Abstract
Coelogyne suaveolens has been used as a traditional medicine for many years, and its potential as a natural source of antibacterial agents is of great interest. This investigation aimed to identify the bioactive compounds in the plant extract and assess their antibacterial properties. To achieve this, we identified the bioactive compounds using Gas chromatography mass spectrometry (GCMS) analysis on the extract's ethyl acetate fraction and used the disc diffusion method to determine the antibacterial effect. Additionally, molecular docking were performed to predict the binding affinities of selected phytochemicals against specific proteins in order to identify the root cause of bacterial inhibition. Our results revealed that the extract exhibited significant antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae, which are common and problematic pathogens. Furthermore, molecular docking studies identified eight best-selected compounds, of which {androstan-17-one, oxime, (5.alpha.)-}, diethofencarb, tetraconazole, {3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran}, and geranyl acetate showed a significant binding affinity with best binding interaction with the target enzymes. This suggests that binding to these specific proteins might lead to the mechanism of action of the evaluated antibacterial action. In conclusion, the present study contributes to the growing body of knowledge on natural antimicrobial agents and could have significant implications for the development of new and effective antibacterial agents.
Keywords: Orchid, Antimicrobial, In-silico, In-vivo, Coelogyne suaveolens
Graphical abstract
Highlights
-
•
The study sheds light on Coelogyne suaveolens and its potential as an antimicrobial agent.
-
•
It explores the therapeutic value of Coelogyne suaveolens in combating bacteria.
-
•
By using GCMS analysis and molecular docking, this research identifies key compounds and their antibacterial potential.
-
•
Significant antibacterial activity was observed against common pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae.
-
•
These findings hold promise for the development of new and effective antibacterial treatments.
1. Introduction
Infectious diseases contribute significantly to global mortality. The burden is greatest in the countries bordering the tropics, but the developed nations feel the effects as well. Antimicrobial resistance has made the problem even harder to solve [1]. Investigating plant life for novel antibacterial candidates is one approach to this issue [2]. Humans have always relied on the healing properties of plants [3]. Over 80 % of the global population, especially people who live in rural regions, rely on medications derived from herbal sources, according to a survey by the World Health Organization (WHO) [4]. According to several studies, herbs are identified as the origin of the majority of standard medications and may lead to the identification of innovative agents against a variety of ailments in the future [5,6]. The bacterium Staphylococcus aureus is a prime example of a microbe that poses a serious risk to human health because it is responsible for a variety of skin diseases and even septicemia [7]. Urology clinics encounter recurrent urinary tract infections (UTIs) more frequently than any other diseases. UTIs can be caused by both Gram-negative and Gram-positive bacteria, with Escherichia coli being the most common [8]. Many different diseases can be caused by the Escherichia coli species, which is also a significant part of the microbiota found in the intestines of humans along with other mammals. Enteric diseases like diarrhea and dysentery are caused by at least six distinct pathotypes of E.coli, while other pathotypes are responsible for extra-intestinal infections [9]. Chloramphenicol, ampicillin, and trimethoprim-resistant Salmonella typhi strains (i.e., MDR or multidrug-resistant strains) have caused multiple epidemics in the Southeast Asia, Indian subcontinent, and Africa since 1989. MDR strains have been identified in an increasing number of immigrant workers in the Arabian Gulf countries and returning travelers in developed countries. To date, all multiple-drug-resistant (MDR) strains analyzed have been found to carry plasmids belonging to the H1 incompatibility group. Since these strains have disseminated so widely, chloramphenicol can no more be considered a first-choice treatment for typhoid fever [10]. In addition, the normal strain of Klebsiella pneumoniae acts as an opportunistic pathogen. Opportunistic pathogens tend to cause nosocomial infections and mostly affect individuals who have compromised immune systems. Some hypervirulent K. pneumoniae serotypes that make more capsule polysaccharides can make people who were healthy before getting life-threatening infections like necrotizing fasciitis, meningitis, pyogenic liver abscess, endophthalmitis, and severe pneumonia. Virulence factors used by K. pneumoniae for infection survival and immune evasion include capsule polysaccharides, fimbriae, lipopolysaccharide, outer membrane proteins, and determinants for iron uptake and nitrogen source usage [11]. Pseudomonas aeruginosa, again, is a common environmental bacterium that can infect humans when they're least protected. This bacterium's remarkable adaptability to different growing environments is due to its wide range of metabolic pathways and regulative genes. These bacteria are notoriously difficult to eliminate from infected persons, particularly lung infections in people with cystic fibrosis, because of their strong antibiotic resistance, wide nutritional tolerance, and high virulence factor count [12]. Invention of novel antibiotics that can treat antibiotic-resistant bacterial strains are urgently needed to combat the resistant strains of the common infection strains of bacteria. This calls for persistent R&D activities, as well as regulations that encourage the creation of novel antibiotics.
Orchids have long been admired for their beauty and are commonly used for decorative purposes in private homes, workplaces, and public settings. Most people simply enjoy them for their aesthetic value, but others have found useful applications for them. Orchids have been used medicinally for long periods of time in many parts of the world. Inadequate study into the efficacy and side effects of orchids has led to a gradual decline in their usage in medicine. Coelogyne suaveolens is a member of the Orchidaceae family and is indigenous to Thailand, the eastern Himalayas, Assam, and central China.We examined the sedative, anxiolytic, and analgesic effects in our earlier work, which produced noteworthy findings in those domains [13]. Since there is no report on the antimicrobial properties of the root, bulb, and leaf extract of Coelogyne suaveolens, the purpose of this study was to investigate its bioactive substances and see if they could be related to the antimicrobial properties of the studied extract by using the molecular docking technique.
2. Material and methods
2.1. Chemicals
The Department of Pharmacy, Faculty of Biological Science, University of Chittagong provided access to all the analytical-grade chemicals.
2.2. Collection and identification of the plant
Coelogyne suaveolens (Lindl.) orchid is newly reported in Bangladesh [14]. Identified then, it was documented in the Herbarium as specimen DPCU/2022/01 by Mr. Md. Owahidul Alam, Assistant horticulturist, Department of Botany, University of Chittagong.
2.3. Preparation of crude extracts
After being washed and sliced, the plant materials (bulb, root, and leaf) were dried in the sun for 7 days in a semi-shed. A mechanical grinder was used to reduce the dried plant materials to powder. Then it was soaked in acetone. The solution was stirred intermittently for 13 days before being filtered. The filtrate was then concentrated using rotary evaporation under reduced pressure and low temperatures (<50 °C).
2.4. Solvent–solvent partitioning
Following the methodology developed by Van Wagenen et al., crude acetonic extracts of the bulb, root, and leaf of Coelogyne suaveolens are subjected to solvent-solvent partitioning [15]. For this purpose, 5 g of dried acetonic extract were triturated with 90 ml of acetone containing 10 ml of distilled water, resulting in the complete dissolution of the crude extract. This solution served as the mother solution, which was subsequently partitioned with ethyl acetate. The acetonic solution was transferred to a separating funnel, and 100 ml of ethyl acetate was added. The funnel was shaken until bubbles formed, after which it was left undisturbed, allowing the ethyl acetate to separate into a beaker. This process was repeated twice. The collected ethyl acetate fraction was then combined and evaporated using a Rota evaporator.
