Abstract
Higher drug loading employed in nanoscale delivery platforms is a goal that researchers have long sought after. But such viewpoint remains controversial because the impacts that nanocarriers bring about on bodies have been seriously overlooked. In the present study we investigated the effects of drug loading on the in vivo performance of PEGylated liposomal doxorubicin (PLD). We prepared PLDs with two different drug loading rates: high drug loading rate, H-Dox, 12.9% w/w Dox/HSPC; low drug loading rate, L-Dox, 2.4% w/w Dox/HSPC (L-Dox had about 5 folds drug carriers of H-Dox at the same Dox dose). The pharmaceutical properties and biological effects of H-Dox and L-Dox were compared in mice, rats or 4T1 subcutaneous tumor-bearing mice. We showed that the lowering of doxorubicin loading did not cause substantial shifts to the pharmaceutical properties of PLDs such as in vitro and in vivo stability (stable), anti-tumor effect (equivalent effective), as well as tissue and cellular distribution. Moreover, it was even more beneficial for mitigating the undesired biological effects caused by PLDs, through prolonging blood circulation and alleviating cutaneous accumulation in the presence of pre-existing anti-PEG Abs due to less opsonins (e.g. IgM and C3) deposition on per particle. Our results warn that the effects of drug loading would be much more convoluted than expected due to the complex intermediation between nanocarriers and bodies, urging independent investigation for each individual delivery platform to facilitate clinical translation and application.

Keywords: drug loading, PEGylated liposomal doxorubicin, in vivo performance, anti-PEG antibody, complement
Introduction
Nanoscale carriers are considered potential tools with which multiple goals can be achieved, such as increased drug solubility, reduced drug toxicity, controllable drug release, and targeted drug delivery [1–3]. Liposomes, as archetypal nanoscale carriers, have been studied for over 50 years [4] and are still being continuously developed. The phospholipid bilayer of liposomes gives them the ability to entrap either hydrophobic or hydrophilic drugs [5]. In 1995, the launch of PEGylated liposomal doxorubicin (PLD) injection (Doxil® in the USA, Caelyx® in Europe) marked the official entry of such drugs into the nanoage. Due to the addition of PEGylated lipids, PLD significantly extended the half-life of doxorubicin (Dox) [6–8]. To date, a total of 21 kinds of liposomal therapeutics have been marketed worldwide, covering antitumor, antiinfection, analgesic and other categories [9, 10].
Moreover, liposomes, as relatively mature nanocarriers, have brought some paradoxical stereotypes during development over several decades. High drug loading has always been pursued in the field of nanomedicine as a benefit for drug delivery systems [11, 12]. First, it is cost-effective to load more of an active pharmaceutical ingredient (API) in fewer carriers. Second, a limited number of nanoscale particles can accumulate in the target sites (especially tumors), and a high drug-loading capacity would lead to more drug distribution in these sites [13]. Third, high drug loading also means that a smaller volume is needed for administration, which is more easily accepted by patients in clinical applications [14–16]. Moreover, it is thought that there would be a relatively low metabolic burden and systemic toxicity in patients who receive fewer carriers [17].
Nevertheless, such viewpoints have not been proven, and blindly pursuing a high drug-loading rate at the nanoscale remains controversial. Drug loading does not seem to be in line with the efficacy of delivery platforms. Nanocarriers are generally not inert but have their own biological activities after entry into the bloodstream [18]. For instance, liposomes are considered highly biocompatible by virtue of their biological membrane-like composition. However, with the deepening of their study in the past three decades, it was revealed that liposomal drugs exhibited complex interactions with the body, which led to a series of subsequent biological effects, such as changes in the type and quantity of plasma protein deposition on the liposomal surface [19–22] and subsequent opsonization and phagocytosis by the mononuclear phagocyte system (MPS) [23–25]. This results in alterations in the pharmacokinetic and pharmacodynamic properties and other unexpected consequences. For example, the PLD formulation (which includes HPSC, cholesterol and mPEG2000-DSPE) successfully prolonged the half-life of Dox from 5 min to 55 h when used as a drug carrier. Additionally, PLD significantly mitigated cardiotoxicity by reducing the free Dox content in circulation and the heart [7, 8]. However, the cutaneous toxicity of PLD is the most dominant and dose-limiting adverse effect reported to date, with specific clinical manifestations of hand-foot syndrome (HFS) [26–28], erythema, desquamation, ulceration and rush, which are clearly related to the liposomal carrier [29–31].
Complex interactions with the body and the subsequent biological effects of the nanocarriers challenge the rationality of the initial ideology in the community that high drug loading is beneficial. Considering that a difference in drug loading would not significantly alter the tolerable dose of the payload, high loading directly indicates a low dose of nanocarriers. In the present work, we took PLD as an example to exploit the effect of Dox loading on the in vivo performance of PLD. PLDs with two different drug loading rates (high drug loading rate (H-Dox) with 12.9% w/w Dox/HSPC and low drug loading rate (L-Dox) with 2.4% w/w Dox/HSPC; L-Dox had approximately 5-fold drug carrier of H-Dox at the same Dox dose) were prepared. In addition to comparing their pharmacokinetics, pharmacodynamics, tissue distribution and other traditional pharmaceutical properties, we paid particular attention to their biological effects, such as plasma protein adsorption, subsequent complement activation and cutaneous accumulation, especially in the presence of anti-PEG antibodies (Abs), which have been widely found in human serum.
Materials and methods
Reagents and antibodies
Doxorubicin hydrochloride, daunorubicin hydrochloride, DiO (DiOC18(3), 3,3’-dioctadecyloxacarbocyanine perchlorate) and DiD (DiIC18(5), 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindodicarbocyanine perchlorate) were purchased from Meilun Biotechnology Co., Ltd. (Dalian, China). HSPC (hydrogenated soy phosphatidylcholine), CHO (cholesterol), and mPEG2000-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-maleimide (polyethylene glycol)-2000) were acquired from A.V.T. Pharmaceutical Co., Ltd. (Shanghai, China). Sephadex® G-50 (G5080) was purchased from Sigma (St. Louis, MO). Tryptone (A650217) and yeast extract (A100850) were purchased from Sangon Biotech (Shanghai, China). HisSep Ni-NTA agarose resin 6FF (20503ES10), ampicillin (60203ES10) and polymyxin B sulfate (60242ES03) were acquired from YEASEN Biotech (Shanghai, China). The HRP-conjugated anti-6× His tag antibody (ab1187), goat anti-rat IgM mu chain (HRP) (ab97180) and recombinant anti-C3 antibody [EPR19394] (ab200999) were purchased from Abcam (Cambridge, MA). APC/Cyanine 7 anti-mouse Ly-6C antibody (cat 128025, clone HK1.4), Brilliant Violet 421™ anti-mouse Ly6G antibody (cat 127628, clone 1A8), APC anti-mouse CD146 (cat 134712, clone ME-9F1), Brilliant Violet 421™ anti-mouse F4/80 (cat 123131, clone BM8), APC anti-mouse CD11c (cat 117309, clone N418), APC anti-mouse CD3 (cat 100236, clone 17A2), and Brilliant Violet 421™ anti-mouse CD19 antibody (cat 115549, clone 6D5) were acquired from BioLegend (San Diego, CA). HRP-labeled mouse anti-rat IgG (H + L), HRP-labeled goat anti-rabbit IgG (H + L), Alexa Fluor 647 donkey anti-rabbit IgG (H + L), TMB (3,3’,5,5’-tetramethylbenzidine) chromogen solution, gradient precast polyacrylamide gels (4–20%) and SDS‒PAGE sample loading buffer (5×) were acquired from Beyotime Biotechnology (Nantong, China).
Animals and cell lines
Male SD rats (4–6 weeks of age), male BALB/c mice (6–8 weeks of age), and female BALB/c mice (6–8 weeks of age) were provided by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and kept under SPF conditions. All animal experiments were carried out in accordance with guidelines evaluated and approved by the ethics committee of Fudan University. 4T1 cells were obtained from Shanghai Institute of Cell Biology. Cells were maintained in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco), 100 U·mL−1 penicillin, and 100 μg·mL−1 streptomycin at 37 °C under a humidified atmosphere containing 5% CO2.
Preparation of tumor-bearing mice
A total of 1.5 million 4T1 cells in 0.1 mL of PBS were subcutaneously injected into the axilla of the female BALB/c mice. Tumor volumes were measured every 2 days from the 7th day after injection until the tumor grew to over 200 mm3 but below 800 mm3.
