Abstract
Type 1 fimbriae, expressed by most Escherichia coli strains, are thought to attach to human uroepithelium as an initial step in the pathogenesis of urinary tract infections (UTI). Numerous reports using both in vitro and murine models support this role for type 1 fimbriae in colonization. Unfortunately, only a limited number of studies have directly examined the expression of fimbriae in vivo. To determine whether type 1 fimbrial genes are transcribed during an acute UTI, we employed a modification of an established method. The orientation (ON or OFF) of the invertible promoter element, which drives transcription of type 1 fimbrial genes, was determined by PCR amplification using primers that flank the invertible element, followed by SnaBI digestion. The orientation of the type 1 fimbrial switch was determined under three experimental conditions. First, E. coli strains from different clinical sources (acute pyelonephritis patients, cystitis patients, and fecal controls) were tested under different in vitro culture conditions (agar versus broth; aerated versus static). The genes in the more-virulent strains (those causing acute pyelonephritis) demonstrated a resistance, in aerated broth, to switching from OFF to ON, while those in fecal strains readily switched from OFF to ON. Second, bladder and kidney tissue from CBA mice transurethrally inoculated with E. coli CFT073 (an established murine model of ascending UTI) was assayed. The switches directly amplified from infected bladder and kidney tissues were estimated to be 33 and 39% ON, respectively, by using a standard curve. Finally, bacteria present in urine samples collected from women with cystitis were tested for type 1 fimbria switch orientation. For all 11 cases, an average of only 4% of the switches in the bacteria in the urine were ON. In 7 of the 11 cases, we found that all of the visible type 1 fimbrial switches were in the OFF position (upper limit of detection of assay, 98% OFF). Strains recovered from these urine samples, however, were shown after culture in vitro to be capable of switching the fimbrial gene to the ON position and expressing mannose-sensitive hemagglutinin. The results from experimental infections and cases of cystitis in women suggest that type 1 fimbrial genes are transcribed both in the bladder and in the kidney. However, those bacteria found in the urine and not attached to the uroepithelium are not transcriptionally active for type 1 fimbrial genes.
Adherence of uropathogenic Escherichia coli to the uroepithelium is traditionally described as the first step in the pathogenesis of urinary tract infection (UTI) (19). Attachment permits bacteria to resist mechanical elimination created by the flow of urine and turnover of epithelium and is mediated by the interaction of a variety of adhesins on the surface of bacteria with their specific ligands found on host uroepithelial cells (44). Because more than 80% of all cases of UTI are caused by E. coli (43, 46), it is important to understand the molecular mechanisms involved in the pathogenesis of these infections. The elucidation of these steps in the pathogenesis of E. coli UTI has been and will be useful in the development of vaccines targeting this species (24, 30, 36).
Type 1 fimbriae, the most common adhesin expressed by E. coli, binds to mannose moieties found on host glycoproteins, including Tamm-Horsfall protein (8, 37). Nearly all E. coli strains possess type 1 fimbrial genes (synonymous designations, pil and fim) and are capable of producing the adhesin. A role for type 1 fimbriae in virulence has been supported by numerous studies (5, 6, 14, 22, 26, 41, 47), some of which used animal models to demonstrate that strains expressing type 1 fimbriae can infect the mouse bladder with greater efficiency than strains deficient in these adhesins (1, 2, 15, 16, 19, 42). While over the last 2 decades emphasis has been placed on P fimbriae as the critical adhesins in UTI (7), these and other investigations have also provided evidence for the importance of type 1 fimbriae as a virulence factor in UTI.
Type 1 fimbrial genes undergo phase variation, which allows for switching between fimbriated and nonfimbriated states (9). A ς70 consensus promoter, upstream of fimA, which encodes the major type 1 fimbrial structural subunit resides on an invertible element that is flanked by inverted repeats. fimB and fimE encode recombinases that are responsible for switching the orientation of an invertible element within the boundaries of inverted repeats and placing a promoter either in a position to drive transcription of fimA (ON) or in the opposite orientation, which prevents fimA transcription (OFF). The position of this promoter element can be used as a means to predict whether transcription of fim genes is active and whether subsequent surface expression of the type 1 fimbria can be expected. We modified (48) a well-established restriction fragment digestion technique (1, 23) to PCR amplify the promoter region for the type 1 fimbria gene cluster from genomic DNA of E. coli strains and digest this fragment by using restriction endonuclease SnaBI. The asymmetric location of the cleavage site within the invertible element allowed us to approximate, based on fragment size and band intensity, the percentage of bacteria predicted to be transcriptionally active with respect to type 1 fimbrial genes.
