Abstract
An inactivated oral enterotoxigenic Escherichia coli (ETEC) vaccine against ETEC diarrhea was given to 25 adult Swedish volunteers. The vaccine consisted of formalin-killed E. coli bacteria expressing the most common colonization factor antigens (CFAs), i.e., CFA/I, -II, and -IV, and recombinantly produced cholera B subunit (CTB). Immunoglobulin A (IgA) antibody responses in intestinal lavage fluid to CTB and CFAs were determined and compared with corresponding responses in stool extracts and serum as well as with IgA antibody-secreting cell (ASC) responses in peripheral blood. Two doses of vaccine induced significant IgA responses to the different CFAs in lavage fluid in 61 to 87% of the vaccinees and in stool in 38 to 81% of them. The most frequent responses were seen against CFA/I. The magnitudes of the antibody responses against CTB and CFA/I in stool correlated significantly (CTB, P < 0.01; CFA/I, P < 0.05) with those in intestinal lavage. Intestinal lavage responses against CFAs were best reflected by the ASC responses, with the sensitivity of the ASC assay being 80 to 85%, followed by stool (sensitivity of 50 to 88%) and serum antibody (sensitivity of 7 to 65%) analyses. CTB-specific immune responses were seen in >90% of the vaccinees in all assays.
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of diarrhea in children in developing countries and in travelers to these areas (4). Nevertheless, there is no ETEC vaccine available for use in humans (25). The bacteria cause disease by colonizing the intestine by means of fimbrial colonization factor antigens (CFAs) and by producing a heat-labile enterotoxin (LT), a heat-stable enterotoxin (ST), or both toxins (9). Three major CFAs—CFA/I, which is a homogeneous protein; CFA/II, which comprises the coli surface (CS) subcomponents CS1, CS2, and CS3; and CFA/IV, which comprises the CS4, CS5, and CS6 antigens (12)—have been found in 50 to 80% of human ETEC strains isolated in different geographic areas (3, 7, 13, 31).
The first prototype of an inactivated oral ETEC vaccine, consisting of formalin-killed E. coli bacteria expressing CFA/I and the CS factors of CFA/II in combination with the cholera toxin B subunit (CTB), provided as the CTB–whole-cell (WC) oral cholera vaccine (5), was shown to be safe and immunogenic when given to adult Swedish volunteers (1, 30). The vaccine induced significant intestinal lavage IgA antibody responses as well as intestinal mucosa-derived IgA antibody-secreting cells (ASCs) in peripheral blood against CTB, CFA/I, and CFA/II in more than 80% of the vaccinees.
A more definitive formulation of the oral ETEC vaccine compared to the prototype formulation has now been developed. This vaccine contains recombinantly produced CTB (rCTB) and formalin-killed E. coli strains expressing high levels of CFA/I and the different CS factors of CFA/II (CS1, CS2, and CS3), as well as E. coli strains expressing the subcomponents of CFA/IV (i.e., CS4, CS5, and CS6). In the present study, this ETEC vaccine was given to adult Swedish volunteers, and immune responses against the CFAs and CTB in intestinal lavage fluid were compared with those in stool extracts and in serum, as well as with ASC responses in peripheral blood, to evaluate whether there is a simpler approach to assess gut mucosal immune responses (e.g., in large trials or in young children).
MATERIALS AND METHODS
Vaccine.
The ETEC vaccine (lot 001) was produced by SBL Vaccin, Stockholm, Sweden. It contains a mixture of rCTB and formalin-killed E. coli bacteria of five different strains expressing CFA/I, CS1, CS2 plus CS3, CS4 plus CS6, and CS5 plus CS6, respectively (17). Each dose of vaccine consisted of approximately 2 × 1010 bacteria of each strain (i.e., a total of 1011 E. coli cells) and 1 mg of rCTB in 4 ml of phosphate-buffered saline.
Subjects and vaccination.
