Abstract
Mitochondria have been primarily considered intracellular organelles that are responsible for generating energy for cell survival. However, accumulating evidence suggests that mitochondria are secreted into the extracellular space under physiological and pathological conditions, and these secreted mitochondria play diverse roles by regulating metabolism, the immune response, or the differentiation/maturation in target cells. Furthermore, increasing amount of research shows the therapeutic effects of local or systemic administration of mitochondria in various disease models. These findings have led to growing interest in exploring mitochondria as potential therapeutic agents. Here, we discuss the emerging roles of mitochondria as extracellularly secreted organelles to shed light on their functions beyond energy production. Additionally, we provide information on therapeutic outcomes of mitochondrial transplantation in animal models of diseases and an update on ongoing clinical trials, underscoring the potential of using mitochondria as a novel therapeutic intervention.
Subject terms: Mitochondria, Drug development
Mitochondrial transplants: a novel approach to disease treatment
Mitochondria, known as the powerhouses of cells, are crucial for maintaining cellular balance. However, the methods by which cells release mitochondria into the extracellular space and how recipient cells utilize these mitochondria are still not fully understood. This study, by Joonho Suh and Yun-Sil Lee at Seoul National University in South Korea, explores the process of mitochondrial secretion and its potential therapeutic applications. The researchers reviewed the current understanding of mitochondrial secretion, focusing on the release of mitochondria as an entire organelle. They discovered that cells can secrete mitochondria via various methods, and these extracellular mitochondria can be absorbed by recipient cells to boost their functions. The study also emphasized the potential of using mitochondrial transplantation as a therapeutic strategy in various diseases. The authors concluded that understanding the methods of mitochondrial secretion and transfer could lead to new therapeutic strategies.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
Mitochondria are multifaceted organelles that perform various functions to regulate cellular homeostasis1. Despite being the most well-known site of energy production or the “powerhouse” of the cell, mitochondria play many other pivotal roles, such as controlling the biosynthesis of molecules needed for cell growth and regulation of apoptosis2, intracellular calcium level3, redox balance4, the immune response5, cell stemness6, and interorganelle communication7. Mitochondria are also highly dynamic organelles, continuously changing their shapes through fusion and fission events8,9. The coordinated remodeling of mitochondrial morphology is tightly coupled with the major mitochondrial functions listed above, and imbalances in mitochondrial dynamics lead to mitochondrial dysfunction and pathological conditions9. A notable feature of mitochondria is their ability to generate mitochondrial-derived vesicles (MDVs) that transport mitochondrial components to lysosomes10, endosomes11, or peroxisomes12 for communication13. Emerging evidence suggests that MDVs and mitochondria may also be involved in intercellular communication or systemic regulation of cellular function14–16, the mechanisms of which are currently under active investigation.
Cells release diverse membrane-bound vesicles into the extracellular space to eliminate or transfer specific compounds or communicate with other cells17. Depending on their size and biogenesis pathway, these extracellular vesicles (EVs) are subcategorized as exosomes (50–150 nm in diameter, multivesicular body-derived), ectosomes (less than 0.1 μm to several μm in diameter, plasma membrane-derived), microvesicles (0.1-1 μm in diameter), large oncosomes (>1 μm in diameter), apoptotic bodies (>1 μm in diameter, apoptotic cell-derived), migrasomes (0.5–3 μm in diameter, migrating cell-derived), and the newly identified exomers (<50 nm in diameter, biogenesis unclear)17–20. However, simple categorization based on size and biogenesis pathway does not fully reflect the heterogeneity of the cellular origins, cargos, and functions of EVs. In this regard, the relatively recent discovery of extracellular mitochondria and EVs containing mitochondrial components that are secreted by many cell types adds a new level of complexity to EV biology21. The characterization, sorting and secretory mechanisms, and biological effects of extracellular mitochondria and EVs containing mitochondrial components under physiological or pathological conditions are currently under intense research.
Accumulating evidence suggests that extracellularly secreted mitochondria are transferred to recipient cells to induce therapeutic responses, suggesting that exogenous supplementation with mitochondria isolated from proper donor cells or tissues could be a therapeutic strategy. Local or systemic delivery of isolated mitochondria or mitochondrial transplantation has shown promising outcomes in animal models under various conditions, and several clinical trials involving mitochondrial transplantation to treat myocardial ischemia, cerebral ischemia, or inflammatory muscle diseases have been initiated. Despite significant attention and efforts aimed at developing mitochondrial transfer/transplantation strategies, research on the mechanisms of mitochondrial transfer is still in its early stages, and many critical questions remain unanswered. Importantly, understanding the mechanisms and biological effects of the extracellular secretion and transfer of mitochondria in vivo, as well as the mechanisms of recipient cell contact and uptake of extracellular mitochondria, will aid in the selection of appropriate sources for mitochondrial isolation and improve target specificity for successful mitochondrial transplantation therapy with minimal adverse effects.
In the first part of the review, we will discuss the evidence, mechanism and outcomes of extracellular mitochondrial secretion, focusing on the release of whole mitochondria due to its relevance with mitochondrial transplantation therapy involving the isolation and administration of intact mitochondria. We will not discuss the release of selective mitochondrial components (mitochondrial proteins, lipids, RNAs and/or DNA) or other modes of intercellular mitochondrial transfer such as tunnelling nanotubes (TNTs) as EVs containing mitochondrial components and TNT-mediated mitochondrial transfer have been previously reviewed21–23. In the second part, we will review the therapeutic effects of mitochondrial transfer in animal models under pathological conditions and current updates on human trials involving mitochondrial transplantation.
Extracellular mitochondrial secretion and its biological effects
Evidence and mechanisms of extracellular mitochondrial secretion
Mitochondria have been reported to be secreted extracellularly by many cell types, including mesenchymal stem cells (MSCs), astrocytes, neural stem cells, platelets, adipocytes, hepatocytes, cardiomyocytes, endothelial progenitor cells, osteoblasts, and various cell lines (Table 1). In this section, we briefly discuss key evidence and major mechanisms of mitochondrial extrusion.
Table 1.
Evidence of extracellular mitochondrial secretion.
| Cell type (species) | Conditions | Secreted form | Mechanism | Biological effect | Methods to visualize mitochondrial transfer | Reference |
|---|---|---|---|---|---|---|
| MSCs (human) | Standard culture conditions | Vesicles containing mitochondria | NR |
Further investigation needed Possibly assists aerobic respiration of recipient cells |
Donor cell mitochondria labeled with the DsRed2-mito transgene Recipient cells unlabeled |
Spees et al., 200624 |
| MSCs (human) |
Standard culture conditions Coculture with macrophages |
Microvesicles or multivesicular bodies containing mitochondria | Undergo mitophagy and use ARMMs to release mitochondria |
Manage intracellular oxidative stress by unloading depolarized mitochondria Enter macrophages to enhance mitochondrial bioenergetics |
Donor cell mitochondria labeled with MitoTracker Red Recipient cell mitochondria labeled with MitoTracker Green |
Phinney et al., 201525 |
| MSCs (human) | Standard culture conditions | Vesicles containing mitochondria | NR | Enter monocyte-derived macrophages and enhance macrophage oxidative phosphorylation to promote phagocytosis and suppress proinflammatory cytokine secretion |
Donor cell mitochondria labeled with MitoTracker Red Recipient cell mitochondria labeled with MitoTracker Green |
Morrison et al., 201740 |
| MSCs (rat) | Standard culture conditions | Vesicles containing mitochondria, MFN2, and PGC-1α | Secretion increased after PGC-1α overexpression | Enter intestinal epithelial cells and promote mitochondrial fusion and biogenesis, thereby improving mitochondrial metabolism and intestinal barrier function |
Donor cells transfected with the RFP-mito plasmid Recipient cell mitochondria labeled with MitoTracker Red |
Zheng et al., 202135 |
| AdMSCs (mouse) | Standard culture conditions | Exosomes containing mitochondria and mtDNA | NR |
Enter alveolar macrophages and improve mitochondrial function Shift macrophages to anti-inflammatory phenotype |
Donor cell mitochondria labeled with MitoTracker Red Recipient cell mitochondria labeled with the HSP60 antibody |
Xia et al., 202241 |
| BMSCs (human) | Coculture with macrophages using transwell system | Vesicles containing mitochondria | NR | Enter macrophages and enhance phagocytosis |
Donor cell mitochondria labeled with MitoTracker Red Recipient cells stained for CD45 |
Jackson et al., 201666 |
| BMSCs (human) | Coculture with macrophages | Vesicles containing mitochondria | nSMase pathway | Enter macrophages and enhance phagocytosis |
Donor cell mitochondria labeled with MitoTracker Green Recipient cells unlabeled |
Ko et al., 202067 |
| BMSCs (human, mouse) |
Standard culture conditions Stimulation with mitochondrial stress-inducing or protective agents |
Vesicles containing mitochondria |
Further investigation needed Agents that affect mitochondrial dynamics and function change the size profiles of secreted vesicles |
Enter stressed chondrocytes and incorporate into host mitochondrial networks |
Donor cells express endogenous mitochondria-specific GFP (derived from PHaM mitoDendra2 mice) Recipient cell mitochondria labeled with MitoTracker Green |
Thomas et al., 202259 |
| Astrocytes (rat) |
Standard culture conditions Focal cerebral ischemia |
Vesicles containing mitochondria | CD38/cADPR/calcium signaling | Enter neurons to promote neuronal survival |
Donor cell mitochondria labeled with MitoTracker Red Recipient cell mitochondria labeled with CellLight Mitochondria-GFP |
Hayakawa et al., 201626 |
| Astrocyte (mouse) | Antidepressant-like effect through the stimulation of sigma-1 receptor | Mitochondria (free or vesicle-enclosed not specified) | Increased CD38 expression | Support neuronal function |