Preparation and characterization of the liposomes
All PEGylated liposomes (sLips) were prepared with a formulation of HSPC/cholesterol/mPEG2000-DSPE (57/38/5 molar ratio). For PEGylated liposomal doxorubicin (PLD), doxorubicin (Dox) was fed into the sLips by the traditional ammonium sulfate gradient loading method [32]. A solution of HSPC, cholesterol and mPEG2000-DSPE in chloroform was made into a thin film by rotary evaporation. Any residual organic solvent was removed by overnight evaporation under vacuum. The dried lipid film was subsequently hydrated with 0.32 M ammonia sulfate at 60 °C for 30 min, and the lipid dispersion was extruded through polycarbonate membranes with pore sizes of 200, 100, and 50 nm. The external liquid phase was replaced with saline using a G-50 column, and then the liposomes were incubated with Dox solution at a Dox/HSPC ratio of 10% (w/w) for H-Dox and 2% (w/w) for L-Dox at 60 °C for 20 min. After cooling to room temperature, free Dox was removed by a G-50 column. DiD- and DiO-labeled liposomes were prepared by the thin-film hydration and extrusion method. A mixture of HSPC, cholesterol, mPEG2000-DSPE and 1% DiD (DiD/HSPC, w/w) or 2% DiO (DiO/HSPC, w/w) in chloroform was processed in the same way as the PLD preparation but hydrated with saline at 60 °C for 30 min. Liposomes were diluted 20-fold with deionized water (DW) and characterized in terms of size and zeta potential by a Zetasizer Nano ZS90 (Malvern, UK). Dox loading efficiency was measured by reversed-phase high-performance liquid chromatography (with 0.1% trifluoroacetic acid in DDW as mobile phase A and acetonitrile as mobile phase B at a flow rate of 0.7 mL/min with isocratic elution with 40% B over 10 min). All liposomes were filtered through a 0.22 μm sterile filter before injection.
Expression and characterization of PEG-scFv (b1)
In our previous work, anti-PEG scFv was prepared and used to ameliorate the ABC effect and separate the protein corona [33, 34], and we introduced anti-PEG scFv (b1) through site-specific mutagenesis to enhance the affinity of PEG-scFv for the PEG unit as described [35]. Here, we applied anti-PEG scFv (b1) to specifically separate PLD and free Dox.
The high-affinity plasmid PEG-scFv was constructed, cloned and inserted into competent E. coli (TOP10). After culturing overnight, several individual clones were selected and amplified. The expressed E. coli were collected by centrifugation at 4 °C, 5000 × g for 30 min. The pellets were resuspended in lysis buffer (0.5 M NaCl and 0.1 M polymyxin B sulfate), and the supernatant was obtained after centrifugation at 4 °C, 12,000 × g for 10 min. PEG-scFv (b1) was purified by passing through Ni-NTA agarose resin and eluted with elution buffer (phosphate-buffered saline (PBS) with 250 mM imidazole, pH adjusted to 8.0). The eluted fractions were concentrated at 4000 × g and washed with chilled PBS at least three times until the storage buffer had been completely replaced. A BCA protein assay kit was used to detect the concentration of PEG-scFv (b1). A gradient polyacrylamide gel (4%–20%) and fast sliver staining kit were applied to validate the purity of PEG-scFv (b1).
The affinity of PEG-scFv (b1) binding to PEG was characterized by ELISA. mPEG2000-DSPE (2 µg per well) was diluted in absolute ethanol and coated on microplates. After blocking with 5% BSA in PBS, serial dilutions of PEG-scFv (b1) in PBS were added for incubation for 1.5 h at 37 °C. HRP-conjugated anti-6× His antibody (1:5000 dilution) was used, and detection was performed at OD at 405 nm with the ABTS substrate.
The affinity precipitation of PLDs by PEG-scFv (b1)
To study PLD precipitation induced by the affinity of PEG-scFv (b1), 10 µL of H-Dox or L-Dox was mixed with 40 µL of PBS or SD rat plasma to study the in vitro and in vivo affinity precipitation profiles, and then the mixtures were incubated with 0–85 µg of anti-PEG scFv (b1) for 30 min. The supernatant was collected by centrifugation at 4 °C and 2000 × g for 10 min. The amount of Dox in the supernatant was quantified by HPLC, and the PLD sedimentation ratio (%) was calculated. The amount of anti-PEG scFv at a sedimentation ratio of 99.9% was used to optimally precipitate the in vitro or in vivo samples.
In vitro release from the PLDs
Dox release from the PLDs in PBS was studied. Briefly, 300 μL of H-Dox or L-Dox (CDox = 0.5 mg/mL) (n = 3) was diluted with 1× PBS buffer as the release medium, and each sample was split into glass tubes and then shaken in a water bath at 37 °C. At 5 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 36 h, 48 h, and 72 h, the glass tubes were removed, and 100 μL of release medium was collected for Dox quantification. To evaluate the drug content in the release medium, 50 μL of each sample was precipitated with anti-PEG scFv, and the Dox in the supernatant was collected for HPLC analysis.
In vivo separation of liposomal Dox and free Dox
SD rats (n = 5) were intravenously injected with H-Dox or L-Dox (2 mg·kg−1), and blood (with anticoagulant EDTA·2Na) was sampled at 5 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h after injection. The plasma was collected by centrifugation at 4 °C and 3000 rpm for 8 min. A volume of 30 µL of plasma sample was added to 70 µL of PEG-scFv (b1) (0.5 mg·mL−1) for incubation at 4 °C for 30 min. Then, 70 μL of the supernatant containing free Dox was collected by centrifugation at 2000 × g for 10 min, and 30 μL of PBS was added. One hundred microliters of each plasma sample was mixed with 100 μL of 50 ng·mL−1 daunorubicin (Dau) in methyl alcohol as an internal standard and 400 μL of chloroform. After 2 min of vortexing and 15 min of centrifugation at 8000 × g, the chloroform phase was transferred to a new tube, and the sample was dried overnight. Samples were redissolved in 50% methanol and centrifuged for 15 min at 12,000 × g to remove insoluble impurities. The concentration of Dox in the supernatant was determined by LC‒MS/MS. For this, 0.1% formic acid in DDW was used as mobile phase A and acetonitrile was used as mobile phase B. Gradient elution was performed as follows at a flow rate of 0.3 mL/min: 16% B for 2 min, 16%–34% B over 6 min, 34%–16% B over 0.1 min, and 16% B for 2.9 min. The transitions of m/z 544.30 → 397.25 for Dox and m/z 528.30 → 321.10 for Dau were used [36].
Pharmacokinetics study
PLDs or DiD-labeled liposomes were injected into SD rats (n = 5) via the tail vein. Blood was sampled at 5 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h after injection. Disodium EDTA was added as an anticoagulant, and then the blood samples were centrifuged at 3000 rpm for 8 min to collect plasma.
For the pharmacokinetic study of PLDs, 100 μL of each plasma sample was mixed with 100 μL of 10 μg/mL Dau in methyl alcohol as an internal standard and 400 μL of chloroform. After 2 min of vortexing and 15 min of centrifugation at 8000 × g, the chloroform phase was transferred to a new tube, and the sample was dried overnight. Then, 120 μL of acetonitrile/DDW (4:6 v:v) was used to dissolve the residue, and the concentration of Dox was determined by HPLC.
For the pharmacokinetic study of DiD-labeled liposomes, the plasma samples were diluted with normal plasma in a 96-well plate for fluorescence measurements. A Spark® multimode microplate reader measured the fluorescence at Ex 630 nm/Em 675 nm. Pharmacokinetic parameters were calculated by DAS 2.0 software.
Characterization of the protein corona
The plasma samples taken for the pharmacokinetic study (n = 3) at 5 min, 1 h, 4 h and 12 h from the H-Dox and L-Dox groups were collected for in vivo PLD protein corona separation. Gravity chromatography columns (3 mL) were prefilled with 400 μL of Ni-NTA resin and equilibrated with 5 mL of PBS. Fresh plasma (20 μL) containing H-Dox and L-Dox was incubated with anti-PEG scFv (final concentration of 0.5 mg·mL−1) at room temperature for 30 min. Then, each mixture was loaded onto the column slowly and incubated with Ni-NTA resin at room temperature for 30 min to allow complete reaction of Ni with His-tagged anti-PEG scFv. Most of the plasma proteins and unbound PLDs were flowed through, and subsequently, the captured PLDs were washed with 4 mL of washing buffer (5 mM imidazole in PBS, pH 7.4). Fractions were collected at a volume of 200 μL per tube (#1 ~ 20). PLDs with protein coronas were eluted by PEG8000 in PBS (100 mg·mL−1) in 100 μL in the first tube, followed by 50 μL in 5 subsequent tubes (#21 ~ 26). The column was further washed with 2 mL of PBS (#27 ~ 36), followed by imidazole (250 mM in PBS) to elute the anti-PEG scFv bound to Ni-NTA (#37 ~ 40). The protein concentrations in all fractions were determined by a BCA kit, and the Dox fluorescence intensity of each tube was measured by a Spark® multimode microplate reader (Ex/Em at 480 nm/550 nm).
The middle protein corona eluate (#23 ~ 24) was collected, the fractions were combined (total 100 μL), and then the amount of phospholipids was adjusted to be the same by detecting the Dox fluorescence intensity in each tube. SDS‒PAGE sample loading buffer (5×) was added to the adjusted eluate. Protein aliquots were separated by size on a 4%–20% gradient polyacrylamide gel. For overall protein corona spotting, the gel was stained with silver. For further specific opsonin detection, protein aliquots were transferred to PVDF membranes. Nonspecific binding sites on the PVDF membrane were blocked by incubation in 0.1% PBST (0.1% Tween 20 in PBS) containing 5% BSA at room temperature for 2 h. For IgM detection, the membrane was incubated overnight at 4 °C with HRP-labeled anti-IgM antibody (1:5000). For C3 detection, the membrane was incubated overnight at 4 °C with recombinant anti-C3 antibody (1:1000). After washing with PBST (6 times, 5 min each) to remove residual primary antibody, HRP-labeled goat anti-rabbit IgG (H + L) (1:1000) was added as a second antibody. The signal was detected (ChemiScope 6000, Clinx Co. Ltd.), and the data were analyzed by ImageJ software.