Twenty-eight healthy Swedish volunteers of both sexes, 21 to 37 years of age, gave informed consent to participate in the study, which had been approved by the Human Research Ethical Committee at the Medical Faculty, Göteborg University, Göteborg, Sweden. None of the volunteers presented a history of diarrheal disease or had traveled outside Scandinavia for the last 6 months prior to the study. The volunteers in the present study constitute a subgroup of volunteers in a parallel study (17) in which the safety and immunogenicity of two different lots (i.e., lots 001 and 003) of the more definitive ETEC vaccine formulation were evaluated. The aim of this study was to evaluate the relationship between immune responses in intestinal lavage fluid and those in stool and in blood. Previous studies have shown, however, that lavage samples with <10 μg of total IgA/ml cannot be reliably assessed (1, 2). For this reason, all volunteers were subjected to intestinal lavage before being given any vaccine, and the three persons presenting a preimmune intestinal lavage with <10 μg of total IgA/ml were excluded from this study.
Twenty-five volunteers were given two oral doses of the ETEC vaccine (lot 001) with a 2-week interval between doses. The vaccine was given as a drink after suspension in 150 ml of a sodium bicarbonate solution (Samarin; Cederroths Nordic AB, Upplands Väsby, Sweden). The volunteers had been instructed not to eat or drink (except water) 1 h prior to and 1 h after ingestion of the vaccine.
Sampling of specimens.
Intestinal lavages and stool samples were obtained from the vaccinees immediately before onset of immunization (day 0) and 9 days after the second dose (day 23). From 11 of the volunteers, stool samples were also collected 3 and/or 6 months after onset of immunization. Thirty milliliters of heparinized venous blood for determination of ASCs was drawn from each of the 25 volunteers before (day 0) and 7 days after the second immunization (day 21). Serum samples were collected simultaneously.
The lavages were performed essentially as described previously (1, 2). After an overnight fast, the volunteers drank 200 ml of an isotonic salt solution containing polyethylene glycol and sulfate (Laxabon; TIKA Pharmaceutical Co., Lund, Sweden) every 10 to 15 min until “clear” watery stools appeared. A portion (100 ml) of this clear lavage specimen was collected, filtered through gauze, and treated with proteolytic enzyme inhibitors essentially as described previously (2). The fluids were frozen in aliquots at −70°C.
Stool samples (≥10 g) were collected from each volunteer as the first solid output after initiation of the lavage procedure and were immediately frozen at −70°C upon collection. Fecal specimens were prepared by extraction of 4 g of feces with 16 ml of an extraction buffer containing proteolytic enzyme inhibitors in the same concentrations as those described for lavage fluid (2). After centrifugation at 20,000 × g for 30 min, the extracts were supplemented with bovine serum albumin (BSA) and NaN3 to final concentrations of 1 mg/ml and 0.02% (vol/vol), respectively, and frozen in aliquots at −70°C until assayed.
Determination of total Ig and specific antibodies.
The total IgA contents in the lavage and fecal samples were determined by a modified microplate enzyme-linked immunosorbent assay (ELISA) method by using a human colostral IgA reference (Sigma Chemical Co., St. Louis, Mo.) as a standard (1, 2). Vaccine-specific CFA antibodies of IgA and IgG isotypes in serum and of the IgA class in lavage fluid and fecal extracts were determined by ELISA methods as previously described (1). Purified CFA/I, CS1, a combined CS1 and CS3 preparation (CS1/3), CS2, and CS4 were used as solid-phase antigens. Antibodies against CTB were analyzed by a GM1 ganglioside-ELISA method (1, 27). Samples were threefold serially diluted in duplicates in microtiter plates, and the endpoint titers were determined as the reciprocal interpolated dilutions of the test samples giving an A450 of 0.4 above the background. The specific IgA antibody titers were divided by the total IgA concentration in the lavage specimen or in the fecal extract. Based on calculation of the methodological error, a ≥twofold increase in the mean IgA titer per total IgA between pre- and postimmunization intestinal lavage samples or fecal extracts was considered as a significant immune response as previously described (1, 16). A ≥twofold increase in serum IgA or IgG titer between samples was considered to signify a significant seroconversion.
Detection of circulating ASCs.
IgA ASCs in peripheral blood against CTB and the CFA or CS antigens of the vaccine were determined by a micromodification of the enzyme-linked immunospot (ELISPOT) assay (8, 17, 30). Vaccinees who developed a ≥twofold increase in vaccine-specific IgA ASCs between pre- and postimmunization specimens were regarded as responders on the condition that the number of ASCs exceeded 10/107 mononuclear cells (MNCs) in the postvaccination specimens. When preimmune samples were missing, volunteers were considered as responders if their postvaccination levels of specific IgA ASCs equaled or exceeded by 2 standard deviations (SDs) the geometric mean of specific IgA ASCs of all individuals examined before immunization. The latter criteria were used to determine CS2 responses for 12 of the volunteers, since the CS2 antigen preparation had to be changed during the study. All postimmunization ASCs were determined, however, with the same CS2 antigen preparation.
