Abstract
Leveraging the specificity of antibody to deliver cytotoxic agent into tumor, antibody-drug conjugates (ADCs) have become one of the hotspots in the development of anticancer therapies. Although significant progress has been achieved, there remain challenges to overcome, including limited penetration into solid tumors and potential immunogenicity. Fully human single-domain antibodies (UdAbs), with their small size and human nature, represent a promising approach for addressing these challenges. Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) is a glycosylated cell surface protein that rarely expressed in normal adult tissues but overexpressed in diverse cancers, taking part in tumorigenesis, progression, and metastasis. In this study, we investigated the therapeutic potential of UdADC targeting CEACAM5. We performed biopanning in our library and obtained an antibody candidate B9, which bound potently and specifically to CEACAM5 protein (KD = 4.84 nM) and possessed excellent biophysical properties (low aggregation tendency, high homogeneity, and thermal stability). The conjugation of B9 with a potent cytotoxic agent, monomethyl auristatin E (MMAE), exhibited superior antitumor efficacy against CEACAM5-expressing human gastric cancer cell line MKN-45, human pancreatic carcinoma cell line BxPC-3 and human colorectal cancer cell line LS174T with IC50 values of 38.14, 25.60, and 101.4 nM, respectively. In BxPC-3 and MKN-45 xenograft mice, administration of UdADC B9-MMAE (5 mg/kg, i.v.) every 2 days for 4 times markedly inhibited the tumor growth without significant change in body weight. This study may have significant implications for the design of next-generation ADCs.
Keywords: solid tumors, antibody-drug conjugate, CEACAM5, single-domain antibody, monomethyl auristatin E
Introduction
Antibody-drug conjugates (ADCs) are a class of promising cancer therapeutic agents that combines the specificity of antibodies and the toxicity of small-molecular payloads through a linker to kill tumor cells. Since Mylotarg was first approved by the United States Food and Drug Administration (FDA) in 2000, so far more than 10 ADCs have gained approval in clinical use [1, 2]. Nevertheless, multiple technical barriers remain for their applications in solid cancers, including tumor penetration, off-target toxicity and drug resistance, urging novel strategies to overcome these obstacles [3].
Target selection is one of the most critical components in ADC design [4]. An ideal target should be highly and exclusively expressed on the surface of tumor cells to maximize intracellular delivery of the payload while avoiding toxicities on healthy tissues. CEACAM5, also known as carcinoembryonic antigen-related cell adhesion molecule 5, is a glycosylated cell surface protein that rarely expressed in normal adult tissues but overexpressed in diverse cancers, including colorectal, pancreatic, lung, and gastric cancers, taking part in tumorigenesis, progression and metastasis [5, 6]. Since its initial discovery in 1965, CEACAM5 has been utilized as an excellent tumor marker for the diagnosis, prognosis and monitoring of various cancers. With rapid development in cancer immunotherapies, CEACAM5 have found new role as a promising therapeutic target in the design of ADCs [7–10], bispecific antibodies [11] and chimeric antigen receptor T cells (CAR-T) [12–14]. Notably, the ADC SAR-408701 developed by Sanofi has been advanced to phase III clinical trials, which is composed of a humanized antibody targeting CEACAM5 conjugated to a cytotoxic maytansinoid DM4 via a cleavable linker N-succinimidyl 4-(2-pyridyldithio) butyrate (SPDB) [7]. However, the clinical efficacy of SAR-408701 in phase I study is less satisfactory than expected, with dose-related toxicity constraining their therapeutic windows, possibly due to limited linker stability and payload toxicity, insufficient tumor penetration, as well as the emergence of anti-therapeutic antibodies [15]. These facts highlight the importance to develop other ADC therapies targeting CEACAM5.
In recent years, single-domain antibodies (sdAbs), which consist of only variable domain of mAb heavy chains, have attracted increasing attention due to their unique properties, such as high stability, low production cost and more accessible binding epitopes [16–18]. Moreover, compared with traditional antibodies, the smaller size of sdAbs allows them to penetrate into tissues more deeply and quickly. Our group have previously established a large phage-display library to identify fully human single-domain antibodies (UdAb) with low level of immunogenicity. Besides, we have demonstrated the superiority of UdAb-based ADC over conventional IgG-based ADCs in accumulation speed at tumor sites as well as tumor uptake and penetration efficiency [19]. In this study, we aim to investigate the therapeutic potential of UdADC targeting CEACAM5. To achieve this, we performed biopanning in our library and obtained an antibody candidate, B9, which bound potently and specifically to CEACAM5 protein and possessed excellent biophysical properties. The conjugation of B9 with a potent cytotoxic agent, monomethyl auristatin E (MMAE), exhibited superior antitumor efficacy in CEACAM5-expressing cell lines as well as in tumor-xenograft mice.
Materials and methods
Cell lines
The human pancreatic carcinoma cell line BxPC-3 and the Chinese hamster ovary cell line FCHO were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The human gastric carcinoma cell line MKN-45 was purchased from Procell Life Science & Technology Co., Ltd. (#CL-0292, Wuhan, China). The human colorectal cell line LS174T was obtained from Prof Wei-guo Hu’s lab (Fudan University, Shanghai, China). All cell lines were validated by short tandem repeat (STR) analysis. All cells were cultured using standard cell culture media as indicated by the providers in a humidified incubator at 37 °C with 5% CO2.
Screening of anti-CEACAM5 single-domain antibody from phage-display library
Panning fully human single-domain antibody phage-displayed library was carried out as described previously [19, 20]. Four rounds of antibody screening were performed on biotinylated human CEACAM5 protein (#11077-H08H-B, Sino Biological Inc, Beijing, China). The enrichment of antigen-specific phages after each round of panning was assessed by polyclonal enzyme-linked immunosorbent assay (ELISA). Positive clones were identified from the enriched rounds of panning using monoclonal ELISA.