Donor cell mitochondria labeled with MitoTracker Red Recipient cells unlabeled |
Wang et al., 202068 |
|
Brain tissue (mouse, human) Fibroblasts (human) |
Down syndrome Mitochondrial damage in vitro |
Mitovesicles (EVs of mitochondrial origin) |
Mitochondrial damage Mitophagy-independent |
May serve as a biomarker to evaluate brain mitochondrial dysfunction in neurodegenerative disorders May eliminate detrimental mitochondrial components from the cell |
Mitochondrial transfer NR | D’Acunzo et al., 202169 |
| Neural stem cells (mouse) |
Standard culture conditions Multiple sclerosis (mouse model) |
Vesicles containing mitochondria Free mitochondria |
NR | Enter mononuclear phagocytes, fuse with the endogenous mitochondrial network, restore oxidative phosphorylation and reduce the expression of proinflammatory markers |
Donor cells constitutively express the mitochondrial MitoDsRed reporter Recipient cell mitochondria stained with MitoTracker Green |
Peruzzotti-Jametti et al., 202136 |
| Platelets (human) | Activated by thrombin |
Microparticles containing mitochondria Free mitochondria |
Actin polymerization independent of microtubules | Induce neutrophil proinflammatory responses through the generation of bioactive mediators (fatty acids, lysophospholipids, and mtDNA) |
Donor mitochondria labeled with MitoTracker Deep Red Recipient cell mitochondria labeled with CellTracker CMTPX |
Boudreau et al., 201416 |
| Platelets (human, mouse) |
Activated Coculture with MSCs |
Vesicles containing mitochondria Free mitochondria |
NR | Enter MSCs and activate de novo fatty acid synthesis and trigger the secretion of pro-angiogenic factors |
Donor mitochondria labeled with MitoTracker Green or isolated from C57BL/6Jsu9-dsRed2 transgenic mice that express RFP in mitochondria Recipient cells stained with WGA |
Levoux et al., 202137 |
| Adipocytes (mouse, human) | Obesity | Vesicles containing oxidatively damaged mitochondria | nSMase pathway | Enter cardiomyocytes and induce transient oxidative stress in cardiac tissue, thereby triggering an antioxidant response |
Donor cell mitochondria from mice that express a mitochondrion-localized Flag tag in adipocytes (adipo-mitoFlag mice) Recipient cardiomyocytes stained for cardiac troponin (CTN1) |
Crewe et al., 202138 |
| Adipocytes (mouse) | Obesity | Not specified, but likely free or EV-associated mitochondria | NR | Enter macrophages through heparan sulfates to regulate metabolic homeostasis |
Donor cell mitochondria from mice that express mitochondria-specific Dendra2 (mtD2 mice) Recipient macrophages labeled with CytoTracker Orange |
Brestoff et al., 202161 |
| Brown adipocytes, BAT (mouse) | Thermogenic stress | Vesicles containing damaged mitochondrial parts/MDVs | PINK1-dependent efflux of mitochondrial proteins into EVs | Negatively affect thermogenesis through AMPK activation when taken up by brown adipocytes |
Donor mitochondria labeled with MitoTracker Green Recipient brown adipocytes unlabeled |
Rosina et al., 202214 |
| Adipocytes (mouse) |
Lean Lard-HFD-induced obesity |
Vesicles containing mitochondria Free mitochondria |
NR |
Enter macrophages and limit the release of adipocyte mitochondria into blood (lean) Circulate systemically and are distributed to distant organs (Lard-HFD-induced obesity) |
Donor cell mitochondria from adipocyte-specific mitochondria reporter (MitoFat) mice Recipient cells unlabeled |
Borcherding et al., 202260 |
|
cFLIP-deficient MEFs Hepatocytes (mouse) |
TNFα stimulation (in vitro) Anti-Fas antibody treatment (in vivo) |
Free mitochondria |
Mitochondrial fragmentation Actin and tubulin polymerization Caspase activation |
Further investigation needed Possibly a source of antigens to trigger autoimmune diseases |
Mitochondrial transfer NR | Nakajima et al., 200831 |
|
Hepatocytes (rat) MEFs (mouse) |
LPS stimulation | Autophagosomal membranes surrounding mitochondria/mitochondrial components | Extrusion via the autophagy‒lysosome pathway | Activate polymorphonuclear leukocytes | Direct mitochondrial transfer not shown | Unuma et al., 201570 |
| Hepatocytes (human) | Obesity |
Microparticles containing mitochondria Free mitochondria (cellular origin unclear) |
NR | Induce proinflammatory responses via TLR9 activation | Direct mitochondrial transfer not shown | Garcia-Martinez et al., 201642 |
| Hepatocytes (mouse) | Chronic-plus-binge ethanol feeding | Microparticles containing mtDNA/possibly mitochondria | ER stress-dependent caspase-1 activation | Activate TLR9 and neutrophilia, thereby causing liver inflammation and hepatocyte injury | Direct mitochondrial transfer not shown | Cai et al., 201771 |
| Cardiomyocytes (mouse) |
Healthy Cardiac stress |
Exophers containing defective mitochondria | Autophagy-driven | Enter cardiac-resident macrophages through the phagocytic receptor MERTK for elimination |
Donor cardiomyocyte mitochondria labeled with mt-Keima through viral transduction Recipient macrophages stained for CD68 |
Nicolas-Avila et al., 202034 |
| Induced pluripotent stem cell-derived cardiomyocytes (human) | Short-term culture conditions | Vesicles containing mitochondria | NR | Improve mitochondrial bioenergetics in hypoxia-injured cardiomyocytes |
Donor mitochondria labeled with BacMam mitochondria-RFP Recipient cell mitochondria with BacMam mitochondria-GFP |
Ikeda et al., 202165 |
| C2C12 myotubes (mouse) | Iron deficiency | Vesicles containing mitochondria |
Possible alternative to mitophagy Independent of BNIP3 and BNIP3l |
Possibly an alternative or additional pathway to clear mitochondria under iron deprivation conditions Further investigation needed |
Mitochondrial transfer NR | Leermakers et al., 202072 |
| Airway MDRCs (human) |
Healthy Asthma |
Vesicles/exosomes containing mitochondria | NR | Enter CD4 + T cells and generate reactive oxygen species |
Donor mitochondria labeled with MitoTracker Green Recipient cells stained for CD4 or MitoTracker Red |
Hough et al., 201839 |
| Endothelial progenitor cells (human) | Brain endothelial damage induced by OGD | Vesicles containing mitochondria | NR | Enter brain endothelial cells and improve brain endothelial energetics, barrier integrity, and angiogenic function |
Donor cell mitochondria labeled with MitoTracker Red Recipient endothelial cells labeled with Rab5A-GFP |
Hayakawa et al., 201815 |
| Osteoblasts (mouse) | Osteogenically differentiated |
Vesicles containing mitochondria Free mitochondria |
Mitochondrial fragmentation CD38/cADPR signaling |
Promote the maturation of osteoprogenitors |
Donor GFP+ mitochondria isolated from Col1a1-Cre; Igs1CKI-mitoGFP/+ osteoblasts Recipient cell mitochondria labeled with MitoTracker Red |
Suh et al., 202327 |
|
FADD-deficient Jurkat cell line (human) L929 fibroblast line (mouse) |
TNF-α-induced necroptosis | Intact mitochondria | RIP1-dependent necroptosis |
Enter macrophages, inducing the secretion of proinflammatory cytokines Enter dendritic cells to induce dendritic cell maturation |
Donor mitochondria labeled with MitoTracker Green Recipient cells labeled with Phalloidin |
Maeda & Fadeel, 201443 |
| THP-1 monocytic cell line (human) | LPS stimulation |
Free mitochondria Microvesicles containing mitochondria |
NR | Trigger proinflammatory responses in endothelial cells | Direct mitochondrial transfer not shown | Puhm et al., 201973 |
|
PC12 cell line (rat) SH-SY5Y cell line HEK293 cell line HeLa cell line Skin fibroblasts (human) |
Mitochondrial stress induction |
Free mitochondria (majority) Vesicles containing mitochondria |
Alternative to mitophagy | Act as an alternative pathway to mitophagy to clear damaged mitochondria | Mitochondrial transfer NR | Choong et al., 202033 |
|
MDA-MB-231 cell line BT-549 cell line (human) |
Chemoresistant | Exosomes containing mitochondria | nSMase pathway | Enter sensitive cancer cells and increase chemoresistance by increasing mutant mtDNA levels | Donor or recipient cells transfected with plasmids containing a mitochondrion-targeted sequence containing RFP or GFP inserts to observe bidirectional mitochondrial transfer | Abad & Lyakhovich, 202274 |
| High-metastatic (A11) and low-metastatic (P29) Lewis lung carcinoma cell lines (mouse) | Coculture system | Vesicles containing mitochondria/mitochondrial components | nSMase pathway |
Enter low-metastatic cancer cells and stromal cells and possibly affect their metastatic ability and protumor activity, respectively Further investigation needed |
Donor or recipient cell mitochondria labeled with MitoTracker Deep Red or CellLight mitochondria-GFP to observe bidirectional mitochondrial transfer | Takenaga et al., 202175 |
| HeLa cell line (human) | Mitochondrial stress induction | Free mitochondria |
PINK1-Parkin-directed mitophagosome formation Autophagic secretion of mitochondria in the absence of mATG8-conjugation |
Activate the cGAS-STING innate immune pathway in recipient cells | Direct mitochondrial transfer not shown | Tan et al., 202232 |
AdMSCs adipose-derived mesenchymal stem cells (MSCs), AMPK adenosine monophosphate-activated protein kinase, ARMMs arrestin domain-containing protein 1 (ARRDC1)-mediated microvesicles, BAT brown adipose tissue, BMSCs bone marrow-derived MSCs, BNIP3 BCL2-interacting protein 3, BNIP3l BNIP3-like, cADPR cyclic adenosine diphosphate (ADP)-ribose, CD38 cluster of differentiation 38, cFLIP cellular FLICE-inhibitory protein, cGAS cyclic GMP-AMP synthase, ER endoplasmic reticulum, EV extracellular vesicle, FADD Fas-associated death domain, GFP green fluorescent protein, HFD high-fat diet, HK-2 human kidney-2, LPS lipopolysaccharide, MDRCs myeloid-derived regulatory cells, MDVs mitochondrial-derived vesicles, MEFs mouse embryonic fibroblasts, MERTK tyrosine-protein kinase Mer, MFN2 mitofusin 2, mtDNA mitochondrial DNA, NR not reported, nSMase neutral sphingomyelinase pathway, OGD oxygen-glucose deprivation, PGC-1α peroxisome proliferator activated receptor gamma coactivator 1 alpha, RFP red fluorescent protein, RIP receptor-interacting protein, STING stimulator of interferon genes, TNF tumor necrosis factor, WGA wheat germ agglutinin.
ARRDC1-mediated microvesicles
Extracellular release of mitochondria was reported as early as 200624 in human MSCs under normal conditions in vitro. Vesicles containing fluorescently labelled mitochondria were released by MSCs onto tissue culture plates and contacted the plasma membranes of nearby cells24. Since then, a number of reports have demonstrated that human, mouse, and rat MSCs actively release microvesicles containing mitochondria into the extracellular space (Table 1). Regarding the mechanism of mitochondrial secretion, Phinney et al. showed that under standard culture conditions, MSCs manage oxidative stress by extracellularly releasing depolarized mitochondria through arrestin domain-containing protein 1 (ARRDC1)-mediated microvesicles (ARMMs)25. Live cell imaging showed that mitochondria travel toward the cell periphery and are included in the outward budding blebs of the plasma membrane25. Electron microscopy confirmed the presence of microvesicles containing mitochondria in MSC-conditioned media25.