RESULTS
Antibody responses in intestinal lavage fluid.
Intestinal lavage responses could be evaluated in 23 of the 25 volunteers after two doses of ETEC vaccine. One person did not present a postimmunization sample, and another presented a preimmune sample with very high and similar preimmune titers against all vaccine antigens, as well as against BSA, indicating nonspecific binding of this sample to the ELISA plates and thus falsely giving too-high preimmune values. None of the postimmune samples showed these high and similar titer values to all vaccine antigens, as well as to unrelated lipopolysaccharide (LPS) and BSA antigens. Pre- and postimmune lavage fluids contained a mean of 85 (±SD [range 51 to 140]) μg of total IgA/ml. The total IgA concentrations in the lavages varied up to 7-fold between the volunteers, whereas the intraindividual differences were <1.5-fold.
Two doses of the ETEC vaccine induced a significant (≥twofold increase in specific IgA titer per total IgA) antibody response in 87% of the vaccinees against CFA/I, in 61 to 70% of them against the different CS components of CFA/II and CFA/IV, and in 96% of them against CTB (Table 1). Nineteen (83%) of the vaccinees developed significant IgA antibody increases in lavage fluid against two or more of the four CFA and CS antigens assayed as well as against CTB.
TABLE 1.
Volunteers with significant responses against CFAs and CTB in intestinal lavage fluid, feces, and serum and significant blood ASC responses after two oral doses of ETEC vaccine
| Vaccine antigen | No. (%) with significant response/no. testeda
|
|||
|---|---|---|---|---|
| Lavage | Feces | ASC | Serum | |
| CFA/I | 20/23 (87) | 17/21 (81) | 19/24 (79) | 14/25 (56) |
| CS1/3 | 16/23 (70) | 11/21 (52) | 19/25 (76) | 5/25 (20) |
| CS2 | 14/23 (61) | 8/21 (38) | 15/23 (65) | 7/25 (28) |
| CS4 | 15/23 (65) | 9/21 (43) | 17/25 (68) | 2/25 (8) |
| CTB | 22/23 (96) | 19/21 (90) | 25/25 (100) | 23/25 (92) |
Increases of ≥twofold in vaccine-specific IgA antibody titer per total IgA (lavage, feces), in number of specific IgA ASCs, and in specific IgA antibody titer (serum) between pre- and postimmune specimens were considered significant responses in the respective assays.
Antibody responses in fecal extracts.
Stool samples from 21 of the 25 vaccinees could be evaluated. One person did not present a preimmune sample, and three volunteers presented stool samples with very high and similar preimmune titers against all vaccine antigens (as well as against unrelated LPS and BSA antigens), indicating unspecific binding to the ELISA plates, and thus they were not possible to evaluate. No such high and similar titers to all vaccine as well as unrelated antigens were found in any of the postimmune stool extracts. The geometric mean total IgA concentration in the fecal extracts was somewhat higher, 160 (±SD [range 60 to 420]) μg/ml, than that in lavage fluids. The IgA concentration in feces varied considerably, up to 23-fold, among the different study persons, whereas the intraindividual differences were less than 4-fold.
Vaccination induced significant IgA antibody titer per total IgA increases against the different vaccine antigens in lower frequencies in feces than in lavage fluids (i.e., 38 to 81% of the vaccinees responded against the CFAs and 90% responded against CTB in stool) (Table 1). Similar to the findings in intestinal lavages, responses to CFA/I were more frequent than responses to the CS factors of CFA/II or CFA/IV.