ELISA
The in vivo anti-PEG IgM mimicking activity (stimulation by empty sLip) and immunogenicity of H-Dox and L-Dox (anti-PEG IgM and IgG triggered by H-Dox and L-Dox) were verified by ELISA. Medium binding ELISA plates were coated with mPEG2000-DSPE (2 µg per well) by dilution in absolute ethanol and volatilization overnight. Coated plates were blocked with 5% BSA in PBS at 37 °C for 1 h and washed with 0.1% PBST (5 times, 1 min each). Plasma samples were mixed with serial dilutions of 0.1% BSA in PBS and added to plates for 1 h of incubation at 37 °C. After rinsing with 0.1% PBST, HRP-labeled anti-mouse IgM and IgG antibodies were added for incubation at 37 °C for 1 h. TMB was added to produce a chromogenic reaction, and after 8 min, the reaction was terminated by the addition of 0.18 M sulfuric acid. The absorbance at 450 nm was measured by a Spark® multimode microplate reader.
Evaluation of PLD skin accumulation
BALB/c mice (n = 8–10) were prestimulated with a low dose of empty sLip (2.3 mg·kg−1), and anti-PEG IgM was detected with an ELISA on Day 5. On Day 6, H-Dox and L-Dox were administered via the tail vein of BALB/c mice at a doxorubicin dose of 3 mg·kg−1. Erythema and ulceration on the mouse skin were observed and recorded. At 24 h after injection, mice were sacrificed and perfused with saline to remove the residual PLDs from the vessels. The truncus skin, ears and paws were dissected and weighed. After homogenization with 5% Triton X-100 in PBS, 100 μL of methanol (containing 100 ng·mL−1 Dau as the internal standard) and 400 μL of chloroform were added to 100 μL of the homogenates to extract Dox. After 2 min of vortexing and 15 min of centrifugation at 8000 × g, the chloroform phase was transferred to a new tube, and the sample was dried overnight. Samples were redissolved in 50% methanol and centrifuged for 15 min at 12,000 × g to remove insoluble impurities. The concentration of Dox in the supernatant was determined by LC‒MS/MS as previously mentioned.
Biodistribution study
H-Dox and L-Dox were injected into 4T1 subcutaneous tumor-bearing mice (n = 4) via the tail vein at a dose of 3 mg·kg−1. The hearts of the mice were perfused with 0.9% NaCl after sacrifice. The tissues were weighed and collected in 2 mL EP tubes. After homogenization with 5% Triton X-100 in PBS, 100 μL of methanol (containing 100 ng·mL−1 Dau as the internal standard) and 400 μL of chloroform were added to 100 μL of the homogenates to extract Dox. After 2 min of vortexing and 15 min of centrifugation at 8000 × g, the chloroform phase was transferred to a new tube, and the samples were dried overnight. Then, 120 μL acetonitrile/DDW (4:6, v:v) was used to dissolve the residue, and the concentration of Dox was determined by HPLC.
Peripheral blood, spleen, and liver cell separation and suborgan distribution
BALB/c mice (n = 4) were intravenously administered DiO-sLips (L-HSPC) or a mixture of DiO-sLips and blank-sLips (H-HSPC with the same fluorescence as L-HSPC) at a dose of 20 or 100 mg HSPC per kg, respectively. At 1 h, 4 h and 12 h postinjection, the blood of the BALB/c mice was sampled using EDTA·2Na as the anticoagulant. After the mice were sacrificed, the spleens and livers were collected.
Peripheral blood
Blood cells were collected by centrifugation at 400 × g and rinsed with PBS. White blood cells were obtained after the erythrocytes were removed by RBC lysis buffer at 4 °C and were divided into two parts. One part was incubated with APC/Cyanine 7-labeled anti-Ly6C antibody (HK1.4, BioLegend) and Brilliant Violet 421™-labeled anti-Ly6G antibody (1A8, BioLegend), and the other part was incubated with APC-labeled anti-CD3 antibody (17A2, BioLegend) and Brilliant Violet 421-labeled anti-CD19 antibody (6D5, BioLegend).
Spleen
Spleens were dissected in chilled dissociation buffer (DMEM with 1 mg·mL−1 DNase I and 1 mg·mL−1 collagenase IV) and sieved through a 70 μm nylon sieve. Spleen cells were collected by centrifugation at 400 × g and rinsed with PBS. Then, spleen cells were obtained after the erythrocytes were removed by RBC lysis buffer at 4 °C and were divided into two parts. One part was incubated with APC-labeled anti-CD11c antibody (HK1.4, BioLegend) and Brilliant Violet 421™-labeled anti-F4/80 antibody (BM8, BioLegend), and the other part was incubated with APC-labeled anti-CD3 antibody (17A2, BioLegend) and Brilliant Violet 421-labeled anti-CD19 antibody (6D5, BioLegend).
Liver
Livers were dissected after perfusion with 0.5 M EGTA and 100 U·mL−1 type IV collagenase via the inferior vena cava and portal vein. The livers were incubated in a water bath for 30 min at 37 °C and sieved through a 70 μm nylon sieve. Liver parenchymal cells were collected by centrifugation (4 °C, 50 × g, 3 min). The supernatant was collected and centrifuged at 650 × g for 7 min. The pellet was collected and resuspended in PBS to obtain a cell suspension prior to 25%/50% Percoll gradient centrifugation (4 °C, 1800 × g, 15 min). The middle cell layer, which contained liver nonparenchymal cells, including Kupffer cells and hepatic sinusoidal endothelial cells, was harvested. The isolated liver parenchymal cells were stained with anti-albumin (Abcam) and Alexa Fluor 647-labeled donkey anti-rabbit secondary antibodies (Abcam) after permeabilization with 0.5% Triton X-100 at room temperature for 20 min. The isolated liver nonparenchymal cells were divided into two parts. One part was incubated with APC-labeled anti-CD146 antibody (ME-9F1, BioLegend) and Brilliant Violet 421™-labeled anti-F4/80 antibody (BM8, BioLegend), and the other part was incubated with APC-labeled anti-CD3 antibody (17A2, BioLegend) and Brilliant Violet 421-labeled anti-CD19 antibody (6D5, BioLegend).
All stained cells were subjected to flow cytometry (Agilent Technologies, Novocyte 3000), and the percentages of DiO-positive cells and mean fluorescence intensities were measured.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) unless otherwise indicated. Comparisons among different groups were analyzed by Student’s t test, one-way ANOVA or two-way ANOVA following their respective applicable principles with GraphPad Prism 8.0.2. In all analyses, P < 0.05 was considered to indicate statistical significance (n.s. indicates nonsignificance at P > 0.05; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
Preparation and characterization of PLDs
PEGylated liposomes (sLips) were prepared by the thin-film hydration and extrusion method. Liposomes with high (12.9% w/w Dox/HSPC, H-Dox) or low (2.4% w/w Dox/HSPC, L-Dox) Dox loading were acquired by feeding different ratios of Dox to lipids [32]. Both populations of liposomes exhibited similar sizes (approximately 79 nm), narrow size distributions (polydisperse indexes, PDIs, of <0.1) after serial extrusion through membranes with predetermined pore sizes and high encapsulation efficiencies (>99.5%) (Fig. 1a). The loading rate of Dox did not affect the zeta potential of the liposomes (−25 to −30 mV). Any residual free Dox was separated from the liposomes using the anti-PEG scFv method (see Methods), which was superior to traditional solid phase extraction (SPE), especially to separate a low concentration of free Dox, according to our previous report [35] (Supplementary Fig. 3a, b). The concentration of free Dox was very low in both populations of PLDs, as expected (less than 0.5% of the total Dox).
Fig. 1. Characterization of H-Dox and L-Dox.

a Content of characterization parameters of H-Dox and L-Dox (n = 3). Representative cryo-transmission electron microscope (Cryo-TEM) images of H-Dox (b) and L-Dox (c). Doxorubicin nanocrystals are labeled by dotted lines, and their widths are labeled by double-headed arrows. Width (d) and volume (calculated as cylinder, V = πr²h) (e) of doxorubicin nanocrystals in H-Dox and L-Dox (n = 16). Data are shown as the means ± SDs.
To investigate whether the loading rate affects the Dox distribution in the liposomes, cryo-transmission electron microscopy (cryo-TEM) was applied to observe the structures of two populations of liposomes (Fig. 1b, c). Both PLDs showed a similar appearance, with Dox nanorod crystals (dotted rectangles) inside the phospholipid bilayer, indicating that the Dox loading rate did not affect the crystalline structure of Dox in the liposomes [37]. Moreover, the width (Fig. 1d) and volume (calculated as a cylinder, V = πr²h, Fig. 1e) of the nanorod crystals were positively correlated with the drug loading rate. The liposomes also maintained the same shape (spherical) in the two PLDs rather than changing to ellipsoids with increasing drug loading rate.