2.5. Identification of compounds by GC MS
The gas chromatograph (model GC-17A; manufacturer: Shimadzu Corporation) and the mass spectrophotometer (model MS, TQ 8040; manufacturer: Shimadzu Corporation; Location: Kyoto, Japan) were utilized to conduct the GC-MS (Gas chromatography-mass spectrometry) analysis. Rxi-5 MS capillary columns (0.25 mm*30 m in length, 0.32 mm in diameter) interfaced with DB-1 (J & W) have been used. Helium gas was used in the column at a flow rate of 0.6 mL/min as the carrier gas. Additional GC-MS parameters include an inlet temperature of 260 °C and an interface temperature of 280 °C with the oven temperature set to start at 70 °C at zero minutes and increase to 150 °C at a rate of 10 °C per second, with a stay duration of 10 min.
2.6. Investigation of antibacterial activity
2.6.1. Disc diffusion method
This study followed the standard practice of making a concentration gradient by diffusing antibiotics from a confined source into nutritional agar gel [16]. Positive and negative controls consist of discs containing a conventional antibiotic (5 g/mL Ciprofloxacin) and blank discs, respectively. Predetermined amounts of test samples are introduced onto sterilized and dried nutritional agar discs (6 mm in diameter), and then the discs are incubated. When the discs are placed in an environment where compounds prevent microbial growth, a zone of inhibition forms around them. Fresh cultures of 6 bacterial strains were received from the University of Chittagong's Department of Microbiology for this investigation (S. aureus, S. typhi, P. vulgaris, E. coli, K. pneumoniae, and P. aeruginosa). The antimicrobial screening was carried out in a laminar flow hood, and all appropriate precautions were taken to prevent any contamination or cross-contamination from the experimental organisms, to guarantee the highest level of accuracy. The UV lights were on for an hour before getting inside the laminar hood. Glassware like micropipette tips, petri dishes, forceps, cotton, and blank discs are autoclaved for 20 min at 121 °C and 15 psi pressure. Discs were submerged into 50 μl of a 1 mg/ml sample solution and then carefully placed in the appropriate wells on agar plates that had already been loaded with test bacteria. The plates were turned upside down and refrigerated at 40 °C for around 24 h to allow the components on the discs to migrate into the agar medium. The plates were then turned upside down and incubated for a full day at 37 °C. The conventional antibiotic ciprofloxacin (5 g/mL) was compared to the plant extract in terms of its ability to suppress growth, namely its zone of inhibition's diameter [17] (see Table 3).
Table 3.
Evaluation of antibacterial activity.
| Bacteria | Zone of Inhibition (mm) |
|||
|---|---|---|---|---|
| Bulb | Leaf | Root | Standard | |
| Staphylococcus aureus | 16 | 14 | 18 | 21 |
| Salmonella typhi | 14 | 13 | NI | 31 |
| Proteus vulgaris | NI | 12 | 17 | 20 |
| E. coli | 18 | 17 | 23 | 25 |
| Klebsiella pneumoniae | 15 | 15 | 16 | 22 |
| Pseudomonas aeruginosa | 14 | 15 | NI | 23 |
NI: Indicates No Zone of Inhibition.
2.7. Molecular docking and post docking analysis
2.7.1. Ligand preparation
A set of compounds has been obtained from the GC-MS profiling table of Coelogyne suaveolens extract [2a, 2b, 2c]. For docking purpose, 29 compounds have been collected from the PubChem database and 9 compounds (4,6,8,9,12,16,27,30,37) [Table 4] were drawn using ACD/ChemSketch (Freeware) (version 2022.1.0) in.sdf and mol format, respectively [18,19]. After that, Open Babel (version 2.3.1) was utilized to convert both the.sdf and mol format. Prior to molecular docking, Gasteiger charge was added and saved the ligand pdb file as.pdbqt format using AutoDock Tools (version 1.5.6) [20]. Furthermore, the rotatable bonds have been fixed by AutoDock Tools. Optimization procedure has been performed by re-docking the co-crystallized ligand into its associated protein. The best-docked conformations have been chosen by comparing the root mean squared deviation (RMSD) values with the actual co-crystallized compound against the protein.
Table 4.
Docking results (kcal/mol)) estimated for all 38 compounds.
| Comp ID | Compound | Binding Affinity (kcal/mol) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| gyrase B |
transpeptidase |
muramyl ligase E |
dihydropteroate synthase |
||||||
| 4URN | 1KZN | 5TW8 | 6NTW | 4C13 | 1E8C | 1AD4 | 5V7A | ||
| 1 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | −6 | −7 | −6.9 | −6.6 | −6.1 | −6.4 | −5.9 | −6.6 |
| 2 | (2R,3R,4aR,5S,8aS)-2-Hydroxy-4a,5-dimethyl-3-(prop-1-en-2-yl)octahydronaphthalen-1(2H)-one | −6.4 | −5.5 | −6 | −6.3 | −6 | −5.8 | −5.5 | −5.6 |
| 3 | (R)-(−)-14-Methyl-8-hexadecyn-1-ol | −4.6 | −5.5 | −4.6 | −4.8 | −4.7 | −4.2 | −4.3 | −4.5 |
| 4 | 1,1,6-trimethyl-3-methylene-2-(3,6,9,13-tetramethyl-6-ethenye-10,14-dimethylene-pentadec-4-enyl)cyclohexane | −6.6 | −5.6 | −6.7 | −5.9 | −6.8 | −5.8 | −5.1 | −5.6 |
| 5 | 1,2–15,16-Diepoxyhexadecane | −3.7 | −4.6 | −4.6 | −4.6 | −4.1 | −4.1 | −4.5 | −4.2 |
| 6 | 1,37-Octatriacontadiene | −3.9 | −4.1 | −4.4 | −3.9 | −4 | −4.3 | −3.6 | −3.5 |
| 7 | 1,6-Octadien-3-ol, 3,7-dimethyl- | −4.4 | −5 | −4.7 | −4.7 | −4.7 | −4.5 | −4.2 | −4.4 |
| 8 | 1-Eicosanol | −4.2 | −4.4 | −3.9 | −4.7 | −4.4 | −4.6 | −4.4 | −4.1 |
| 9 | 1-Heptatriacotanol | −4.2 | −4.5 | −4.3 | −4.1 | −4.2 | −3.6 | −4 | −4.1 |
| 10 | 17-Octadecynoic acid | −4.7 | −5 | −5.1 | −4.4 | −4.1 | −4.8 | −4.5 | −4.2 |
| 11 | 2-(4-Hydroxybutyl)cyclohexanol | −5.1 | −5.8 | −5.4 | −5.1 | −6.2 | −5.2 | −5.1 | −4.9 |
| 12 | 2-Methyltetracosane | −3.6 | −4.3 | −4.5 | −4.3 | −4.1 | −5.7 | −4.3 | −4 |
| 13 | 2-Octylcyclopropene-1-heptanol | −4.4 | −5.5 | −5.1 | −4.9 | −6.6 | −4.4 | −4.2 | −4.2 |
| 14 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | −5.6 | −5.3 | −5.5 | −5.4 | −6.2 | −6.1 | −4.6 | −5.3 |
| 15 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | −4.5 | −5.6 | −4.9 | −5.6 | −6.9 | −4.1 | −4.1 | −4.5 |
| 16 | 3-Chloropropionic acid, heptadecyl ester | −4.4 | −4.3 | −4.7 | −5 | −4.7 | −4 | −3.9 | −4.5 |