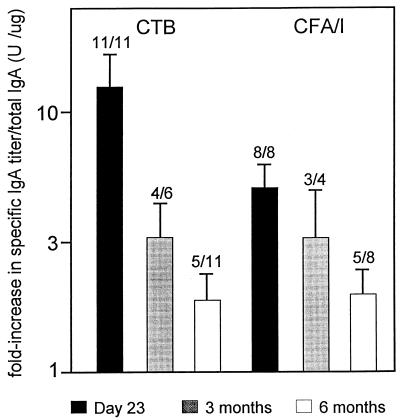
From 11 arbitrarily selected volunteers who had responded to either or both CTB and CFA/I in feces 9 days after the second immunization, stool samples were also collected 3 and/or 6 months after the onset of vaccination. The geometric means of the fold increases of responses against CTB and CFA/I for this subgroup on day 23 did not differ from those of the whole group of responders. Although the vaccine-specific IgA antibody titers per total IgA decreased in stool during the study period, 63% (5 of 8) of the volunteers still had significantly elevated anti-CFA/I antibody levels and 45% (5 of 11) had elevated antitoxin titers 6 months after immunization (Fig. 1). The volunteers with long-lasting responses were generally those with the highest responses on day 23.
FIG. 1.
Geometric mean (+ standard error) fold increases of the specific IgA titer per total IgA 9 days, 3 months, and 6 months after two oral doses of ETEC vaccine. The number of responders/total number of volunteers is given above each bar.
Reflection of intestinal lavage responses in feces.
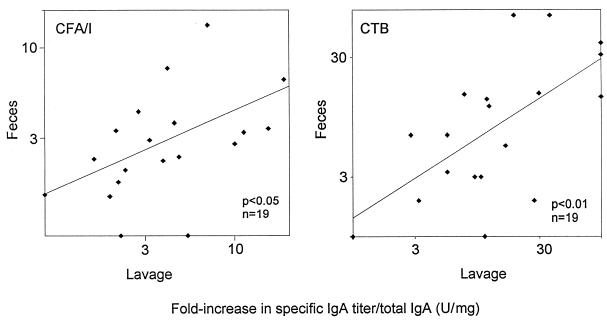
For the 19 volunteers fulfilling our inclusion criteria and presenting both lavage and stool samples that were evaluable, as determined above, the individual immune responses against CFA/I and CTB in the two types of samples were compared. A significant correlation was found between the magnitudes of the specific IgA per total IgA responses in fecal extracts and in lavage fluids against CFA/I (r = 0.48; P < 0.05) (Fig. 2), as well as those against CTB (r = 0.64; P < 0.01), after two doses of ETEC vaccine. Significant correlations between the individual CTB-specific postvaccination titers (without adjustment for total IgA) (r = 0.60; P < 0.01) as well as the individual CFA/I-specific IgA titers (r = 0.58; P < 0.01) in lavage fluids and in stools were also observed. The magnitudes of the immune responses against the CS antigens were comparable to those against CFA/I both in lavage fluids and in feces.
FIG. 2.
Correlation between increases in IgA antibody titer per total IgA against CTB and CFA/I, respectively, in fecal extracts and in lavage fluid after two oral immunizations with ETEC vaccine.
The sensitivities of the fecal antibody analyses in comparison with intestinal lavage esponses were 50 to 88% for the different CFA or CS antigens and 94% for CTB (Table 2); the positive predictive values for stool analyses were even higher. In those instances in which significant rises in titer were seen in lavage fluid but not in corresponding stool specimens, the magnitudes of the lavage responses were comparatively low.
TABLE 2.
Comparison of immune responses in stool with those in intestinal lavage after two oral immunizations with ETEC vaccinea
| Parameter | Value (%) for vaccine antigen
|
||||
|---|---|---|---|---|---|
| CFA/I | CS1/3 | CS2 | CS4 | CTB | |
| Sensitivity | 88 | 54 | 50 | 62 | 94 |
| Positive predictive value | 93 | 70 | 71 | 100 | 100 |
Intestinal lavage samples and fecal extracts from 19 volunteers collected 9 days after the second dose were assayed for specific IgA per total IgA responses to the respective antigens. The percentage of volunteers responding in both stool and lavage in relation to those responding in lavage (sensitivity) and in stool (positive predictive value) is given for each antigen.
Reflection of intestinal lavage responses in blood.
