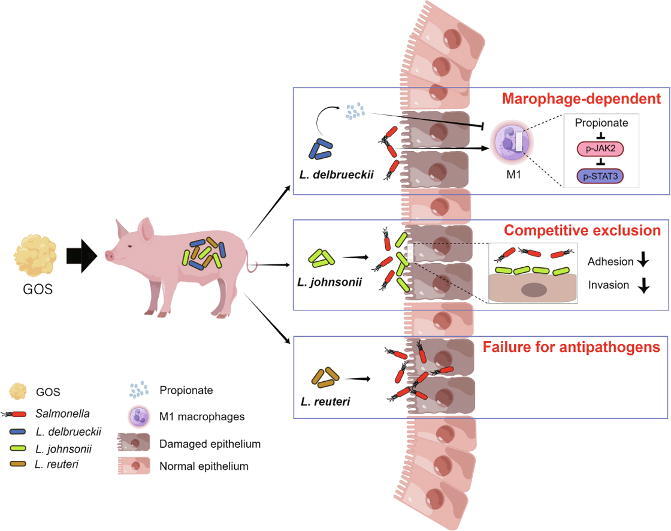
Graphical abstract
Keywords: Galactooligosaccharides, Lactobacillus, Salmonella, Intestinal inflammation
Highlights
-
•
GOS selectively enriched intestinal L. delbrueckii, L. johnsonii and L. reuteri in piglets and mice.
-
•
Strain-specific properties of GOS-enriched Lactobacilli were identified for the promotion of gut health.
-
•
Preventative administration of L. delbrueckii strain alleviated intestinal barrier damage by reducing the inflammation in macrophages.
-
•
Continuous administration of L. johnsonii strain inhibited Salmonella adhesion and invasion through competitive exclusion.
-
•
L. reuteri strain failed to protect against Salmonella infection in this study.
Abstract
Introduction
Galactooligosaccharides (GOS) are lactogenic prebiotics that exert health benefits by stimulating the growth of different Lactobacillus strains in the gastrointestinal (GI) tract.
Objectives
This study aimed to investigate the mechanism of action of different GOS-enriched lactobacilli in intestinal health.
Methods
Piglets and mice were supplemented with GOS to identify specific enrichment of Lactobacillus. The protective effects of individual GOS-enriched lactobacilli were investigated in Salmonella-infected mice. Macrophage depletion and transcriptome analysis were further performed to assess the involvement of macrophages and the underlying mechanisms of individual lactobacilli. An in vitro cell co-culture system was also used to evaluate the anti-adhesive and anti-invasive activities of lactobacilli against Salmonella in epithelial cells.
Results
GOS markedly increased the relative abundance of three lactobacilli including L. delbrueckii, L. johnsonii, and L. reuteri in both piglets and mice. Supplementation with GOS further alleviated Salmonella infection in mice. L. delbrueckii (ATCC®BAA 365™), but not L. johnsonii or L. reuteri, enhanced propionate production in the intestinal tract and ameliorated Salmonella-induced intestinal inflammation and barrier dysfunction by suppressing the JAK2-STAT3 signaling and M1 macrophage polarization. L. johnsonii (BNCC 186110), on the other hand, inhibited Salmonella adhesion and invasion of epithelial cells through competitive exclusion. However, L. reuteri (BNCC 186135) failed to protect mice against Salmonella infection.
Conclusion
GOS-enriched lactobacilli show a differential role in protecting against Salmonella-induced intestinal barrier dysfunction and inflammation. Our results provide novel insights into the mechanism of action of GOS and individual Lactobacillus strains in the control and prevention of intestinal inflammatory disorders.
Introduction
Galactooligosaccharides (GOS) are well-known prebiotics for selective stimulation of the growth of Lactobacillus and Bifidobacterium [1], [2], [3]. We previously demonstrated that GOS promotes intestinal Lactobacillus colonization and proliferation in piglets, leading to an improvement in intestinal barrier functions and immune development [4]. In addition, GOS enhances the competitive exclusion of intestinal pathogens, thereby reducing the inflammatory response [5], [6]. As a result, GOS has been widely used to improve intestinal function and health [7].
Lactobacillus is known to exert a broad spectrum of health benefits to the host such as immune modulation and intestinal barrier promotion [8], [9]. However, different Lactobacillus species appear to work through different modes of action. For example, certain Lactobacillus strains inhibit the adhesion of pathogenic bacteria to epithelial cells [10], [11], while a few others are capable of reprogramming the polarization of immune cells such as CD4+ T cells and macrophages [12], [13]. Additionally, several lactobacilli improve intestinal health by producing novel antimicrobial metabolites, reinforcing intestinal epithelial barriers, and modulating host immune response [14], [15].
Although GOS is known to regulate the microbiota and intestinal health, the underlying mechanisms and particularly the roles of different GOS-enriched lactobacilli in intestinal health remain largely unknown. The present study aimed to investigate the specific effect of three GOS-enriched Lactobacillus strains on intestinal barrier integrity and immune response in a mouse model of Salmonella Typhimurium infection.
Materials and Methods
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the Institutional Animal Care and Use Committee of China Agricultural University, China (Approval No. AW07040202-1).