| 17 | 3-Tetradecyn-1-ol | −4.5 | −4.9 | −4.7 | −4.4 | −5.9 | −4.5 | −4.2 | −4.6 |
| 18 | 6-epi-shyobunol | −5.5 | −6.2 | −5.7 | −5.2 | −5.3 | −5.4 | −4.9 | −5.2 |
| 19 | 7-Hexadecenal, (Z)- | −4.5 | −4.6 | −4.7 | −4.4 | −5.5 | −4.2 | −4.2 | −4.1 |
| 20 | 7-Hexadecyn-1-ol | −4.2 | −4.8 | −4.9 | −4.8 | −4.6 | −4.6 | −4.8 | −3.9 |
| 21 | 7-Tetradecene | −4.2 | −5 | −4.5 | −4.6 | −3.9 | −4.1 | −4.4 | −4 |
| 22 | 9,12-Octadecadienoic acid (Z,Z)- | −5.3 | −5.6 | −5.5 | −4.8 | −4.8 | −5.2 | −4.9 | −4.7 |
| 23 | 9,12-Tetradecadien-1-ol, acetate, (Z,E)- | −4.5 | −5 | −5.3 | −4.8 | −5.3 | −5.2 | −4.4 | −4.9 |
| 24 | Androstan-17-one, oxime, (5.alpha.)- | −7.2 | −7.5 | −6.8 | −6.9 | −7.3 | −7.2 | −7.2 | −7 |
| 25 | alpha-Linolenic acid | −4.4 | −5.9 | −5.8 | −5.4 | −5.1 | −5.9 | −4.1 | −4.8 |
| 26 | Cyclobarbital | −6.7 | −6.1 | −5.5 | −6.5 | −6.1 | −6.2 | −6 | −5.9 |
| 27 | Cyclopropaneoctanoic acid, 2-[[2-[(2-ethylcyclopropyl)methyl]cyclopropyl]methyl]-, methyl ester | −4.6 | −5.9 | −5.2 | −5.5 | −6.6 | −5.1 | −5.1 | −5.2 |
| 28 | Diethofencarb | −5.9 | −6.6 | −6.2 | −5.8 | −7.4 | −7.1 | −5.6 | −5.8 |
| 29 | E,E,Z-1,3,12-Nonadecatriene-5,14-diol | −4.8 | −4.7 | −5.2 | −4.7 | −5.9 | −4.9 | −4.5 | −5.4 |
| 30 | Ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)- | −4.2 | −5 | −5 | −4.5 | −5.5 | −5 | −4.8 | −4.2 |
| 31 | Geranyl Acetate | −5.3 | −5.7 | −5.6 | −5.1 | −7.1 | −6 | −4.8 | −5 |
| 32 | Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- | −5.1 | −4.9 | −5.2 | −5.3 | −4.9 | −4.8 | −5.1 | −5.5 |
| 33 | Isopulegol | −5.1 | −5.9 | −5.2 | −4.9 | −6.3 | −5.6 | −4.6 | −4.8 |
| 34 | Naphthalene, decahydro-1-pentadecyl- | −4 | −4.9 | −5 | −5.2 | −5.1 | −4.8 | −5.1 | −5.6 |
| 35 | Tetraconazole | −6.6 | −6.6 | −6.6 | −6.7 | −6.5 | −6.7 | −6.4 | −7.2 |
| 36 | Thunbergol | −6 | −5.7 | −5.8 | −6.6 | −6 | −6 | −5.7 | −5.8 |
| 37 | Undec-10-ynoic acid, decyl ester | −3.9 | −5 | −5.3 | −4.5 | −4.4 | −5.3 | −4 | −4.4 |
| 38 | Z,Z-3,13-Octadecedien-1-ol | −4.4 | −4.9 | −5.2 | −4.8 | −5 | −5.9 | −4 | −4.6 |
N.B. Binding affinity marked bold are selected as potential inhibitor against the mentioned protein.
2.7.2. Protein preparation
For inspecting the antibacterial activity of the isolated compounds, four target proteins from Gram (+ve) S. aureus and Gram (-ve) E. coli were chosen, which include transpeptidase (PDB ID: 5TW8 and 6NTW), gyrase B (PDB ID: 4URN and 1KZN), dihydropteroate synthase (PDB's: 1AD4 and 5V7A), and muramyl ligase E (MurE) (PDB ID: 4C13 and 1E8C). The existing research indicates that these proteins are crucial for the survival of the corresponding bacteria. Therefore, altering or inhibiting any of the proteins can result in the annihilation of microorganisms by interfering with their nucleic acid cleavage, assembly, and replication, or by disrupting the components and functions of their cell walls [21].
These 8 PDB structures of four respective proteins were collected from RCSB Protein Data Bank (rscsb.org). BIOVIA Discovery Studio 16.1 was used to clean and prepare all of the protein structures by taking out the water molecules and complexed co-structures [20,22]. Using AutoDock Tools version 1.5.6, polar hydrogens were included, and non-polar hydrogens were combined. Then, missing atoms were checked and repaired before applying Kollman charges, and the cleaned protein is subjected to energy minimization using the Steepest Descent algorithm in SwissPdbViewer (version 4.1.0) [23]. Energy computations were done in vacuo with the GROMOS96 43B1 parameters set, with the implementation of the Swiss-PDBViewer. The complexed ligands were then taken out of the crystal structures and used as control ligands. The resulting macromolecule was generated using Autodock tools version 1.5.6 and then saved as a combined PDBQT file for later use [20].
2.7.3. Docking procedure
A cubic grid box (60 × 60 × 60, 1 Å spacing) with the coordinates shown in Table 1 was made at each protein's active site using AutoDockTools 1.5.6. To determine the binding interactions and binding affinities between the identified metabolites and previously chosen proteins, docking was performed using AutoDock Vina (version 1.1.2) [20,24] (see Table 2).
Table 1.
List of molecular docking targets, PDB IDs, bacterial strains, coordinates, and control ligands.
| PROTEIN | PDB ID | Coordinates |
Control | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| gyrase B | G + ve | 4URN | −31.161 | 9.134 | −3.919 | Novobiocin |
| G-ve | 1KZN | 18.509 | 29.903 | 34.385 | Clorobiocin | |
| transpeptidase | G + ve | 5TW8 | 22.167 | −59.861 | 38.111 | Ceftaroline |
| G-ve | 6NTW | 21.667 | −32.371 | 41.914 | Vimirogant | |
| muramyl ligase E | G + ve | 4C13 | −23.959 | −1.254 | 9.795 | C05892 |
| G-ve | 1E8C | 12.316 | 47.458 | 97.224 | DB02314 | |
| dihydropteroate synthase | G + ve | 1AD4 | 34.588 | 8.016 | 42.32 | DB04047 |
| G-ve | 5V7A | −16.643 | 8.094 | 105.445 | 8Y7 | |
3. Statistical analysis
The data were shown as “mean ± SEM (Standard Error of Mean)”. “Statistical Package for the Social Sciences” (SPSS, Version 25.0, IBM Corporation, New York) was used for statistical analysis, and one-way ANOVA and Dunnett's test for post hoc comparisons were performed. Statistical significance was defined as a difference from the control group with a p-value of 0.05 or less, 0.01 or less, or 0.001 or less.
4. Results
4.1. GCMS profiling
The ethyl acetate fraction, which is rich in phytoconstituents, of acetonic extract of the bulb, root, and leaf of C. suaveolens were subjected to GC-MS analysis. Sixteen compounds with diverse phytochemical activity were found in the bulb extract, sixteen such compounds were found in the root extract as well, and twelve compounds were found in the leaf extract. The chromatogram is shown in Fig. 1a, Fig. 1b, Fig. 1ca, b, and 1c, and the molecular weight (MW), molecular formula, retention time (RT), and concentration (%) of each chemical component are shown in Table 2a, Table 2b, Table 2ca, 2b, and 2c.
Fig. 1a.
Coelogyne suaveolens ethyl acetate fraction's GC-MS chromatogram (Bulb).