IgA ASC responses in peripheral blood, as well as IgA and IgG antibody responses in serum against the various vaccine antigens, were also determined (17) (Table 1). The geometric mean number of vaccine-specific IgA ASCs/107 MNCs 7 days after the second dose was 55 (±SD [range, 23 to 130]) for responders against CFA/I. Similar means were found for the CS antigens, whereas the mean against CTB was 840 (±SD [range, 190 to 3,600]) IgA ASCs/107 MNCs. Preimmune numbers of ASCs were generally low or negligible (i.e., a mean of 1 to 5 IgA ASCs/107 MNCs for the respective antigens). Serum antibody responses against the CFAs were mainly of the IgA type, although some increases in IgG could also be recorded. The highest geometric means of the increase in individual IgA titer in serum among responders were seen against CFA/I and CS2, i.e., 3.2-fold (±SD [range, 2.0 to 4.9]) and 3.9-fold (±SD [range, 2.1 to 7.1]), respectively, but were still considerably lower than that against CTB, which was 18-fold (±SD [range, 3.9 to 89]) after two doses of vaccine.
The individual immune responses to two doses of ETEC vaccine in intestinal lavages were also compared with corresponding IgA ASC responses in peripheral blood as well as with IgA and IgG antibody responses in serum. Intestinal lavage responses were better reflected by the ASCs than by stool or serum antibody responses (Tables 2 to 4). The sensitivities of the ASC assay were about 80% for CFA responses and 100% for CTB responses. However, no direct correlations between the magnitudes of the intestinal lavage and the ASC responses were observed. The sensitivities of serum antibody analyses to reflect responses against the different CFA or CS factors in intestinal lavage were rather low; on the other hand, the positive predictive values were high (Table 4). There was also a 100% correlation between intestinal lavage responses and serum (both IgA and IgG) responses against CTB.
TABLE 4.
Comparison of immune responses in serum with those in intestinal lavage after two oral immunizations with the ETEC vaccinea
| Parameter | Value (%) for vaccine antigen
|
||||
|---|---|---|---|---|---|
| CFA/I | CS1/3 | CS2 | CS4 | CTB | |
| Sensitivity | 65 | 27 | 36 | 7 | 100 |
| Positive predictive value | 93 | 80 | 83 | NCb | 100 |
Samples collected 7 days (serum) and 9 days (lavage) after the second dose from 22 or 23 volunteers were assayed for specific IgA antibodies per total IgA responses in lavage and serum IgA responses to the respective antigens. The percentage of volunteers responding both in serum and lavage in relation to those responding in lavage (sensitivity) and in serum (positive predictive value) is given for each antigen.
NC, not calculated because the numbers in the denominator were too low.
We also investigated the relationship between IgA ASC responses and corresponding IgA responses in stool or serum after two doses of vaccine. The sensitivities of fecal IgA analyses to reflect ASCs were 83% for CFA/I and 42 to 53% for CS1/3, CS2, and CS4 responses. The sensitivities of serum IgA analyses were lower: 55% for CFA/I and 6 to 40% for responses against the CS factors of CFA/II and -IV. The CTB-specific ASC response, on the other hand, was well reflected in serum (sensitivity of 92%).
DISCUSSION
This study of a new inactivated oral vaccine against ETEC diarrhea confirms and extends the findings from our previous studies of a prototype ETEC vaccine (1). Significant intestinal IgA responses against the CFA and CS components of the vaccine, as well as against enterotoxin, were seen in a majority of the vaccinees. Although responses to CFA/I were seen somewhat more frequently (87%) than those to the other vaccine CS antigens (60 to 70%), the magnitudes of the intestinal immune responses to the different CFA or CS components were similar and equalled those obtained after two doses of the prototype vaccine (1). The magnitudes of the vaccine-specific responses were also comparable to those of the anti-CTB and antibacterial responses obtained after oral immunization with two doses of a closely related inactivated CTB-WC cholera vaccine (16, 26, 28) with documented protective efficacy (5). Interestingly, significant intestinal immune responses against CFA/I could still be detected in stool extracts from more than 60% of the volunteers studied 6 months after the onset of immunization.