Mammalian and bacterial cell culture
Murine macrophage RAW264.7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37℃ and 5% CO2. L. delbrueckii (ATCC®BAA 365™), L. johnsonii (BNCC 186110), L. reuteri (BNCC 186135), and Salmonella Typhimurium SL1344 (DSM 24522) were obtained from China Committee for Culture Collection of Microorganisms. All lactobacilli were cultured in MRS medium (Aladdin, Shanghai, China), while Salmonella Typhimurium was cultured in LB medium (Aladdin, Shanghai, China) at 37℃.
Galactooligosaccharides (GOS)
GOS was provided by Beijing Sanyuan Foods Co., Ltd (Beijing, China) and produced through transgalactosylation of galactose, which was originally converted from lactose by β-galactosidases. The end product was obtained after a series of processing procedures including purification, decoloration, filtration, and concentration. The glycosidic linkages of the GOS product used in this study are β(1 → 3) and β(1 → 4), and the degree of polymerization ranged from 2 to 7. Detailed analysis of the GOS product in this study was shown in Fig. S1, Supplementary Table S1 and Table S2.
Administration of GOS to piglets
A total of 12 newborn piglets with similar birth weights (Duroc × Landrace × Yokshire, 1.53 ± 0.04 kg) were divided into two groups, and administered orally with 2 mL saline in the presence or absence of GOS (1 g/kg BW) daily for 7 days. All piglets had free access to sows’ milk till the end of the trial on d 21, when the feces and colonic segments were collected for further analysis.
Administration of GOS and lactobacilli to Salmonella-infected mice
Seven-week-old, specific pathogen-free C57BL/6 mice were purchased from SPF (Beijing) Biotechnology and provided with ad libitum access to water and standard laboratory chow for one week of acclimation prior to experimentation. For prevention of intestinal infection and inflammation, mice were orally gavaged with L. delbrueckii (ATCC®BAA 365™), L. johnsonii (BNCC 186110), or L. reuteri (BNCC 186135) daily for two weeks prior to Salmonella infection. For treatment of intestinal infection, after two weeks’ pretreatement with lactobacilli, mice were orally administered with Salmonella Typhimurium SL1344 (DSM 24522), followed by oral gavage with L. johnsonii (BNCC 186110) or L. reuteri (BNCC 186135) daily for one week. In a separate trial, mice were supplemented with 5% GOS in the diet together with oral gavage with L. reuteri (BNCC 186135) daily throughout the trial. Individual Lactobacillus strains were administered to mice by oral gavage in 0.2 mL PBS containing 109 CFU/mL bacteria. Salmonella infection was conducted by oral gavage with 0.2 mL PBS containing 107 CFU/mL Salmonella Typhimurium. After euthanasia, blood samples, the colonic segments, and feces were collected from each animal at the end of each trial.
Administration of propionate to Salmonella-infected mice
Seven-week-old, specific pathogen-free C57BL/6 mice were infected with 0.2 mL PBS containing 107 CFU/mL Salmonella Typhimurium, followed by administration of 1% sodium propionate (ProNa) in drinking water. On d 3 post-infection, blood samples, the colonic segments, and feces were collected from all mice after euthanasia.
Depletion of macrophages in mice
Macrophage depletion was achieved by intraperitoneal injection with clodronate liposomes (0.2 mL/mouse, Biohub International Trade Co., Shanghai, China) 3 days prior to and then every third day during Salmonella Typhimurium infection as previously described [16]. ProNa (1%, w/v) was provided in drinking water throughout the trial. On d 3 post-infection, blood samples, the colonic segments, and feces of all mice were collected after euthanasia.
LPS and ProNa treatment of RAW264.7 macrophages
Mouse RAW264.7 macrophages were seeded in six-well plates at a density of 1 × 106 cells. After overnight growth, cells were treated with or without 1 mM ProNa for 24 h, followed by 100 ng/mL LPS stimulation for another 12 h. Whenever necessary, RAW264.7 cells were incubated with AT9283, Stattic, or GLPG0974 (MedChem Express, Shanghai, China) for specific inhibition of JAK2, STAT3, or GPR43, respectively, for 1 h prior to an addition of ProNa and LPS.
Anti-adhesion and anti-invasion of Salmonella to epithelial cells by lactobacilli
Anti-adhesion and anti-invasion assays were performed according to a previous study [17]. For the anti-adhesion assay, human HT-29 intestinal epithelial cell monolayers were pretreated with 109 CFU L. johnsonii (BNCC 186110) or L. reuteri (BNCC 186135) for 1 h prior to infection with 107 CFU Salmonella Typhimurium. After 1 h, the monolayers were washed three times with PBS and lysed with Triton X-100. Adhered Salmonella Typhimurium were enumerated by serial plating. The same procedures were applied to measure the invasion of Salmonella Typhimurium, except that HT-29 cells were treated with 100 μg/mL gentamicin for 30 min prior to lysis with Triton X-100. Intracellular Salmonella Typhimurium were enumerated by serial plating.
