived cells expressed no CD3 or TCR αβ on their surfaces, whereas the gut-derived cells have been described as CD3+ (14). This characteristic may have a fundamental impact on the potential function of the cells. However, we believe that these cells are T-cell-like cells. No correlations between the proportion of B cells (B220+) or macrophages (mac-1+) and the proportion of double-positive cells could be detected (data not shown). The double-positive cells did not express TCR αβ and may thus have expressed TCR γδ. However, pulmonary γδ T cells have been reported to be composed of CD4− CD8− rather than of double-positive cells (13). Furthermore, we have not detected more than 5% γδ T cells in the lungs during C. pneumoniae infection in NIH/S mice (data not shown).
The lung-derived double-positive cells, although lacking a major part of the TCR complex, were activated (shown by proliferation) by the T-cell mitogen ConA. However, after stimulation they did not secrete IFN-γ and probably did not secrete any IL-10 or IL-4 either. They may have secreted some other cytokines (e.g., inflammatory cytokines), or they may even have acted as cytotoxic cells, as a gut-derived intraepithelial CD4+ CD8+ T-cell line has been suggested to do (15). The function of the double-positive cells may also have to be examined more broadly than that of traditional αβ T cells. Their accumulation at the sites of the infected epithelium may have effects that, perhaps in addition to effects that lead to the destruction of infected cells, support the reconstitution of the lung epithelium. Such function has been described for another group of extrathymic T cells, the epidermal γδ T cells, that are able to secrete keratinocyte growth factor upon stimulation (5). Further, because the CD4+ CD8+ cells lack major parts of the TCR complex, they may react to changes other than conventional antigen presentation on the surfaces of the infected cells (1). One example of such T cells (Vγ9+ Vδ2+) with broad specificity was described recently for humans (6). These cells are stimulated by phosphorylated nonpeptidic metabolites that may be released by living extracellular or intracellular bacteria or by damaged cells of the body, and they are proposed to be part of the innate response to pathogens and/or tissue damage.
We found differences between NIH/S and BALB/c mice in the extents of their CD4+ CD8+ cell responses, and in C57BL/6 mice no expansion of the double-positive cell population was detected during C. pneumoniae infection. We believe that these cells are actually exhibited differently in these three mouse strains and that this difference is not due to a technical artifact, such as differential levels of adherence of the double-positive cells to the extracellular matrix in the lungs, since similar numbers of mononuclear cells could be isolated from the different mouse strains by the method employed (data not shown).
Sensitivity to many intracellular infections, as well as the severeness of symptoms, has been shown to be under genetic control, with differences between mouse strains being associated with activation of different types of CMI responses. The most studied and best-understood example of this type of genetic control occurs in leishmaniasis (10), but such genetic control has been also described for the development of the severe sequelae of C. trachomatis infections (18). All three mouse strains were able to control C. pneumoniae infection independently of the proportion of double-positive cells detected in infected lungs. However, we have observed differences in the levels of severeness of inflammation reactions and in CMI responses induced during infection (14a). Such differences might be related to differences in the levels of expansion of the herein-described pulmonary CD4+ CD8+ lymphocyte population at the infection site. However, more work is needed to identify the function of this new cell type before its role in infection can be specified. It would, for example, be interesting to use immunohistochemical staining to study whether the double-positive cells accumulate at the inflammatory sites or if they are widely distributed across lung tissue.
ACKNOWLEDGMENTS
This work was partly supported by the Academy of Finland.
We thank Carola Andersson-Parkkonen, Outi Rautio, Irene Viinikangas, and Anja Villberg for their skillful technical assistance.
REFERENCES
- 1.Abo T, Watanabe H, Sato K, Iiai T, Moroda T, Takeda K, Seki S. Extrathymic T cells stand at an intermediate phylogenetic position between natural killer cells and thymus-derived T cells. Nat Immun. 1995;14:173–187. [PubMed] [Google Scholar]
- 2.Abreu-Martin M T, Targan S R. Regulation of immune responses of the intestinal mucosa. Crit Rev Immunol. 1996;16:277–309. doi: 10.1615/critrevimmunol.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 3.Augustin A, Kubo R T, Sim G-K. Resident pulmonary lymphocytes expressing the γ/δ T-cell receptor. Nature. 1989;340:239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 4.Beatty W L, Byrne G I, Morrison R P. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 5.Boismenu R, Havran W. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 6.De Libero G. Sentinel function of broadly reactive human gamma-delta T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 7.Dowdle W R, Schild G C. Laboratory propagation of human influenza viruses, experimental host range, and isolation from clinical material. In: Kilbourne E D, editor. The influenza viruses and influenza. New York, N.Y: Academic Press Inc.; 1975. pp. 243–268. [Google Scholar]
- 8.Ekman M-R, Grayston J T, Visakorpi R, Kleemola M, Kuo C-C, Saikku P. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin Infect Dis. 1993;17:420–425. doi: 10.1093/clinids/17.3.420. [DOI] [PubMed] [Google Scholar]
- 9.Goodman T, Lefrançois L. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 10.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaukoranta-Tolvanen S-S, Laurila A L, Saikku P, Leinonen M, Liesirova L, Laitinen K. Experimental infection of Chlamydia pneumoniae in mice. Microb Pathog. 1993;15:293–302. doi: 10.1006/mpat.1993.1079. [DOI] [PubMed] [Google Scholar]
- 12.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin T, Matsuzaki G, Kenai H, Nakamura T, Nomoto K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes. Eur J Immunol. 1993;23:1968–1974. doi: 10.1002/eji.1830230836. [DOI] [PubMed] [Google Scholar]
- 14.Mosley R L, Styre D, Klein J R. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int Immunol. 1990;2:361–365. doi: 10.1093/intimm/2.4.361. [DOI] [PubMed] [Google Scholar]
- 14a.Penttilä, J. M., M. Anttila, M. Puolakkainen, A. Laurila, K. Varkila, M. Sarvas, P. H. Mäkelä, and N. Rautonen. Local immune responses to Chlamydia pneumoniae in the lungs of BALB/c mice during primary infection and reinfection. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 15.Sasahara T, Tamauchi H, Ikewaki N, Kubota K. Unique properties of a cytotoxic CD4+CD8+ intraepithelial T-cell line established from the mouse intestinal epithelium. Microbiol Immunol. 1994;38:191–199. doi: 10.1111/j.1348-0421.1994.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 16.Seki S, Abo T, Ohteki T, Sugiura K, Kumagai K. Unusual αβ-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991;147:1214–1221. [PubMed] [Google Scholar]
- 17.Tabata A, Kaneda K, Watanabe H, Abo T, Yano I. Kinetics of organ-associated natural killer cells and intermediate CD3 cells during pulmonary and hepatic granulomatous inflammation induced by mycobacterial cord factor. Microbiol Immunol. 1996;40:651–658. doi: 10.1111/j.1348-0421.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 18.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fert. 1992;95:31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]