Studies of the compartmentalization of local immune responses indicate that even though responses can be recorded at distant sites from that of the antigen administration, the highest responses are generally seen at the actual site of immunization (14, 15, 22). Thus, mucosal immune responses in the gut after oral immunization should most reliably be assessed by determining intestinally produced IgA antibody responses. The intestinal lavage procedure offers a noninvasive method by which antibodies produced in the entire intestine are determined, and it has therefore been considered one of the “gold standards” for evaluation of intestinal immune responses. However, since the method is labor-intensive, time-consuming, and sometimes considered unpleasant, it is suitable only for studies of a limited number of volunteers. Aiming at a simpler approach, determination of ASCs in peripheral blood has become a commonly used method to assess intestinal immunity after oral immunization with enteric vaccines (11, 17, 19–21, 24, 30). The results from such ASC studies, as well as recent work to determine different homing receptors on ASCs induced by different routes of immunization, suggest that ASCs of the IgA isotype identified approximately 7 days after oral antigen administration largely represent cells of gut origin (23). Very few studies, however, have addressed the actual relationship between the antibody responses observed in intestine and the ASC responses in peripheral blood. In this study, we have evaluated this relationship in volunteers receiving two oral doses of ETEC vaccine. To obtain optimal responses in the respective assays after two doses of vaccine, ASC and lavage responses were determined 7 and 9 days after the second dose, respectively. Previous studies of the ETEC vaccine as well as the related CTB-WC oral cholera vaccine have shown that intestinal IgA responses to enterotoxin and to antigens expressed by killed bacteria (e.g., LPS and CFAs), peak first after the second dose of vaccination and then as late as on ≥9 days after this dose (1, 26, 28). Optimal IgA ASC responses to CTB and CFAs after two oral doses are seen on days 5 to 7 and start to decline already on day 9 (18, 29). When analyzing ASC and intestinal lavage responses 7 and 9 days after the second dose of vaccine, respectively, we found that in ≥80% of the vaccinees, ASC responses to the CFAs were seen when intestinal lavage responses were present and vice versa. For CTB responses, there was an almost 100% concordance between the two methods. Blood IgA ASC responses have been reported to peak already 7 days after primary vaccination with live or attenuated vaccine strains (19–21), which also was the case when the ETEC vaccine was given to adults endemically exposed to ETEC infection (24). However, previous and ongoing studies show comparable ASC responses after the first and the second doses of vaccine in a nonprimed population (18, 30). These findings suggest that blood ASC responses are less suitable in reflecting immunity after booster immunization for both live and killed vaccine strains (19, 21).
In numerous previous studies, determination of antibody responses in serum and other easily accessible secretions such as saliva and breast milk has proved to be relatively insensitive in reflecting intestinal immune responses after oral immunization, with the exception of immune responses to CTB. In this study, only 7 to 36% of the vaccinees developed IgA (and even lower frequencies of IgG) antibody responses in serum to the respective CS antigens despite demonstrable intestinal IgA responses. Determination of antibody responses in urine may be an alternative approach to assess intestinal immune responses, especially in young children, since such responses have been shown to have a high degree of specificity after natural Shigella infection or vaccination; however, the sensitivity has been lower than that of serum analyses (6). On the other hand, recent studies have shown that oral immunization with the killed CTB-WC cholera vaccine only rarely results in significant anti-CTB responses in urine (32).
Since both the intestinal lavage procedure and ASC analyses, at least in their present form, are unsuitable methods for large-scale immunogenicity studies, particularly in children, we evaluated the possibility of determining ETEC vaccine-induced intestinal immune responses in feces. Stool extracts have been widely used to assess intestinal immunity, but it has also been argued that this approach may not be adequate (10). In this study, we show that analyses of immune responses in stool were not as sensitive as determinations of ASC increases in reflecting intestinal lavage responses. However, 50 to 80% of the vaccinees who responded in lavage fluid to a CFA and 94% of them who responded to enterotoxin also had increased antibody levels to the corresponding antigen in simultaneously collected stool samples. The magnitudes of responses against CTB and CFA/I in stool correlated significantly with those in lavage fluid. The total IgA concentrations varied considerably between individuals in both lavage and stool, which also was reflected in the corresponding specific postvaccination titers. Thus, a single postvaccination titer value in lavage or stool does not indicate the actual immune response, unless it has been compared with the titer in a corresponding preimmune sample.