Fig. 1b.
Coelogyne suaveolens ethyl acetate fraction's GC-MS chromatogram (Root).
Fig. 1c.
Coelogyne suaveolens ethyl acetate fraction's GC-MS chromatogram (Leaf).
Table 2a.
Compounds identified in the bulb of Coelogyne suaveolens.
| SL No | Compound Name | Molecular Formula | Molecular Weight (g/mol) | RT (min) |
|---|---|---|---|---|
| 1 | Naphthalene, decahydro-1-pentadecyl- | C25H48 | 348.6 | 25.978 |
| 2 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | C16H22O2S | 278.4 | 25.186 |
| 3 | 4,4′-Dimethoxy-2,2′-dimethylbiphenyl | C16H18O2 | 242.31 | 26.861 |
| 4 | 2-Methyltetracosane | C25H52 | 352.7 | 27.555 |
| 5 | 7-Hexadecenal, (Z)- | C16H30O | 238.41 | 29.381 |
| 6 | .alpha.-Linolenic acid, TMS derivative | C21H38O2Si | 350.6 | 36.110 |
| 7 | Undec-10-ynoic acid, decyl ester | C21H38O2 | 322.5 | 14.017 |
| 8 | [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | C21H38O2 | 322.5 | 14.833 |
| 9 | 9,12-Tetradecadien-1-ol, acetate, (Z,E)- | C16H28O2 | 252.39 | 15.246 |
| 10 | E,E,Z-1,3,12-Nonadecatriene-5,14-diol | C19H34O2 | 294.5 | 16.697 |
| 11 | (R)-(−)-14-Methyl-8-hexadecyn-1-ol | C17H32O | 252.4 | 19.100 |
| 12 | 2-Octylcyclopropene-1-heptanol | C18H34O | 266.5 | 20.520 |
| 13 | Ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)- | C20H38O2 | 310.5 | 20.941 |
| 14 | 2-(4-Hydroxybutyl)cyclohexanol | C10H20O2 | 172.26 | 22.132 |
| 15 | 7-Hexadecyn-1-ol | C16H30O | 238.41 | 22.475 |
| 16 | (2R,3R,4aR,5S,8aS)-2-Hydroxy-4a,5-dimethyl-3-(prop-1-en-2-yl)octahydronaphthalen-1(2H)-one | C15H24O2 | 236.35 | 34.891 |
RT: Retention time.
Table 2b.
Compounds identified in the root of Coelogyne suaveolens.
| SL No | Compound Name | Molecular Formula | Molecular Weight (g/mol) | RT (min) |
|---|---|---|---|---|
| 1 | 1,6-Octadien-3-ol, 3,7-dimethyl- | C10H18O | 154.25 | 9.537 |
| 2 | Geranyl Acetate | C12H20O2 | 196.29 | 13.812 |
| 3 | 7-Tetradecene | C14H28 | 196.37 | 14.027 |
| 4 | 1-Eicosanol | C20H42O | 298.5 | 16.705 |
| 5 | Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- | C10H16O | 152.23 | 17.702 |
| 6 | 7-Hexadecenal, (Z)- | C16H30O | 238.41 | 19.103 |
| 7 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280.4 | 22.359 |
| 8 | Isopulegol | C10H18O | 154.25 | 22.988 |
| 9 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | C10H16O | 152.23 | 25.171 |
| 10 | Androstan-17-one, oxime, (5.alpha.)- | C19H31NO | 289.5 | 25.969 |
| 11 | 4-Biphenyltrimethylsiloxane | C15H18OSi | 242.39 | 26.849 |
| 12 | 5.beta.-Androstan-17-one, 3.alpha.-(trimethylsiloxy)-, O-methyloxime | C23H41NO2Si | 391.7 | 27.493 |
| 13 | Cyclobarbital | C12H16N2O3 | 236.27 | 28.838 |
| 14 | Diethofencarb | C14H21NO4 | 267.32 | 30.278 |
| 15 | Tetraconazole | C13H11Cl2F4N3O | 372.14 | 29.309 |
| 16 | 3-Chloropropionic acid, heptadecyl ester | C20H39ClO2 | 347 | 19.103 |
RT: Retention time.
Table 2c.
Compounds identified in the leaf of Coelogyne suaveolens.
| SL No | Compound Name | Molecular Formula | Molecular Weight (g/mol) | RT (min) |
|---|---|---|---|---|
| 1 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296.5 | 19.594 |
| 2 | 1,2–15,16-Diepoxyhexadecane | C16H30O2 | 254.41 | 19.657 |
| 3 | 3-Tetradecyn-1-ol | C14H26O | 210.36 | 21.264 |
| 4 | 2-Octylcyclopropene-1-heptanol | C18H34O | 266.5 | 22.471 |
| 5 | Z,Z-3,13-Octadecedien-1-ol | C18H34O | 266.5 | 22.471 |
| 6 | Cyclopropaneoctanoic acid, 2-[[2-[(2-ethylcyclopropyl)methyl]cyclopropyl]methyl]-, methyl ester | C22H38O2 | 334.5 | 22.940 |
| 7 | 1,1,6-trimethyl-3-methylene-2-(3,6,9,13-tetramethyl-6-ethenye-10,14-dimethylene-pentadec-4-enyl)cyclohexane | C33H56 | 452.8 | 24.553 |
| 8 | 17-Octadecynoic acid | C18H32O2 | 280.4 | 24.842 |
| 9 | 1-Heptatriacotanol | C37H76O | 537.0 | 25.200 |
| 10 | Thunbergol | C20H34O | 290.5 | 25.705 |
| 11 | 6-epi-shyobunol | C15H26O | 222.37 | 25.810 |
| 12 | 1,37-Octatriacontadiene | C38H74 | 531.0 | 30.290 |
RT: Retention time.
4.2. Evaluation of antibacterial effect
4.2.1. Disc diffusion method
The antibacterial activity of extracts derived from the bulb, root, and leaf of Coelogyne suaveolens was compared with the standard antibiotic ciprofloxacin.
Starting with the bulb extract, it exhibited a Zone of Inhibition of 16 mm against Staphylococcus aureus and 18 mm against E.coli, indicating a moderate level of effectiveness compared to ciprofloxacin. However, the bulb extract did not show any inhibitory effect against Proteus vulgaris.
Moving on to the root extract, it demonstrated a satisfactory Zone of Inhibition of 23 mm against E.coli, and 17 mm inhibition zone against Proteus Vulgaris indicating a moderate level of effectiveness in comparison to ciprofloxacin. Additionally, the root extract exhibited an 18 mm inhibition zone against Staphylococcus aureus. However, no inhibition was observed against Salmonella typhi.
Regarding the leaf extract, it also displayed a satisfactory Zone of Inhibition of 17 mm against E.coli, suggesting a moderate level of susceptibility. The leaf extract showed a slightly lower inhibitory effect of 14 mm against Staphylococcus aureus compared to the bulb extract. However, for Salmonella typhi and Proteus vulgaris, the inhibition zones were considerably lower compared to the bulb and root extracts.
Comparatively, the standard antibiotic ciprofloxacin exhibited varying degrees of effectiveness against different bacterial strains. The extracts from Coelogyne suaveolens, specifically the bulb and leaf extracts, demonstrated similar or slightly lower efficacy compared to the root, suggesting the presence of potential antimicrobial properties in the plant.