Expression and purification of single-domain antibody and variant
The human single-domain antibody B9 was cloned into the pComb3x vector with N-terminal OmpA signal peptide (MKKTAIAIAVALAGFATVAQA) and C-terminal hexahistidine and Flag tag. Variants of B9 were generated using a site-directed mutagenesis kit (#11003ES10, Yeasen, Shanghai, China). The expression was performed in Escherichia coli (E. coli) HB2151 bacteria culturing at 37 °C for 2 h and 30 °C for 14 h under the induction of 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG, #10902ES60, Yeasen, Shanghai, China). The E. coli was harvested and resuspended by phosphate buffered saline solution (PBS) with 500 mM NaCl and disrupted by ultrasonication. The supernatant was obtained by centrifugation at 8000 r/min for 20 min and purified by Ni-NTA (#20503ES10, Yeasen, Shanghai, China) resin according to the manufacture’s introduction. For cysteine-variant B9-S84C, washing buffer and elution buffer were added with 1 mM tris(2-carboxyethyl) phosphine hydrochloride (TCEP, #ST045, Beyotine, Shanghai, China) to maintain free cysteines. The collected pure fractions were immediately buffer exchanged into PBS and concentrated using an Amicon ultra centrifugal concentrator (Merck Millipore Ltd, MA, USA) with a molecular weight cut-off of 3 kDa. Purity was evaluated by SDS-polyacrylamide gel electrophoresis, and the protein concentration was measured using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
ELISA
The CEACAM5 antigen was coated in 96 well half-area microplate (#3690, Corning, NY, USA) at 100 ng per well over night at 4 °C. After washing by three times of PBST (PBS with 0.05% Tween 20), the plate was blocked with PBS containing 3% skim milk (w/v, #36120ES76, Yeasen, Shanghai, China) for 1 h at 37 °C. Then, the plate was again washed by PBST for three times and incubated with 50 μL three-fold serially diluted antibody solutions for 1.5 h at 37 °C, followed by PBST washing for three times. The bound antibodies were detected with anti-FLAG-HRP (#A8592-1MG, Sigma-Aldrich Corp., St. Louis, MO, USA). The plate was washed with PBST for five times and added with 50 μL ABTS substrate (#002024, Thermo Fisher Scientific Waltham, MA, USA) for measurement of absorbance at 405 nm.
Size exclusion chromatography (SEC)
The antibody samples were prepared to a concentration of 0.2 mg/mL and injected onto the Superdex™ 200 10/300 GL column (GE Healthcare, San Diego, CA, USA) connected to a FPLC AKTA BASIC pH/C system (GE Healthcare, San Diego, CA, USA). The flow rate was 0.5 mL/min using PBS as the running buffer. Absorbance at 280 nm was monitored. Molecular mass determination was calculated by reference to a protein standard mix (Sigma-Aldrich Corp., St. Louis, MO, US).
Circular dichroism (CD) spectroscopic analysis
The CD spectra of antibodies were collected using a J-815 spectropolarimeter (Jasco International, Tokyo, Japan). Antibodies were prepared at a concentration of 0.25 mg/mL in PBS. The molar ellipticity signals were recorded at 216 nm while the solutions were heated from 20 °C to 100 °C at 1 °C intervals. The data analysis was performed by GraphPad Prism and the thermal denaturation (Tm) value was extrapolated by fitting the data to a Boltzmann sigmoidal.
Dynamic light scattering (DLS)
The B9 protein was prepared by centrifuging at 12,000 r/min for 10 min, filtering through a 0.22-μm filter (Merck Millipore Ltd, MA, US) and adjusting the concentration to 1 mg/mL. Then, the size distribution of protein particles was measured for three times in polystyrene cuvettes at 25 °C with a Zetasizer Nano ZSZEN 3600 (Malvern Instruments Ltd, Worcestershire, UN).
Bio-layer interferometry (BLI) binding assay
The binding kinetics of antibodies to CEACAM5 were measured by BLI on an Octet-RED96 (ForteBio, CA, USA). The biotinylated CEACAM5 antigen was loaded onto streptavidin-coated (SA) biosensors (#18-5019, Sartorius, Hamburg, Germany) at 10 μg/mL, followed by incubation with three-fold serially diluted antibodies starting at 3000 nM in PBST (PBS with 0.02% Tween 20) for 400 s for association, and then immersed into PBST for another 400 s for dissociation. All the curves were fitted by 1:1 binding model using the Data Analysis software 10.0. KD values were determined with R2 values of greater than 95% confidence level.
Cell binding by flow cytometry
To evaluate CEACAM5 expression on the surfaces of different cell lines, 1 × 106 logarithmic phase cells were incubated with Alexa Fluor 647 conjugated anti-human CEACAM5 mouse antibody (#392805, BioLegend, San Diego, CA, USA) for 0.5 h on ice, followed by washing with FPBS (PBS containing 2% fetal bovine serum) twice. To determine the binding capacity of B9 and B9-MMAE to these cell lines, 1 × 106 logarithmic phase cells were incubated with biotinylated antibodies at 200 nM for 1 h on ice, followed by washing with FPBS twice. Then, the cells were incubated with APC-conjugated streptavidin (#405207, BioLegend, San Diego, CA, USA) for 0.5 h on ice and washed three times with FPBS. Staining was analyzed by flow cytometry (Thermo Fisher Scientific, Waltham, MA, USA) and data analyses were performed with Flowjo 10.4.