PLDs with different drug loadings show a high degree of stability
The in vitro release profiles from H-Dox and L-Dox were first investigated in phosphate-buffered saline (pH = 7.4) in a 37 °C water bath (Supplementary Fig. 1) using the anti-PEG scFv method (see Methods). Less than 2% of the encapsulated Dox was released from both H-Dox and L-Dox after 72 h of incubation. No significant difference in the Dox release profile was observed between the two populations of PLDs, suggesting that the Dox loading rate did not affect the release profile in vitro.
To study the effect of the Dox loading rate on the in vivo pharmacokinetic profiles of the liposomes, both H-Dox and L-Dox were intravenously injected into the tail vein of SD rats at the same dose of Dox (2 mg·kg−1 Dox) but different doses of HSPC (20 and 100 mg·kg−1 HSPC, respectively). Blood was sampled at predetermined time points (5 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h). As shown in Fig. 2a, b, L-Dox showed an extended circulation time that was accompanied by a significant enhancement in the AUC0-24h in comparison to H-Dox (1.17-fold) but a similar half-life (9.964 h for L-Dox, 9.092 h for H-Dox, Supplementary Table 1). To better understand the effect of the Dox loading rate on drug release from the liposomes, in vivo Dox release was evaluated using anti-PEG scFv to separate the free Dox in plasma at different time points (see Methods). Both PLDs showed similar, small amounts of leakage (<200 μg·mL−1, <2%) at each time point, as shown in Fig. 2c, d, which was consistent with the in vitro results.
Fig. 2. Pharmacokinetic profiles of PEGylated liposomes (sLips) with or without Dox administered to SD rats.
H-Dox and L-Dox were intravenously injected into the tail veins of SD rats at the same dose of Dox (2 mg·kg−1 Dox) but different doses of HSPC (20 and 100 mg·kg−1 HSPC, respectively). The total Dox concentrations (a) and areas under the curve (AUCs) (b) of H-Dox and L-Dox in SD rats (n = 4–5). The free Dox concentration (c) indicates the in vivo release of H-Dox and L-Dox. The free Dox percentage (d) is the specific free Dox concentration compared to the total Dox concentration at each time point. Pharmacokinetic profiles (e) and AUCs (f) of DiD-labeled liposomes administered to SD rats (n = 5). The liposomal contents were quantitated using DiD. The HSPC doses were 20 mg·kg−1 (H-Dox) and 100 mg·kg−1 (L-Dox). The HSPC ratio (g) is the specific lipid concentration at each time point compared to that at 5 min (5 min was taken as 100%). Data are shown as the means ± SDs. Statistical significance was evaluated by Student’s t test (**P < 0.01).
Moreover, DiD, a fluorescent dye that anchors into the liposomal bilayer was used to label liposomes at different doses (20, 50, and 100 mg·kg−1 HSPC), and the pharmacokinetic profiles of the lipid carriers were evaluated in SD rats. The blood concentrations (Fig. 2e) and AUC0-48h values (Fig. 2f) of HSPC (calculated according to the fluorescence of DiD at different time points) were proportional to the dose (near 2-fold and 5-fold, respectively; Supplementary Table 2), which was in line with the calculated lipid concentration in H-Dox and L-Dox. In addition, as shown in Fig. 2g, when calculating the ratio of lipid concentration at each time point to that at 5 min (5 min was taken as 100%) for different liposomes with or without Dox, the percentage of HSPC was identical. The combination of the above results indicated that PLDs with different drug loadings had high stability both in vitro and in vivo. In other words, almost all of the Dox in systemic circulation (>99%) was included in liposomes.
More carriers resisting the preexisting anti-PEG Abs enables extended blood circulation
As nanomedicines continue to improve, the conjugation of polyethylene glycols (PEGs) to nanocarriers has become a widely accepted method to prolong systemic circulation [38]. However, these drugs and agents are often immunogenic partially owing to their triggering of anti-PEG Abs. In addition to those found postimmunization, anti-PEG Abs are also observed in healthy subjects without any prior exposure to PEGylated drugs or agents [39, 40]. Moreover, these Abs have been shown to account for efficacy loss due to the accelerated blood clearance (ABC) phenomenon of the drug by our group [34, 41, 42] and many other research groups [43–45].
To verify our hypothesis that more PEGylated liposomes in blood circulation (due to lower drug loading) could consume more anti-PEG Abs to ameliorate the ABC phenomenon, SD rats were prestimulated with a low dose of empty sLips (2.3 mg·kg−1 without Dox loading) as previously described [34]. The detection of anti-PEG IgM on Day 5 after stimulation suggested that anti-PEG IgM was produced, as expected (Supplementary Fig. 2), which is the causal factor for the ABC phenomenon. On Day 6 after stimulation, H-Dox and L-Dox were intravenously administered into the tail veins of naïve and stimulated rats, and the pharmacokinetic profiles were evaluated. As shown in Fig. 3a, b, prestimulation (which led to higher blood anti-PEG Ab levels due to increased IgM) resulted in the rapid clearance of H-Dox with a 45.7% reduction in AUC0-24h, while the clearance of L-Dox dropped by only 15.3% (Supplementary Table 3). However, as shown in Fig. 3c, d, the amount of free Dox did not increase (<200 μg·mL−1, <2%) at each time point, even in the presence of preexisting anti-PEG Abs.
Fig. 3. Pharmacokinetic profiles and protein coronas of H-Dox and L-Dox in SD rats with or without the presence of anti-PEG antibodies (Abs).
H-Dox and L-Dox were intravenously injected into the tail veins of SD rats at the same dose of Dox (2 mg·kg−1) but different doses of HSPC (20 and 100 mg·kg−1, respectively). The total Dox concentrations (a) and AUCs (b) of H-Dox and L-Dox in SD rats prestimulated with empty sLips (2.3 mg·kg−1, without Dox loading) or unstimulated (n = 4–5). The free Dox concentration (c) detected in the in vivo H-Dox and L-Dox release experiment. The free Dox percentage (d) is the ratio of the free Dox concentration compared to total Dox concentration at each time point. Protein coronas were separated from pharmacokinetic plasma samples (taken at 5 min, 1 h, 4 h, and 12 h) by anti-PEG scFv-based affinity chromatography (AfC) and quantified by Western blot analysis (n = 3) after normalization to the same amount of phospholipids (same number of recovered liposomes) determined by measuring the fluorescence of Dox. IgM (e, f), total C3 (g, h), and iC3b (i) deposited on the protein corona of prestimulated rats treated with H-Dox and L-Dox were calculated by ImageJ software and normalized by setting the intensity of the same band in the control plasma (1:2000 dilution) panel to 1. Data are shown as the means ± SDs. Statistical significance was evaluated by ordinary one-way ANOVA and two-way ANOVA (n.s. indicates nonsignificance, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
To delve into the mechanism by which a higher drug loading rate (in other words, with a lower dose of the nanocarrier) resulted in faster clearance with the same level of anti-PEG IgM, protein (especially opsonins) adsorption on the liposomal surface and the following effects were explored. Complement has been recognized as the pivotal opsonin. Generally, complement can be activated via three pathways: the classical pathway, lectin pathway and alternative pathway. All three pathways converge at the same point, factor C3 [23, 46, 47]. After activation, phagocytes can recognize liposomes quickly, thereby accelerating the clearance of liposomes by the MPS [48, 49]. Moreover, natural immunoglobulins (such as IgG and IgM) are classed as potent opsonins that can be used to determine the efficiency of C3 deposition on liposomes, thus modulating complement activation and even stimulating the immune response [50–54]. In our previous studies, natural IgM was found to be a negative regulator of the in vivo delivery of liposomes. Enhanced natural IgM adsorption on the liposomal surface led to accelerated clearance from and shortened circulation in rats [42, 55]. Since IgM and C3 are the key proteins affecting the pharmacokinetic parameters of liposomes, we focused on these two components of the protein corona. Protein coronas on the surface of liposomes in plasma at different time points (5 min, 1 h, 4 h, and 12 h) were collected by anti-PEG scFv-based affinity chromatography (AfC) (see Methods and Supplementary Fig. 4) [33] and then adjusted to have the same amount of phospholipids by measuring the fluorescence of Dox. The most abundant proteins in the characteristic bands in the stained SDS‒PAGE gel (Supplementary Fig. 5a) were identified as IgM, albumin, and IgG. The abundance of these three proteins (Supplementary Fig. 5b–d) in prestimulated rats treated with H-Dox was much higher than that in those treated with L-Dox. Specifically, IgM and C3 were quantified by Western blot analysis. As shown in Fig. 3e, f, the IgM gray values from prestimulated rats treated with H-Dox were also higher than those treated with L-Dox, which was consistent with the intuitive results of SDS‒PAGE. As shown in Fig. 3g, h, C3 showed the same tendency as IgM. Notably, phagocytosis is mediated by the binding of iC3b (on the protein corona of nanoparticles) to CR3 (on phagocytes). The results of the deposition of iC3b (Fig. 3i) were also in line with those of the other proteins, accounting for complement activation leading to accelerated blood clearance. Above all, more carriers (carriers with a lower Dox loading rate) could prolong blood circulation due to less opsonin (especially IgM and C3) deposition on the liposomal surface.