In an effort to assess the transcriptional status of E. coli strains infecting the urinary tract, we measured the orientation of the invertible promoter element in the chromsome of bacteria infecting tissue of experimentally infected mice and from bacteria directly isolated from the urine of women with the clinical symptoms of cystitis. While some of the switches were ON and some were OFF in promoters from the bladders and kidneys of experimentally infected mice, we found that the switch was primarily in the OFF position in the urine of women with acute cystitis. These experiments demonstrated that type 1 fimbrial genes are transcribed by E. coli within the uroepithelium but that those bacteria found in the urine are not transcriptionally active for type 1 fimbrial genes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli strains were chosen from an established strain library (20). Three collections of E. coli strains have been established by culturing isolates from humans with appropriate clinical syndromes. The first collection consists of 61 isolates from urine or blood of patients (41 women and 20 men) who were admitted to the University of Maryland Medical Systems with acute pyelonephritis (bacteriuria of ≥105 CFU/ml, pyuria, fever, and no other source of infection) (31). The second collection is of 38 isolates from the urine of women with cystitis. Isolates were kindly provided by A. Stapleton (University of Washington) and B. Foxman (University of Michigan [10]). The third collection is of 28 control strains composed of E. coli from the feces of healthy women (20 to 50 years old) who had not had a symptomatic UTI or known bacteriuria within the previous 6 months and who had not experienced diarrhea or received antibiotics within the preceding 1 month. All of the strains came from epidiemiologically distinct episodes of bacteriuria or from fecal flora. No two strains were cultured from the same patient or volunteer.
Each strain was grown both on agar plates and in liquid culture medium. For evaluation on solid medium, individual strains were streaked onto Luria agar plates and incubated at 37°C for 18 h. For each strain studied, one isolated colony was selected and used for DNA extraction. For aerated broth culture, strains were inoculated into Luria broth (100 ml) and incubated at 37°C for 18 h with aeration (200 rpm/min). For static broth culture, bacteria were passaged three times for 48 h each passage in Luria broth at 37°C with no shaking. Bacteria were harvested by centrifugation (10,000 × g; 5 min; 4°C) from 2 ml of culture. The supernatant was discarded, and the bacterial pellet was used for DNA extraction.
For measurement of the effect of aliphatic amino acids on the position of the invertible element in a population of bacteria, strains were cultured in MOPS (morpholinepropanesulfonic acid) minimal liquid medium in the presence or absence of aliphatic amino acids (alanine, isoleucine, leucine, or valine) (11).
Murine model of ascending UTI.
Female (20- to 22-g, 6- to 8-week-old) CBA mice were anesthetized with methoxyflurane and inoculated with 107 CFU of E. coli CFT073 through a sterile polyethylene catheter inserted through the urethra into the bladder as described previously (32). After 2 days, mice were sacrificed by administration of a lethal dose of methyoxyflurane. From each mouse, urine was collected and immediately frozen and the bladder and both kidneys were removed aseptically and immediately immersed in ice-cold 50% ethanol and held at 4°C for PCR template preparation.
Human urine samples.
A total of 11 women (18 to 29 years old) who voluntarily attended a student health clinic at the University of Maryland College Park with complaints of symptoms consistent with cystitis were diagnosed as having a UTI caused by E. coli as determined by (i) the presence of >103 CFU of E. coli/ml, with a single type of bacterium isolated; (ii) pyuria; and (iii) appropriate symptoms. Clean-catch urine samples (2 to 30 ml) collected from these patients were held at −20°C for up to 4 weeks and then were transported to University of Maryland Baltimore on ice. Specimens were then thawed, aliquoted, and stored at −80°C until used. All identification of the infecting bacterial species was determined at University of Maryland College Park University Health Center. All specimens were provided without patient identifiers. Frozen samples were thawed on ice, and bacteria were recovered by centrifugation (10,000 × g; 10 min; 4°C) and washed one time in phosphate-buffered saline, pH 7.4 (per liter, 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4). Bacterial pellets were used directly for DNA extraction.
Preparation of DNA template.
Bacteria were harvested from urine samples and from overnight culture by centrifugation (10,000 × g; 10 min; 4°C) and were resuspended in digestion buffer (50 mM Tris [pH 8.5], 1 mM EDTA, 0.5% Tween 20) containing proteinase K (200 μg/ml). Samples were incubated for 1 h at 55°C, followed by deactivation of proteinase K for 10 min at 96°C. For mouse samples, tissues were removed from 50% ethanol and homogenized in a glass grinder. Homogenate was digested with proteinase K as described above. DNA was purified for PCR amplification for all samples by ethanol precipitation or by using a Qiagen DNA purification kit.
PCR amplification and restriction enzyme digestion.