Antibody conjugation
After adjusting the concentration of B9-S84C to 2 mg/mL, B9-S84C was reduced by 3 molar equivalents TCEP and 1 mM diethylene triamine pentaacetic acid (DTPA, #HY-B1335, MedChemExpress, New Jersey, USA) for 2 h at 25 °C. The reduced B9-S84C was then linked with 5 molar equivalents of vcMMAE (mc-vc-PAB-MMAE, #HY-15575, MedChemExpress, New Jersey, USA) at 25 °C for 1 h with gentle shaking. Buffer of conjugates was exchanged into PBS through overnight dialysis. The conjugation efficiency was analyzed using a high performance liquid chromatography time-of-flight mass spectrometry (HPLC-TOF/MS) system comprising of an Agilent 1290 Infinity II coupled to an Agilent 6230 LC/TOF mass spectrometer. Analytes were separated with an Agilent ZORBAX SB-C3 column. The mobile phase was composed of 0.1% formic acid (A) and acetonitrile (B) by using the gradient program as follows: 5%–70% B at 0–16 min, equilibration 5 min. The column was maintained at a temperature of 60 °C. The sample was injected at a volume of 5 μL and introduced into the mass spectrometer at a flow rate of 0.2 mL/min. The acquired HPLC-TOF/MS data were analyzed by Agilent MassHunter BioConfirm Software 10.0 and the average drug-to-antibody ratio (DAR) was determined based on peak areas.
In vitro cytotoxicity
Logarithmically growing cell lines MKN-45, BxPC-3, LS174T, and FCHO were seeded in a 96 well cell culture plate at 5000 cells/well for 12 h. After the culture supernatant was discarded, the 200 µL three-fold diluted UdADC was added to cells, with native antibody B9 and free payload vcMMAE used as control. The concentration of each group started from 1 µM and was repeated in triplicate. Cells were cultured for 72 h at 37 °C, then the culture medium was removed and 100 µL CCK8 solution (10 µL CCK8 + 90 µL medium) was added into each well, using CCK8 solution without cells as reference wells. After cells were incubated at 37 °C for 1–3 h, absorbance was measured at 450 nm using a 96-well microplate reader. Cell viability (%) = [(ODtreated – ODreference well)/(ODnon-treated – ODreference well)] × 100%.
Animal study
All of the procedures related to animal handling, care, and treatment were performed and approved by the ethics committee of the School of Basic Medical Sciences at Fudan University. In the ADC treatment efficacy experiment, BALB/c athymic (nu/nu) mice purchased from Bikai Co., Ltd (Shanghai, China) were selected to establish BxPC-3 and MKN-45 xenograft models. Cells (5 × 106) were suspended in PBS and Matrigel (1:1, v/v, #354234, Corning, NY, USA) at a volume of 100 µL and injected subcutaneously into the right flank of mice. In the BxPC-3 tumor model, mice were randomly divided into 3 groups and were injected intravenously with B9-MMAE (5 mg/kg), B9 alone (5 mg/kg) and the PBS blank control every 2 days for a total of 4 doses. The dosage frequency was determined based on our previous work of another UdADC [19]. In the MKN-45 tumor model, mice were randomly divided into 4 groups and intravenously injected with B9-MMAE (1 or 5 mg/kg), vcMMAE (0.41 mg/kg, equimolar to 5 mg/kg B9-MMAE) and the PBS blank control every 2 days for a total of 4 doses. Tumor volumes and body weights of mice were monitored every 2 days and tumor volumes were calculated using the formula, length × width2/2. At the end of the observation period, mice were scarified to record the tumor weights.
In the ADC safety experiment, healthy BALB/c mice were intravenously injected with B9-MMAE at 5 mg/kg or PBS every 2 days for four times. The body weights of mice were monitored every day. After the administration, blood was collected through the eye socket to analyze peripheral blood components (WBC, white blood cell; RBC, red blood cell; PLT, platelet; neutrophils, and lymphocytes) and parameters about liver and kidney functions (ALT, alanine aminotransferase; AST, aspartate aminotransferase; UA, uric acid; UN, urea nitrogen; CR, creatinine). After sacrificing the mice, the heart, liver, spleen, lung and kidney were fixed in 4% paraformaldehyde for hematoxylin and eosin (H&E) staining.
Statistical analyses
Statistical analyses were conducted using Prism software (Version 8.2.1, GraphPad Software, San Diego, CA, USA). Data were presented as mean ± SD from three independent experiments. Individual or multiple group comparisons were performed by the 2-tailed unpaired Student’s t test. Statistically significant difference was defined as P < 0.05 and different levels of significance were set as *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Isolation of a single domain antibody targeting CEACAM5
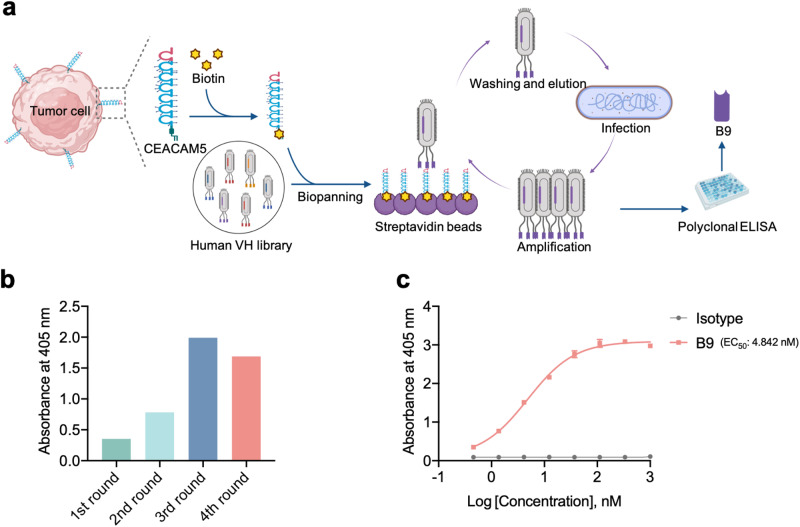
Previously, we have constructed a large phage-display single-domain antibody library by identifying a stable heavy-chain variable fragment as scaffold and then grafting diverse complementarity-determining regions from naïve human antibody library, whose quality has been validated by successful biopanning selection of candidates against multiple types of antigens, including viral spike proteins [20, 21] and tumor targets [19]. Aiming to develop novel antitumor biomolecules, in this study, we set out to isolate a CEACAM5-specific single-domain antibody (Fig. 1a). After four rounds of bio-panning against biotinylated human CEACAM5 protein, significant enrichment was observed by polyclonal phage ELISA (Fig. 1b) and a panel of seven genetically unique antibodies were selected for binding affinity detection by monoclonal ELISA. Finally, a single-domain antibody, designated as B9, was found to bind potently to CEACAM5 at low nanomolar concentration (EC50: 4.84 nM, Fig. 1c) as measured by enzyme-linked immunosorbent assay (ELISA), while the isotype control did not bind to the CEACAM5 antigen. Meanwhile, B9 did not exhibit any binding to two unrelated viral antigens, namely the receptor-binding domain of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the domain III of Zika virus envelope glycoprotein (Supplementary Fig. S1), confirming the binding specificity of B9 to the target antigen.