CD38/cADPR/calcium signaling
In addition to MSCs, astrocytes have been shown to secrete mitochondria into the extracellular space through a calcium-dependent mechanism (Table 1). In 2016, Hayakawa et al. reported the presence of extracellular particles (0.3-1.1 μm in diameter) containing functional mitochondria released from rat cortical astrocytes26. A high percentage of extracellular mitochondria-containing particles were β1-integrin- and CD63-positive and were released via CD38/cyclic ADP-ribose (cADPR)/calcium signaling in astrocytes26. Similarly, our group recently demonstrated that mature osteoblasts secrete CD63-positive mitochondria-containing vesicles (>0.2 μm in diameter) into the extracellular space partly through CD38/cADPR signaling27. CD38 is highly expressed in osteoblasts28 and has been suggested to play a critical role during bone formation, and Cd38-knockout mice exhibit an osteoporotic phenotype29,30. Our group showed that the Cd38 expression pattern in differentiating osteoblasts coincided with the pattern of mitochondrial secretion, and knockdown of Cd38 significantly impaired mitochondrial release27. However, whether CD38/cADPR signaling is specific to mitochondrial release or regulates mitochondrial secretion by other cell types requires further investigation.
Actin polymerization
Activated platelets can release functional mitochondria into the extracellular space through actin dynamics (Table 1). Boudreau et al. demonstrated that intact free mitochondria or microparticles containing mitochondria were present in the supernatant of thrombin-activated human platelets16. The group used actin polymerization inhibitors (cytochalasin B, D, E, or latrunculin A) or tubulin polymerization inhibitor (nocodazole) and found that the extrusion of free mitochondria and microparticles containing mitochondria was significantly decreased by the addition of actin inhibitors but not the tubulin inhibitor, suggesting that mitochondrial secretion requires intact actin but not microtubule dynamics16. However, the release of microparticles without mitochondria also significantly decreased in response to the actin inhibitors and not the tubulin inhibitor, suggesting that the mechanism may not be mitochondria-specific but may apply to microparticle secretion in general. The involvement of actin polymerization in mitochondrial extrusion was also demonstrated in cellular FLICE-like inhibitory protein (cFLIP)-deficient mouse embryonic fibroblasts (MEFs) stimulated with tumor necrosis factor alpha (TNF-α)31. Unlike in platelets, exposure to an actin polymerization inhibitor (cytochalasin D) or tubulin destabilizer (paclitaxel) impaired cytoplasmic vacuole formation and the subsequent secretion of free mitochondria by MEFs, indicating that both intact actin and tubulin dynamics are essential for mitochondrial release by MEFs31.
Changes in mitochondrial morphology
Specific alterations in mitochondrial morphology have been suggested as a mechanism leading to extracellular mitochondrial secretion27,31. Nakajima et al. showed that cytoplasmic vacuoles within cFLIP-deficient MEFs engulfed fragmented mitochondria but not elongated mitochondria and released them into the extracellular space in response to TNF-α stimulation, indicating that mitochondrial fragmentation is a prerequisite for their extracellular release31. Likewise, our group recently reported that mitochondrial fragmentation and donut formation, which actively produce MDVs, stimulated mitochondrial extrusion from osteoblasts27. We demonstrated that inducing mitochondrial fission and donut formation by knocking down Opa1, which mediates mitochondrial fusion, or overexpressing Fis1, which promotes mitochondrial fission and MDV formation8, significantly increased the extracellular release of mitochondria, while treatment with the mitochondrial fusion promoter M1 prevented mitochondrial secretion by osteoblasts27. These results indicate that mitochondrial dynamics may play direct and critical roles in mediating mitochondrial extrusion. Close examination of mitochondrial morphology in different cell types that secrete mitochondria will help determine whether this mechanism applies universally.
Secretory autophagy
Extracellular release of mitochondria may be a mitochondrial quality control (MQC) process alternative to mitophagy32–34. Nicolas-Avila et al. reported that healthy or stressed mouse cardiomyocytes ejected defective mitochondria into the extracellular space through LC3-positive membrane vesicles called exophers, which are distinct from classical EVs in that they are larger in size (3.5 0.1 μm in mean diameter), contain large organelles such as mitochondria and are driven by the autophagy machinery34. The group showed that extracellular secretion of damaged mitochondria through exophers was a mechanism of mitochondrial quality control34. In the same year, Choong et al. suggested that extracellular release of mitochondria was an alternative MQC system to maintain mitochondrial homeostasis in rat PC12 cells and several human cell lines33. In support of this conclusion, genetic deletion or knockdown of autophagy/mitophagy genes significantly increased mitochondrial secretion into the extracellular environment through direct budding from the plasma membrane to compensate for defective mitophagy33. Likewise, Tan et al. reported that during PINK1-Parkin-mediated mitophagy, damaged mitochondria could still be cleared (through extracellular secretion) without the mammalian ATG8 (mATG8)-conjugation system, which is a crucial step in autophagy that leads to lysosomal degradation32. The group suggested that mitochondria were extruded through the secretory autophagy pathway that releases secretory cargos within autophagosomes, which was supported by data showing that genetic inhibition of autophagosome formation or autophagosome-plasma membrane fusion decreased mitochondrial secretion32. Through biochemical analysis and proteinase K protection analysis of the EV fraction of ATG7-knockout HeLa cells, the group demonstrated that secreted mitochondria were present as free organelles and were not enclosed by EVs32. Overall, these findings indicate that different mechanisms of mitochondrial secretion exist in a variety of cell types. However, further investigation is necessary to determine whether the various mechanisms discussed above act in conjunction with each other or if there is a distinct molecular mechanism that is specific to the secretion of mitochondria other than general EV secretion.
Biological effects of extracellular mitochondria on target cells
Extracellularly secreted mitochondria have been shown to target recipient cells to modulate various metabolic, immune, or differentiation/maturation responses (Table 1). In the following section, we review the major biological effects of secreted mitochondria on recipient cells.
Metabolic effects
The most widely reported biological effects of extracellular mitochondria are their incorporation into recipient cells and subsequent regulation of metabolism, such as mitochondrial bioenergetics and the oxidative stress response. For instance, extracellular mitochondria secreted by MSCs are taken up by macrophages through phagocytosis and fuse with host mitochondria to enhance the macrophage oxygen consumption rate (mitochondrial respiration)25. MSC-derived extracellular mitochondria are also internalized by IEC-6 intestinal epithelial cells and fused with host mitochondria to improve mitochondrial function35. Hayakawa and colleagues demonstrated that extracellular mitochondria secreted by astrocytes entered neurons and enhanced intracellular ATP levels and neuronal viability26. Similarly, mitochondria extruded by neural stem cells were taken up by mononuclear phagocytes by endocytosis and integrated into the endogenous mitochondrial network to restore oxidative phosphorylation36. Furthermore, activated platelets secrete functional mitochondria that are subsequently incorporated into MSCs to stimulate the tricarboxylic acid (TCA) cycle and de novo fatty acid synthesis, thereby triggering the secretion of proangiogenic factors37. In addition to regulating mitochondrial bioenergetics in recipient cells, extracellular mitochondria have been shown to regulate the oxidative stress response. Small EVs containing oxidatively damaged mitochondria released by adipocytes were internalized into cardiomyocytes to induce a burst of reactive oxygen species (ROS), triggering a compensatory antioxidant response and protecting cardiomyocytes through hormesis38. Likewise, exosomes containing polarized mitochondria (MitoTracker Green-positive) secreted by proinflammatory human leukocyte antigen-antigen D related (HLA-DR)-positive airway myeloid-derived regulatory cells (MDRCs) were taken up by peripheral T cells, and MitoTracker Green-positive mitochondria were integrated into the host mitochondrial network, possibly affecting T-cell differentiation and function through ROS generation39.
Anti- or proinflammatory effects
In addition to modulating metabolism, extracellular mitochondria have been shown to trigger anti- or proinflammatory responses in recipient cells. The transfer of EVs containing mitochondria derived from MSCs to human macrophages enhanced phagocytosis and downregulated proinflammatory cytokine secretion by macrophages40. Similarly, mitochondria and mitochondrial DNA (mtDNA) transfer through exosomes released by adipose-derived MSCs restored mitochondrial integrity in macrophages and induced their shift to an anti-inflammatory phenotype by suppressing proinflammatory cytokine (IL-1β and TNF-α) secretion and upregulating anti-inflammatory cytokine (IL-10 and Arg-1) production41. The expression of proinflammatory genes (Il1β, Nos2, and Il6) was also significantly downregulated in mononuclear phagocytes in response to extracellular mitochondria released by neural stem cells36. Although further research is necessary, the reported anti-inflammatory effects of extracellular mitochondria appear to be secondary responses to improvements in mitochondrial integrity and function.
Secreted mitochondria have also been reported to stimulate proinflammatory responses in recipient cells. For example, extracellular mitochondria released by activated platelets were shown to act as endogenous substrates of secreted phospholipase A2 IIA (sPLA2-IIA), which hydrolyzes the mitochondrial membrane and leads to the generation of inflammatory mediators such as lysophospholipids, fatty acids, and mtDNAs that induce a proinflammatory response in neutrophils16. Furthermore, extracellular mitochondria or mtDNA enclosed by microparticles released from hepatocytes activate Toll-like receptor 9 (TLR9) and the proinflammatory response in lysozyme-expressing cells such as neutrophils, monocytes, and macrophages42. Damaged mitochondria released extracellularly by HeLa cells via the secretory autophagy pathway induced a proinflammatory response in recipient HeLa cells by activating the cGAS-STING pathway, possibly through mtDNA32. Based on these reports, mtDNA, which is a well-known and potent damage-associated molecular pattern (DAMP), appears to be largely responsible for the proinflammatory response triggered by extracellular mitochondria. Importantly, specific mechanisms that induce or prevent the proinflammatory effects of mtDNAs on recipient cells warrant further investigation.
Cell differentiation/maturation effects
Extracellular mitochondria have been shown to regulate target cell differentiation or maturation. Recently, Rosina and colleagues reported that brown adipocytes released EVs containing MDVs with damaged mitochondrial parts, which were incorporated into recipient brown adipocytes and activated AMP-activated protein kinase (AMPK) to suppress adipocyte differentiation and thermogenic potential by downregulating the expression of Cd36, Fabp4, and Ucp114. The group suggested that oxidized materials and high levels of AMP contained within EVs could trigger AMPK in target cells to prevent adipogenesis14. Regarding the effects of extracellularly secreted mitochondria on stimulating target cell maturation, mitochondria released by necroptotic, Fas-associated protein with death domain (FADD)-deficient Jurkat cells were engulfed by human monocyte-derived dendritic cells (MDDCs) and promoted their maturation, inducing the cell surface markers CD80, CD83, and CD8643. Furthermore, our group recently reported that mitochondria secreted from mature osteoblasts enhanced the osteogenic maturation of osteoprogenitor cells without affecting mitochondrial respiration through the delivery of cargo proteins27. We showed that the incorporation of intact extracellular mitochondria into recipient cells was not required for this effect as treatment with secreted mitochondria after a repeated freeze/thaw cycle did not abrogate the increase in the maturation of osteoprogenitors. Instead, exposure of extracellular mitochondria to proteinase K abolished their ability to stimulate osteoprogenitor maturation, indicating that specific proteins within mitochondria were responsible for this effect. Whether the proteins activate surface receptors or are taken up by target cells needs further investigation, but the results suggest a mechanism that does not involve direct metabolic effects mediated by the integration of intact mitochondria into recipient cells27.