In vitro inhibition of Salmonella by L. reuteri
A bacterial co-culture system was performed as previously described [18]. Briefly, 5% GOS was added to a mixture of LB and MRS media containing 107 CFU of L. reuteri (BNCC 186135) and 106 CFU of Salmonella Typhimurium and incubated at 37 °C for 24 h. Salmonella were then enumerated by serial plating.
Fecal microbiota analysis and RT-qPCR validation of dominant lactobacilli
Total genomic DNA was extracted from the feces using QIAamp® Fast DNA Stool Mini Kit (Qiagen, Tübingen, Germany). The V3-V4 region of the 16S rRNA gene was amplified using universal primers, pooled in equimolar ratios, and sequenced on the Illumina MiSeq platform to generate paired-end reads. Raw reads were analyzed using QIIME. Briefly, high-quality sequences were clustered into OTUs with 97% similarity and taxonomically classified with an RDP classifier against the SILVA132 16S rRNA gene database with a confidence threshold of 0.70. Data analysis was performed on the Majorbio Cloud Platform (https://www.majorbio.com) as previously described with slight modifications [19]. Principal coordinate analysis (PCoA) was based on the unweighted unifrac distance. The microbial α-diversity analysis included richness (Sobs Index), Shannon Index, and Simpson Index. Wilcoxon rank-sum test was applied for the analysis of microbial differences. Spearman rank correlation analysis was used for the evaluation of correlation between various parameters and the microbiota composition. The results were adjusted by the false discovery rate (FDR) analysis (q < 0.05). Statistical significance was considered if p < 0.05. Differential enrichment of dominant lactobacilli in piglets and mice were further verified by RT-qPCR as previously described [20] using species-specific primers (Supplementary Table S3).
Quantification of short-chain fatty acids (SCFAs) in the feces
The fecal samples of piglets and mice were homogenized in ultrapure water and centrifuged at 5,000 × g for 10 min. Supernatants were diluted and filtered through a 0.22 µm membrane. The filtrates were subjected to Ion Chromatography System (Thermo Fisher Scientific, Massachusetts, USA) for measurement of SCFAs as previously described [21].
Haematoxylin and eosin (H&E) stain and histological score evaluation
Colonic segments of piglets and mice were collected and fixed in 4% phosphate-buffered formalin, processed, and stained with hematoxylin and eosin. The histological score was evaluated under bright fields (100 × and 200 × ) on a Zeiss Axio Imager microscope (Carl Zeiss Microscopy, New York, USA) based on a previous study [22].
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA of the colonic segments was extracted using Trizol (Invitrogen, California, USA), and cDNA was obtained using Prime ScriptTM RT Kit (Takara, Shiga, Japan). RT-qPCR was performed using SYBR Premix Ex Taq TM II (Takara, Shiga, Japan) on a Light Cycler® System (Roche, California, USA) using gene-specific primers (Supplementary Table S4). PCR amplification was performed in duplicate for each sample. The relative expression level of each target gene was calculated using the 2−ΔΔCt method and β-actin as the reference gene as we previously described [23].
RNA-seq and RT-qPCR validation of gene expression
Total RNA was extracted from the colonic segments for subsequent paired-end sequencing on the Illumina HiSeq platform to generate short reads of 150-bp. High-quality clean data were obtained by SeqPrep and Sickle with default parameters and then aligned to the Mus_musculus reference genome (GRCm39) in the orientation mode using HISAT2. Mapped reads were assembled by StringTie. Differentially expressed genes (DEGs) were identified through pairwise comparisons using DESeq2. DEGs with |log2(Fold Change)| > 1.5 and q ≤ 0.05 were considered. Functional enrichment analyses of KEGG were performed at q ≤ 0.05 using Goatools and KOBAS. The expressions of selected DEGs were further validated with RT-qPCR using gene-specific primers (Supplementary Table S5). Data analysis was performed on the Majorbio Cloud Platform (https://www.majorbio.com).
Enzyme-linked immune-sorbent assay (ELISA)
The plasma of mice was obtained from blood samples by centrifuging at 3,000 × g at 4 °C for 10 min. The concentrations of TNF-α, IL-1β, IL-6, and IL-10 were determined using cytokine-specific ELISA kits (Invitrogen, California, USA) according to the manufacturer’s instructions.
Flow cytometry
The colonic segments of mice were minced and digested to individual cells. After centrifugation, the cells were harvested and incubated with antibodies specific to F4/80, CD11b, CD11c, or CD206 (Thermo Fisher Scientific, Carlsbad, USA) for 30 min. Cells were then washed three times and subjected to flow cytometry on BD FACSAria II Cell Sorter (BD Biosciences, New Jersey, USA). F4/80+CD11b+ cells were identified as macrophages and F4/80+CD11b+CD206+ cells as M2 macrophages, while F4/80+CD11b+CD11c+ cells were identified as M1 macrophages.