Fig. 1. Isolation of a human single-domain antibody targeting CEACAM5.
a The extracellular domain of CEACAM5 antigen that was expressed on the surface of tumor cells was biotinylated and used to biopanning in the human single-domain antibody library. b The phage enrichment during four rounds of bio-panning, as measured by polyclonal ELISA. c Binding of B9 to the CEACAM5 antigen, as measured by ELISA.
The candidate UdAb possesses favorable biophysical properties
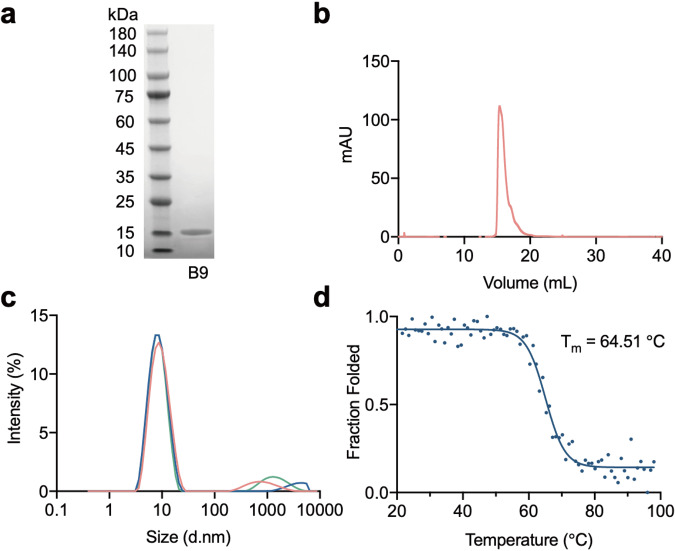
In recent studies, the superior stability of UdAbs has been well demonstrated by our and other groups [16, 19, 20]. Here, to characterize the biophysical properties of the selected candidate, we first examined the protein purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As shown in Fig. 2a, the B9 protein obtained from the E. coli expression system maintained a pure monomeric form in PBS buffer. This result was further confirmed by SEC (Fig. 2b). The aggregation propensity of B9 was assessed by DLS. As shown in Fig. 2c, at the concentration of 1 mg/mL in PBS buffer, the majority of B9 particles were distributed in a small range of diameters, indicating the prominent quality of aggregation resistance of B9. Besides, the thermal stability was evaluated according to the changes of circular dichroism ellipticity at 216 nm in response to increasing temperature. Results indicated that the midpoint transition (melting) temperature of B9 was 64.51 °C, comparable to the UdAbs we previously developed (Fig. 2d). Taken together, the low aggregation tendency, high homogeneity and thermal stability of B9 demonstrate its great potential for further biopharmaceutical applications.
Fig. 2. Biophysical characteristics of UdAb B9.
a SDS-PAGE shows the purity of the UdAb B9. b Size exclusion chromatography profile of B9. c Aggregation propensity of B9 as measured by DLS, three different colors represent three repeats. d Thermal stability of B9 as measured by CD spectroscopy.
UdAb B9 efficiently binds to CEACAM5-expressing cells
To further characterize the binding kinetics of B9 to CEACAM5, we performed the biolayer interferometry assay. The biotinylated CEACAM5 antigen was immobilized onto SA biosensors, followed by incubation with threefold antibody dilutions. According to the results, the equilibrium dissociation constant (KD) of B9 was 77.40 ± 2.55 nM with on-rate (kon) of 4.03 × 103 M−1·s−1 and off-rate (koff) of 3.12 × 10−4 s−1, indicating a slow-on and slow-off binding mode (Fig. 3a).
Fig. 3. Molecular and cellular binding profile of UdAb B9 to the CEACAM5 protein.
a Binding kinetics of the UdAb B9 to CEACAM5, as measured by BLI. b CEACAM5 expression levels on the surfaces of MKN-45, BxPC-3, LS174T, and FCHO cell lines, as determined by flow cytometry. c Binding of B9 and B9-MMAE to the surfaces of MKN-45, BxPC-3, LS174T, and FCHO cell lines, as determined by flow cytometry.