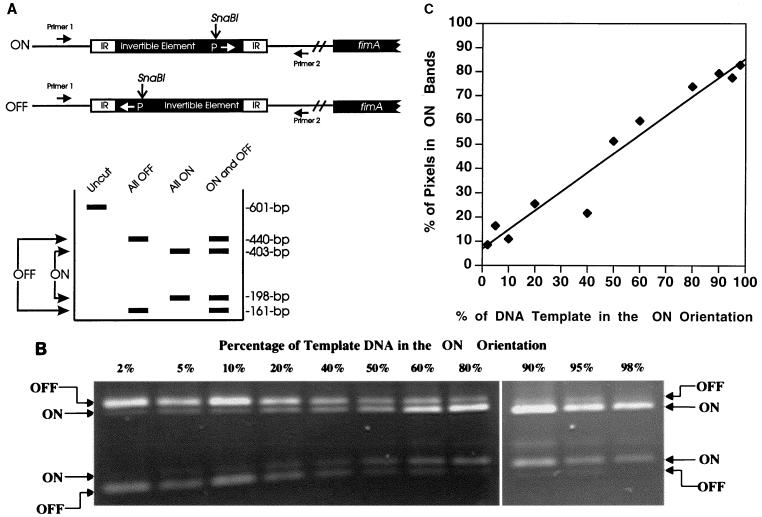
The nucleotide sequence for E. coli type 1 fimbrial genes was obtained from GenBank (accession no. Z37500) (25). Oligonucleotide primers were designed to flank the 314-bp invertible element, which contains the promoter element upstream of the fimA structural gene. Primers were synthesized by the phosphorimidite method on an automated DNA synthesizer (model 380B; Applied Biosystems). The sequence for the upstream primer (primer 1, Fig. 1A) was 5′GTTGTTCTGTCGGCTCTGTC3′, and that for the downstream primer (primer 2, Fig. 1A) was 5′AGTAATGCTGCTCGTTTGC3′. Genomic DNA (0.1 to 1 μg) was amplified in a reaction mix consisting of 50 pM each primer; 100 μM (each) dATP, dGTP, dCTP, and dTTP; 1.5 mM MgCl2; 1 U of Taq polymerase (Boehringer Mannheim Corp.); and 1× reaction buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 0.1% Triton X-100). Reactions were carried out in a Minicycler (model PTC-150-16; MJ Research, Inc.) thermocycler, programmed for 30 cycles of denaturing at 94°C for 45 s, annealing at 65°C for 30 s, and extension at 72°C for 45 s. The reaction product is predicted to be a 601-bp fragment. Amplified product (8 μl, approximately 0.1 μg) was digested with SnaBI (New England Biolabs), which cuts the fragment asymmetrically at one location to reveal the orientation of the promoter element (the ON orientation is indicated by fragments of 403 and 198 bp, and the OFF orientation is indicated by fragments of 440 and 161 bp) (Fig. 1A). Digested products were electrophoresed on 2% agarose or 6% polyacrylamide gels, stained with ethidium bromide, and visualized by UV illumination.
FIG. 1.
Sensitivity of the fim invertible switch assay. (A) Two primers were used to PCR amplify a 601-bp fragment carrying the fim invertible element. When this element is digested with SnaBI, the two possible orientations can be differentiated. The switch in the OFF position yields fragments of 403 and 198 bp; the switch in the ON position yields fragments of 440 and 161 bp. IR, inverted repeat. (B) The lower limit of detection of the type 1 fimbria invertible switch in a given orientation (either ON or OFF) was determined. Template DNA (plasmids pPM36 and pPM34, which have the invertible element locked in either the ON or OFF position, respectively) was mixed in various ratios, PCR amplified, digested with SnaBI, and electrophoresed on a 2% agarose gel. The ratio of DNA is given as the percentage of template DNA in the ON orientation (e.g., 1:50 = 2%). (C) To establish a standard curve, the data in panel B were subjected to densitometry and are presented as the proportion of pixels in the bands representing switches in the ON position to the total pixels in the bands representing switches in the ON and OFF positions. The formula for the best-fit curve is as follows: y = 0.79x + 6.93; the correlation coefficient, r, is 0.98.
Densitometric analysis (sensitivity of assay).
For E. coli strains cultured in vitro or present in urine or tissue of infected animals or humans, there is a ratio of the number of bacteria with the type 1 switch in the ON orientation to the number in the OFF orientation. When the DNA from these bacteria are pooled and PCR amplified, digested with restriction endonucleases, and electrophoresed, a certain ratio of band intensity (ON/OFF ratio) is also evident. To determine the sensitivity of this assay (i.e., the lowest level of detection of ON versus OFF and OFF versus ON switches), we prepared template from plasmids pPM36 and pPM34, which have the invertible element locked in either the ON or OFF position, respectively (kindly provided by Ian Blomfield, Bowman Gray School of Medicine [27]). These templates, mixed such that the fraction of template in the ON orientation varied from 0.001 to 1,000 (0.1 to 99.9%), were used for PCR amplification, restriction enzyme digestion, and agarose gel electrophoresis. The intensities of the bands representing ON switches and those representing OFF switches were quantitated by using densitometric software (The Eagle Sight Software, version 3.0; Stratagene) on a gel documentation system (Stratagene). This calibration allowed us to determine quantitatively what percentages of the template were in the ON and OFF orientations, when analyzing bacteria sampled from UTIs. As well, the lower limit of detection was also determined.
RESULTS
Sensitivity of the invertible switch PCR assay.
The invertible switch assay measures the percent of the bacterial population that has the fimA promoter in the ON or OFF orientation. To quantitate the switch orientation and determine the lower limit of detection, we prepared mixtures (0.1 to 99.9% of each plasmid) of plasmids pPM34 and pPM36, which carry the invertible promoter element locked in the OFF or ON positions, respectively, as templates for the PCR assay (Fig. 1B). When equal amounts of each template were present (50% ON), equal amounts of the respective products were amplified. When one orientation represented as little as 2% of the template, a product was still identifiable by an observer or by densitometry (Fig. 1B). However, when one orientation represented 1% or less, a product was not reproducibly visualized (data not shown). Thus, lack of bands in one orientation indicates that <2% of the template (i.e., bacterial population) is in that orientation. To establish a standard curve, the gel was recorded as a digital image and the band intensities were measured by densitometry. A standard curve was prepared from these data, with which values for the percent of switches that are ON in a given bacterial population could be estimated (Fig. 1C).