Western blot
After treatment, RAW264.7 macrophages were lysed in cold RIPA buffer containing a cocktail of protease and phosphatase inhibitors, followed by centrifugation. The protein concentration of each supernatant was quantified by the BCA method and 20 µg proteins were subjected to SDS-PAGE and then transferred onto a PVDF membrane. After blocking in 5% skim milk for 1 h at 37 °C, the membrane was incubated sequentially with a primary antibody (1:2,000) overnight at 4 °C and an HRP-conjugated secondary antibody (1:5,000 dilution) for 1 h at 37 °C. Protein bands were visualized using an enhanced chemiluminescence kit on a FluorChem system (Proteinsimple, California, USA).
Statistical analysis
Data were analyzed using SPSS 20.0 (IBM, USA). Results are reported as means ± SEM (standard errors of the mean). For parametric data, Student’s t-test was used for two-group analysis, and one-way ANOVA was used for multiple groups. For nonparametric data, Mann-Whitney U test or Kruskal-Wallis H test was used for two and multiple groups, respectively. All statistical analyses were considered significant at P < 0.05.
Results
GOS enriches specific lactobacilli in piglets and alleviates Salmonella-induced intestinal disorders in mice
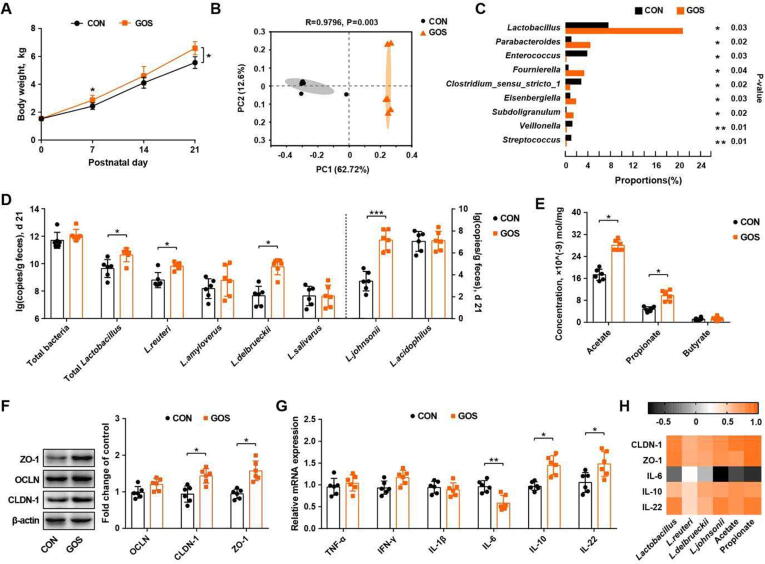
To examine the effect of GOS on growth, intestinal microbiome, and barrier function, newborn piglets was orally administered with GOS daily for a week. GOS increased the body weight of piglets on d 7 and d 21 (Fig. 1 A) and also markedly altered the fecal microbial community of piglets (Fig. 1 B). GOS increased the microbial diversity (Fig. S2 A) and relative abundances of lactobacilli (Fig. 1 C). Among dominant Lactobacillus species, L. delbrueckii, L. reuteri, and L. johnsonii were significantly enriched in GOS-supplemented piglets on both d 8 (Fig. S2 B) and d 21 (Fig. 1 D). Acetate and propionate were also increased in the feces of GOS-fed piglets on d 8 and d 21(Fig. 1 E, Fig. S2 C). Additionally, the intestinal barrier integrity was improved after GOS intervention as evidenced by an upregulation of claudin-1 and ZO-1 mRNAs (Fig. 1 F). The IL-6 expression level was decreased, while IL-10 and IL-22 were increased in the colon of GOS-supplemented piglets (Fig. 1 G). Further, Spearman correlation analysis indicated a positive correlation of relative abundances of L. delbrueckii, L. johnsonii, and L. reuteri with the intestinal barrier and immune-related parameters (Fig. 1 H).
Fig. 1.
Galactooligosaccharides (GOS) enriches specific Lactobacillus species and improves intestinal barrier function of neonatal piglets. (A) Body weight of piglets. (B) Principal coordinates analysis plot of unweighted unifrac distances of the fecal microbiota among different groups of piglets. (C) Differential enrichment analysis of the fecal microbial community. (D) Relative abundances of dominant fecal lactobacilli on d 21. (E) Fecal concentrations of SCFAs on d 21. The expression levels of tight junction proteins (F) and mRNA expression levels of cytokines (G) in the colonic segments are shown. (H) Spearman correlation analysis of lactobacilli, SCFAs, and intestinal barrier parameters. CON, piglets administered saline; GOS, piglets administered with galactooligosaccharides; OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occludin-1, TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; IL-22, interleukin-22. * P<0.05, ** P<0.01.
To directly evaluate the role of GOS-enriched lactobacilli in intestinal health, a combination of L. delbrueckii, L. johnsonii, and L. reuteri strains were supplemented to piglets for 7 days (Fig. S3 A). Similar to GOS-supplemented piglets, body weight, the fecal propionate level, intestinal barrier, and immune response of piglets were all improved (Fig. S3 B-E), suggesting that the benefical effect of GOS is largely mediated through enrichment of lactobacilli.