More carriers reduced the cutaneous accumulation of PLDs in the presence of anti-PEG Abs
The complement activation-related biological effects of nanoparticles, such as pseudoallergy, were considered to be closely associated with the deposition of adsorbed iC3b [56]. It was also found that the skin extravasation of PLDs was correlated with iC3b-related complement activation (unpublished). Considering that more carriers attenuated the adsorption of opsonins in a certain pool of plasma proteins, we further examined whether the extra carriers could reduce the cutaneous accumulation of PLDs in the presence of preexisting anti-PEG Abs. BALB/c mice were intravenously administered H-Dox and L-Dox at a Dox dose of 3 mg·kg−1 (30 and 150 mg·kg−1 HSPC) after stimulation as previously described. At 24 h after administration, red spots (Dox accumulation) on mouse skin were observed and recorded. The mice were then sacrificed, and the accumulation of Dox on the dorsal skin, ears and paws was quantified by LC‒MS/MS (see Methods). As shown in Fig. 4a, b, the number of red spots (Fig. 4c) and the incidence rate (Fig. 4d) in prestimulated mice treated with H-Dox were much higher than those in prestimulated mice treated with L-Dox. As expected, quantitative analysis of the concentration of Dox in skin tissue extracts (Fig. 4e–h) from different groups showed that prestimulated mice treated with L-Dox had significantly less Dox accumulation in skin. However, there were no significant differences between the H-Dox- and L-Dox-treated unstimulated groups in terms of Dox skin accumulation. Nevertheless, our results clearly revealed that more carriers (carriers with lower drug loading) reduced PLD-related Dox cutaneous accumulation in the presence of preexisting anti-PEG Abs, which are widespread in humans.
Fig. 4. Cutaneous accumulation of H-Dox and L-Dox in BALB/c mice with or without prestimulation with anti-PEG Abs.
a Red spots (red circles) indicate the accumulation of PLDs in dorsal skin. b Enlarged photos of the red spots on BALB/c mice prestimulated with empty sLips (2.3 mg·kg−1) followed by treatment with H-Dox and L-Dox. The numbers of dots (c) and incidence rates (d) in the H-Dox- or L-Dox-treated groups with or without prestimulation. Concentrations of Dox in the forepaw (e), hindpaw (f), ear (g), and dorsal (h) tissue extracts. Data are shown as the mean ± SD (n = 8–10). Statistical significance was evaluated by ordinary one-way ANOVA (n.s. indicates nonsignificance, *P < 0.05, **P < 0.01, ***P < 0.001).
PLDs with different Dox loadings exhibit little immunogenicity
To investigate the immunogenicity of different phospholipid doses with or without Dox encapsulation, empty sLips (20 and 100 mg·kg−1 HSPC) and PLDs (H-Dox and L-Dox, 2 mg·kg−1 Dox and equal to 20 and 100 mg·kg−1 HSPC, respectively) were injected into SD rats every 7 days for a total of 3 injections on Days 0, 7, and 14, and titers of anti-PEG IgM and IgG were detected on Day 0, 7, 14, and 21. As shown in Fig. 5a, b, e, f, for the empty sLips, the fewer carriers there were, the higher titers of anti-PEG IgM and IgG were detected in the 7-day period, respectively.
Fig. 5. Immunogenicity of H-Dox and L-Dox in SD rats.
Empty sLips (20 and 100 mg·kg−1 HSPC) and PLDs (H-Dox and L-Dox, 2 mg·kg−1 Dox, equal to 20 and 100 mg·kg−1 HSPC, respectively) were intravenously injected into the tail veins every 7 days for a total of 3 injections (Day 0, 7, and 14). Specific anti-PEG IgM (a–d) and IgG (e–h) were detected by ELISAs before and 7 days after each administration (Day 0, 7, 14, and 21). Data are shown as the means ± SDs (n = 4–5).
As shown in Fig. 5c, d, g, h, PLDs with different phospholipid doses displayed a homogenous immunogenicity effect with rather low titers of anti-PEG IgM and IgG, respectively, compared to empty sLips, which suggests that the immunogenicity of Dox loaded into PEGylated liposomes is weak. This might be because therapeutic doses of PLDs suppressed anti-PEG IgM production as a result of toxicity to marginal zone B cells, as there have been no reports that PLDs would be immunogenic or cause efficacy loss because of the ABC phenomenon [29, 57, 58]. Our results reconfirmed this and extended the conclusion to a PLDs with a higher dose of phospholipids.
H-Dox and L-Dox show equivalent antitumor effects, and L-Dox eases splenic augmentation in 4T1 tumor-bearing mice
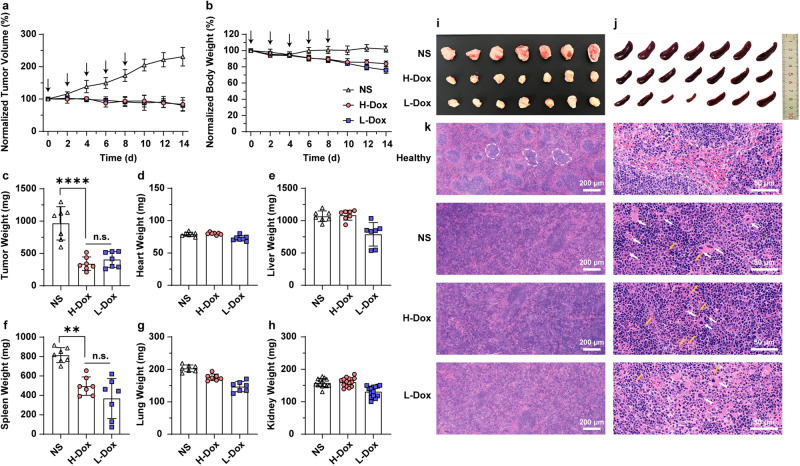
To investigate the antitumor effects and biosafety of liposomes with different Dox loadings, H-Dox, L-Dox and normal saline (NS) as a control were given to 4T1 subcutaneous tumor-bearing BALB/c mice at a dose of 2 mg·kg−1 Dox every 2 days for a total of 5 treatments (see Methods). The tumor volume (Fig. 6a) and body weight (Fig. 6b) variations in animals revealed that both PLDs showed equivalent antitumor effects. However, L-Dox treatment caused slightly more weight loss, which was specific during administration. Additionally, no significant differences were found in the appearances of the heart, lung or liver and routine blood data compared to the NS-treated group, which revealed that PLDs with different loading rates had no obvious toxicity (Supplementary Fig. 8).
Fig. 6. Pharmacodynamics and biosafety of H-Dox and L-Dox in 4T1 subcutaneous tumor-bearing BALB/c mice.
Tumor growth (a) and weight changes (b) in mice treated with NS and H-Dox and L-Dox (n = 7) at a dose of 2 mg·kg−1 Dox (20 and 100 mg·kg−1 HSPC) 5 times (10 mg·kg−1 Dox in total). The black arrows show the times of injection. The weights of the tumors (c), hearts (d), livers (e), spleens (f), lungs (g) and kidneys (h) were measured after the mice were sacrificed (n = 7, n = 14 for kidney). The appearance of the tumor (i) and spleen (j). k Hematoxylin-eosin staining of spleens from healthy mice and tumor-bearing mice treated with NS, H-Dox and L-Dox. Marginal zones are labeled with white dotted lines to distinguish red pulps from white pulps. Megakaryocytes are labeled with white arrows, and hemosiderin is labeled with yellow arrows. Data are shown as the means ± SDs. Statistical significance was evaluated by ordinary one-way ANOVA (n.s. indicates nonsignificance, **P < 0.01, ****P < 0.001).
After treatment and recovery, the tumor-bearing mice were sacrificed, and then the tumors and major organs were weighed. The tumor weights in both PLD-treated groups were reduced to half (400 mg) that in the control (1000 mg), as shown in Fig. 6c, i, while no significant differences or weight changes were found in the heart (Fig. 6d), liver (Fig. 6e), lung (Fig. 6g), or kidney (Fig. 6h). However, there were differences in spleen weight between the three groups (Fig. 6f). The spleen is the largest peripheral immune organ and plays important roles in tumor immunology. As tumors develop, the role of the spleen gradually changes from positive to negative immune regulation, which promotes the growth and metastasis of the tumor. Excessive enlargement of the spleen marks the stage of negative immune regulation, especially in solid tumor courses [59–61]. As shown in Fig. 6f, j (and the spleen indices in Supplementary Fig. 9), spleen augmentation was most obvious in the control group (800 mg), followed by the H-Dox group (500 mg) and the L-Dox group (400 mg), which would partly explain the extra weight loss in the L-Dox group. Moreover, to explore what changes occurred in the spleen structure in tumor-bearing mice, the spleens were sliced and stained with hematoxylin-eosin (HE). As shown in Fig. 6k, in the normal spleen, the stroma was composed mainly of a network of reticular connective tissue. This mesh provided support for blood cells and cells of the immune system (lymphocytes, macrophages, and dendritic cells). The parenchyma of the spleen was divided into two functionally and morphologically distinct compartments (red pulp and white pulp) by a tissue layer called the marginal zone. Outside the marginal zone is the perifollicular zone, which contains sheathed capillaries and blood-filled spaces without endothelial lining [62]. In the control and H-Dox groups, the normal physiological structures of the spleen were damaged, showing a lack of clear boundaries between red pulp and white pulp and more megakaryocytes (white arrows), white blood cells (violet nucleus) and red blood cells (red cytoplasm). However, the L-Dox group displayed milder structural changes. The reduced spleen augmentation in tumor-bearing mice indicated the feasibility of using PLDs with a low drug loading rate to ensure the relative preservation of spleen function.