4.3. Molecular docking analysis
The crystal structures of four bacterial target proteins representing both G(+ve) and G(-ve) bacteria were successfully docked with a total number of 38 reported compounds from Coelogyne suaveolens. Gyrase b, transpeptidase, muramyl ligase E (MurE), and dihydropteroate synthase targets were included in this study. The results are presented as the lowest energy of binding(LEB) for each compound as shown in Table 4. The higher binding affinity is indicated by the lower binding affinity values. Among them, eight compounds are further analyzed based on their active site residue interactions. Hydrogen bonds, hydrophobic bonds (i.e., Pi-sigma, Pi-Pi, Pi-alkyl), electrostatic bonds (i.e., Pi-cation/anion), and Pi-Sulfur, etc. were also analyzed and inspected.
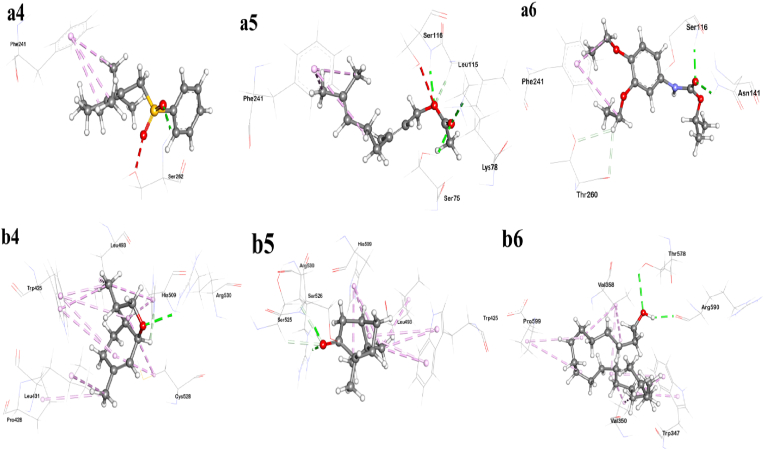
In our molecular docking study, a range of binding energy from −3.6 to −7.2 kcal/mol and −4.1 to −7.5 kcal/mol were observed against Bacterial DNA gyrase B subunit of S. aureus, and E. coli respectively. Comp31 (Geranyl Acetate) shows binding affinity of −5.3 kcal/mol and forms three hydrogen bonds (ARG79, GLY80, GLY78), comp28 (Diethofencarb) shows LEB of −5.9 kcal/mol and forms three hydrogen bond (with GLU53, THR168, PRO82), comp14 (3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran) shows LEB of −5.6 kcal/mol and forms two hydrogen bonds (ASN49, THR168). These three compounds appear to be moderate but show good active site binding affinity compared to the control ligand Novobiocin which shows LEB of −7.4 kcal/mol against S. aureus. Whereas comp1 ((2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene) shows LEB of −7 kcal/mol and forms one hydrogen bond (ASN46), comp35 (tetraconazole) shows LEB of −6.6 kcal/mol and forms three hydrogen bond (ASN46, GLU50), comp28 (Diethofencarb) shows LEB of −6.6 kcal/mol and forms three h-bond (with ASP73, GLY77, THR165). These three also showed moderate affinity in comparison to the control ligand Clorobiocin showing LEB of −8.1 kcal/mol against E. coli [Table 5a, Table 5ba and 5b] [Fig. 2].
Table 5a.
Interaction with amino acid residue with selected 3 ligand with 4URN.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 28 | Diethofencarb | −5.9 | Glu 53, Thr 168, Pro 82 | Pro 82 (2), Ile 96, Val 170, Met 81 | Met 81 | |||||
| 31 | Geranyl Acetate | −5.3 | Arg 79, Gly 80 | Gly 78 | Ile 96, Pro 82, Met 81 | |||||
| 14 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | −5.6 | Asn 49, Thr 168 | Pro 82, Met 81 (4), Val 170 | ||||||
Table 5b.
Interaction with amino acid residue with selected 3 ligand with 1KZN.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 1 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | −7 | Asn 46 | Ile 78 (4), Pro 79 (2), Ile 90 | ||||||
| 35 | Tetraconazole | −6.6 | Asn 46 | Asn 46, Glu 50 | Val 71, Ala 47, Val 43, Val 167, Ile 78, Pro 79, Ile 90 | |||||
| 28 | Diethofencarb | −6.6 | Asp 73 | Gly 77, Thr 165 | Pro 79, Arg 76, Ala 47, Val 71, Ile 78 | Glu 50 | ||||
Fig. 2.
In Silico non-bonding interactions of six best-selected ligands with DNA gyrase B protein (for S. aureus (lettered a) and E.coli (lettered b) (a1: Diethofencarb, a2: Geranyl acetate, a3: 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran, b1: 2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene, b2: Tetraconazole, b3: Diethofencarb).
Additionally, a range of binding energy from −3.9 to −7.2 kcal/mol and −3.9 to-6.9 kcal/mol were observed against bacterial transpeptidase of S. aureus, and E. coli respectively. Comp1 ((2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene) shows LEB of −6.9 kcal/mol and forms one H-bond (with SER262), Comp31 (Geranyl Acetate) shows LEB of −5.6 kcal/mol and shows four H-bond (with SER75, LYS78, SER116, LEU115), comp28 shows LEB of −6.2 kcal/mol and forms three H-bond (with ASN141, SER116, THR260). All compound shows moderate to strong interaction whereas the aforementioned three compound shows moderate interaction with active site residue compared to the control ligand Ceftaroline shows LEB of −7.2 kcal/mol against S. aureus. On the other part, comp8 shows LEB of −4.6 kcal/mol and forms two H-bond (with THR578, ARG590), comp14 shows LEB of −5.4 kcal/mol and forms two H-bond (ARG530, HIS509), comp32 (Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)-) shows LEB of −5.2 kcal/mol and forms three H-bond (ARG530, SER526, SER 525). All compounds showed moderate but have good active site binding compared to the control ligand Vimirogant shows LEB of −6.6 kcal/mol against E. coli [Table 5c, Table 5dc and 5d] [Fig. 3].
Table 5c.
Interaction with amino acid residue with selected 3 ligands with 5TW8.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 1 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | −6.9 | Ser 262 | Phe 241 (3) | ||||||
| 31 | Geranyl Acetate | −5.6 | Ser 75, Lys 78, Ser 116 | Leu 115 | Phe 241 (3) | |||||
| 28 | Diethofencarb | −6.2 | ASN141, SER116 | THR260 | PHE241 | |||||
Table 5d.
Interaction with amino acid residue with selected 3 ligand with 6NTW.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 14 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | −5.4 | Arg 530 | His 509 | Leu 493,His 509(2), Trp 425 (3), Cys 528(2), Leu 431, Pro 428 | |||||
| 32 | Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- | −5.3 | Arg 530, Ser 526 | Ser 525 | Trp 425 (2), Leu 493, His 509(3) | |||||
| 8 | 1-Eicosanol | −4.7 | Thr 578, Arg 590 | Trp 347(2), Val 350 (2), Val 358(3), Pro 599 (2) | ||||||
Fig. 3.
In Silico non-bonding interactions of six best-selected ligands with transpeptidase protein of S. aureus (denoted a) and E.coli (denoted b) [a4: (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene, a5: Geranyl acetate, a6: Diethofencarb, b4: 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran, b5: Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)-, b6: 1-Eicosanol].