Mitochondrial transplantation therapy
Therapeutic effects of mitochondrial transplantation on animal models
The beneficial effects of extracellularly secreted mitochondria on the metabolism and function of recipient cells indicate that exogenous supplementation with mitochondria could induce curative responses through similar mechanisms. As with those secreted extracellularly by donor cells, injected mitochondria have been shown to enter recipient cells to regulate target cell metabolism, inflammatory response, or their differentiation/maturation in vivo (Table 2). Through these effects, mitochondrial transplantation has been shown to repair damaged tissues, including but not limited to the heart, brain, spinal cord, liver, lungs, kidney, musculoskeletal tissues, and intestine, in animal models of critical illnesses (Table 2). Currently, several clinical trials are recruiting or selecting patients to investigate the effects of mitochondrial treatment on myocardial ischemia, cerebral ischemia, or inflammatory muscle diseases such as polymyositis and dermatomyositis (Table 3). Thus, we will briefly review the major therapeutic effects of mitochondrial transplantation on animal models of cardiac ischemia, cerebral ischemia, and decline in skeletal muscle function or mass. Descriptions of mitochondrial transplantation in animal models of other diseases are provided in Table 2.
Table 2.
Mitochondrial transfer in animal models.
| Tissue | Disease/Injury (animal model) | Intervention | Mitochondrial source | Mitochondrial target/distribution | Target/uptake mechanism | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Heart | Cardiac ischemia (Rabbits subjected to regional ischemia) | Injection of mitochondria directly into the ischemic zone | Nonischemic heart | Interfibrillar space near the epicardial surface | Distribution aided by interfibrillar separation after myocardial ischemia and myocardial contraction | Improved regional and global function recovery | McCully et al., 200944 |
| Autologous pectoralis major muscle | Cardiomyocytes |
Internalization within hours of transplantation Possible extracellular effects without internalization Viable mitochondria required |
Increased oxygen consumption rate, ATP production, and cardioprotective cytokine induction Enhanced postinfarct cardiac function |
Masuzawa et al., 201345 | |||
| Cardiac ischemia (Rabbits subjected to regional/global ischemia) | Infusion of mitochondria through the coronary artery (less invasive than direct injection into the ischemic zone) | Autologous liver | Widespread myocardial dispersion | Rapid widespread distribution of mitochondria throughout the myocardium |
Reduced infarct size Enhanced cardiac function in regionally ischemic hearts |
Cowan et al., 201676 | |
| Cardiac ischemia (Pigs subjected to regional ischemia) | Injection of mitochondria into the ischemic area | Autologous pectoralis major muscle | Cardiomyocytes | Internalization possibly through actin-dependent endocytosis |
Decreased infarct size Enhanced myocardial cell viability |
Kaza et al., 201746 | |
| Heterotopic heart transplantation (Mice subjected to heart transplantation) | Injection of mitochondria into the coronary ostium | Gastrocnemius muscle | Heart | Diffuse distribution |
Prolonged cold ischemia time Decreased neutrophil infiltration and necrosis Enhanced heart graft tissue viability and function |
Moskowitzova et al., 201977 | |
| Cardiac ischemia (Rats subjected to warm global ischemia) | Administration of mitochondria to the coronary artery through an aortic cannula | Autologous pectoralis major muscle |
Heart tissue Myocardial fibers |
Internalization possibly through actin-dependent endocytosis | Increased myocardial function and myocellular survival in diabetic heart | Doulamis et al., 202047 | |
| Cardiac ischemia (Pigs subjected to regional ischemia) | Preischemic intracoronary injection of mitochondria | Autologous pectoralis major muscle | Cardiac cells | Internalization possibly through actin-dependent endocytosis |
Increased global and regional myocardial function Decreased infarct size |
Guariento et al., 202078 | |
| Right heart failure (Piglets subjected to pulmonary artery banding) | Intramyocardial injection of mitochondria | Autologous gastrocnemius muscle | Cardiomyocytes | Internalization of viable mitochondria |
Preserved contractile function Decreased cardiomyocyte apoptosis and fibrosis |
Weixler et al., 202179 | |
| Myocardial infarction (Mice subjected to surgical ligation of LAD) | Intramyocardial injection of mitochondria-rich EVs | EVs from human iCM | Cardiomyocytes | Fusion of the EV lipid bilayer with the cell membrane to release mitochondria into the cytoplasm | Improved bioenergetics and mitochondrial biogenesis in the peri-infarct area | Ikeda et al., 202165 | |
| Retro-orbital injection of small EVs containing mitochondria | Small EVs from adipocytes | Cardiomyocytes | Integration into the host mitochondrial network |
Decreased infarct size Reduced cardiac hypertrophy Increased cardiac function |
Crewe et al., 202138 | ||
| Heart donated after circulatory death (Pediatric and neonatal pigs) | Ex situ heart perfusion of mitochondria | Autologous skeletal muscle | Heart |
Not specified A mechanism previously reported by the group |
Improved myocardial function and viability | Alemany et al., 202380 | |
| Sepsis (Rats subjected to cecal ligation and puncture procedure) | Tail vein injection of mitochondria | Pectoralis major muscle | Heart | Internalization into heart tissue |
Improved survival rate Enhanced mitochondrial function and biogenesis in the heart |
Mokhtari et al., 202381 | |
| Brain | Cerebral ischemia (Rats subjected to MCAO) |
Injection of mitochondria into the ischemic striatum Infusion of mitochondria into the femoral artery |
BHK-21 cell line |
Neurons Astrocytes Microglia |
Internalization may not be solely responsible for the effect Intact mitochondria required |
Restored motor performance Reduced infarct area and neuronal cell death |
Huang et al., 201648 |
| Intravenous injection of multipotent MSCs | Multipotent MSCs | Neural cells | Mitochondrial transfer through Miro 1-mediated TNTs | Improved recovery of neurological functions | Babenko et al., 201882 | ||
| Intracerebroventricular injection of mitochondria | Autologous pectoralis major muscle | Neurons | Distribution and internalization in penumbra areas |
Decreased oxidative stress and cell death Increased neurogenesis Decreased infarct volume |
Zhang et al., 201949 | ||
| Injection of mitochondria into the internal carotid artery |
Neuro-2a cell line Mouse neural stem cell |
Unspecified Mitochondria could not pass the blood‒brain barrier |
Internalization into host cells and possible separation of mitochondrial components |
Improved neurobehavioral deficits Reduced infarct size |
Xie et al., 202152 | ||
| Intracerebroventricular injection of mitochondria | Human umbilical cord-derived MSCs | Brain cells (possibly neurons and astrocytes) | Internalization into recipient cells |
Decreased infarct area Improved neurobehavioral functions |
Pourmohammadi-Bejarpasi et al., 202083 | ||
| Injection of mitochondria into the ischemic striatum | Astrocytes | Neurons | Internalization into recipient cells | Decreased infarct size | Lee et al., 202351 | ||
| Cerebral ischemia (Mice subjected to MCAO) | Intravenous infusion of mitochondria | Placenta |
Brain Peripheral organs (lung, liver, kidney, heart) |
Further investigation needed | Decreased infarct size | Nakamura et al., 202050 | |
| Traumatic brain injury (Rats subjected to weight-drop injury) | Stereotaxic injection of mitochondria into the right lateral ventricle | Human umbilical cord-derived MSCs | Brain cells | Internalization of intact mitochondria |
Improved motor function Increased brain cell survival Alleviated astrogliosis and microglia activation |
Bamshad et al., 202384 | |
| Parkinson’s disease (6-hydroxydopamine-induced rats) | Injection of mitochondria into the medial forebrain bundle |
PC12 cell line (allogeneic) Human osteosarcoma cybrids (xenogeneic) |
Neurons | Internalization into recipient cells |
Improved locomotion Decreased oxidative stress and dopaminergic neuron loss |
Chang et al., 201685 | |
| Intranasal infusion of mitochondria | Liver | Rostral migratory stream neurons | Internalization into recipient cells |
Improved rotational and locomotor behaviors and neuronal survival Decreased plasma inflammatory cytokine levels |
Chang et al., 202186 | ||
| Alzheimer’s disease (Amyloid-beta-injected mice) | Intravenous injection of mitochondria | HeLa DsRed2-mito cells |
Liver Possibly the brain |
Further investigation needed |
Improved cognitive performance Decreased neuronal loss and gliosis Increased liver mitochondrial activity |
Nitzan et al., 201987 | |
| Depression (LPS-injected mice) | Intravenous injection of mitochondria | Hippocampus | Possibly brain cells | Further investigation needed |
Attenuated depression-like behaviors Decreased oxidative stress and neuroinflammation |
Wang et al., 201988 | |
| Age-associated cognitive decline (Aged mice) | Tail vein injection of mitochondria | Liver of young mice | Possibly the brain and skeletal muscle | Further investigation needed | Improved cognitive and motor performance | Zhao et al., 202053 | |
| Injection of mitochondria into the hippocampus | Liver of young mice | Neural progenitors | Internalization into recipient cells |
Improved cognitive performance Enhanced neurogenesis |
Zhang et al., 202289 | ||
| Spinal cord | Spinal cord injury (Rats subjected to contusion) | Injection of mitochondria into the spinal cord |
PC12 cell line Soleus |
Brain macrophages, endothelial cells, pericytes, astrocytes, and oligodendrocytes |
Internalization into recipient cells May exert their effects extracellularly |
Partial recovery of acute bioenergetics No improvement in long-term functional recovery Further investigation needed |
Gollihue et al., 201890 |
| BMSCs | Neurons, astrocytes, macrophages |
Internalization into recipient cells BMSCs transfer mitochondria to neurons through gap junctions |
Enhanced locomotor functional recovery | Li et al., 201991 | |||
| Spinal cord injury (Rats subjected to compression) | Transplantation of mitochondria into the spinal cord using a microsyringe pump | Soleus | Extracellular spaces | Interstitial localization rather than internalization into recipient cells |
Improved locomotor functions Decreased mitochondrial fragmentation, apoptosis, inflammation, and oxidative stress |
Lin et al., 202292 | |
| Liver | Partial liver ischemia (Rats subjected to partial hepatic I/R injury) | Splenic injection of mitochondria | Liver | Hepatocytes | Internalization of intact mitochondria |
Decreased serum aminotransferase level Decreased hepatocyte death, oxidative stress, and tissue injury |
Lin et al., 201393 |
| Nonalcoholic fatty liver disease (Mice subjected to high-fat and high-cholesterol diet) | Intravenous injection of mitochondria | HepG2 cell line | Liver, lung, brain, muscle, and kidney | Internalization into various tissue cells |