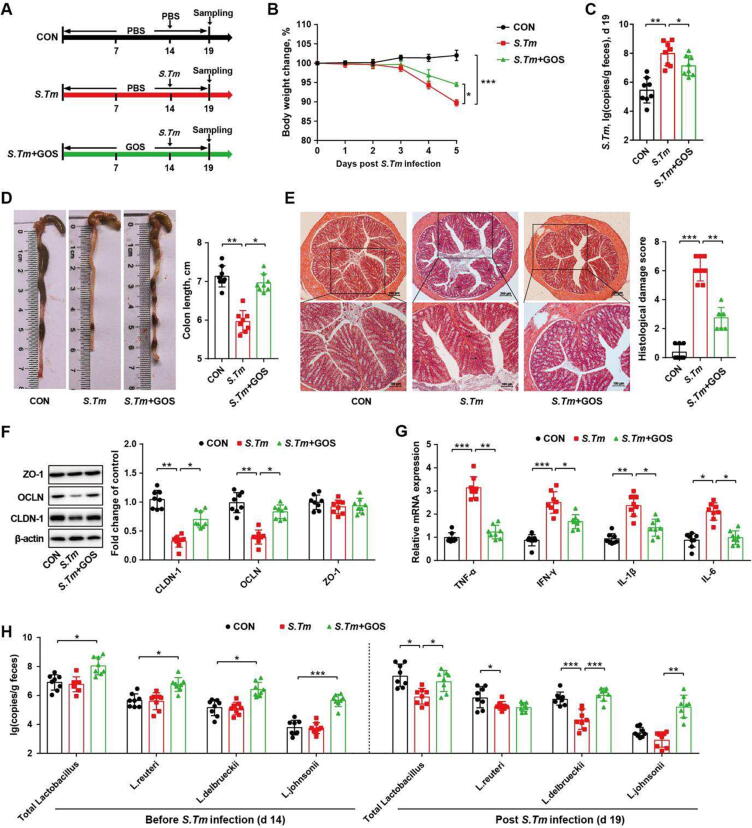
A mouse model of Salmonella infection was further employed to assess the effect of GOS on immune modulation and intestinal barrier function (Fig. 2 A). Consistently, GOS reversed Salmonella-induced weight loss (Fig. 2 B) and the Salmonella burden in infected mice (Fig. 2 C). GOS also alleviated the intestinal pathology in Salmonella-infected mice as evidenced by an increased colon length (Fig. 2 D), reduced histological score (Fig. 2 E), and upregulation of several tight junction protein genes such as claudin-1 and occludin (Fig. 2 F). Dietary GOS also dampened the gene expression of pro-inflammatory cytokines in Salmonella-infected mice (Fig. 2 G). Consistent with GOS-fed piglets, the fecal abundance of L. delbrueckii, L. johnsonii, and L. reuteri were all increased after 14 days of GOS supplementation (Fig. 2 H), while other dominant bacteria were not significantly altered (Fig. S4 A-C). Lactobacilli remained elevated in GOS-fed mice on d 19 (Fig. S4 D, E). The fecal abundance of L. delbrueckii and L. johnsonii (Fig. 2 H) as well as the fecal acetate and propionate levels (Fig. S4 F-I) were increased in GOS-supplemented mice. Furthermore, L. delbrueckii, L. johnsonii, and L. reuteri were positively correlated with enhanced intestinal barrier and immune-related parameters in mice (Fig. S4 J). Collectively, these results suggested that GOS enriches lactobacilli, which in turn promote intestinal health, barrier function, and homeostasis.
Fig. 2.
Dietary GOS enriches specific lactobacilli and alleviates Salmonella infection in mice. (A) Experimental scheme. (B) Body weight changes. (C) Fecal titers of Salmonella on d 19. The colon length (D), histological score (E), tight junction protein expression (F), and cytokine mRNA expression (G) in the colonic segments on d 19 were shown. (H) Relative abundances of different dominant lactobacilli on d 19. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; GOS, mice fed GOS and infected with Salmonella. OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occludin-1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6. * P<0.05, ** P<0.01, *** P<0.001.