Next, we selected a panel of cell lines to identify the CEACAM5 expression on their surfaces with an Alexa Fluor 647 conjugated mouse anti-human CEACAM5 antibody by flow cytometry. In agreement with previously reported data [7, 9], the human gastric cancer cell line MKN-45 displayed the highest level of CEACAM5 expression, human pancreatic carcinoma cell line BxPC-3 and human colorectal cancer cell line LS174T possessed moderate levels of CEACAM5, while the Chinese hamster ovary cell line FCHO displayed no CEACAM5 expression on their surfaces (Fig. 3b). To further characterize the binding ability of B9 to these cells, logarithmic-phase cells were exposed sequentially to biotinylated B9 and APC-conjugated streptavidin. By analyzing APC fluorescence on the cells, it was found that B9 can bound to highest percent of MKN-45 cells, followed by BxPC-3 and LS174T, whereas no FCHO cells, which was in accordance with CEACAM5 expression levels on these cells (Fig. 3c).
Site-specific conjugation of MMAE to UdAb inhibited growth of CEACAM5-expressing human cancer cell lines
To endow the selected single-domain antibody with target cell killing functionality, we set out to prepare B9 into an antibody-drug conjugate format. Previously, we have investigated a novel site-specific conjugation strategy by introducing cysteine mutations at specific residues of UdAbs, which overwhelmed conventional random labeling of lysine side-chain amines in terms of conjugation quantifiability and homogeneity [22]. Here, based on the predicted structure of B9 by AlphaFold2 (Supplementary Fig. S2a) [23], we selected four sites in the framework 3 region of B9 (S62, S65, S70, and S84) that were out of the antigen binding surface. Considering the protein purity by SDS-PAGE, yields in E.coli expression system as well as binding affinity to CEACAM5 of different variants (Supplementary Fig. S2b, c), we finally chose the S84C mutant for further conjugation. As to the cytotoxic payload, we employed a classic tubulin inhibitor MMAE, which was linked to the antibody via the lysosomally cleavable dipeptide, valine-citrulline (vc) (Fig. 4a). The successful conjugation was validated by SDS-PAGE (Fig. 4b) and HPLC-TOF/MS (Fig. 4c), with the average DAR being 0.88 (Supplementary Fig. S3). The resulted UdADC, B9-MMAE, maintained the binding capacity to recombinant CEACAM5 protein, with similar EC50 (Fig. 4d) and binding kinetics (KD = 86.10 ± 1.48 nM, kon = 2.86 × 103 M−1·s−1, koff = 2.46 × 10−4 s−1, Fig. 4e) to the unconjugated antibody. Meanwhile, the conjugation of MMAE to the B9 antibody did not impact the binding to the surface of tumor cells mentioned above (Fig. 3c).
Fig. 4. In vitro cell cytotoxicity of the UdADC B9-MMAE.
a Schematic representation of the conjugation of UdAb B9 to the payload MMAE via the linker vc. b SDS-PAGE analysis of B9-S84C and B9-MMAE. c Deconvoluted HPLC-TOF/MS of B9-S84C and B9-MMAE. d Binding of the UdADC B9-MMAE to the CEACAM5 antigen, as measured by ELISA. e Binding kinetics of the UdADC B9-MMAE to the CEACAM5 antigen, as measured by BLI. f Cell cytotoxicity assays were performed on MKN-45, BxPC-3, LS174T, and FCHO cell lines, which were treated with the parent antibody B9, the free payload vcMMAE or the UdADC B9-MMAE.
Next, we evaluated the in vitro killing activity of the drug-conjugated antibody against cell lines with different levels of CEACAM5-expression on their surfaces, respectively. The non-conjugated antibody B9 and the free linker-drug vcMMAE were used as control. As shown in Fig. 4f, B9-MMAE displayed robust killing activity against MKN-45, BxPC-3, and LS174T cells in a dose-dependent manner, with estimated half-maximal inhibitory concentration (IC50) values at 38.14 nM, 25.60 nM, and 101.4 nM, respectively. By contrast, the native antibody B9 and free payload vcMMAE did not cause obvious cell toxicity. It is interesting that B9-MMAE showed slightly stronger cytotoxicity against BxPC-3 than MKN-45, although the latter possesses more antigen expression, implying the existence of other factors than antigen density that may contribute to the sensitivity to antibody-drug conjugates. For the CEACAM5- FCHO cells, no killing activity was observed with B9, free drug, or B9-MMAE treatment. These results demonstrate that the single-domain antibody-drug conjugate can deliver potent cell killing activity with target specificity.