Furthermore, a range of binding energy from −3.9 to −7.4 kcal/mol and −3.6 to −7.2 kcal/mol were observed against bacterial muramyl ligase E (MurE) of S. aureus, and E. coli respectively. Comp28 (Diethofencarb) shows LEB of −7.4 kcal/mol and forms four H-bond (GLY113, LYS114, HIS205, ASN112), comp31 (Geranyl Acetate) shows LEB of −7.1 kcal/mol and forms four H-bond (LYS114, GLY113, THR115, ASN112), comp35 (Tetraconazole) shows −6.5 kcal/mol and forms three H-bond (with THR137, LYS114, HIS353). These three have shown moderate to strong interaction comp control ligand Uridine 5′diphospho N-Acetyl Muramoyl-L-Alanyl-D-Glutamyl- l-Lysine (C05892) shows LEB of −8.9 kcal/mol against S. aureus, whereas, comp1 ((2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl) benzene) shows LEB of −6.4 kcal/mol and forms one H-bond (GLY47), comp31 (Geranyl Acetate) shows LEB of −6 kcal/mol and forms three H-bond (LYS119, THR120, ASN117), comp35 (tetraconazole) shows LEB of −6.7 kcal/mol and forms five conventional H-bond (ARG389, ARG416, TYR357, THR157, THR142, HIS359, GLU182, SER184). The preceding compound showed good active site interaction with a good binding score compared to the control ligand Uridine-5′-diphosphate-N-acetylmuramoyl-l-alanine-d-glutamate (DB02314) shows LEB of −8.2 kcal/mol against E. coli [Table 5e, Table 5fe and 5f] [Fig .4].
Table 5e.
Interaction with amino acid residue with selected 3 ligands with 4C13.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 28 | Diethofencarb | −7.4 | Gly 113, Lys 114 | His 205, Asn 112 | His 353, Arg 335, Tyr 351, His 205 | Lys 114 | ||||
| 31 | Geranyl Acetate | −7.1 | Lys 114, Gly 113, Thr 115 | Asn 112 | His 353, Ala 352, Phe 300, Leu 336, Leu 361 | |||||
| 35 | Tetraconazole | −6.5 | Thr 137, Lys 114 | His 353 | His 353 | Lys114 | His 353 | |||
Table 5f.
Interaction with amino acid residue with selected 3 ligand with 1E8C.
| Comp No | Compound Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 1 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | −6.4 | Gly 47 | Tyr 50, Leu 26, Val 40 | Asp 27 | |||||
| 31 | Geranyl Acetate | −6 | Lys 119, Thr 120 | Asn 117 | Lys 366, Ala 363, Phe 306, Arg 341, Ala 367, Mse 342, Ala 358 | |||||
| 35 | Tetraconazole | −6.7 | Arg 389, Arg 416, Tyr 357, Thr 157, Thr 142 | His 359, Glu 182, Ser 184 | His 210, His 186 | Lys 119 | Glu 468, His 369 | |||
Fig. 4.
In Silico non-bonding interactions of six best-selected ligands with muramyl ligase E protein of S. aureus (denoted a) and E.coli (denoted b) [a7: Diethofencarb, a8: Geranyl acetate, a9: Tetraconazole, b7: (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene, b8: Geranyl acetate, b9: Tetraconazole].
Finally, a range of binding energy from from −3.6 to −7.2 kcal/mol and −3.5 to −6.6 kcal/mol were observed against bacterial dihydropteroate synthase of S. aureus, and E. coli respectively. Comp1 ((2,6,6-Trimethyl cyclohex-1-enylmethanesulfonyl)benzene) shows LEB of −5.9 kcal/mol and forms two H-bond (with ARG52), comp24 (Androstan-17-one, oxime, (5.alpha.)-) shows LEB of −7.2 kcal/mol and forms two H-bond (with VAL49, ARG239), comp35 (tetraconazole) shows LEB of −6.4 kcal/mol and forms six conventional H-bond (ARG52, ASN 103, LYS 203, SER50, ARG52). These three have shown good active side interaction though having less binding affinity compared to the control ligand 6-Hydroxymethylpterin-diphosphate (DB04047) which shows LEB of −6.8 kcal/mol against S. aureus. Whereas comp7 (1,6-Octadien-3-ol, 3,7-dimethyl-) shows LEB of −4.4 kcal/mol and forms two H-bond (SER222, GLY189), comp14 (3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran) shows LEB of −5.3 kcal/mol and forms one H-bond (SER222), and comp35 (tetraconazole) shows LEB of −7.2 kcal/mol and forms five H-bond (ARG255, LYS221, GLY58, THR62, HIS257). In comparison with all the compounds, these three compounds showed good active site interaction having moderate binding affinity in response to the control ligand [(2-amino-9-methyl-6-oxo-6,9-dihydro-1H-purin-8-yl) sulfanyl]acetic acid (8Y7) which shows LEB of −6.3 kcal/mol against E. coli [Table 5g, Table 5hg and 5h] [Fig. 5].
Table 5g.
Interaction with amino acid residue with selected 3 ligand with 1AD4.
| Comp No | Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 1 | (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene | −5.9 | Arg 52 | Arg 52 | Lys 203, His 241, Phe 172 | Arg 239 | ||||
| 24 | Androstan-17-one, oxime, (5.alpha.)- | −7.2 | Val 49, Arg 239 | Pro 216, Arg 202 | ||||||
| 35 | Tetraconazole | −6.4 | Arg 52, Asn 103, Lys 203 | Ser 50 (2), Arg 52, | Lys 203 (2), Ala 199, Asp 84 | Met 128 | Arg 239 | val 49 (2) | ||
Table 5h.
Interaction with amino acid residue with selected 3 ligand with 5V7A.
| Comp No | Name | Docking Score (kcal/mole) | Interactions by H-Bond |
Hydrophobic Bonds |
Electrostatic |
fluorine | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Carbon–Hydrogen | Pi–Alkyl/Alkyl | Pi–Pi/Pi–Sigma/Amide–Pi | Pi–Sulfur | Pi-Anion | Pi-cation | ||||
| 7 | 1,6-Octadien-3-ol, 3,7-dimethyl- | −4.4 | Ser 222, Gly 189 | Phe 190, Lys 221, Arg 63, Pro 64 (2) | ||||||
| 14 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | −5.3 | Ser 222 | Lys 221 (4), Phe 190, His 257 | ||||||
| 35 | Tetraconazole | −7.2 | Arg 255, Lys 221 | Gly 58, Thr 62, His 257 | Phe 190, Lys 221 | Asp 96 (2) | ||||
Fig. 5.
In Silico non-bonding interactions of six best-selected ligands with dihydropteroate synthase protein of S. aureus (denoted a) and E.coli (denoted b) [a10: (2,6,6-Trimethylcyclohex-1-enylmethanesulfonyl)benzene, a11: Androstan-17-one, oxime, (5.alpha.)-, a12: Tetraconazole, b10: 1,6-Octadien-3-ol, 3,7-dimethyl-, b11:3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran, (1.alpha.,2.beta.,5.alpha.)-, b12: Tetraconazole].