Type 1 fimbria invertible element orientation in fecal, cystitis, and pyelonephritis strains of E. coli cultured in vitro.
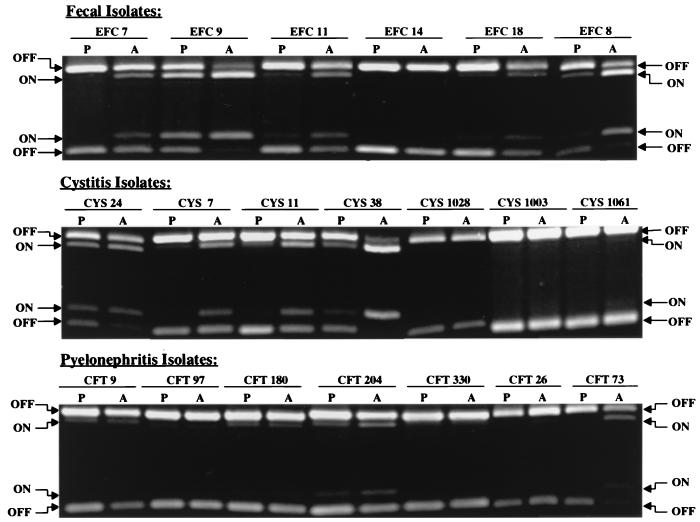
In our first experiments, we cultured strains of E. coli isolated from the feces of healthy adult women or from patients with cystitis or acute pyelonephritis, on both Luria agar plates (18 h) and in aerated Luria broth (18 h) and determined the orientation of the type 1 fimbria promoter element (Fig. 2). Analysis of six fecal strains revealed that these strains can readily switch the orientation of the invertible element with changing culture conditions. Five of six strains demonstrated a higher percentage of switches in the ON position when cultured under conditions that favored the expression of type 1 fimbriae (40% ON after growth in aerated broth versus 11% ON on agar plates). For cystitis strains, these bacterial strains showed a decreased switching potential when bacteria cultured in aerated broth (29% ON) and on agar plates (8% ON) were compared. Four of seven strains showed a higher percentage of switches in the ON position when grown in aerated broth. For pyelonephritis strains, the inability to switch from the OFF position to the ON position was more pronounced. No visible ON bands were observed in four of seven strains when grown on agar plates and three of seven strains when grown in aerated broth. Only two of six strains showed a significant (greater-than-twofold) increase in the percentage of switches in the ON position when cultures grown on agar plates and in aerated broth were compared. In addition, the percentage of switches in the ON position averaged only 2% for agar conditions and 8% for aerated broth cultures of the seven strains, values that were significantly less than those of fecal and cystitis strains (P < 0.001).
FIG. 2.
Orientation of the type 1 fimbria invertible switch in fecal, cystitis, and pyelonephritis strains of E. coli after in vitro culture. E. coli strains isolated from the feces of healthy adult women (Fecal Isolates) or strains recovered from the urine of women with cystitis (Cystitis Isolates) or acute pyelonephritis (Pyelonephritis Isolates) were cultured on Luria agar plates (P) or in aerated (shaken at 200 rpm) Luria broth (A). Strain designations are given along the top of each gel. The switch orientation was determined as described in Materials and Methods. Restriction fragments corresponding to switches in the ON and OFF positions are described in the legend to Fig. 1.
A subset of the strains from the previous experiments were also grown in the presence or absence of aliphatic amino acids (alanine, isolecuine, leucine, and valine) to observe the effect on promoter switching. It has been shown that aliphatic amino acids stimulate the switching of the type 1 fimbrial promoter, probably through the action of lrp (11). Strains that were nonresistant to switching, such as EFC7 and EFC8, demonstrated some sensitivity to switching induced by aliphatic amino acids. Pyelonephritis strain CFT073 also demonstrated sensitivity to aliphatic amino acid-induced switching. However other pyelonephritis strains, CFT097 and CFT330, were shown to be resistant to switching induced by the aliphatic amino acids (data not shown). It is difficult therefore to conclude that resistance to switching could be uniformly overcome by the action of lrp.
In vivo phase variation of type 1 fimbrial genes in a CBA mouse model of ascending UTI.
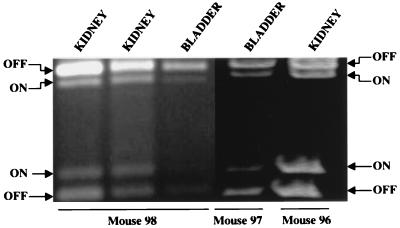
To determine the switch position of an E. coli strain during an acute experimental UTI, E. coli CFT073, a pyelonephritis strain (32), was transurethrally inoculated into the bladders of CBA mice. After 2 days, animals were sacrificed and the bladder and kidneys of each mouse were isolated and used to isolate total DNA preparations for use in PCR amplification. Successful amplification was achieved from the tissue of three of five infected mice (Fig. 3). In all samples, the E. coli population had the switch both in the ON and OFF positions. The switches in the two bladder samples averaged 33% ON, and those in the three kidney samples averaged 39% ON. Mouse urine from three different mice inoculated with CFT073 was collected, and the type 1 fimbrial promoter switch position of the bacteria present was assayed. All three samples had the switch predominantly in the OFF position.