UdADC possesses potent in vivo antitumor efficacy
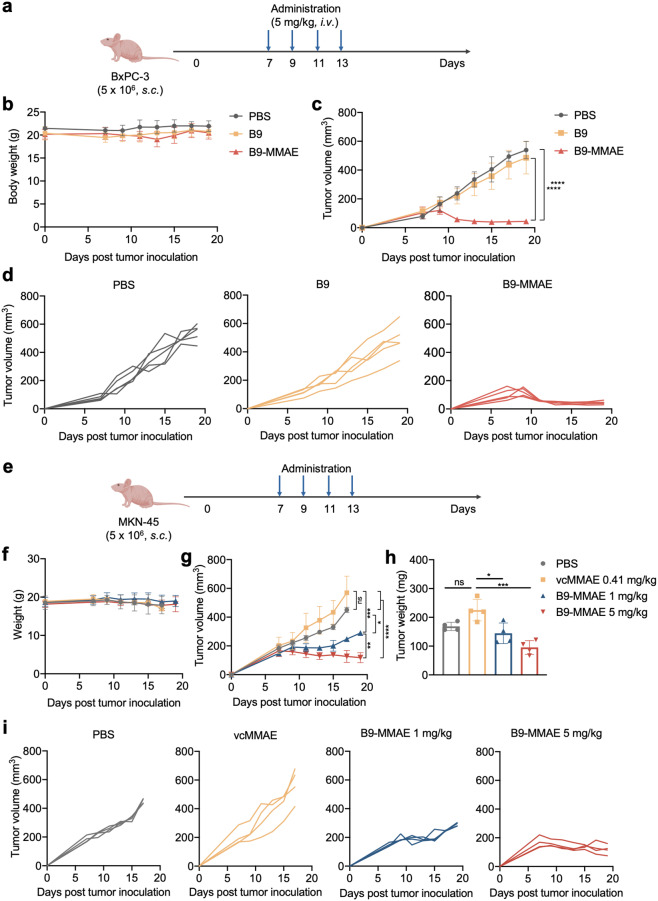
To further demonstrate the in vivo antitumor activity of B9-MMAE, we constructed two human tumor xenograft mouse models by subcutaneously inoculating nude BALB/c mice with 5 × 106 pancreatic cancer cells BxPC-3 or gastric cancer cells MKN-45 (Fig. 5a, e). In the BxPC-3 tumor model, the therapeutic activity was compared among groups treated with PBS, B9, and B9-MMAE, which received four doses of treatment at 5 mg/kg every 2 days. As expected, the tumor growth of mice treated with B9-MMAE was significantly inhibited compared with that of the PBS and B9-treated groups (Fig. 5c, d), while no significant change in body weight was witnessed among these groups throughout the observation period, demonstrating the safety of different treatment schedules (Fig. 5b). In the MKN-45 xenograft model, both low and high doses of B9-MMAE were well tolerated based on body weight curve (Fig. 5f) and led to statistically significant tumor regression compared with groups treated with PBS or the free payload vcMMAE (Fig. 5g–i). Of note, administration at 5 mg/kg induced a more robust and prolonged antitumor response than the low dose group of 1 mg/kg, indicating the dose-dependent tumor regression effect of B9-MMAE. In total, these results indicated that B9-MMAE possesses effective antitumor efficacy in vivo.
Fig. 5. In vivo anti-tumor efficacy of UdADC B9-MMAE.
UdADC B9-MMAE exhibited potent antitumor effect in BxPC-3 (a–d) and MKN-45 (e–i) xenograft mice. a Nude BALB/c mice bearing BxPC-3 xenograft tumors were treated intravenously 4 times every 2 days with PBS, parent antibody B9 (5 mg/kg) or UdADC B9-MMAE (5 mg/kg). The first treatment started at 7 days after tumor inoculation. Each group consisted of five mice. b, f Body weight of mice, which were monitored before tumor inoculation and every 2 days after the first treatment. Data are shown as mean ± SEM. c, g Tumor volumes of mice which were monitored every 2 days since the first treatment. Tumor volumes were calculated as (length × width2)/2. d, i Individual tumor growth curves of mice in different treatment groups. e Nude BALB/c mice were implanted with 5 × 106 MKN-45 tumor cells to establish MKN-45 xenograft model. Seven days after tumor inoculation, mice were intravenously administered PBS, vcMMAE (0.41 mg/kg, equimolar to 5 mg/kg B9-MMAE), or B9-MMAE (1 or 5 mg/kg) 4 times every 2 days. Each group consisted of four mice. h Weight of MKN-45 xenograft tumors. Statistical analysis was performed using two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
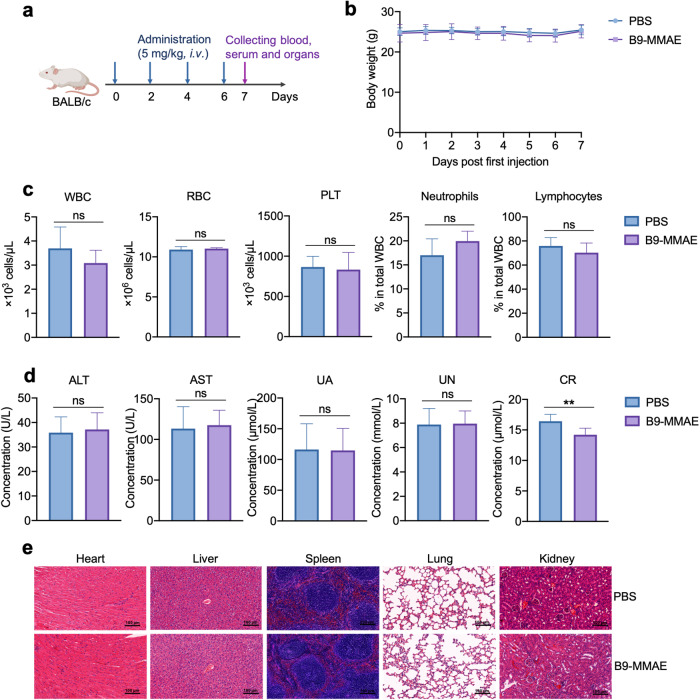
In addition, the safety profile of B9-MMAE was further confirmed by administering healthy immune-competent mice with B9-MMAE at 5 mg/kg or PBS every other day for four times (Fig. 6a). The body weight of mice did not witness significant change throughout the treatment (Fig. 6b). By analyzing the peripheral blood and serum of mice at the end of the dosage, no significant difference was found between two groups in peripheral blood components (Fig. 6c) and parameters about liver and kidney functions (Fig. 6d), expect creatinine whose reduction suggested improved kidney function. Moreover, H&E staining of heart, liver, spleen, lung, and kidney also revealed no abnormal changes after B9-MMAE administration, providing more evidence about the safety of the candidate drug (Fig. 6e).
Fig. 6. Safety profile of the UdADC B9-MMAE in BALB/c mice.
a Treatment schedule of B9-MMAE at 5 mg/kg in Balb/c mice, using PBS as control group. Each group consisted of six mice. b Body weight of mice treated with PBS and B9-MMAE. c Peripheral blood constituents (WBC, white blood cell; RBC, red blood cell; PLT, platelet; neutrophils, and lymphocytes) of mice treated with PBS and B9-MMAE. d Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), urea nitrogen (UN), creatinine (CR) in mice treated with PBS and B9-MMAE. e Hematoxylin & eosin (H&E) staining of the heart, liver, spleen, lung and kidney of BALB/c mice treated with PBS and B9-MMAE. Statistical analysis was performed using unpaired t test. **P < 0.01.