Decreased serum aminotransferase and cholesterol levels Decreased lipid accumulation Increased energy production and hepatocyte function |
Fu et al., 201794 | |
| Acetaminophen-induced liver injury (Acetaminophen-injected mice) | Intravenous injection of mitochondria | HepG2 cell line | Hepatocytes, brain, lung, kidney, muscle | Internalization through endocytosis |
Increased energy supply Decreased oxidative stress and tissue injury |
Shi et al., 201895 | |
| Acetaminophen-induced liver injury (Acetaminophen-administered rats) | Injection of mitochondria into the subcapsular region of spleen | Rat MSC cell line | Hepatocytes | Internalization of intact mitochondria |
Improved liver structure Decreased serum aminotransferase level, cell death, and oxidative stress |
Ulger et al., 202196 | |
| Liver ischemia (Mice subjected to liver I/R injury) | Intravenous injection of EVs containing mitochondria | EVs from human umbilical cord-derived MSCs | Intrahepatic neutrophils | Internalization of functional mitochondria |
Improved liver I/R injury Decreased local neutrophil extracellular trap formation |
Lu et al., 202297 | |
| Lungs | Acute lung injury (LPS-induced mice) | Intranasal instillation of wild-type BMSCs | BMSCs | Alveolar epithelial cells | Mitochondria-containing microvesicles through connexin 43-containing gap junctional channels |
Increased alveolar ATP concentrations Increased mouse survival |
Islam et al., 201298 |
| Tail vein injection of exosomes containing mitochondrial components | Exosomes from AdMSCs | Alveolar macrophages |
Exosomes containing mitochondria and mtDNA Internalized through clathrin and caveolae-mediated endocytosis |
Alleviated lung damage through decreased inflammation and alveolar wall thinning Improved mitochondrial function and immune homeostasis of alveolar macrophages |
Xia et al., 202241 | ||
| Intratracheal injection of MSCs | MSCs | PMVECs | Mitochondrial transfer through TFAM-mediated TNTs | Alleviated LPS-induced lung edema and improved permeability barrier of PMVECs | Zhang et al., 202399 | ||
| Acute lung injury (Ischemia‒reperfusion injury-induced mice) | Pulmonary artery injection or aerosol delivery via the trachea (nebulization) of mitochondria | Gastrocnemius muscle | Lung alveoli and connective tissue |
Not specified Possible internalization through actin-dependent endocytosis |
Improved lung mechanics and decreased lung tissue injury | Moskowitzova et al., 2020100 | |
| Pulmonary fibrosis (Bleomycin-treated mice) | Tail vein injection of hMSCs or mitochondria isolated from hMSCs | hMSCs | Alveolar epithelial cells | Mitochondrial transfer through connexin 43-containing gap junctional channels | Mitigation of fibrotic progression through restoring mitochondrial function | Huang et al., 2021101 | |
| Kidney | Renal artery stenosis (Mice subjected to surgical placement of arterial cuff) | Intra-arterial injection of EVs containing mitochondria | EVs from STC-like cells isolated from pig kidneys | Tubular epithelial cells | Internalization of viable mitochondria |
Improved kidney perfusion and oxygenation Decreased fibrosis |
Zou et al., 2018102 |
| Diabetic nephropathy (Streptozotocin-induced rats) | Injection of mitochondria directly under the renal capsule | BMSCs |
Proximal tubular epithelial cells Renal tubules |
Internalization into recipient cells |
Improved histology of proximal tubules Improved structure of tubular basement membrane and brush border |
Konari et al., 2019103 | |
| Acute kidney injury (Rats subjected to renal I/R injury) | Injection of mitochondria into the renal artery | Pectoralis major muscle | Kidney | Further investigation needed |
Increased regenerative potential of renal cells Decreased renal cell death |
Jabbari et al., 2020104 | |
| Acute kidney injury (Pigs subjected to renal I/R injury) | Intra-arterial injection of mitochondria | Sternocleidomastoid muscle |
Kidney Tubular epithelium of cortex and medulla |
Internalization possibly through actin-dependent endocytosis | Increased renal function and tissue recovery | Doulamis et al., 2020105 | |
| Acute kidney injury (Mice subjected to renal I/R injury) | Tail vein injection of EVs containing mitochondrial components | EVs from BMSCs | Liver, lung, kidney, and spleen | Internalization into renal tubular epithelial cells and recover TFAM protein and mtDNA stability | Attenuated renal dysfunction, mitochondrial damage, and inflammatory response | Zhao et al., 2021106 | |
| Diabetic nephropathy (Streptozotocin-induced mice) | Tail vein injection of mitochondria-transferred macrophages | MSCs | Macrophages (artificially transferred in vitro) | Internalization into recipient cells (artificially transferred in vitro) |
Suppressed inflammatory response Improved kidney injury |
Yuan et al., 2021107 | |
| Skeletal muscle | Age-associate decline in muscle function (Aged mice) | Tail vein injection of mitochondria | Liver of young mice | Possibly brain and skeletal muscle | Further investigation needed | Enhanced skeletal muscle function (swim and rotarod test) | Zhao et al., 202053 |
| Acute limb ischemia (Tourniquet-applied mice) | Injection of mitochondria into hindlimb muscles | Gastrocnemius or vastus medialis muscles | Myofibers in soleus, vastus medialis, and gastrocnemius | Internalization into recipient cells |
Decreased infarct size Enhanced hindlimb function |
Orfany et al., 202054 | |
| Muscle injury (BaCl2-injected mice) | Injection of mitochondria-transferred C2C12 cells into the gastrocnemius muscle | C2C12 cell line | C2C12 cell line (artificially transferred in vitro) | Internalization via endocytosis (artificially transferred in vitro) | Improved muscle regeneration and function in response to injection of mitochondria-transferred C2C12 cells | Sun et al., 2022108 | |
| Tail vein injection of mitochondria | Liver | Damaged myofibers | Internalization of intact mitochondria (possibly through damaged myofiber membrane) |
Improved muscle regeneration Restored muscle function |
Alway et al., 202355 | ||
| Muscle atrophy (Dexamethasone-injected rats) | Injection of mitochondria into soleus muscles | Umbilical cord-derived MSCs | Soleus muscle |
Possible internalization into muscle-resident cells Further investigation needed |
Increased muscle mass | Kim et al., 202356 | |
| Bone/Cartilage | Bone defect (Rats subjected to cranial defect surgery) | Local treatment of mitochondria-transferred BMSCs | BMSCs | BMSCs (artificially transferred in vitro) | Internalization into recipient cells (artificially transferred in vitro) | Enhanced bone regeneration due to increased osteogenic activity of BMSCs | Guo et al., 2020109 |
| Osteoarthritis (Monosodium iodoacetate-induced rats) | Intra-articular injection of mitochondria into the right knee | L6 myoblast cell line | Chondrocytes (in vitro) | Internalized into recipient cells (in vitro) | Improved pain, cartilage destruction, and bone loss | Lee et al., 2022110 | |
| Bone defects (Mice subjected to cranial defect surgery) | Local treatment with extracellular mitochondria | Extracellular mitochondria released from mature osteoblasts | Osteoprogenitors (in vitro) |
Effect mediated by protein cargo within mitochondria (in vitro) Intact mitochondria may be unnecessary |
Enhanced bone regeneration | Suh et al., 202327 | |
| Intestine | Sepsis (Rats subjected to cecal ligation and puncture procedure) | Intravenous injection of vesicles containing mitochondria/mitochondrial proteins | Extracellular microvesicles from MSCs | Intestinal epithelial cells | Internalization into recipient cells and delivery of mitochondrial proteins, promoting mitochondrial fusion and biogenesis | Improved mitochondrial function and intestinal barrier function | Zheng et al., 202135 |
AdMSCs adipose-derived mesenchymal stem cells, BaCl2 barium chloride, BMSCs bone marrow-derived MSCs, EV extracellular vesicle, hMSCs human MSCs, iCM induced pluripotent stem cell-derived cardiomyocytes, I/R ischemia/reperfusion, LAD left anterior descending coronary artery, LPS lipopolysaccharide, MCAO middle cerebral artery occlusion, mtDNA mitochondrial DNA, PC12 adrenal pheochromocytoma, PMVECs pulmonary microvascular endothelial cells, STCs scattered tubular cells, TFAM transcription factor A, mitochondrial, TNTs tunneling nanotubes.
Table 3.
Clinical trials involving mitochondrial transplantation.
| Conditions | Interventions | Location | Mitochondria donor | Status | Year | NCT number |
|---|---|---|---|---|---|---|
|
Low ovarian reserve Poor quality oocytes |
Injection of mitochondria into oocytes | Hadassah University Hospital, Israel | Autologous granulosa cells | Withdrawn (phase 1/2) |
Start: 2012 Primary completion: 2015 Study completion: 2015 |
NCT01631578 |
| Infertility | Microinjection of mitochondria into oocytes as a complementary ICSI technique | IVI Valencia, Spain | Autologous ovarian stem cells | Completed (phase NA) |
Start: 2015 Primary completion: 2017 Study completion: 2017 |
NCT02586298 |
| Extracorporeal membrane oxygenation complication | Injection or infusion of mitochondria into the ischemic myocardium | Boston Children’s Hospital, USA | Autologous skeletal muscle of the chest wall | Recruiting (phase NA) |
Start: 2017 Estimated primary completion: 2024 Estimated study completion: 2025 |
NCT02851758 |
| Repetition failure | Injection of mitochondria into oocytes |
Location not specified Sponsored by Sun Yat-sen University |
BMSCs | Unknown |
Estimated start: 2018 Estimated primary completion: 2020 Estimated study completion: 2021 |
NCT03639506 |
|
Mitochondrial diseases Pearson syndrome |
Transplantation of mitochondria-transplanted hematopoietic stem cells | Sheba Medical Center Hospital-Tel Hashomer, Israel | Normal and healthy blood cells | Completed (phase 1/2) |
Start: 2019 Primary completion: 2021 Study completion: 2021 |
NCT03384420 |
| Cerebral ischemia | Infusion of mitochondria into the brain artery via microcatheter during reperfusion | Harborview Medical Center, USA | Autologous muscle tissue adjacent to the surgical access site | Recruiting (phase 1) |
Start: 2021 Estimated primary completion: 2024 Estimated study completion: 2024 |
NCT04998357 |
|
Polymyositis Dermatomyositis |
Intravenous administration of a single-dose of allogeneic mitochondria (PN-101) |
Seoul National University, Korea Soonchunhyang University Seoul Hospital, Korea Hanyang University Seoul Hospital, Korea |
Allogeneic umbilical cord-derived mesenchymal stem cells | Enrolling by invitation (phase 1/2) |
Start: 2021 Estimated primary completion: 2023 Estimated study completion: 2023 |
NCT04976140 |
|
Myocardial infarction Myocardial ischemia Myocardial stunning |
Intracoronary and intramyocardial injection of mitochondria and/or MSC-derived exosomes | Tehran University of Medical Sciences, Iran | MSCs for exosomes and autologous tissues for mitochondria | Recruiting (phase 1/2) |
Start: 2022 Estimated primary completion: 2023 Estimated study completion: 2024 |
NCT05669144 |
BMSC bone marrow-derived mesenchymal stem cell, ICSI intracytoplasmic sperm injection, NA not applicable.