L. delbrueckii, but not L. johnsonii or L. reuteri, elevates the intestinal propionate level and alleviates Salmonella infection
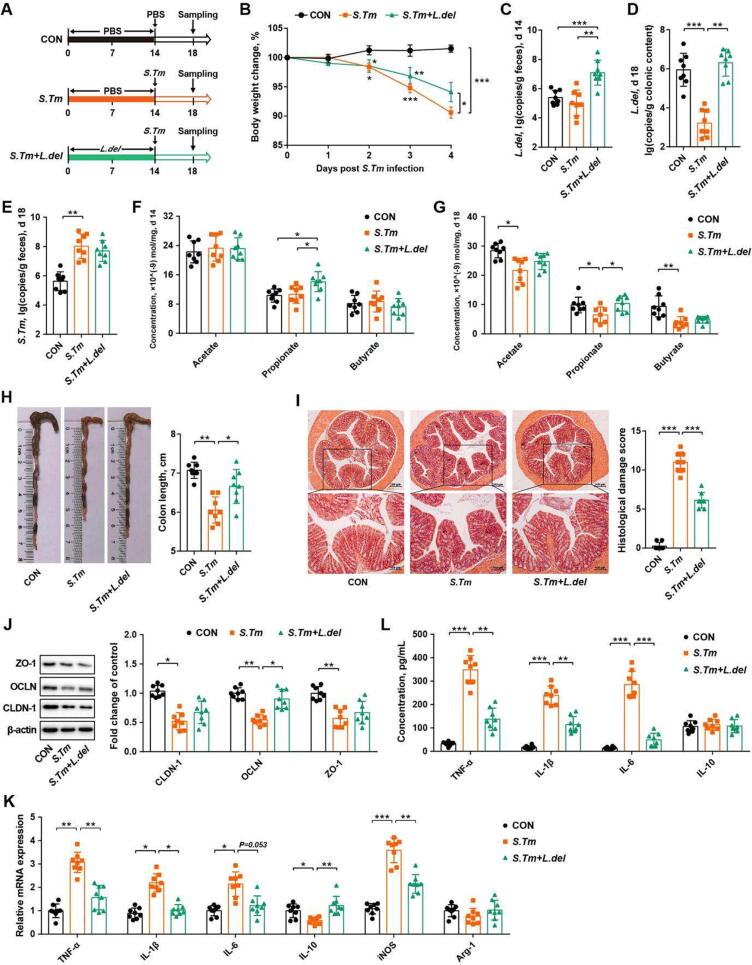
To reveal the impact of individual GOS-enriched lactobacilli on intestinal homeostasis, L. delbrueckii strain (ATCC®BAA 365™, L. del, Fig. 3 A), L. johnsonii strain (BNCC 186110, L. john, Fig. S5 A), or L. reuteri strain (BNCC 186135, L. reu, Fig. S5 C) were administered individually to mice two weeks prior to Salmonella infection. L. delbrueckii (Fig. 3 B), but not L. johnsonii or L. reuteri (Fig. S5 B, D), was found to alleviate weight loss of Salmonella-infected mice. Fecal L. delbrueckii was enriched before (d 14) and after infection (d 19) in L. delbrueckii-supplemented mice (Fig. 3 C, D). Although L. delbrueckii failed to reduce the luminal Salmonella burden in the feces (Fig. 3 E), norank_f_Muribaculaceae and Duboslella were increased (Fig. S5 E, F). Fecal propionate was increased in L. delbrueckii-supplemented mice before and after Salmonella infection (Fig. 3 F, G). Additionally, L. delbrueckii attenuated Salmonella-induced colonic atrophy, intestinal histological damage, and tight junction dysfunction (Fig. 3 H-J). Furthermore, both colonic mRNA expressions and plasma concentrations of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) were reduced by L. delbrueckii (Fig. 3 K, L). The results indicated that L. delbrueckii prevents Salmonella-induced intestinal barrier dysfunction and inflammation mainly by promoting the synthesis of propionate.
Fig. 3.
L. delbrueckii promotes intestinal propionate synthesis and alleviates Salmonella infection in mice. (A) Experimental scheme. (B) Body weight changes. Abundances of L. delbrueckii in the feces on d 14 (C) and d 18 (D) were shown. (E) Fecal titers of Salmonella on d 18. (F) Fecal SCFA levels on d 14. (G) Fecal SCFA levels on d 18. The colon length (H), histological score (I), tight junction protein expression levels (J), and cytokine mRNA expression levels (K) in the colon as well as plasma immune cytokine levels (L) were indicated. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; L. delbrueckii, mice administered with L. delbrueckii for two weeks prior to Salmonella infection. OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occludin-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase. * P<0.05, ** P<0.01, *** P<0.001.
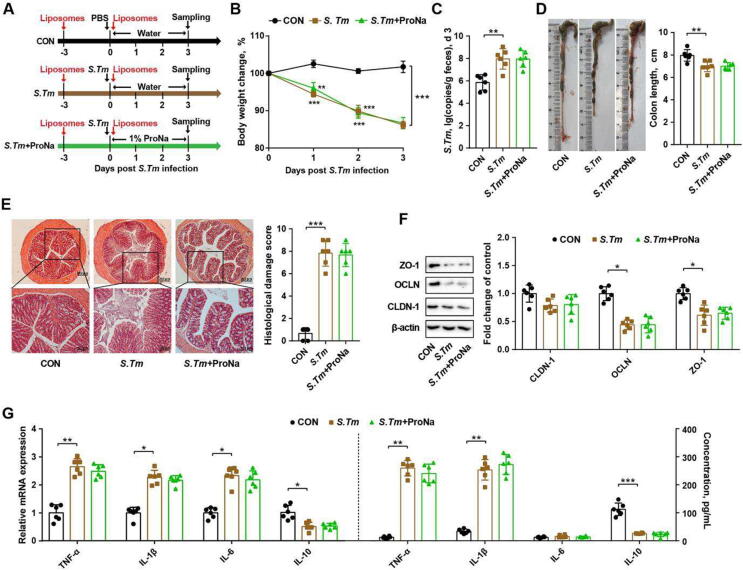
Propionate maintains intestinal homeostasis in a macrophage-dependent manner
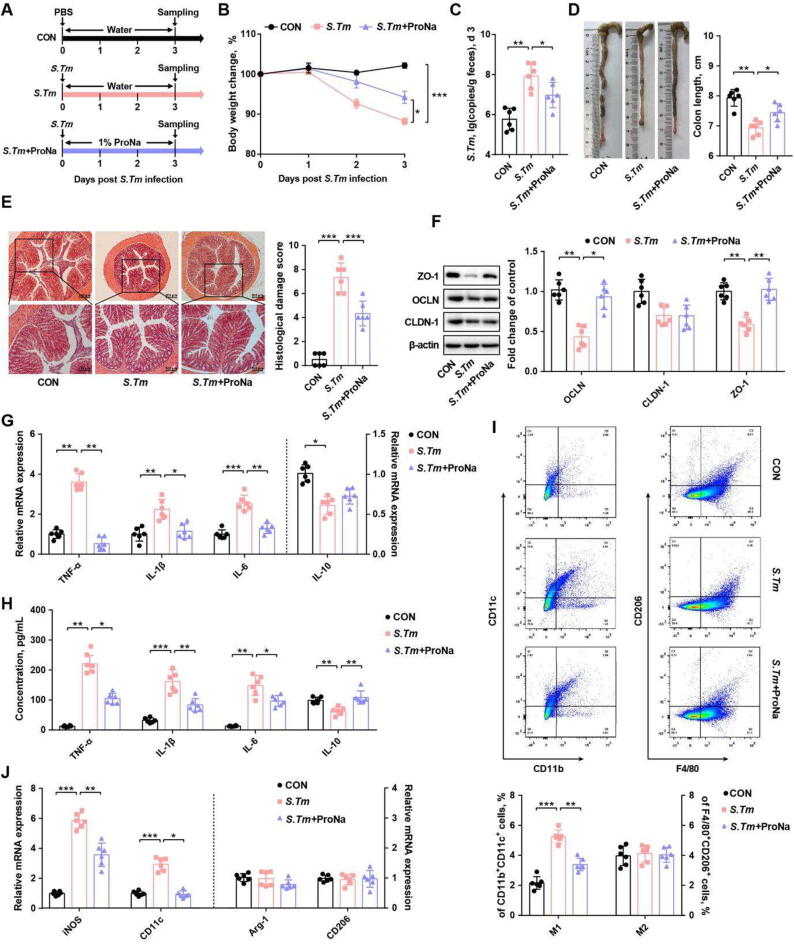