Discussion
The growing numbers of ADCs in clinics, in clinical trials and in academic research have well demonstrated the significant confidence and potential of these therapeutic agents against cancer. From different generations of ADCs, it can be seen the expansion of target ranges, optimization of linker technologies and innovation of new payloads. Nevertheless, there remain a number of unmet needs in the development of anti-cancer ADCs, especially for solid cancers, such as insufficient therapeutic windows due to off-target toxicity, rapid clearance of ADCs with high immunogenicity, as well as drug resistance.
One development strategy is to design novel forms of ADCs, coupling the payload to peptide, small molecule, or single chain variable region fragment instead of traditional mAbs [24–26]. The smaller molecular weight of ADCs is intended for better penetration and payload delivery efficiency. PEN-221 is a miniaturized peptide-drug conjugate targeting somatostatin receptor 2 with a molecular weight of ~2 kDa [24]. Compared with conventional ADCs, PEN-221 exhibited rapid tumor accumulation, quick plasma clearance and deep tumor penetration. Multiple dosing schedules of PEN-221 treatment showed antitumor activity, indicating the potential for a large therapeutic window in the clinic. Our group previously investigated the potential of employing a UdAb n501 targeting the oncofetal antigen 5T4 to deliver toxic payload SN38 to tumors [19]. The smaller size of UdAb-based ADC endows itself with excellent features including rapid distribution to tumor sites, deep tumor penetration and high tumor uptake, resulting in potent antitumor efficacy. In this study, similar design strategy was adopted and the UdADC B9-MMAE displayed significant inhibition of tumor growth, confirming the therapeutic potential of UdADCs in the treatment of solid tumors. Besides, it is interesting to find more evident antitumor effect of B9-MMAE against BxPC-3 than MKN-45 cells both in vitro and in vivo, although the latter possesses higher CEACAM5 expression levels, possibly due to varied internalization efficiency and MMAE sensitivity of different cells. Further work to in-detail characterize cell metabolic kinetics and pharmacokinetics of B9-MMAE as well as comparison with other competitive ADC candidates are required.
In addition to the advantages of smaller size, the antibody we isolated here also possessed favorable biological properties, with low aggregation tendency and high melting temperature at 64.51 °C. This may result from our unique UdAb design, which was chosen for its superior thermo stability compared to other VH domains [27]. This design laid foundation for the efficient obtainment of highly stable candidates. Besides, the fully human nature of our library imply that the antibodies identified from the library may have lower immunogenicity than those from immunized mice or camelids, thus reducing the risk of the emergence of anti-therapeutic antibodies [28]. Indeed, besides B9, we have successfully isolated high-affinity UdAbs targeting multiple antigens from our library, including SARS-CoV-2 RBD and 5T4 with excellent biological characteristics [19, 20]. Taken together, these results provided further evidence for the excellent biological characteristics of fully human single-domain antibodies.
It is notable that the smaller size of UdADCs also has other characteristics to minimize systematic toxicity, such as the short plasma half-life, resulting in limited circulation time of drug in vivo. Nevertheless, a major concern lies in the short half-lives of these drugs, which means frequent administration when applied in clinical use. To overcome this obstacle, strategies to extend their half-lives are being investigated, such as fusion to albumin-binding domain (ABD) [29] or Fc fragment [30]. These strategies should be noted that it cannot interfere the stability or antigen binding of the antibody.
In conclusion, this work brings up a novel human single-domain antibody with high binding affinity and specificity towards tumor CEACAM5 antigen. Conjugation of the antibody with the tubulin inhibitor MMAE can deliver potent antitumor efficacy in CEACAM5-expressing tumor cells as well as in BxPC-3 and MKN-45 xenograft mice. Besides, similar to the UdAbs we previously developed, B9 possesses favorable biological properties, suggesting its potential in biopharmaceutical applications.
Supplementary information
Acknowledgements
This project was supported by National Key R&D Program of China (2019YFA0904400), and National Natural Science Foundation of China (32270984).
Author contributions
XYZ and QXL performed the experiments, analyzed the data and conceived the manuscript with the help of YK, KKH, GW, and YJW. TLY and YLW initiated, planned, and supervised the project. JL and GQH revised and edited the final version of the manuscript. All authors reviewed and approved the submission version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiao-yi Zhu, Quan-xiao Li
Contributor Information
Yan-ling Wu, Email: yanlingwu@fudan.edu.cn.
Tian-lei Ying, Email: tlying@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01200-9.