FIG. 3.
Type 1 fimbria promoter orientation of E. coli infecting the bladders and kidneys of transurethrally infected CBA mice. CBA mice (n = 5) were transurethrally inoculated with 107 CFU of E. coli CFT073. After 2 days, mice were sacrificed and the bladder and kidneys from each animal were aseptically removed and placed immediately in ice-cold isopropanol. Total DNA was isolated from infected tissue and used for PCR determination of switch orientation. PCR products were obtained from the infected tissue of three of five mice. The first three samples (from the left and right kidneys and the bladder of one mouse) were run on a 2% agarose gel; the last two samples (from the bladder of one mouse and the kidney of another mouse) were run on a 6% polyacrylamide gel. All samples that yielded PCR products are shown.
The inoculum was prepared by culture on L agar. When an inoculum was prepared in this manner it was found that the invertible element within the bacterial population was almost exclusively in the OFF position (data not shown).
In vivo phase variation of type 1 fimbrial genes in human urine samples.
In an attempt to correlate the animal model studies with human UTIs, we measured the orientation of the type 1 fimbrial switch directly from the urine collected (and flash frozen) from women attending a student health clinic with complaints consistent with the diagnosis of cystitis. In each of the 11 cases, duplicate urine samples were used to confirm E. coli bacteriuria (>105 CFU of E. coli/ml of urine). Urine samples from 11 women with E. coli bacteriuria, evaluated for type 1 fimbria switch position (Fig. 4), revealed that these bacteria primarily had their switch in the OFF position. For all 11 cases, bacteria in the urine averaged only 4% ON. In 7 of the 11 cases, we found that all of the visible type 1 fimbrial switches were in the OFF position (upper limit of detection of assay, >98% OFF). In the remaining 4 cases, the position of the promoter element for type 1 fimbriae averaged only 9% ON. These results are uniform and show that the majority of E. coli bacteria expelled in the urine are not transcriptionally active with respect to expression of type 1 fimbriae.
FIG. 4.
Type 1 fimbria promoter orientation of E. coli recovered from the urine of women with cystitis. Urine samples, collected from women attending a student health clinic with complaints consistent with the clinical symptoms of cystitis, were frozen immediately. Significant E. coli bacteriuria (>105 CFU of E. coli/ml of urine) was verified in the clinical laboratory, with duplicate urine samples. DNA template prepared from the frozen urine samples was used to determine the orientation of the invertible element. Sample numbers are given along the top of the gel. The positions of the invertible element were determined as described in the legend to Fig. 1.
Analysis of E. coli strains isolated from urine samples.
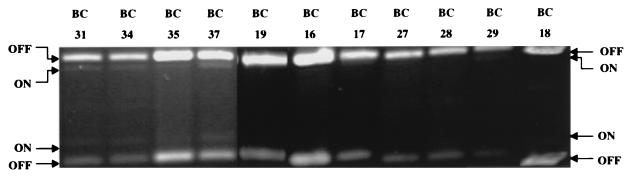
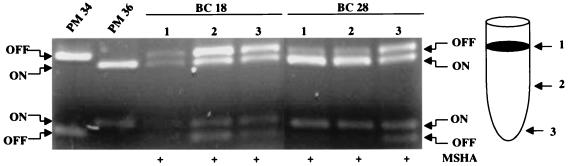
Since the invertible switch from the E. coli strains infecting the urine of women with cystitis was found overwhelmingly to be in the OFF position, we questioned whether these strains were capable of turning on transcription of the fim genes. That is, were these bacteria capable of expressing type 1 fimbriae or were these genes permanently locked in the OFF position? We examined two of these strains, BC18 and BC28, by culturing them in Luria broth without shaking (static conditions). Samples were taken from the pellicle (top surface, in which type 1 fimbria synthesis is most favored) and the middle and bottom of the culture tubes. Both of these strains were shown to have the ability to switch the promoter element to the ON position under these conditions (Fig. 5). Indeed, the switches in the three BC18 samples averaged 49% ON (as compared to <2% ON in urine) and the BC28 samples revealed the majority of the switch to be in the ON orientation (69% ON), as compared to <2% ON in urine. In addition, suspensions of both of these strains used for template preparation were also capable of causing mannose-sensitive hemagglutination of guinea pig erythrocytes (Fig. 5), a phenotype of type 1 fimbriated bacteria. The hemagglutination strength of a single cystitis strain, CYS38, was also assayed for two different populations, one having nearly 100% of the promoters in the ON position (cultured by static passage) and the other with nearly 100% of the promoters in the OFF position (cultured on agar). The bacterial population with promoters in the ON position demonstrated strong mannose-sensitive hemagglutination. The bacterial population with promoters in the OFF position demonstrated little or no mannose-sensitive hemagglutination. Therefore, while E. coli strains isolated from the urine of women with cystitis have the capability of expressing type 1 fimbriae, in general, they do not appear to actively transcribe type 1 fimbrial genes when present in the urine.