5. Discussion
The two fundamental mechanisms by which an antibiotic exerts its antimicrobial effect are chemical interference with the synthesis or function of important bacterial components and/or evasion of the common mechanisms of antibacterial resistance. Inhibiting bacterial protein synthesis by interfering with their ribosomal subunits is an efficient way of combating infections caused by bacteria. Antibiotics with this method of action include macrolides, aminoglycosides, tetracyclines, and oxazolidinones [25]. Several antibiotics, including ciprofloxacin, block cell division by interfering with the synthesis of nucleic acids. Specifically, ciprofloxacin blocks the activity of a type II topoisomerase (DNA gyrase) and topoisomerase IV, enzymes required for the separation of bacterial DNA [26]. Some antibiotics, including penicillin, vancomycin, and cephalosporins, work by preventing the production of the cell walls of bacteria. Ideal targets for these kinds of bactericidal antibiotics include the bifunctional enzymes transglycosylase and transpeptidases, which both play crucial roles in the construction of the bacterial cell wall [27]. By covalently attaching to crucial penicillin-binding proteins (enzymes that are engaged in the final phases of peptidoglycan cross-linking in gram-negative as well as gram-positive bacteria), beta-lactam antibiotics disrupt bacterial cell wall construction. Bacterial production of beta-lactamase enzymes, which hydrolyze the beta-lactam ring and render the medicine ineffective, is the primary cause of resistance to beta-lactams. At least for the time being, the development of new classes of beta-lactamase inhibitors is the most promising development in the fight against resistance. These inhibitors will safeguard some of the most useful antibiotics used in clinical practice [28]. MurC, MurD, MurE, and MurF are amide ligases that catalyze the synthesis of non-ribosomal peptide bonds, which are necessary for the attachment of the peptide moiety to the peptidoglycan building blocks. These enzymes are great targets for antibiotics since they are vital to the survival of the bacteria. Because of their unique properties, researchers can focus on them while designing new types of antibiotics [29].
In the current investigation, we looked for evidence of bioactive compounds and antibacterial activity in the bulb, root, and leaf extract of Coelogyne suaveolens. From the GCMS analysis, several compounds were identified, and some of them such as {7-hexadecenal, (Z)-} [30,31], {1,6-octadien-3-ol, 3,7-dimethyl-} [32], {geranyl acetate} [33], {1-eicosanol} [34], {bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)-} [35], {3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran} [36] and a few other compounds displayed antimicrobial behaviors in previous investigations.
Amongst the 38 compounds examined, {androstan-17-one, oxime, (5.alpha.)-} (comp24) displayed a strong binding affinity (−6.7 to −7.3 kcal/mol) towards all the studied proteins, and with the S. aureus dihydropteroate synthase, formed two hydrogen bonds with Val 49 and Arg 239 residues. The S. aureus muramyl ligase E was found to have the highest binding affinity (−7.4 kcal/mol) for the molecule diethofencarb (comp28), which formed two hydrogen bonds with the His 205 and Asn 112 residues. It also exhibited a significant binding affinity (−5.9 to −6.6 kcal/mol) for S. aureus gyrase B and E. coli gyrase B, as well as for S. aureus transpeptidase, by forming multiple hydrogen bonds with (Glu 53, Thr 168, pro 82), (Asp 73, Gly 77, Thr 165), and (Asn 141, Ser 116 Thr 260) residues respectively. Tetraconazole (comp35) has the highest binding affinity (−7.2 kcal/mol) for the E. coli dihydropteroate synthetase, making several hydrogen bonds with residues including Arg 255, Lys 221, Gly 58, Thr 62, and His 257. Furthermore, this compound exhibited substantial binding affinity towards S. aureus dihydropteroate synthase, S. aureus muramyl ligase E, E. coli muramyl ligase E, and E. coli gyrase B. It is interesting to note that comp35 formed the largest number of hydrogen bonds with the residues of muramyl ligase E in E. coli (Arg 389, Arg 416, Tyr 357, Thr 157, Thr 142, His 359, Glu 182, and Ser 184). Again, geranyl acetate (comp31) showed a significant binding affinity (−5.3 to −7.1) towards gyrase B and muramyl ligase E of both S. aureus and E. coli, as well as transpeptidase of S. aureus. This compound displayed better interactions with muramyl ligase E of E. coli through the formation of hydrogen bonds with Lys 119, Thr 120, and Asn 117 residues alongside hydrophobic bonds with Lys 366, Ala 363, Phe 306, Arg 341, Ala 367, Mse 342, and Ala 358 residues. Another molecule, known as 3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran (comp14), depicted good binding affinity and a better interaction profile in comparison to the other compounds that were investigated. Compared to the other proteins, it had an affinity for the gyrase B of S. aureus, as well as the transpeptidase and dihydropteroate synthase of E. coli. (−5.6, −5.4, and −5.3 kcal/mol, respectively) having a binding interaction profile consisting of amino acid residues (Asn 49, Thr 168), (Arg 530, His 509), and Ser 222, respectively.
Based on the results obtained, it appears that the compounds in question may have the ability to inhibit the examined proteins. However, in our study, the comparison was made between the antimicrobial activity of Coelogyne suaveolens and that of ciprofloxacin. This comparison was undertaken to provide context and insight into the relative efficacy of the natural extract compared to a well-established synthetic antibiotic. However, it is important to note that such a comparison comes with inherent limitations. Ciprofloxacin, being a synthetic antibiotic, may have different mechanisms of action, pharmacokinetics, and resistance patterns compared to the bioactive compounds identified in Coelogyne suaveolens. Therefore, the direct comparison between the two should be interpreted cautiously. Future research is encouraged to delve deeper into understanding the nuanced differences in the antimicrobial mechanisms of the extract and synthetic antibiotics.
6. Conclusion
The observed antibacterial effects in our in vitro experiments and molecular docking analysis suggest the presence of bioactive compounds within Coelogyne suaveolens, and the identified compounds show significant binding affinity with target enzymes. However, it is important to note that the specific bioactive compounds responsible for the antibacterial activity remain unidentified in this study and it is imperative to note the absence of direct biological evidence, particularly in vivo bacteria-killing assays, in our research. Studies on molecular docking discovered eight best-selected compounds as such, Androstan-17-one, oxime, (5.alpha.)-, Diethofencarb, Tetraconazole, 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran, and Geranyl Acetate presented noteworthy binding affinity with the target enzymes. While the exact bioactive compounds responsible for the antibacterial activity were not identified in this study, the molecular docking analysis provides a foundation for future investigations to identify and isolate these compounds. Nevertheless, to establish Coelogyne suaveolens as a viable antibacterial agent, comprehensive biological evidence is imperative. Future research should prioritize conducting in vivo experiments to gain a more comprehensive understanding of the pharmacological properties of Coelogyne suaveolens. These experiments will contribute crucial insights into its efficacy, safety, and potential side effects as an antibacterial agent. As we advance in exploring the therapeutic potential of Coelogyne suaveolens, the integration of biological evidence will be instrumental in substantiating its antibacterial properties.
Institutional review Board statement
The department of pharmacy, University of Chittagong, Ethics Committee provided the rules and regulations that were used in the design and implementation of the experiments.
CRediT authorship contribution statement
S. M. Moazzem Hossen: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. Taslima Akter Eva: Software, Visualization, Writing – original draft. Md Sifytul Karim: Software, Visualization, Writing – original draft. Husnum Mamurat: Software, Visualization, Writing – original draft. Md Habibul Hasan Rahat: Software, Visualization, Writing – original draft. Tanzina Sharmin Nipun: Software, Visualization, Writing – original draft.
Declaration of competing interest
The authors declare that they have no conflict of interest regarding the publication of this.
Acknowledgments
We highly acknowledge University Grant Commission (UGC), Bangladesh for support during research work.
Contributor Information
S. M. Moazzem Hossen, Email: hossen.pharmacy@cu.ac.bd.
Taslima Akter Eva, Email: eva663300@gmail.com.
Md Sifytul Karim, Email: sifytul.karim.cu@gmail.com.
Husnum Mamurat, Email: husnum.mamurat@gmail.com.
Md Habibul Hasan Rahat, Email: habibrahat.bpharmcu@gmail.com.
Tanzina Sharmin Nipun, Email: tsn.np99@gmail.com.
Data availability
Data will be made available on request.