Because the intestinal propionate level was increased in L. delbrueckii-supplemented mice, exogenous propionate (ProNa) was administered to determine whether it could attenuate Salmonella infection (Fig. 4 A). ProNa indeed alleviated Salmonella-induced weight loss and the fecal burden of Salmonella (Fig. 4 B, C). ProNa also markedly increased colon length, mitigated histological damage, and upregulated tight junction protein (claudin-1 and ZO-1) gene expression in Salmonella-infected mice (Fig. 4 D-F). In addition, ProNa attenuated Salmonella-induced inflammation by reducing both colonic gene expression and plasma levels of pro-inflammatory cytokines (Fig. 4 G, H).
Fig. 4.
Propionate ameliorates Salmonella infection in mice and suppresses M1 macrophage polorization. (A) Experimental scheme. (B) Body weight changes. (C) Fecal titers of Salmonella on d 3. The colon length (D), histological score (E), tight junction protein expression levels (F), and cytokine mRNA expression levels (G) in the colon as well as plasma immune cytokine levels (H) were indicated. (I) Percentages of M1 and M2 macrophages in colonic segments. (J) Relative mRNA expression of macrophage polarization markers. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; ProNa, mice received 1% sodium propionate (ProNa) in water after Salmonella infection. OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occludin-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; Arg-1, arginase-1. * P<0.05, ** P<0.01, *** P<0.001.
Upon infection, Salmonella can invade and survive inside macrophages [24]. Macrophage activation and polarization is known to be involved in intestinal pathogen invasion and inflammation [25]. In this study, ProNa suppressed Salmonella-triggered polarization of pro-inflammatory M1 macrophage cells (CD11b+CD11c+, Fig. 4 I) and the gene expression of associated biomarkers (iNOS and CD11c, Fig. 4 J). To further examine whether macrophages are required for propionate to attenuate Salmonella-induced intestinal inflammation, macrophages were depleted in mice by intraperitoneal injection with clodronate liposomes (Fig. 5 A, Fig. S6 A). The protective effect of ProNa on attenuating weight loss and reducing fecal Salmonella burden was abrogated in macrophage-depleted mice (Fig. 5 B, C). Salmonella-induced colonic shortening, intestinal histological damage, and impaired tight junction also failed to be reversed by ProNa treatment in the absence of macrophages (Fig. 5 D-F). In addition, ProNa failed to suppress colonic and plasma pro-inflammatory cytokine expression in macrophage-depleted mice (Fig. 5 G). These results highlighted an essential role of macrophages in propionate-mediated alleviation of Salmonella-induced intestinal disorders and inflammation.
Fig. 5.