PLDs with different drug loadings possess similar tissue and cell distribution profiles
The pharmacokinetics and biodistribution of the two PLDs were studied in 4T1 subcutaneous tumor-bearing mice. H-Dox and L-Dox were intravenously injected via the tail vein at a dose of 3 mg·kg−1 Dox (30 and 150 mg·kg−1 HSPC). The blood and the main organs were sampled at 1 h, 4 h, 8 h, and 24 h. L-Dox showed an extended circulation time (Fig. 7a), similar to the results in healthy rats (Fig. 2a). H-Dox and L-Dox also revealed similar distributions in the heart (Fig. 7b), lung (Fig. 7c), and kidney (Fig. 7d). Unexpectedly, L-Dox exhibited a greater distribution (approximately 2-fold at 4 h, 1.5-fold at 8 h, and 1.5- to 3-fold at 24 h) in the tumor (Fig. 7e), liver (Fig. 7f), and spleen (Fig. 7g). The increase in the number of particles of L-Dox would be responsible for the greater drug accumulation in certain tissues due to the prolonged circulation time along with increased blood concentration at each time point, offering more chances to enter tissues. These particles (approximately 80 nm) could be easily captured by macrophages, which are considered to lack metabolic capability compared to parenchymal cells [63, 64]. The more particles there were, the more time macrophages would take to degrade them or transfer them to other cells; thus, macrophage-rich tissues such as the liver, spleen and tumor displayed relatively slow rates of degradation.
Fig. 7. Pharmacokinetic profiles and tissue distribution of H-Dox and L-Dox in 4T1 subcutaneous tumor-bearing BALB/c mice.
Pharmacokinetic profiles (a) and Dox distribution in the heart (b), lung (c), kidney (d), tumor (e), liver (f), and spleen (g) in H-Dox- and L-Dox-treated 4T1 tumor-bearing BALB/c mice. Mice were treated with H-Dox and L-Dox at the same dose of 3 mg·kg−1 Dox (30 and 150 mg·kg−1 HSPC). Data are shown as the means ± SDs (n = 4). Statistical significance was evaluated by two-way ANOVA (n.s. indicates nonsignificance, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
To further confirm whether the extra carriers would be phagocytosed by macrophages, DiO-labeled liposomes were used as a marking tool to analyze phagocytosis by different cells (especially mononuclear phagocyte lines) in the blood, liver and spleen (see Methods). To compare the semiquantitative flow cytometry data, the DiO fluorescence of the two kinds of formulations was adjusted to the same level, while the HSPC concentration was adjusted to a 5-fold difference by adding 4 times the number of blank-sLips (without DiO labeling) to DiO-sLips (Supplementary Table 4).
As shown in Fig. 8a and 8e, both types of liposomes demonstrated a full cell proportion (near 100%) in Kupffer cells at each time point with an increasing mean fluorescence intensity (from 120 to 200). H-HSPC retained 4 times more carriers than L-HSPC, indicating that excess carriers at the current dosage did not saturate the Kupffer cells in the liver. Moreover, the higher the phospholipid dose was, the more phagocytosis by hepatic sinusoidal endothelial cells and hepatocytes was observed (Supplementary Fig. 10). This same result was observed in the spleen and blood, in which splenic macrophages (Fig. 8b, f) and dentritic cells (Fig. 8c, g) and peripheral monocytes (Fig. 8d, h) also showed slightly more phagocytosis. The additional capture by phagocytes would be conducive to the accumulation of Dox in relevant tissues.
Fig. 8. In vivo cellular uptake of carriers with different lipid concentrations in BALB/c mice.
Mice were treated with H-HSPC and L-HSPC (30 and 150 mg·kg−1 HSPC) with the same DiO fluorescence intensity. The mean variations in DiO fluorescence in Kupffer cells (a), splenic macrophages (b), splenic dendritic cells (c), and peripheral monocytes (d). Percentage of each cell type that took up the liposomes (e–h) at 1 h, 4 h, and 24 h. Data are shown as the means ± SDs (n = 3–4). Statistical significance was evaluated by one-way ANOVA (n.s. indicates nonsignificance, *P < 0.05, **P < 0.01).
Since the additional storage of Dox in such phagocytic tissues was just 1% (1 ng/mg versus 100 ng/mg) of that in the blood, the impact of Dox accumulation in these tissues seemed to be limited and controllable on our current research scale.
Discussion
In previous studies, more attention has been given to APIs in high-end formulations at the nanoscale because these formulations were constructed for the sake of delivering drugs to their intended site. However, the potential biological effects of nanocarriers have been neglected in many cases. This absence may bring about consequences beyond our control. Therefore, the aim of our study was to go back and carefully investigate the biological effects of the nanocarriers on the body with further consideration of the protein deposition on the liposomal surface, cellular phagocytosis, immunologic safety, etc. PLD was selected as our research object because it has a certain representativeness as the first liposomal drug to be launched, standing the test of nearly 30 years in the clinic [8].
In the present work, we prepared two populations of PLDs with high (H-Dox) and low (L-Dox) Dox loading to understand the biological effects of the liposomes. Considering that the loading rate would not significantly alter the tolerable dose of Dox, all experiments were performed with the same amount of Dox but different amounts of carrier (a 5-fold difference according to the difference in loading rate). The concentration of free Dox was very low in both populations of PLDs (less than 0.5% of the total Dox, which is close to the marketed PLD formulation), suggesting that the successful encapsulation of Dox could avoid the influence of free Dox in the following studies. In the investigation of pharmaceutical properties, including pharmacokinetics, pharmacodynamics and tissue distribution, there were three special points we would like to note (Fig. 9). First, these PLDs were extremely stable. Both PLDs showed similar, small amounts of leakage (<200 μg·mL−1, <2%) at each time point (Fig. 2c, d) in vivo, which was consistent with the results in vitro (<2% in 72 h) (Supplementary Fig. 1). Second, the presence of more carriers did not reduce Dox accumulation in tumors and thus affect efficacy. Generally, the structural basis of the enhanced penetration and retention (EPR) effect in tumors is the pores through the endothelial gaps with diameters between 100 nm and 2 μm [65, 66]. The size of liposomes (approximately 80 nm) is suitable so that they can seep through the pores and produce effects theoretically [67]; however, they might not be able to penetrate throughout the entire tumor and instead be distributed near the leaky blood vessels only as a result of the high tissue osmotic pressure in the tumor microenvironment, where a threshold of particles that could accumulate might exist. However, we did not witness Dox saturation in tumor sites (Fig. 7e) on the current scale. This might be because the prolonged circulation contributed to extra phagocytosis by phagocytic cells (Fig. 8), which are partly deficient in metabolic capacity, as we analyzed in the results. Third, there were some paradoxes that carriers bring about in terms of cell and tissue distribution. The Chan group previously discussed that an increase in the number of nanocarrier particles would result in decreased liver storage and enhanced tumor storage, and thus reinforced efficacy [68]. In their study, they used 9-fold more empty sLips as enhancers along with a therapeutic dose of Caelyx® to treat 4T1 tumor-bearing mice, and the objective was to reduce uptake by increasing the number of particles that encounter the limited number of receptor binding sites on MPS cells (mainly Kupffer cells). However, in our study, the same amount of drug was distributed evenly across more carriers, and even when using more carriers, the phagocytic cells did not become saturated (Fig. 8). The possible reason for this was that in the previous study, they used inorganic molecules as tracers for demonstration in the liver, while there have been only pharmacodynamic studies on liposomes to verify the previous hypothesis, but an enormous gap was found between the actual behaviors of organic and inorganic carriers. In addition, as early as 1983 [69], the issue of blocking the MPS by adding blank conventional liposomes was raised and taken into consideration, as phospholipids are safe reagents for mainstream administration, and several studies reported that blocking the MPS with high doses of conventional liposomes increased the accumulation of subsequently injected nanoparticles at target sites [70]. However, the effects of empty sLips on nanoparticle internalization by target cells are still unclear.
Fig. 9.

Overall comparison of H-Dox and L-Dox in terms of their pharmaceutical properties (black) and biological effects (purple).