Cardiac ischemia
The first therapeutic evaluation of mitochondrial transplantation in an animal model dates back to 2009 when McCully et al. demonstrated that directly injecting viable mitochondria isolated from a nonischemic heart into the ischemic zone of cardiac tissue promoted myocardial functional recovery and cell viability in rabbits subjected to ischemia/reperfusion (I/R) injury44. The authors emphasized that the isolated mitochondria must be fresh, viable, and respiration-competent to induce cardioprotective effects, since frozen mitochondria or separated mitochondrial components failed to do so44. Since then, McCully’s team has actively investigated mitochondrial transplantation therapy for cardiac ischemia in rabbits, pigs, and rats (Table 2). Masuzawa et al. reported that injecting mitochondria isolated from autologous skeletal muscle into the ischemic heart attenuated myocardial injury and improved cardiac function in rabbits subjected to I/R injury45. The group showed that the injected mitochondria were taken up by cardiomyocytes 2 hours after administration, and this uptake resulted in an increase in the oxygen consumption rate and ATP production, while also leading to a significant decrease in serum inflammatory markers45. Notably, the authors suggested that the transplanted mitochondria also exerted extracellular effects (without internalization by cardiomyocytes) as cardioprotection was apparent within 10 minutes of reperfusion when mitochondria were present in the interstitial space outside of cardiomyocytes45. The authors also highlighted the importance of transplanting viable mitochondria45. A few years later, McCully’s team reported similar findings in a porcine model of I/R, suggesting that injecting mitochondria derived from autologous skeletal muscle into the ischemic area of cardiac tissue improved myocardial cell viability46, and recently reported the cardioprotective effects of mitochondrial transplantation on diabetic rat hearts subject to warm global ischemia47.
Cerebral ischemia
Mitochondrial transplantation has also exerted beneficial effects on animal models of cerebral ischemia. In 2016, Huang et al. first demonstrated that local intracerebral or systemic intra-arterial injection of xenogenic hamster mitochondria (isolated from the BHK-21 hamster cell line) significantly rescued motor performance and attenuated infarct size and neuronal cell death in a rat ischemic stroke model induced by middle central artery occlusion (MCAO)48. The group detected the internalization of transplanted mitochondria into neurons and astrocytes but with low efficacy, suggesting that cellular uptake of exogenous mitochondria may not be necessary for their neuroprotective effects48. A few years later, Zhang et al. used the same rat stroke model of MCAO and showed that intracerebroventricular (ICV) injection of mitochondria isolated from autologous skeletal muscle led to their internalization by neurons, especially in the ischemic penumbra, thereby increasing ATP levels and neurogenesis while decreasing oxidative stress, apoptosis, reactive astrogliosis, and brain infarct volume49. Nakamura et al. also showed that in a mouse MCAO model, intravenous infusion of mitochondria isolated from cryopreserved placentas significantly reduced infarction volume50. The infused mitochondria crossed the blood‒brain barrier and were distributed in the ischemic brain and peripheral organs such as the lung, liver, kidney, and heart, the effects of which are yet to be determined50. Xie et al. and Lee et al. further confirmed the therapeutic potential of mitochondrial transplantation, which decreased infarct volume and improved cell survival in a rat MCAO model of stroke51,52.
Loss of skeletal muscle function or mass
The therapeutic effects of mitochondrial transplantation on skeletal muscle tissues have only recently been investigated. In 2020, Zhao et al. demonstrated that tail vein injection of mitochondria isolated from young mouse livers significantly improved skeletal muscle function in aged mice (18-month-old mice with compromised muscle function), which was assessed by the forced swimming test for muscular endurance and rotarod test for muscular coordination53. Mitochondrial treatment significantly increased pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, NADH dehydrogenase, and ATP levels in aged skeletal muscles while decreasing ROS levels53. In the same year, McCully’s group evaluated the therapeutic potential of mitochondrial transplantation in a mouse model of acute limb ischemia (ALI), which leads to loss of muscle viability and function54. The injection of mitochondria isolated from donor mouse skeletal muscle into the hindlimb muscles of ALI-induced mice resulted in their distribution within myofibers, a decrease in apoptosis, and an increase in hindlimb function and power, as measured by DigiGait54. Recently, Alway et al. showed that in a mouse model of muscle injury induced by BaCl2, injecting liver-derived mitochondria through the tail vein resulted in preferential uptake by injured myofibers, which was possibly facilitated by the damaged sarcolemma. This uptake significantly improved muscle regeneration, increased muscle weight and fiber size and restored maximal muscle force55. Kim et al. also reported that intramuscular injection of mitochondria obtained from human umbilical cord-derived mesenchymal stem cells (UC-MSCs) significantly increased muscle mass by 1.5-fold and reduced the expression of muscle-specific ubiquitin E3-ligases in a dexamethasone-induced rat model of muscle atrophy56. Although research on skeletal muscle-targeted mitochondrial transplantation is still in its early stage, the results thus far suggest that mitochondrial treatment is a promising therapeutic strategy to accelerate the recovery of muscle function and mass.
Clinical trials
The encouraging results of mitochondrial transplantation in animal models of diseases have led to several human trials. Here, we identified registered clinical trials based on the information provided by ClinicalTrials.gov with search terms for intervention/treatment, including “mitochondria transplantation” or “mitochondria injection”. At the time that this manuscript was revised, another review discussing clinical trials using mitochondrial transplantation has been published57. To date, two human trials involving mitochondrial transplantation have been completed, which include the microinjection of mitochondria into oocytes to treat infertility and the infusion of mitochondria-transplanted/augmented hematopoietic stem cells to treat Pearson syndrome (Table 3). More importantly, four trials involving systemic or tissue-specific injection of isolated mitochondria, which align more closely with the focus of this review, are currently recruiting or enrolling by invitation (Table 3). We will introduce these 4 clinical trials in the following paragraphs.
A clinical trial sponsored by Boston Children’s Hospital is recruiting participants to assess the effects of autologous mitochondrial transplantation on myocardial I/R injury and extracorporeal membrane oxygenation (ECMO) complications (NCT02851758). The mitochondria will be isolated from the skeletal muscle of the chest wall and administered to the ischemic myocardium by direct injection (for surgical reoperation subjects) or intracoronary infusion (for catheterization subjects). The outcomes will be determined by safety, improvements in ventricular function, and the ability to be removed from ECMO support.
A clinical trial held at the University of Washington is recruiting subjects to investigate the effects of autologous mitochondrial transplantation on brain ischemia for the first time (NCT04998357). The mitochondria will be isolated from muscle tissues adjacent to the surgical access site and will be infused into an artery in the brain by a microcatheter during standard-of-care endovascular reperfusion therapy. For the outcome measures, investigators will monitor adverse effects associated with mitochondrial infusion and check for a reduction in infarct volume.
A human trial in South Korea sponsored by Paean Biotechnology Inc. is enrolling by invitation to evaluate the safety, tolerability, and efficacy of the transplantation of PN-101 (mitochondria isolated from allogeneic UC-MSCs) in subjects with refractory polymyositis or dermatomyositis (NCT04976140). In mice, PN-101 was shown to reduce pathological inflammatory responses by blocking the nuclear factor kappa B (NFκB) signaling pathway58. The mitochondria will be administered intravenously, and the primary outcome will be measured by dose-limiting toxicity (DLT) and International Myositis Assessment and Clinical Studies Group-total improvement score (IMACS-TIS).
Finally, investigators at Tehran University of Medical Sciences are recruiting patients to determine the therapeutic effects of transplanting autologous mitochondria and/or MSC-derived exosomes on myocardial infarction, ischemia, and stunning (NCT05669144). Exosomes-only, mitochondria-only, exosomes plus mitochondria, or placebo will be administered through intracoronary and intramyocardial injection. The primary outcome measures include left ventricle ejection fraction and allergic reactions.
Conclusion and future perspectives
Over the past several years, it has become evident that mitochondria are spontaneously secreted by various cells into the extracellular space and are transferred to recipient cells (Fig. 1). The field of extracellular mitochondrial secretion and transfer has increasingly gained attention partly because (1) a significant advancement in imaging technology has led to a more qualitative way to detect extracellular mitochondrial release by different cell types; (2) the development of various mitochondrial reporter mice, such as PHaM mitoDendra259, C57BL/6Jsu9-dsRed237, adipo-mitoFlag38, MitoFat60, and Col1a1-Cre; Igs1CKI-mitoGFP/+27 (Table 1), which has allowed greater experimental and technical convenience in the analysis of mitochondrial secretion and transfer in vivo; and 3) the potential therapeutic applications because mitochondria released by donor cells have been shown to enter recipient cells to enhance mitochondrial bioenergetics and cellular functions. Similar to those secreted extracellularly, mitochondria that are transferred exogenously have been shown to regulate mitochondrial metabolism, the inflammatory response, or the differentiation and maturation of recipient cells. This regulation can occur through their integration into the host mitochondrial network, signaling by mitochondrial cargos, or other unspecified extracellular effects (Fig. 1). The promising outcomes of exogenous mitochondrial transfer in animal models of diseases have led to the development of clinical trials with mitochondrial transplantation-based therapeutic interventions. However, research on the mechanisms of the secretion and transfer of mitochondria is still in its early stages, and several critical questions remain to be answered in future investigations.
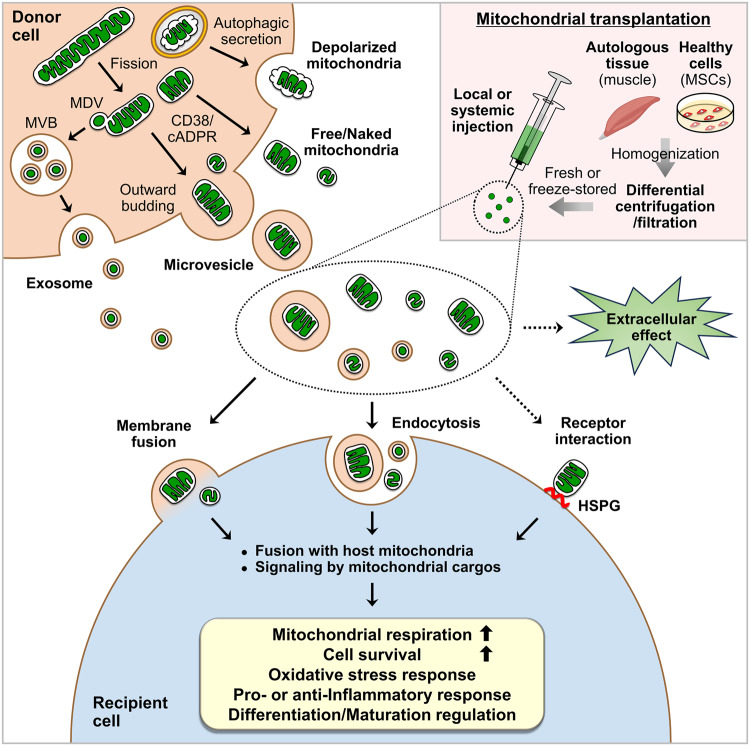
Fig. 1. Mitochondrial secretion, transplantation, and biological effects on target cells.