References
- 1.Ceci C, Lacal PM, Graziani G. Antibody-drug conjugates: resurgent anticancer agents with multi-targeted therapeutic potential. Pharmacol Ther. 2022;236:108106. doi: 10.1016/j.pharmthera.2021.108106. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Schladetsch MA, Huang X, Balunas MJ, Wiemer AJ. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917. doi: 10.1016/j.pharmthera.2021.107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7:93. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boni V, Sharma MR, Patnaik A. The resurgence of antibody drug conjugates in cancer therapeutics: novel targets and payloads. Am Soc Clin Oncol Educ Book. 2020;40:1–17. doi: 10.1200/EDBK_281107. [DOI] [PubMed] [Google Scholar]
- 5.Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643–71. doi: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 6.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 7.Decary S, Berne P-F, Nicolazzi C, Lefebvre AM, Dabdoubi T, Cameron B, et al. Preclinical activity of SAR408701: a novel anti-CEACAM5–maytansinoid antibody–drug conjugate for the treatment of CEACAM5-positive epithelial tumors. Clin Cancer Res. 2020;26:6589–99. doi: 10.1158/1078-0432.CCR-19-4051. [DOI] [PubMed] [Google Scholar]
- 8.DeLucia DC, Cardillo TM, Ang L, Labrecque MP, Zhang A, Hopkins JE, et al. Regulation of CEACAM5 and therapeutic efficacy of an anti-CEACAM5–SN38 antibody–drug conjugate in neuroendocrine prostate cancer. Clin Cancer Res. 2021;27:759–74. doi: 10.1158/1078-0432.CCR-20-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindan SV, Cardillo TM, Moon S-J, Hansen HJ, Goldenberg DM. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009;15:6052–61. doi: 10.1158/1078-0432.CCR-09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharkey RM, Govindan SV, Cardillo TM, Donnell J, Xia J, Rossi EA, et al. Selective and concentrated accretion of SN-38 with a CEACAM5-targeting antibody–drug conjugate (ADC), labetuzumab govitecan (IMMU-130) Mol Cancer Ther. 2018;17:196–203. doi: 10.1158/1535-7163.MCT-17-0442. [DOI] [PubMed] [Google Scholar]
- 11.Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin Cancer Res. 2016;22:3286–97. doi: 10.1158/1078-0432.CCR-15-1696. [DOI] [PubMed] [Google Scholar]
- 12.Han ZW, lyv ZW, Cui B, Wang Y. The old CEACAMs find their new role in tumor immunotherapy. Invest New Drugs. 2020;38:1888–98. doi: 10.1007/s10637-020-00955-w. [DOI] [PubMed] [Google Scholar]
- 13.Baek D-S. A highly-specific fully-human antibody and CAR-T cells targeting CD66e/CEACAM5 are cytotoxic for CD66e-expressing cancer cells in vitro and in vivo. Cancer Lett. 2022;525:97–107. doi: 10.1016/j.canlet.2021.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Li W, Zhelev DV, Mellors JW, Dimitrov DS, Baek D-S. Chimeric antigen receptor-T cells are effective against CEACAM5 expressing non-small cell lung cancer cells resistant to antibody-drug conjugates. Front Oncol. 2023;13:1124039. doi: 10.3389/fonc.2023.1124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzah A. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol. 2022;33:416–25. doi: 10.1016/j.annonc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Verhaar ER, Woodham AW, Ploegh HL. Nanobodies in cancer. Semin Immunol. 2021;52:101425. doi: 10.1016/j.smim.2020.101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Jiang S, Ying T. Single-domain antibodies as therapeutics against human viral diseases. Front Immunol. 2017;8:1802. doi: 10.3389/fimmu.2017.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao C, Gao Q, Li LL, Han L, Zhang B, Ding Y, et al. The application of nanobody in CAR-T therapy. Biomolecules. 2021;11:238. doi: 10.3390/biom11020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Li Q, Kong Y, Wang Z, Lei C, Li J, et al. A highly stable human single-domain antibody-drug conjugate exhibits superior penetration and treatment of solid tumors. Mol Ther. 2022;30:2785–99. doi: 10.1016/j.ymthe.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Li C, Xia S, Tian X, Kong Y, Wang Z, et al. Identification of human single-domain antibodies against SARS-CoV-2. Cell Host Microbe. 2020;27:891–8. doi: 10.1016/j.chom.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhan W, Yang Z, Tu C, Hu G, Zhang X, et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell. 2022;185:1389–401. doi: 10.1016/j.cell.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pleiner T, Bates M, Trakhanov S, Lee C-T, Schliep JE, Chug H, et al. Nanobodies: site-specific labeling for super-resolution imaging, rapid epitope-mapping and native protein complex isolation. eLife. 2015;4:e11349. doi: 10.7554/eLife.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whalen KA, White BH, Quinn JM, Kriksciukaite K, Alargova R, Au Yeung TP, et al. Targeting the somatostatin receptor 2 with the miniaturized drug conjugate, PEN-221: a potent and novel therapeutic for the treatment of small cell lung cancer. Mol Cancer Ther. 2019;18:1926–36. doi: 10.1158/1535-7163.MCT-19-0022. [DOI] [PubMed] [Google Scholar]
- 25.Jung S, Jiang L, Zhao J, Shultz LD, Greiner DL, Bae M, et al. Clathrin light chain-conjugated drug delivery for cancer. Bioeng Transl Med. 2022;8:e10273. doi: 10.1002/btm2.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nessler I, Khera E, Vance S, Kopp A, Qiu Q, Keating TA, et al. Increased tumor penetration of single-domain antibody–drug conjugates improves in vivo efficacy in prostate cancer models. Cancer Res. 2020;80:1268–78. doi: 10.1158/0008-5472.CAN-19-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewert S, Huber T, Honegger A, Plückthun A. Biophysical properties of human antibody variable domains. J Mol Biol. 2003;325:531–53. doi: 10.1016/S0022-2836(02)01237-8. [DOI] [PubMed] [Google Scholar]
- 28.Rossotti MA, Bélanger K, Henry KA, Tanha J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022;289:4304–27. doi: 10.1111/febs.15809. [DOI] [PubMed] [Google Scholar]
- 29.Xenaki KT, Dorresteijn B, Muns JA, Adamzek K, Doulkeridou S, Houthoff H, et al. Homogeneous tumor targeting with a single dose of HER2-targeted albumin-binding domain-fused nanobody-drug conjugates results in long-lasting tumor remission in mice. Theranostics. 2021;11:5525–38. doi: 10.7150/thno.57510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huo J, Le Bas A, Ruza RR, Duyvesteyn HME, Mikolajek H, Malinauskas T, et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat Struct Mol Biol. 2020;27:846–54. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.