Even more striking, we made additional inquiries into the carrier effect in the presence of anti-PEG Abs and complement activation. All of the reported liposome-related adverse impacts on the body (drug leakage, ABC, HSR, etc.) could not transpire without a unifying key link: a specific protein adsorbed on the liposomes, among which the role of anti-PEG Abs and complement is the most nonnegligible [23, 46, 56]. Complement has been recognized as a key factor in immunogenicity and HSR (especially complement activation-related pseudoallergy, CARPA) in studies over the past 20 years [46, 56, 71]. In the present work, anti-PEG Abs were not found to trigger the sudden release of PLDs in vivo. There was no increase in free Dox (<200 μg·mL−1 and <2%) in the pharmacokinetic study at each time point (Fig. 3c, d) in the presence of preexisting anti-PEG Abs, which were previously reported by the Roffler group as culprits leading to the formation of the membrane attack complex (C5b-9) in the liposomal membrane, thus causing the rapid release of encapsulated Dox from the liposomes [72, 73]. In other words, our findings showed that preexisting Abs did not leakage from the PLDs in vivo. This discordance could be attributed to three reasons. (1) The way to mimic in vivo anti-PEG Abs was different in the two studies. Purified rat anti-PEG IgG was injected immediately before PLD injection in the previous study, while in our work, we prestimulated rats with empty sLips to allow them to undergo an immune process and produce anti-PEG Abs by themselves. (2) The separation of Dox in the liposomal form and free form was also not the same. According to the reference, Dox separation was performed by solid-phase extraction (SPE), which risks damage to the liposome due to their passage through the hydrophobic stationary phase, prompting the leakage and interception of carrier drugs, as we discussed in our previous work [35]. We developed a facile method to simultaneously measure the encapsulation and release of drugs in liposomes in biological medium by anti-PEG scFv, which introduces less bias to our results, especially with trace levels of free Dox. (3) The half-life of Dox is only 5 min. Once Dox is released, it is metabolized, and this form makes monitoring Dox over a long period of time difficult.
We also found that the increase in the number of carriers was able to counteract the ABC phenomenon. In our findings, L-Dox exhibited a moderate loss (approximately 15%) in Dox concentration, while H-Dox suffered a steep drop of nearly 50% at each time point (Fig. 3a) in prestimulated rats. Although there have been no explicit conclusions on the observed ABC phenomenon in patients due to the variety of therapeutic doses and physical conditions [47], the influence of anti-PEG Abs on Dox pharmacokinetics deserves more attention. In addition, L-Dox effectively reduced cutaneous toxicity. However, there were no significant differences between the H-Dox- and L-Dox-treated unstimulated groups (Fig. 4a) in terms of the skin accumulation of Dox, probably due to the limitations of the relatively low dose without repeated injections used and the inadequacy of the observation time. Our results still clearly revealed that a low Dox loading rate reduced cutaneous accumulation in the presence of preexisting anti-PEG Abs (Fig. 4e–h). It is notable that this could be theoretically closely related to complement activation, which anti-PEG Abs has a good chance of taking part in. However, this point has not been clarified directly in our work, but it merits further discussion in subsequent research. Back to view in its enlightenment on clinical practice, the application of PLDs with low Dox loading rate could reduce the risk of severe cutaneous accumulation in patients, possessing the potential to pave a new way for those who are forced to terminate treatment.
Nevertheless, there are also limitations in using PLDs as an entry point. Liposomes cannot fully represent all nanomedicines at the current research scale. Second, Dox has unique properties. The colloidal precipitation of ammonium Dox sulfate in PLDs is very certain [37], and the metabolism of Dox in vivo is too rapid to cause particular differences or changes in the pharmacological properties of the two different PLDs [74]. Moreover, Dox, as a cytotoxic drug, can kill immune cells (e.g., B cells), which restricts the exploration of its immunogenicity [29]. Third, the drug loading rate (from 2% to 10%) in our work does not cover a broad range. However, our findings can still shed light on the study of nanomedicines, as there are a few products that possess properties similar to PLDs (stable and long circulation) on the market, such as Onivyde® (PEGylated liposomal irinotecan) and Cynviloq® (paclitaxel polymeric micelles) [75]. Our results show that Dox is very stable when encapsulated in PLDs. The free form of Dox released in vitro versus in vivo is rather finite, and the detection of Dox can indicate the distribution of carriers to a certain extent. Moreover, ADRs (cutaneous toxicity, HSR, etc.) caused by nanomedicines are of common interest, and the related effects of carriers are worthy of independent and in-depth exploration [76]. Furthermore, our findings have unveiled the relationship between the amount of opsonin deposited on the protein corona and the pharmacokinetics and cutaneous toxicity, which would provide a new point of view for probing the mechanism of these ADRs.
However, precisely because the particular carrier (liposome), subjective drug (Dox), and limited drug loading rate in our research have issued singular interesting results, the necessity of translating specific preparations from bench to bedside is even more prominent. As the ideas of drug delivery continue to broaden and the synthetic means and technologies continue to display breakthroughs, the varieties of materials and carriers have exploded in the past decades. Currently, some policy makers have taken action and adopted specific debates on the study of specific nanomedicines. For instance, the FDA has introduced documentation guidance for liposomal drug products in industry in terms of chemistry, manufacturing, controls, pharmacokinetics, bioavailability and labeling [77]. In addition, there are 4 liposomal projects with special discussions, including biopharmaceutical studies on the pharmacokinetics and mass balance of liposomes, lipid-protein interactions, etc., which urge us to explore more questions in the design and clinical applications of drug delivery systems and continue to challenge and optimize the traditional views in this field.
Conclusion
In brief, we herein compared the pharmaceutical properties and biological effects of two PLDs with different drug loading rates. The in vivo drug release and pharmacokinetic profiles indicated that L-Dox and H-Dox were both stable in blood circulation, but L-Dox exhibited extended circulation time when exposed to high levels of anti-PEG Abs in plasma due to less opsonin being adsorbed on the surface of an equivalent number of carriers. Furthermore, we found that more carriers could alleviate cutaneous Dox accumulation and thus reduce the incidence of adverse skin reactions. Both H-Dox and L-Dox exhibited little immunogenicity with relatively low levels of anti-PEG IgM and IgG. In the pharmacodynamic study, although L-Dox showed a similar antitumor effect, it relieved spleen augmentation to some extent when compared with H-Dox. Moreover, L-Dox allowed more Dox to be stored in macrophage-rich tissues, such as the tumor, liver and spleen, and since the extra accumulation was only 1% of the blood concentration at 24 h, the impact of Dox accumulation in these tissues seemed to be limited and controllable.
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82273866, 82125035, 81973245) and Shanghai Education Commission Major Project (2021-01-07-00-07-E00081).
Data availability
The data that support the findings of this study are available within the paper and the supplementary information. All other data are available from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Contributor Information
Jun Qian, Email: qianjun@fudan.edu.cn.
Kuan Jiang, Email: jiangkuan@fudan.edu.cn.
Chang-you Zhan, Email: cyzhan@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01169-5.
References
- 1.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 2.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 3.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–8. doi: 10.1016/S0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriadis G, Wills EJ, Swain CP, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1:1313–6. doi: 10.1016/S0140-6736(74)90682-5. [DOI] [PubMed] [Google Scholar]
- 6.Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–8. doi: 10.4103/0973-1482.139267. [DOI] [PubMed] [Google Scholar]
- 7.Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71:2531–58. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Akhtar N, Mohammed SA, Singh V, Abdellatif AA, Mohammad HA, Ahad A, et al. Liposome-based drug delivery of various anticancer agents of synthetic and natural product origin: a patent overview. Pharm Pat Anal. 2020;9:87–116. doi: 10.4155/ppa-2019-0020. [DOI] [PubMed] [Google Scholar]
- 10.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 11.Maiti P. Drug-delivery vehicles and their efficiency toward cancer treatment. Nanomed (Lond) 2020;15:1637–40. doi: 10.2217/nnm-2020-0152. [DOI] [PubMed] [Google Scholar]
- 12.Kita K, Dittrich C. Drug delivery vehicles with improved encapsulation efficiency: taking advantage of specific drug–carrier interactions. Expert Opin Drug Deliv. 2011;8:329–42. doi: 10.1517/17425247.2011.553216. [DOI] [PubMed] [Google Scholar]
- 13.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–75. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 14.Gerecke C, Edlich A, Giulbudagian M, Schumacher F, Zhang N, Said A, et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11:267–77. doi: 10.1080/17435390.2017.1292371. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Park K. Biocompatibility issues of implantable drug delivery systems. Pharmacol Res. 1996;13:1770–6. doi: 10.1023/A:1016012520276. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Wang X, Li J, Nie Y, Liao G, Yu Y, et al. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don’t-Eat-Us” strategy. ACS Nano. 2019;13:13015–26. doi: 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Tang I, Wainberg ZA, Meng H. Safety considerations of cancer nanomedicine—a key step toward translation. Small. 2020;16:e2000673. doi: 10.1002/smll.202000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand W, Noorlander CW, Giannakou C, De Jong WH, Kooi MW, Park MV, et al. Nanomedicinal products: a survey on specific toxicity and side effects. Int J Nanomed. 2017;12:6107–29. doi: 10.2147/IJN.S139687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caracciolo G. Liposome-protein corona in a physiological environment: challenges and opportunities for targeted delivery of nanomedicines. Nanomedicine. 2015;11:543–57. doi: 10.1016/j.nano.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Zahednezhad F, Saadat M, Valizadeh H, Zakeri-Milani P, Baradaran B. Liposome and immune system interplay: challenges and potentials. J Control Release. 2019;305:194–209. doi: 10.1016/j.jconrel.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Onishchenko N, Tretiakova D, Vodovozova E. Spotlight on the protein corona of liposomes. Acta Biomater. 2021;134:57–78. doi: 10.1016/j.actbio.2021.07.074. [DOI] [PubMed] [Google Scholar]
- 22.Yang K, Reker-Smit C, Stuart MCA, Salvati A. Effects of protein source on liposome uptake by cells: Corona composition and impact of the excess free proteins. Adv Health Mater. 2021;10:e2100370. doi: 10.1002/adhm.202100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghimi SM, Hamad I. Liposome-mediated triggering of complement cascade. J Liposome Res. 2008;18:195–209. doi: 10.1080/08982100802309552. [DOI] [PubMed] [Google Scholar]
- 24.Yan X, Scherphof GL, Kamps JA. Liposome opsonization. J Liposome Res. 2005;15:109–39. doi: 10.1081/LPR-64971. [DOI] [PubMed] [Google Scholar]
- 25.Clogston JD. The importance of nanoparticle physicochemical characterization for immunology research: what we learned and what we still need to understand. Adv Drug Deliv Rev. 2021;176:113897. doi: 10.1016/j.addr.2021.113897. [DOI] [PubMed] [Google Scholar]
- 26.Luo R, Li Y, He M, Zhang H, Yuan H, Johnson M, et al. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int J Pharm. 2017;519:1–10. doi: 10.1016/j.ijpharm.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXILI/CAELYXI) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37:870–7. doi: 10.1016/S0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 28.Shafei A, El-Bakly W, Sobhy A, Wagdy O, Reda A, Aboelenin O, et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother. 2017;95:1209–18.. doi: 10.1016/j.biopha.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 29.DOXIL® (doxorubicin HCl liposome injection) for intravenous infusion, https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050718s029lbl.pdf (2007).