Donor cells extracellularly secrete microvesicles containing mitochondria through outward budding, exosomes containing mitochondrial-derived vesicles (MDVs) through the fusion of multivesicular bodies (MVBs) with the plasma membrane, free/naked mitochondria through an unclarified mechanism, or depolarized mitochondria through the secretory autophagy pathway. Mitochondrial fission and CD38/cADPR signaling have been suggested to mediate extracellular mitochondrial secretion. Mitochondrial transplantation involves the isolation of mitochondria from autologous tissues such as skeletal muscle or healthy cells such as mesenchymal stem cells (MSCs) via differential centrifugation or filtration methods and subsequent local or systemic administration. Although the administration of freeze-stored mitochondria has been described, the injection of freshly isolated mitochondria appears to be ideal. Mitochondria that are secreted extracellularly or introduced exogenously can be taken up by recipient cells through membrane fusion or endocytosis. Extracellular mitochondria may also interact with recipient cell surface receptors such as heparan sulfate proteoglycans (HSPGs) for uptake. Once inside the cells, the mitochondria integrate with the host mitochondrial network or activate signaling pathways mediated by their cargo. Although further investigation is needed, secreted or transplanted mitochondria have also been suggested to exert extracellular effects. Overall, these reactions elicit major biological effects on recipient cells, including increases in mitochondrial respiration and cell survival and the regulation of the oxidative stress response, the inflammatory response, and cell differentiation or maturation.
First, the molecular mechanisms specific to extracellular mitochondrial secretion are largely unknown. While CD38/cADPR signaling has been shown to promote extracellular mitochondrial release in astrocytes and osteoblasts26,27, whether it plays the same role in other cells that highly express CD38, such as immune cells, needs further examination. Future research could also focus on identifying additional pathways that commonly regulate mitochondrial secretion by different cell types. Furthermore, mechanisms that differentiate the secretion of free/naked mitochondria from vesicle-enclosed mitochondria also require additional investigation.
Second, it is still unclear whether there are mechanisms that allow extracellularly released mitochondria to target specific cell types. Recently, mitochondria released by adipocytes were shown to preferentially target macrophages among various clusters of cells in adipose tissues60, and cellular heparan sulfates were thought to act as receptors to mediate the specific uptake of extracellular mitochondria by macrophages61 and human HepG2 cells62 (Fig. 1). However, it is also possible that specific surface molecules or proteins are expressed on extracellularly secreted mitochondria to direct their interaction with the recipient cell membrane. Identifying these molecules may allow for the modification or genetic engineering of isolated mitochondria to improve the target specificity of mitochondrial transplantation therapies and minimize off-target effects.
Third, future research should focus on developing techniques to enhance the purity of isolated mitochondria for biological/biochemical analysis and exogenous delivery. While differential centrifugation and differential filtration are the most popular methods used to isolate mitochondria for therapeutic transfer because they are simple and quick processes, they likely also yield nonmitochondrial particles that may contribute to undesired effects. Flow cytometry-based sorting of mitochondria significantly increases purity and allows precise quantification63 but takes relatively longer to obtain a sufficient number of mitochondria and requires a specialized device, making it applicable for biochemical analysis of pure mitochondria but suboptimal for clinical situations where fresh mitochondria are expected to be rapidly isolated. The development of strategies to enhance the long-term storage of viable mitochondria for off-the-shelf use would partly overcome this limitation.
Finally, mitochondrial transplantation protocols, including methods, time point, frequency, and dose of administration, needs further establishment and optimization for long-term efficacy. Whether treatment with mitochondria plus other drugs can enhance mitochondrial transfer efficiency or function may also be investigated. The transplantation of cells that are naturally capable of mitochondrial transfer may prolong the effects of mitochondrial delivery and minimize the rejection of exogenous mitochondria. In this regard, in vivo transplantation of CD34+ hematopoietic stem and progenitor cells (HSPCs) augmented with normal exogenous mitochondria ex vivo induced long-term persistence (up to 4.5 months post transplantation) of exogenous mitochondrial transfer from HSPCs to myeloid and B cells in a mouse model of mitochondrial dysfunction64. Transplantation of CD34+ stem cells enriched with mitochondria is currently being examined in a clinical trial in pediatric patients with Pearson syndrome (Table 3). Additionally, whether artificially packaging mitochondria in vesicles for transplantation enhances mitochondrial stability or uptake may be tested in the future as mitochondria inside EVs have been suggested to be more resistant to the extracellular environment containing high levels of Ca2+ and oxidative stress than free isolated mitochondria65.
In conclusion, extracellular mitochondrial secretion, their transfer to recipient cells, and mitochondrial transplantation are increasingly gaining attention for their potential in a variety of therapeutic settings. Understanding the mechanisms and biological effects of the extracellular secretion and transfer of mitochondria, which still require extensive research, serves as a theoretical basis for the development of successful mitochondrial transplantation strategies. Therefore, future efforts should focus on unraveling the molecular and cellular mechanisms of extracellular mitochondrial secretion and transfer, as well as methods to improve the efficiency and efficacy of mitochondrial transplantation therapy.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants (NRF-2020R1A2C1010359, NRF-2018R1A5A2024418, and RS-2023-00246115).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Monzel AS, Enriquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5:546–562. doi: 10.1038/s42255-023-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science359, eaao6047 (2018). [DOI] [PubMed]
- 3.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 6.Dohla J, et al. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat Cell Biol. 2022;24:148–154. doi: 10.1038/s41556-021-00837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csordas G, Weaver D, Hajnoczky G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and signaling functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleele T, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–439. doi: 10.1038/s41586-021-03510-6. [DOI] [PubMed] [Google Scholar]
- 9.Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 10.Soubannier V, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 11.McLelland GL, Lee SA, McBride HM, Fon EA. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol. 2016;214:275–291. doi: 10.1083/jcb.201603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanty A, Zunino R, Soubannier V, Dilipkumar S. A new functional role of mitochondria-anchored protein ligase in peroxisome morphology in mammalian cells. J Cell Biochem. 2021;122:1686–1700. doi: 10.1002/jcb.30114. [DOI] [PubMed] [Google Scholar]
- 13.Chaiyarit S, Thongboonkerd V. Mitochondria-derived vesicles and their potential roles in kidney stone disease. J Transl Med. 2023;21:294. doi: 10.1186/s12967-023-04133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosina M, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;34:533–548 e512. doi: 10.1016/j.cmet.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa K, et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36:1404–1410. doi: 10.1002/stem.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudreau LH, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 18.Dixson AC, Dawson TR, Di Vizio D, Weaver AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 2023;24:454–476. doi: 10.1038/s41580-023-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25:24–38. doi: 10.1038/cr.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, et al. MitoEVs: A new player in multiple disease pathology and treatment. J Extracell Vesic. 2023;12:e12320. doi: 10.1002/jev2.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, et al. The existence and function of mitochondrial component in extracellular vesicles. Mitochondrion. 2020;54:122–127. doi: 10.1016/j.mito.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Sun Y, Qi Z, Cao L, Ding S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12:66. doi: 10.1186/s13578-022-00805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh J, et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 2023;35:345–360 e347. doi: 10.1016/j.cmet.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Sun L, et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 2002;16:302–314. doi: 10.1096/fj.01-0705com. [DOI] [PubMed] [Google Scholar]
- 29.Moridera K, et al. Skeletal unloading reduces cluster of differentiation (CD) 38 expression in the bone marrow and osteoblasts of mice. J Orthop Sci. 2020;25:331–337. doi: 10.1016/j.jos.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, et al. Disordered osteoclast formation and function in a CD38 (ADP-ribosyl cyclase)-deficient mouse establishes an essential role for CD38 in bone resorption. FASEB J. 2003;17:369–375. doi: 10.1096/fj.02-0205com. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima A, Kurihara H, Yagita H, Okumura K, Nakano H. Mitochondrial Extrusion through the cytoplasmic vacuoles during cell death. J Biol Chem. 2008;283:24128–24135. doi: 10.1074/jbc.M802996200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan HWS, et al. A degradative to secretory autophagy switch mediates mitochondria clearance in the absence of the mATG8-conjugation machinery. Nat Commun. 2022;13:3720. doi: 10.1038/s41467-022-31213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choong CJ, et al. Alternative mitochondrial quality control mediated by extracellular release. Autophagy. 2021;17:2962–2974. doi: 10.1080/15548627.2020.1848130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas-Avila JA, et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell. 2020;183:94–109 e123. doi: 10.1016/j.cell.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Zheng D, et al. Mesenchymal stem cell-derived microvesicles improve intestinal barrier function by restoring mitochondrial dynamic balance in sepsis rats. Stem Cell Res Ther. 2021;12:299. doi: 10.1186/s13287-021-02363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peruzzotti-Jametti L, et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021;19:e3001166. doi: 10.1371/journal.pbio.3001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levoux J, et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021;33:283–299 e289. doi: 10.1016/j.cmet.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Crewe C, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33:1853–1868 e1811. doi: 10.1016/j.cmet.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hough KP, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64. doi: 10.1016/j.redox.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison TJ, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia L, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12:2928–2947. doi: 10.7150/thno.69533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Martinez I, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-alpha-induced necroptosis act as danger signals. Cell Death Dis. 2014;5:e1312. doi: 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCully JD, et al. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296:H94–H105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuzawa A, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H966–H982. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaza AK, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153:934–943. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 47.Doulamis IP, et al. Mitochondrial transplantation for myocardial protection in diabetic hearts. Eur J Cardiothorac Surg. 2020;57:836–845. doi: 10.1093/ejcts/ezz326. [DOI] [PubMed] [Google Scholar]