However, despite the comparatively high sensitivity of the stool analyses in this study, fecal analyses have limitations. In the present study, stool samples were collected under ideal conditions, i.e., in the initiation phase of a lavage, which probably reduced the intestinal transit time of the stool; furthermore, samples were frozen at −70°C immediately upon collection. Our continued studies have indicated that even a short delay in freezing stool samples may quickly cause degradation of Igs by digestive enzymes and bacterial proteases. Furthermore, high antibody levels were recorded in a few stool samples, not only against ETEC vaccine antigens, but also against unrelated antigens, in the absence of increased antibody levels in the corresponding lavage fluids, suggesting nonspecific binding of some of the stool samples to the ELISA plates. Such nonspecific binding is most likely due to dietary products in the stool, since consecutive preimmune samples from the same individual do not show the same unspecific binding to the plates (18). In many studies in which intestinal antibody responses in stool have been analyzed, antibody levels have been expressed per gram of fresh stool and the water content of the sample has not been taken in consideration. To circumvent this problem, we have chosen to adjust the specific vaccine titers in relation to the total IgA content of the fecal extracts, as we do for lavage specimens. In this study, we show that the IgA concentration in consecutive lavage specimens from the same subject is very consistent, whereas the intraindividual differences in IgA concentration in stool extracts were considerably higher. By using “ideal” stool collection procedures, the difference in total IgA content between pre- and postimmune samples from the same subject was less than fourfold. In this and continued studies, we have seen that when the difference in total IgA concentration between pre- and postimmune stool samples is more than 10-fold, vaccine-specific titer increases adjusted in relation to the total IgA cannot be reliably assessed.
In conclusion, this study shows that a majority (≥80%) of the vaccinees responded to CTB as well as to the CFA and CS antigens after two doses of the oral ETEC vaccine. The intestinal lavage responses against the CFAs were best reflected by circulating ASC responses, followed by stool and serum analyses; CTB responses were seen in ≥90% of the vaccinees in all assays.
TABLE 3.
Comparison of circulating ASC responses with immune responses in intestinal lavage after two oral immunizations with ETEC vaccinea
| Parameter | Value (%) for vaccine antigen
|
||||
|---|---|---|---|---|---|
| CFA/I | CS1/3 | CS2 | CS4 | CTB | |
| Sensitivity | 85 | 81 | 83 | 80 | 100 |
| Positive predictive value | 89 | 72 | 71 | 80 | 96 |
Samples collected 7 days (blood) and 9 days (lavage) after the second dose from 22 or 23 vaccinees were assayed for specific IgA antibodies per total IgA responses in lavage and for blood IgA ASC responses against the respective antigens. The percentage of volunteers mounting both ASC and lavage responses in relation to those responding in lavage (sensitivity) and to those mounting ASC responses (positive predictive value) is given for each antigen.
ACKNOWLEDGMENTS
We are grateful to I. Ahlstedt, K. Andersson, and G. Wiklund for skilled technical assistance and to SBL Vaccin, Stockholm, Sweden, for providing the ETEC vaccine.
This study was supported by grants from the Swedish Medical Research Council (grant 16 × 09084), the Swedish Agency for Research Cooperation with Developing Countries, the World Health Organization, and the Medical Faculty, Göteborg University.
REFERENCES
- 1.Åhrén C, Wennerås C, Holmgren J, Svennerholm A-M. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine. 1993;11:929–934. doi: 10.1016/0264-410x(93)90380-g. [DOI] [PubMed] [Google Scholar]
- 2.Åhrén C, Andersson K, Wiklund G, Wennerås C, Svennerholm A-M. Optimization of the intestinal lavage procedure for determination of intestinal immune responses. Vaccine. 1995;13:1754–1758. doi: 10.1016/0264-410x(95)00153-r. [DOI] [PubMed] [Google Scholar]