- 30.Lotem M, Hubert A, Lyass O, Goldenhersh MA, Ingber A, Peretz T, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475–80. doi: 10.1001/archderm.136.12.1475. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Lofchy L, Wang G, Gaikwad H, Fujita M, Simberg D. PEGylated liposomes accumulate in the areas relevant to skin toxicities via passive extravasation across “Leaky” Endothelium. ACS Nano. 2022;16:6349–58. doi: 10.1021/acsnano.2c00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151:201–15. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 33.Chu Y, Tang W, Zhang Z, Li C, Qian J, Wei X, et al. Deciphering protein Corona by scFv-based affinity chromatography. Nano Lett. 2021;21:2124–31. doi: 10.1021/acs.nanolett.0c04806. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Chu Y, Li C, Tang W, Qian J, Wei X, et al. Anti-PEG scFv corona ameliorates accelerated blood clearance phenomenon of PEGylated nanomedicines. J Control Release. 2021;330:493–501. doi: 10.1016/j.jconrel.2020.12.047. [DOI] [PubMed] [Google Scholar]
- 35.Tang W, Zhang Z, Li C, Chu Y, Qian J, Ying T, et al. Facile separation of PEGylated liposomes enabled by anti-PEG scFv. Nano Lett. 2021;21:10107–13. doi: 10.1021/acs.nanolett.1c03946. [DOI] [PubMed] [Google Scholar]
- 36.Choi WG, Kim DK, Shin Y, Park R, Cho YY, Lee JY, et al. Liquid chromatography-tandem mass spectrometry for the simultaneous determination of doxorubicin and its metabolites doxorubicinol, doxorubicinone, doxorubicinolone, and 7-deoxydoxorubicinone in mouse plasma. Molecules. 2020;25:1254. [DOI] [PMC free article] [PubMed]
- 37.Wei X, Shamrakov D, Nudelman S, Peretz-Damari S, Nativ-Roth E, Regev O, et al. Cardinal role of intraliposome doxorubicin-sulfate nanorod crystal in doxil properties and performance. ACS Omega. 2018;3:2508–17. doi: 10.1021/acsomega.7b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen BM, Su YC, Chang CJ, Burnouf PA, Chuang KH, Chen CH, et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–6. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 40.Povsic TJ, Lawrence MG, Lincoff AM, Mehran R, Rusconi CP, Zelenkofske SL, et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol. 2016;138:1712–5. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Ding T, Guan J, Liu X, Wang J, Jin P, et al. Interrogation of Folic Acid-functionalized nanomedicines: the regulatory roles of plasma proteins reexamined. ACS Nano. 2020;14:14779–89. doi: 10.1021/acsnano.0c02821. [DOI] [PubMed] [Google Scholar]
- 42.Ding T, Guan J, Wang M, Long Q, Liu X, Qian J, et al. Natural IgM dominates in vivo performance of liposomes. J Control Release. 2020;319:371–81. doi: 10.1016/j.jconrel.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–24. doi: 10.1080/14686996.2019.1627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655–77. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163–75. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Tan LA, Yu B, Sim FC, Kishore U, Sim RB. Complement activation by phospholipids: the interplay of factor H and C1q. Protein Cell. 2010;1:1033–49. doi: 10.1007/s13238-010-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawanishi M, Hashimoto Y, Shimizu T, Sagawa I, Ishida T, Kiwada H. Comprehensive analysis of PEGylated liposome-associated proteins relating to the accelerated blood clearance phenomenon by combination with shotgun analysis and conventional methods. Biotechnol Appl Biochem. 2015;62:547–55. doi: 10.1002/bab.1291. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Yan X, Jacobson O, Sun W, Wang Z, Tong X, et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 2017;7:319–28. doi: 10.7150/thno.18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci USA. 2017;114:E10871–E80. doi: 10.1073/pnas.1713390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blandino R, Baumgarth N. Secreted IgM: new tricks for an old molecule. J Leukoc Biol. 2019;106:1021–34. doi: 10.1002/JLB.3RI0519-161R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadjidemetriou M, McAdam S, Garner G, Thackeray C, Knight D, Smith D, et al. The human in vivo biomolecule Corona onto PEGylated liposomes: a proof-of-concept clinical study. Adv Mater. 2019;31:e1803335. doi: 10.1002/adma.201803335. [DOI] [PubMed] [Google Scholar]
- 53.Ju Y, Kelly HG, Dagley LF, Reynaldi A, Schlub TE, Spall SK, et al. Person-specific biomolecular Coronas modulate nanoparticle interactions with immune cells in human blood. ACS Nano. 2020;14:15723–37. doi: 10.1021/acsnano.0c06679. [DOI] [PubMed] [Google Scholar]
- 54.Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260–8. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan J, Shen Q, Zhang Z, Jiang Z, Yang Y, Lou M, et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982. doi: 10.1038/s41467-018-05384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neun B, Barenholz Y, Szebeni J, Dobrovolskaia M. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules. 2018;23:1700. [DOI] [PMC free article] [PubMed]
- 57.Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115:251–8. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–32. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- 59.Han Y, Liu Q, Hou J, Gu Y, Zhang Y, Chen Z, et al. Tumor-induced generation of splenic Erythroblast-like Ter-cells promotes tumor progression. Cell. 2018;173:634–48. doi: 10.1016/j.cell.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka K, Koga Y, Taniguchi K, Kamikaseda K, Nihashi Y, Nomoto K. T-cell recruitment from the thymus to the spleen in tumor-bearing mice. II. Functional characteristics of recruited T -cells. J Natl Cancer Inst. 1986;77:733–8. doi: 10.1093/jnci/77.3.733. [DOI] [PubMed] [Google Scholar]
- 61.Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4:eaau6085. [DOI] [PMC free article] [PubMed]
- 62.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 63.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 64.Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19:384–408. doi: 10.1038/s41423-021-00791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–92. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 68.Ouyang B, Poon W, Zhang YN, Lin ZP, Kingston BR, Tavares AJ, et al. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19:1362–71. doi: 10.1038/s41563-020-0755-z. [DOI] [PubMed] [Google Scholar]
- 69.Proffitt RT, Williams LE, Presant CA, Tin GW, Uliana JA, Gamble RC, et al. Liposoinal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science. 1983;220:502–5. doi: 10.1126/science.6836294. [DOI] [PubMed] [Google Scholar]
- 70.Liu T, Choi H, Zhou R, Chen IW. RES blockade: a strategy for boosting efficiency of nanoparticle drug. Nano Today. 2015;10:11–21. doi: 10.1016/j.nantod.2014.12.003. [DOI] [Google Scholar]
- 71.Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res. 2002;12:165–72. doi: 10.1081/LPR-120004790. [DOI] [PubMed] [Google Scholar]
- 72.Estape Senti M, de Jongh CA, Dijkxhoorn K, Verhoef JJF, Szebeni J, Storm G, et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Control Release. 2022;341:475–86. doi: 10.1016/j.jconrel.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 73.Chen E, Chen BM, Su YC, Chang YC, Cheng TL, Barenholz Y, et al. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano. 2020;14:7808–22. doi: 10.1021/acsnano.9b07218. [DOI] [PubMed] [Google Scholar]
- 74.Gabizon A. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–36. [DOI] [PubMed]
- 75.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, et al. To PEGylate or not to PEGylate: immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079. doi: 10.1016/j.addr.2021.114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liposome Drug Products: Chemistry, manufacturing, and controls; human pharmacokinetics and bioavailability; and labeling documentation, https://www.fda.gov/media/70837/download (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the paper and the supplementary information. All other data are available from the authors upon reasonable request.