- 48.Huang PJ, et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transpl. 2016;25:913–927. doi: 10.3727/096368915X689785. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, et al. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav Brain Res. 2019;356:322–331. doi: 10.1016/j.bbr.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y, Lo EH, Hayakawa K. Placental Mitochondria Therapy for cerebral ischemia-reperfusion injury in mice. Stroke. 2020;51:3142–3146. doi: 10.1161/STROKEAHA.120.030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EH, et al. Primary astrocytic mitochondrial transplantation ameliorates ischemic stroke. BMB Rep. 2023;56:90–95. doi: 10.5483/BMBRep.2022-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie Q, et al. Mitochondrial transplantation attenuates Cerebral Ischemia-Reperfusion Injury: Possible involvement of mitochondrial component separation. Oxid Med. Cell Longev. 2021;2021:1006636. doi: 10.1155/2021/1006636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Z, Yu Z, Hou Y, Zhang L, Fu A. Improvement of cognitive and motor performance with mitotherapy in aged mice. Int J Biol Sci. 2020;16:849–858. doi: 10.7150/ijbs.40886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orfany A, et al. Mitochondrial transplantation ameliorates acute limb ischemia. J Vasc Surg. 2020;71:1014–1026. doi: 10.1016/j.jvs.2019.03.079. [DOI] [PubMed] [Google Scholar]
- 55.Alway SE, et al. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J Cachexia Sarcopenia Muscle. 2023;14:493–507. doi: 10.1002/jcsm.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim, MJ., Lee, JM, Min, K & Choi, YS. Xenogeneic transplantation of mitochondria induces muscle regeneration in an in vivo rat model of dexamethasone-induced atrophy. J Muscle Res Cell Motil10.1007/s10974-023-09643-7. (2023) [DOI] [PubMed]
- 57.Kim JS, Lee S, Kim WK, Han BS. Mitochondrial transplantation: an overview of a promising therapeutic approach. BMB Rep. 2023;56:488–495. doi: 10.5483/BMBRep.2023-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu SH, et al. Human umbilical cord mesenchymal stem cell-derived mitochondria (PN-101) attenuate LPS-induced inflammatory responses by inhibiting NFkappaB signaling pathway. BMB Rep. 2022;55:136–141. doi: 10.5483/BMBRep.2022.55.3.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas MA, et al. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front Bioeng Biotechnol. 2022;10:870193. doi: 10.3389/fbioe.2022.870193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borcherding N, et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 2022;34:1499–1513 e1498. doi: 10.1016/j.cmet.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brestoff JR, et al. Intercellular Mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 2021;33:270–282 e278. doi: 10.1016/j.cmet.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kesner EE, Saada-Reich A, Lorberboum-Galski H. Characteristics of mitochondrial transformation into human cells. Sci Rep. 2016;6:26057. doi: 10.1038/srep26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDonald JA, et al. A nanoscale, multi-parametric flow cytometry-based platform to study mitochondrial heterogeneity and mitochondrial DNA dynamics. Commun Biol. 2019;2:258. doi: 10.1038/s42003-019-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacoby E, et al. Mitochondrial augmentation of CD34(+) cells from healthy donors and patients with mitochondrial DNA disorders confers functional benefit. NPJ Regen Med. 2021;6:58. doi: 10.1038/s41536-021-00167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikeda G, et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic Myocardium. J. Am Coll Cardiol. 2021;77:1073–1088. doi: 10.1016/j.jacc.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson MV, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko JH, Kim HJ, Jeong HJ, Lee HJ, Oh JY. Mesenchymal stem and stromal cells harness macrophage-derived amphiregulin to maintain tissue homeostasis. Cell Rep. 2020;30:3806–3820 e3806. doi: 10.1016/j.celrep.2020.02.062. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, et al. Activation of astrocytic sigma-1 receptor exerts antidepressant-like effect via facilitating CD38-driven mitochondria transfer. Glia. 2020;68:2415–2426. doi: 10.1002/glia.23850. [DOI] [PubMed] [Google Scholar]
- 69.D’Acunzo, P et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv7, eabe5085 (2021). [DOI] [PMC free article] [PubMed]
- 70.Unuma K, Aki T, Funakoshi T, Hashimoto K, Uemura K. Extrusion of mitochondrial contents from lipopolysaccharide-stimulated cells: Involvement of autophagy. Autophagy. 2015;11:1520–1536. doi: 10.1080/15548627.2015.1063765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai, Y et al. Mitochondrial DNA-enriched microparticles promote acute-on-chronic alcoholic neutrophilia and hepatotoxicity. JCI Insight2, e92634 (2017). [DOI] [PMC free article] [PubMed]
- 72.Leermakers PA, et al. Iron deficiency-induced loss of skeletal muscle mitochondrial proteins and respiratory capacity; the role of mitophagy and secretion of mitochondria-containing vesicles. FASEB J. 2020;34:6703–6717. doi: 10.1096/fj.201901815R. [DOI] [PubMed] [Google Scholar]
- 73.Puhm F, et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce Type I IFN and TNF responses in endothelial cells. Circ Res. 2019;125:43–52. doi: 10.1161/CIRCRESAHA.118.314601. [DOI] [PubMed] [Google Scholar]
- 74.Abad E, Lyakhovich A. Movement of Mitochondria with mutant DNA through extracellular vesicles helps cancer cells acquire Chemoresistance. ChemMedChem. 2022;17:e202100642. doi: 10.1002/cmdc.202100642. [DOI] [PubMed] [Google Scholar]
- 75.Takenaga K, Koshikawa N, Nagase H. Intercellular transfer of mitochondrial DNA carrying metastasis-enhancing pathogenic mutations from high- to low-metastatic tumor cells and stromal cells via extracellular vesicles. BMC Mol Cell Biol. 2021;22:52. doi: 10.1186/s12860-021-00391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cowan DB, et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One. 2016;11:e0160889. doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moskowitzova K, et al. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transpl. 2019;38:92–99. doi: 10.1016/j.healun.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guariento A, et al. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg. 2020;160:e15–e29. doi: 10.1016/j.jtcvs.2019.06.111. [DOI] [PubMed] [Google Scholar]
- 79.Weixler V, et al. Autogenous mitochondria transplantation for treatment of right heart failure. J Thorac Cardiovasc Surg. 2021;162:e111–e121. doi: 10.1016/j.jtcvs.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Alemany, VS et al. Mitochondrial transplantation preserves myocardial function and viability in pediatric and neonatal pig hearts donated after circulatory death. J Thorac Cardiovasc Surg.167, e6–e21 (2023). [DOI] [PubMed]
- 81.Mokhtari B, Hamidi M, Badalzadeh R, Mahmoodpoor A. Mitochondrial transplantation protects against sepsis-induced myocardial dysfunction by modulating mitochondrial biogenesis and fission/fusion and inflammatory response. Mol Biol Rep. 2023;50:2147–2158. doi: 10.1007/s11033-022-08115-4. [DOI] [PubMed] [Google Scholar]
- 82.Babenko, VA et al. Miro1 enhances mitochondria transfer from multipotent Mesenchymal Stem Cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules23, 687 (2018). [DOI] [PMC free article] [PubMed]
- 83.Pourmohammadi-Bejarpasi Z, et al. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res Bull. 2020;165:70–80. doi: 10.1016/j.brainresbull.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 84.Bamshad C, et al. Human umbilical cord-derived mesenchymal stem cells-harvested mitochondrial transplantation improved motor function in TBI models through rescuing neuronal cells from apoptosis and alleviating astrogliosis and microglia activation. Int Immunopharmacol. 2023;118:110106. doi: 10.1016/j.intimp.2023.110106. [DOI] [PubMed] [Google Scholar]
- 85.Chang JC, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res. 2016;170:40–56 e43. doi: 10.1016/j.trsl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Chang JC, et al. Intranasal delivery of mitochondria for treatment of Parkinson’s Disease model rats lesioned with 6-hydroxydopamine. Sci Rep. 2021;11:10597. doi: 10.1038/s41598-021-90094-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nitzan K, et al. Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in Alzheimer’s disease mice. J Alzheimers Dis. 2019;72:587–604. doi: 10.3233/JAD-190853. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, et al. Mitochondrial transplantation attenuates lipopolysaccharide- induced depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:240–249. doi: 10.1016/j.pnpbp.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, et al. Hippocampal mitochondrial transplantation alleviates age-associated cognitive decline via enhancing Wnt signaling and neurogenesis. Comput Intell Neurosci. 2022;2022:9325302. doi: 10.1155/2022/9325302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gollihue JL, et al. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma. 2018;35:1800–1818. doi: 10.1089/neu.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9:2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin MW, et al. Mitochondrial transplantation attenuates neural damage and improves locomotor function after traumatic spinal cord injury in rats. Front Neurosci. 2022;16:800883. doi: 10.3389/fnins.2022.800883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin HC, Liu SY, Lai HS, Lai IR. Isolated mitochondria infusion mitigates ischemia-reperfusion injury of the liver in rats. Shock. 2013;39:304–310. doi: 10.1097/SHK.0b013e318283035f. [DOI] [PubMed] [Google Scholar]
- 94.Fu A, Shi X, Zhang H, Fu B. Mitotherapy for fatty liver by intravenous administration of exogenous mitochondria in male mice. Front Pharmacol. 2017;8:241. doi: 10.3389/fphar.2017.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi X, et al. Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice. Transl Res. 2018;196:31–41. doi: 10.1016/j.trsl.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Ulger O, et al. The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats. Life Sci. 2021;279:119669. doi: 10.1016/j.lfs.2021.119669. [DOI] [PubMed] [Google Scholar]
- 97.Lu T, et al. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284:121486. doi: 10.1016/j.biomaterials.2022.121486. [DOI] [PubMed] [Google Scholar]
- 98.Islam MN, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang F, et al. TFAM-Mediated mitochondrial transfer of MSCs improved the permeability barrier in sepsis-associated acute lung injury. Apoptosis. 2023;28:1048–1059. doi: 10.1007/s10495-023-01847-z. [DOI] [PubMed] [Google Scholar]
- 100.Moskowitzova K, et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2020;318:L78–L88. doi: 10.1152/ajplung.00221.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang T, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci Adv. 2021;7:eabj0534. doi: 10.1126/sciadv.abj0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou X, et al. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep. 2018;8:1263. doi: 10.1038/s41598-018-19750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Konari N, Nagaishi K, Kikuchi S, Fujimiya M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9:5184. doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jabbari H, et al. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys. Acta Mol. Basis Dis. 2020;1866:165809. doi: 10.1016/j.bbadis.2020.165809. [DOI] [PubMed] [Google Scholar]
- 105.Doulamis IP, et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Ren Physiol. 2020;319:F403–F413. doi: 10.1152/ajprenal.00255.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao M, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. 2021;15:1519–1538. doi: 10.1021/acsnano.0c08947. [DOI] [PubMed] [Google Scholar]
- 107.Yuan Y, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1alpha activation. Stem Cells. 2021;39:913–928. doi: 10.1002/stem.3375. [DOI] [PubMed] [Google Scholar]
- 108.Sun J, et al. High-efficiency quantitative control of mitochondrial transfer based on droplet microfluidics and its application on muscle regeneration. Sci Adv. 2022;8:eabp9245. doi: 10.1126/sciadv.abp9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo Y, et al. Mitochondria transfer enhances proliferation, migration, and osteogenic differentiation of bone marrow mesenchymal stem cell and promotes bone defect healing. Stem Cell Res Ther. 2020;11:245. doi: 10.1186/s13287-020-01704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee AR, et al. Mitochondrial transplantation ameliorates the development and progression of Osteoarthritis. Immune Netw. 2022;22:e14. doi: 10.4110/in.2022.22.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]