FIG. 5.
Promoter orientation in two E. coli strains, isolated from urine samples of women with cystitis, grown under conditions favoring expression of type 1 fimbria. E. coli BC18 and BC28, for which promoter orientation was determined (Fig. 4), were cultured from the urine of women with cystitis. These strains were cultured statically (no shaking) in Luria broth for three passages of 48 h each, and the promoter orientation of each strain was determined. Samples of bacteria for PCR template preparation were taken from the pellicle at the top surface of the culture (1), the center of the culture (2), and the bottom of the culture tube (3). Suspensions of bacteria from these cultures were used for mannose-sensitive hemagglutination (MSHA) of a 3% suspension of guinea pig erythrocytes. A plus sign indicates a positive hemagglutination reaction.
DISCUSSION
We have examined for the first time, the orientation of the transcription-regulating invertible element that controls expression of type 1 fimbrial genes in E. coli during active UTIs in mice and humans. While most E. coli strains are capable of undergoing phase variation between a transcriptionally active and inactive state by inversion of a promoter-containing element, bacterial strains isolated from more severe UTIs, that is, acute pyelonephritis, are recalcitrant to switching the invertible element to the ON position when cultured in vitro. However, the switch directly amplified from infected bladder and kidney tissue from experimentally infected mice was estimated at 33% ON and 39% ON, respectively, indicating that the genes are transcribed in vivo. In contrast, bacteria collected from the urine of women with cystitis were found to be 96% transcriptionally inactive, as indicated by the invertible element being primarily in the OFF position; the urine samples from experimentally infected mice corroborated this finding.
Perhaps there is a simple explanation for the latter findings. Bacteria collected directly from urine samples from women with cystitis and subjected to PCR amplification of the switch revealed that the switch was almost always OFF and thus that the fim gene cluster was transcriptionally inactive. Quantitation of the assay indicates that only 4% of these bacteria are transcriptionally active. If we accept that adherence is required for colonization, bacteria not expressing fimbriae may not be adherent and thus may be subject to elimination in the next urination. There are, however, some complications to this logic. Uroepithelial cells bearing adherent bacteria and previously adherent bacteria whose fimbrial attachments have been sheared would also be present in urine and would have been included in the PCR amplification and would have given a positive ON signal. These bacteria may, however, represent a minority of bacteria present and thus not be detected. In addition, there would be a delay from the time that a switch inverted to the OFF position until expression of fim genes ceased and a further delay from the time fimbriae were assembled on the surface until all such structures were turned over or sheared from the surface. Likewise, bacteria that had just turned on transcription could be afimbriate until gene expression and fimbrial assembly occurred. Therefore, one would expect bacteria expelled in the urine to be more representative of the total population than the first simple explanation implies. That is, all bacteria in the urine probably do not simply represent “losers” that are nonadherent and thus washed out of the urinary tract. This premise is supported by immunofluorescence studies by Pere and colleagues (34, 38), who showed that 5 out of 20 urine specimens from young girls with UTI contained bacteria that reacted with type 1 fimbria antiserum (the proportion of stained bacteria in each specimen, however, was not given for this antiserum).
We could also conclude that type 1 fimbriae play no role in UTI; however, there is substantial evidence that this adhesin contributes significantly to certain stages of infection. First, immunological approaches have suggested that type 1 fimbriae are involved in colonization of the urinary tract. Antiserum raised against fimbriae from type 1-fimbriated strains, administered passively or raised by direct immunization, protected rats from colonization by transurethrally introduced E. coli (45). Similarly, hydbridoma antibodies directed against type 1 fimbriae administered intraperitoneally prevented colonization by E. coli instilled into the bladder (1). Recently, a FimH-FimC (adhesin-chaperon) complex was used to vaccinate mice (24); significant protection was afforded by the vaccine against homologous challenge, suggesting that type 1 fimbriae are produced by strains attempting to colonize the urinary tract. Secondly, genetic evidence supports a role for type 1 fimbriae in UTI. Adhesin-negative mutants constructed by mutation of adhesin genes fimH (5) or pilE (21) were recovered in significantly lower numbers than parent strains after transurethral challenge of mice and rats, respectively. Another group (18) also demonstrated that adherence mutants defective in their ability to agglutinate Candida albicans (a phenotypic assay for mannose-sensitive agglutination) were unable to adhere to mouse bladder epithelial cells or infect mouse bladders. Third, direct observation supports a role for type 1 fimbriae. Three-fourths of bacterial isolates collected from the urine of women with UTI were shown to be able to produce type 1 fimbriae (42). In experimental infections, when piliated or nonpiliated variants were inoculated into the bladders of mice, the type 1 piliated organisms established infection more readily (16). The urine of these mice, however, often showed no growth, and when organisms were cultured, they revealed only 30% of the colonies to be hemagglutinating, suggesting that urine may not necessarily reflect the bacteriologic status of the bladder mucosa (35). This is supported by our studies, in which isolates from urine predict very low transcription of fim genes whereas bladder and kidney samples predict significant fim transcription. Finally, evidence for the contribution of type 1 fimbriae to UTI was provided by the use of inhibitors that specifically block attachment of type 1 fimbriae to its mannosylated receptor. Levels of bacteriuria (CFU of bacteria per milliliter of urine) in experimentally infected rats and mice were lower when the inoculum included d-mannose (29) or α-d-mannopyranoside (2); d-glucose did not exert this effect. This story, however, is complicated by the fact that low-molecular-weight manno-oligosaccharides have been identified in the urine as natural inhibitors of lectin binding (37). Collectively, these experiments strongly suggest that at least initial attachment of bacteria to uroepithelium by type 1 fimbriae is important to establish infection.