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhury M.M.H., Kubra K., Ahmed S.R. Antimicrobial, phytochemical and Toxicological evaluation of Lawsonia Inermis extracts against clinical isolates of pathogenic bacteria. Res. J. Med. Plant. 2014;8:187–195. [Google Scholar]
- 3.Petrovska B. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012;6(11):1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekor M. The growing Use of herbal medicines: Issues relating to Adverse Reactions and Challenges in Monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sithranga Boopathy N., Kathiresan K. Anticancer drugs from marine flora: an Overview. J Oncol. 2010;2010 doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural Products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 7.Adhikari R.P., Ajao A.O., Aman M.J., Karauzum H., Sarwar J., Lydecker A.D., Johnson J.K., Nguyen C., Chen W.H., Roghmann M.-C. Lower Antibody levels to Staphylococcus aureus Exotoxins are associated with Sepsis in Hospitalized Adults with Invasive S. Aureus infections. J. Infect. Dis. 2012;206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 8.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment Options. Nat. Rev. Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaper J.B., Nataro J.P., Mobley H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 10.Rowe B., Ward L.R., Threlfall E.J. Multidrug-resistant Salmonella typhi: a Worldwide epidemic. Clin. Infect. Dis. 1997;24(Suppl 1):S106–S109. doi: 10.1093/clinids/24.supplement_1.s106. [DOI] [PubMed] [Google Scholar]
- 11.Li B., Zhao Y., Liu C., Chen Z., Zhou D. Molecular Pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2014;9(9):1071–1081. doi: 10.2217/fmb.14.48. [DOI] [PubMed] [Google Scholar]
- 12.Wu W., Jin Y., Bai F., Jin S. Pseudomonas aeruginosa. Molecular Medical Microbiology. 2015:753–767. doi: 10.1016/B978-0-12-397169-2.00041-X. [DOI] [Google Scholar]
- 13.Eva T.A., Mamurat H., Rahat M.H.H., Hossen S.M. Unveiling the pharmacological potential of Coelogyne suaveolens: an investigation of its diverse pharmacological activities by in vivo and computational studies. Food Sci. Nutr. 2023 doi: 10.1002/fsn3.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huda M.K., Hoque M.M., Alam M.W. New report of three orchid species to the flora of Bangladesh. Indian For. 2021;147(5):508–511. [Google Scholar]
- 15.VanWagenen B.C., Larsen R., Cardellina J.H., Randazzo D., Lidert Z.C., Swithenbank C. Ulosantoin. A potent Insecticide from the Sponge Ulosa Ruetzleri. J. Org. Chem. 1993;58(2):335–337. doi: 10.1021/jo00054a013. [DOI] [Google Scholar]
- 16.Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Antibiotic Susceptibility testing by a Standardized Single Disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 17.Hossain M.R., Alam R., Chung H.J., Eva T.A., Kabir M.F., Mamurat H., Hossen S.M. In vivo, in vitro and in silico study of Cucurbita moschata flower extract: a promising source of natural analgesic, anti-inflammatory, and antibacterial agents. Molecules. 2023;28(18):6573. doi: 10.3390/molecules28186573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Free Chemical Drawing Software for Students | ChemSketch | ACD/Labs. Available online: https://www.acdlabs.com/resources/free-chemistry-software-apps/chemsketch-freeware/.
- 19.Hadni H., Mazigh M., Charif E., Bouayad A., Elhallaoui M. Molecular modeling of Antimalarial agents by 3D-QSAR study and molecular docking of two Hybrids 4-Aminoquinoline-1,3,5-Triazine and 4-Aminoquinoline-Oxalamide Derivatives with the Receptor protein in its both Wild and Mutant types. Biochem Res Int. 2018;2018 doi: 10.1155/2018/8639173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S., Tuli H.S., Varol M., Agarwal P., Rani A., Abbas Z., Kumar M. Antimicrobial screening to molecular docking of newly Synthesized Ferrocenyl-Substituted Pyrazole. Int. J. Health Sci. 2022;16(4):3–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Saqallah F.G., Hamed W.M., Talib W.H., Dianita R., Wahab H.A. Antimicrobial activity and molecular docking screening of bioactive components of Antirrhinum Majus (Snapdragon) Aerial parts. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian Genetic algorithm and an Empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14%3C1639::AID-JCC10%3E3.0.CO;2-B. [DOI] [Google Scholar]
- 23.Guex N., Peitsch M.C. SWISS-MODEL and the Swiss-pdb Viewer: an environment for Comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 24.Trott O., Olson A.J. AutoDock Vina: Improving the speed and Accuracy of docking with a new scoring function, efficient Optimization, and Multithreading. J. Comput. Chem. 2009;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaou N., Stavropoulou E., Voidarou C., Tsigalou C., Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: a review study on Challenges and future Perspectives. Microorganisms. 2021;9:2041. doi: 10.3390/microorganisms9102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell A. DNA gyrase as a drug target. Trends Microbiol. 1997;5(3):102–109. doi: 10.1016/S0966-842X(96)10085-8. [DOI] [PubMed] [Google Scholar]
- 27.Schneider T., Sahl H.-G. An Oldie but a Goodie – cell wall Biosynthesis as antibiotic target pathway. Int J Med Microbiol. 2010;300(2–3):161–169. doi: 10.1016/j.ijmm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Bush K., Bradford P.A. β-Lactams and β-lactamase inhibitors: an Overview. Cold Spring Harb Perspect Med. 2016;6(8) doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouidmi I., Levesque R.C., Paradis-Bleau C. The Biology of Mur ligases as an antibacterial target. Mol. Microbiol. 2014;94(2):242–253. doi: 10.1111/mmi.12758. [DOI] [PubMed] [Google Scholar]
- 30.Aljaafari M.N., Alkhoori M.A., Hag-Ali M., Cheng W.-H., Lim S.-H.-E., Loh J.-Y., Lai K.-S. Contribution of Aldehydes and their Derivatives to antimicrobial and Immunomodulatory activities. Molecules. 2022;27:3589. doi: 10.3390/molecules27113589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisignano G., Laganà M.G., Trombetta D., Arena S., Nostro A., Uccella N., Mazzanti G., Saija A. In vitro antibacterial activity of some Aliphatic Aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001;198:9–13. doi: 10.1111/j.1574-6968.2001.tb10611.x. [DOI] [PubMed] [Google Scholar]
- 32.PubChem Linalool. https://pubchem.ncbi.nlm.nih.gov/compound/6549
- 33.Mastelic J., Politeo O., Jerkovic I., Radosevic N. Composition and antimicrobial activity of Helichrysum Italicum Essential Oil and its Terpene and Terpenoid fractions. Chem. Nat. Compd. 2005;41:35–40. doi: 10.1007/s10600-005-0069-z. [DOI] [Google Scholar]
- 34.Chatterjee S., Karmakar A., Azmi S.A., Barik A. Antibacterial activity of long-Chain primary Alcohols from Solena Amplexicaulis Leaves. Proc. Zool. Soc. 2017;71:313–319. doi: 10.1007/s12595-017-0208-0. [DOI] [Google Scholar]
- 35.Pirnia M., Tabatabaee Yazdi F., Mortazavi S.A., Mohebbi M. Comparison and survey of chemical Composition and antimicrobial effects Hyssopus Officinalis and Frankincense (Boswellia Carteri) Oils against some of Food infectious and Spoiling microorganisms in vitro. Food Science and Technology. 2020;17:15–30. [Google Scholar]
- 36.Miao Y., Hu Y., Yang J., Liu T., Sun J., Wang X. Natural source, Bioactivity and synthesis of Benzofuran Derivatives. RSC Adv. 2019;9(47):27510–27540. doi: 10.1039/c9ra04917g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.