- 3.Binsztein N, Jouve M J, Viboud G I, López-Moral L, Rivas M, Ørskov I, Åhrén C, Svennerholm A-M. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J Clin Microbiol. 1991;29:1893–1898. doi: 10.1128/jcm.29.9.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R E. The epidemiology of diarrheal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 5.Clemens J D, Sack D A, Harris J R, Van Loon F, Chakraborty J, Ahmed F, Rao M R, Khan M R, Yunus M D, Huda N, Stanton B F, Kay B A, Walter S, Eeckels R, Svennerholm A-M, Holmgren J. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 6.Cohen D, Orr N, Robin G, Slepon R, Ashkenazi S, Ashkenazi I, Shemer J. Detection of antibodies to Shigella lipopolysaccharide in urine after natural Shigella infection or vaccination. Clin Diagn Lab Immunol. 1996;3:451–455. doi: 10.1128/cdli.3.4.451-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cravioto A, Reyes R E, Ortega R, Fernández G, Hernández R, Lopéz D. Prospective study of diarrheal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988;101:123–124. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerkinsky C, Nilsson L-Å, Nygren H, Ouchterlony Ö, Tarkowski A A. Solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 9.Evans D J, Jr, Evans D G. Determinants of microbial attachment and their genetic control. In: Farthing M J G, Keusch G T, editors. Enteric infection. Mechanisms, manifestations and management. London, United Kingdom: Chapman & Hall; 1989. pp. 15–30. [Google Scholar]
- 10.Ferguson A, Humphreys K A, Croft N M. Technical report: results of immunological tests on fecal extracts are likely to be extremely misleading. Clin Exp Immunol. 1995;90:70–75. doi: 10.1111/j.1365-2249.1995.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest B D. Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet. 1988;i:81–83. doi: 10.1016/s0140-6736(88)90284-x. [DOI] [PubMed] [Google Scholar]
- 12.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 13.Gothefors L, Åhrén C, Stoll B, Barua D K, Ørskov F, Salek M, Svennerholm A-M. Presence of colonization factor antigens on fresh isolates of fecal Escherichia coli: a prospective study. J Infect Dis. 1985;152:1128–1133. doi: 10.1093/infdis/152.6.1128. [DOI] [PubMed] [Google Scholar]
- 14.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins S, Kraehenbuhl J-P, Schödel F, Potts A, Peterson D, De Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jertborn M, Svennerholm A-M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jertborn M, Åhrén C, Holmgren J, Svennerholm A-M. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine. 1998;16:255–260. doi: 10.1016/s0264-410x(97)00169-2. [DOI] [PubMed] [Google Scholar]
- 18.Jertborn, M., C. Åhrén, J. Holmgren, and A.-M. Svennerholm. Unpublished data.
- 19.Kantele A, Kantele J M, Arvilommi H, Mäkelä P H. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine. 1991;9:428–431. doi: 10.1016/0264-410x(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Pal T, Forsum U, Lindberg A A. Safety and immunogenicity of the live oral autotrophic Shigella flexneri SFL 124 in volunteers. Vaccine. 1992;10:395–404. doi: 10.1016/0264-410x(92)90070-z. [DOI] [PubMed] [Google Scholar]
- 21.Losonsky G A, Tacket C O, Wasserman S S, Kaper J B, Levine M M. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: changes with time and lack of correlation with protection. Infect Immun. 1993;61:729–733. doi: 10.1128/iai.61.2.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quiding-Järbrink M, Nordström I, Granström G, Killander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric and nasal immunisations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savarino S J, Brown F M, Hall E, Bassily S, Youssef F, Wierzba T, Peruski L, El-Masry N A, Safwat M, Rao M, Jertborn M, Svennerholm A-M, Lee Y J, Clemens J D. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli cholera toxin B subunit vaccine in Egyptian adults. J Infect Dis. 1998;177:796–799. doi: 10.1086/517812. [DOI] [PubMed] [Google Scholar]
- 25.Svennerholm A-M, Åhrén C, Jertborn M. Oral inactivated vaccines against enterotoxigenic Escherichia coli. In: Levine M M, Woodrow G C, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 865–873. [Google Scholar]
- 26.Svennerholm A-M, Holmgren J, Sack D A, Bardhan P K. Intestinal antibody responses after immunization with cholera B subunit. Lancet. 1982;i:305–307. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- 27.Svennerholm A-M, Holmgren J, Black R, Levine M, Merson M M. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis. 1983;147:514–522. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 28.Svennerholm A-M, Jertborn M, Gothefors L, Karim A M M M, Sack D A, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 29.Tarkowski A, Czerkinsky C, Nilsson L Å. Simultaneous induction of rheumatoid factor and antigen specific antibody secreting cells during secondary immune responses in man. Clin Exp Immunol. 1985;61:379–387. [PMC free article] [PubMed] [Google Scholar]
- 30.Wennerås C, Svennerholm A-M, Åhrén C, Czerkinsky C. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect Immun. 1992;60:2605–2611. doi: 10.1128/iai.60.7.2605-2611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf M K, Taylor D N, Boedeker E C, Hyoms K C, Maneval D R, Levine M M, Tamura K, Wilson R A, Echeverria P. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J Clin Microbiol. 1993;31:851–856. doi: 10.1128/jcm.31.4.851-856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wullt, B., W. Månsson, S. Colleen, C. Svanborg, and A.-M. Svennerholm. Unpublished data.