Additional studies support a role for type 1 fimbriae in the bladder rather than in the kidney. Mice challenged transurethrally with a mixture of type 1-fimbriated and P-fimbriated bacteria revealed that the type 1-fimbriated bacteria predominated in the bladder while the P-fimbriated bacteria predominated in the kidney (13, 14). Other studies have also supported this result (16, 17). Bacteria that remained attached to the bladder epithelium were type 1 fimbriated, while those washed out in the urine were not (16). Although we provide only limited data (five observations based on three mice), the levels of fim transcription do not appear to be significantly different in the bladders and kidneys of experimentally infected mice.
Interestingly in our studies, the more virulent strains (those causing pyelonephritis and cystitis) appeared to be more resistant in vitro than fecal strains to turning the invertible element to the ON position. The position of the invertible element was assayed for each strain after in vitro culture on both solid agar plates and in broth under aerated conditions. Fecal strains showed the most plasticity in the ability to switch the invertible element from the OFF position after culture on agar to the ON position in aerated broth. In contrast, pyelonephritis-causing strains were relatively resistant to phase variation (switching the invertible element from the OFF to the ON position) of the promoter element that controls type 1 fimbriae expression. Ritter and colleagues (39) recently provided a possible explanation for this phenomenon, at least for one virulent strain. In E. coli 536, one of the two pathogenicity islands is inserted very near the leuX tRNA gene. When pathogenicity island sequences spontaneously delete, which occurs at a high frequency in this strain, this tRNA gene is disrupted. Since one of the recombinase genes (fimB) that turns on transcription of type 1 fimbrial genes contains five TTG (Leu) codons recognized by the tRNA gene that is disrupted, expression of type 1 fimbriae in these variants is greatly reduced. While we do know that most uropathogenic strains carry pathogenicity island sequences (20) and that these strains appear to resist turning on type 1 fimbrial genes (Fig. 2), we do not know if the disrupted tRNA gene can explain this phenomenon in all strains. Indeed, since pathogenicity island sequences have been described to insert in different sites in different strains (28), it is not likely that this explanation is suitable for all strains.
The results obtained with E. coli differ dramatically from those of studies of another uropathogen in which fimbrial expression is also controlled by an invertible element. Proteus mirabilis, associated most significantly with development of renal and bladder stones (12) and bacteriuria in patients with long-term catheterization (33), also produces a fimbria whose regulation is controlled by inversion of a promoter-containing element (48). The MR/P (mannose-resistant Proteus-like) fimbria is clearly associated with the development of UTI by this organism. The fimbriae bind to renal tissue (40) and elicit an antibody response following experimental UTI (3), and isogenic mutants lacking these structures are recovered in significantly lower numbers than the parent strain 1 week after mice are transurethrally challenged with bacterial suspensions of each isolate (4). Unlike the results presented in this report, the P. mirabilis transcriptional switch was >98% ON when PCR amplified directly from the urine, bladder, and kidney of infected mice (unless a bladder stone was present). The switch is ON in vivo in spite of the fact that no in vitro culture condition has yet been identified under which the switch in more than about 50% of the population is ON (48). In contrast, in this study the transcriptional switch in E. coli in the urine of women with cystitis was primarily OFF. While significant transcriptional activity (33 to 39% ON) of type 1 fimbrial genes was evident in the bladder and kidney for E. coli, this was still in sharp contrast to the apparent >98% ON values observed for P. mirabilis infecting the bladder and kidney. Additional studies that examine transcriptional switch position in E. coli during different phases of infection or from different sites, including bladder and kidney tissue, may allow us to more accurately assess whether transcription of E. coli fim genes occur in a significant proportion of the bacterial population during acute UTI.
ACKNOWLEDGMENTS
J.K.L. and N.W.G. contributed equally to this study.
This work was supported in part by Public Health Service grant DK47920 from the National Institutes of Health.
We thank Virginia Lockatell for animal inoculations and Michael Donnenberg for discussions regarding assay sensitivity.
REFERENCES
- 1.Abraham S N, Babu J P, Giampapa C S, Hasty D L, Simpson W A, Beachey E H. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary d-mannose receptors. Infect Immun. 1985;48:625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl α-D-mannopyranoside. J Infect Dis. 1979;139:329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani F K, Johnson D E, Robbins D, Mobley H L T. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991;59:3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani F K, Massad G, Lockatell C V, Johnson D E, Russell R G, Warren J W, Mobley H L T. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan K, Falkow S, Hull R A, Hull S I. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985;162:799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell H, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: ASM Press; 1996. pp. 135–174. [Google Scholar]
- 8.Duncan J L. Differential effect of Tamm-Horsfall protein or adherence of Escherichia coli to transitional epithelial cells. J Infect Dis. 1988;158:1379–1382. doi: 10.1093/infdis/158.6.1379. [DOI] [PubMed] [Google Scholar]
- 9.Eisenstein B I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981;214:337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- 10.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 11.Gally D L, Bogan J A, Eisenstein B I, Blomfield I C. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith D P, Musher D M, Itin C. Urease: the primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 13.Hagberg L, Hull R, Hull S, Falkow S, Freter R, Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983;40:265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagberg L, Jodal U, Korhonen T, Lidin-Janson G, Lindberg U, Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981;31:564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultgren S J, Porter T N, Schaeffer A J, Duncan J L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985;50:370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultgren S J, Schwan W R, Schaeffer A J, Duncan J L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun. 1986;54:613–620. doi: 10.1128/iai.54.3.613-620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwahi T, Abe Y, Nakao M, Imada A, Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by Escherichia coli in mice. Infect Immun. 1983;39:1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–125. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao J-S, Stucker D M, Warren J W, Mobley H L T. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keith B R, Maurer L, Spears P A, Orndorff P E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986;53:693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisielius P V, Schwan W R, Amundsen S K, Duncan J L, Schaeffer A J. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infection. Infect Immun. 1989;57:1656–1662. doi: 10.1128/iai.57.6.1656-1662.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986;5:1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Leath S, Hones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 25.Marc D, Dho-Moulin M. Analysis of the fim cluster of an avian O2 strain of Escherichia coli: serogroup-specific sites within fimA and nucleotide sequence of fimI. J Med Microbiol. 1996;44:444–452. doi: 10.1099/00222615-44-6-444. [DOI] [PubMed] [Google Scholar]
- 26.Marild S, Jodal U, Orskov I, Orskov F, Svanborg Eden C. Special virulence of the Escherichia coli O1:K1:H7 clone in acute pyelonephritis. J Pediatr. 1989;115:40–45. doi: 10.1016/s0022-3476(89)80326-9. [DOI] [PubMed] [Google Scholar]
- 27.McClain M S, Blomfield I C, Eisenstein B I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991;173:5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaels E K, Chmiel J S, Plotkin B J, Schaeffer A J. Effect of D-mannose and D-glucose on Escherichia coli bacteriuria in rats. Urol Res. 1983;11:97–102. doi: 10.1007/BF00256954. [DOI] [PubMed] [Google Scholar]
- 30.Mobley H L T. Vaccines against Escherichia coli and Proteus urinary tract infections. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 945–962. [Google Scholar]
- 31.Mobley H L T, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobley H L T, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of αGal(1-4)βGal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 33.Mobley H L T, Warren J W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki B, Rhen M, Vaisanen-Rhen V, Pere A, Korhonen T K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984;160:691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofek I, Mosek A, Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981;34:708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hanley P. Prospects for urinary tract infection vaccines. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: ASM Press; 1996. pp. 405–425. [Google Scholar]
- 37.Parkkinen J, Virkola R, Korhonen T K. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect Immun. 1988;56:2623–2630. doi: 10.1128/iai.56.10.2623-2630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pere A, Nowicki B, Saxen H, Siitonen A, Korhonen T K. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J Infect Dis. 1987;156:567–574. doi: 10.1093/infdis/156.4.567. [DOI] [PubMed] [Google Scholar]
- 39.Ritter A, Gally D L, Olsen P B, Bobrinndt U, Friedrich A, Klemm P, Hacker J. The Pai-associated leuX specific tRNA5Leu affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression. Mol Microbiol. 1997;25:871–882. doi: 10.1111/j.1365-2958.1997.mmi517.x. [DOI] [PubMed] [Google Scholar]
- 40.Sareneva T, Holthofer H, Korhonen T K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990;58:3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaeffer, A. J. 1991. Potential role of phase variation of type 1 pili in urinary tract infection and bacterial prostatitis. Infection 19(Suppl. 3):144–149. [DOI] [PubMed]
- 42.Schaeffer A J, Schwan W R, Hultgren S J, Duncan J L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoolnik G K. How Escherichia coli infects the urinary tract. N Engl J Med. 1989;320:804–805. doi: 10.1056/NEJM198903233201211. [DOI] [PubMed] [Google Scholar]
- 44.Siitonen A. What makes Escherichia coli pathogenic? Ann Med. 1994;26:229–231. doi: 10.3109/07853899409147895. [DOI] [PubMed] [Google Scholar]
- 45.Silverblatt F J, Cohen L S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979;64:333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren J W. Clinical presentations and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: ASM Press; 1996. pp. 3–27. [Google Scholar]
- 47.Wold A E, Thorssén M, Hull S, Svanborg Edén C. Attachment of Escherichia coli via mannose- or Galα1→4 Galβ-containing receptors to human colonic epithelial cells. Infect Immun. 1988;56:2531–2537. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H, Li X, Johnson D E, Blomfield I, Mobley H L T. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]