Macrophage depletion abrogates the protective effect of propionate against Salmonella infection in mice. (A) Experimental scheme. (B) Body weight changes. (C) Fecal titers of Salmonella on d 3. The colon length (D), histological score (E), tight junction protein expression levels (F), and cytokine mRNA expression levels (G) in the colon as well as plasma immune cytokine levels (H) were indicated. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; ProNa, mice received 1% sodium propionate (ProNa, w/v) in water after Salmonella infection. OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occluden-1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10. * P<0.05, ** P<0.01, *** P<0.001.
To directly verify whether macrophages are also responsible for L. delbrueckii-mediated alleviation of Salmonella infection, L. delbrueckii was supplemented to mice prior to macrophage depletion and Salmonella challenge (Fig. S6 B). The results indicated that L. delbrueckii failed to reverse weight loss and the fecal Salmonella burden in macrophage-depleted mice (Fig. S6 C, D). L. delbrueckii also failed to attenuate Salmonella-induced tight junction impairment and pro-inflammatory cytokine expression in the absence of macrophages (Fig. S6 E, F). Additionally, L. delbrueckii lost the ability to reverse Salmonella-triggered suppression of propionate when macrophages were depleted (Fig. S6 G). These results indicated that L. delbrueckii-induced propionate alleviates Salmonella infection in a macrophage-dependent manner.
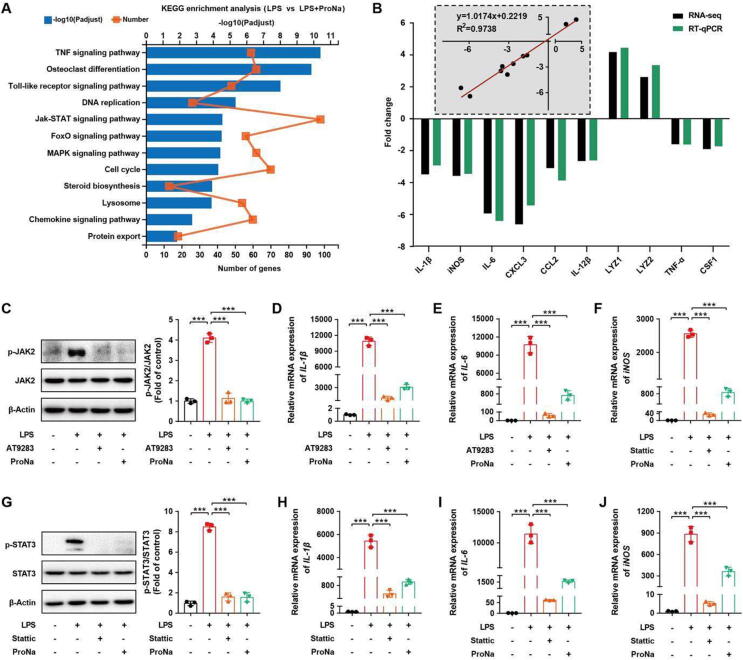
Propionate mitigates inflammation in macrophages via inhibiting JAK2-STAT3 signaling
To understand anti-inflammatory signaling mechanisms of propionate, RNA-seq was performed on LPS-challenged RAW264.7 macrophages. A number of differentially expressed genes (DEGs) were identified in response to propionate (Fig. S7 A-C). KEGG pathway annotations revealed that major DEGs belonged to the JAK-STAT signaling pathway (Fig. 6 A, Fig. S7 D). Furthermore, RT-qPCR validated the RNA-seq results and showed that DEGs in LPS-challenged macrophages were markedly reversed by ProNa (Fig. 6 B; Fig. S7 E, F). To confirm the role of the JAK-STAT signaling pathway in ProNa-mediated anti-inflammation, RAW264.7 macrophages were treated with AT9283 or Stattic, two specific inhibitors for JAK2 and STAT3, respectively, prior to LPS challenge. Similar to AT9283 or Stattic, ProNa inhibited the phosphorylation of JAK2 (p-JAK2) and STAT3 (p-STAT3) and induction of pro-inflammatory cytokines, IL-1β, IL-6, and iNOS (Fig. 6 C-J). These results indicated that ProNa attenuates LPS-triggered inflammation mainly by inhibiting the JAK2-STAT3 signaling in macrophages.
Fig. 6.
Propionate suppresses LPS-induced inflammation in RAW264.7 macrophages via the JAK2-STAT3 signaling pathway. (A) KEGG analysis of differentially enriched pathways by propionate. (B) Fold change of selected genes by RNA-Seq and the correlation analysis of selected genes between the RT-qPCR and RNA-Seq results. Western blot analysis of p-JAK2 (C) and p-STAT3 (G) as well as the relative mRNA expression of pro-inflammatory cytokines (D-F, H-J). IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; IL-6, interleukin-6. *** P< 0.001.
The gene expression of GPR43, a major SCFA receptor, was further evaluated and found to be decreased in Salmonella-infected mice and LPS-treated macrophages, but reversed by propionate (Fig. S8 A, B). In the presence of a GPR43 antagonist (GLPG0974), propionate failed to suppress the JAK2-STAT3 signaling and pro-inflammatory cytokine gene expression (Fig. S8 C-F), suggesting that GPR43 is mainly involved in regulating propionate-mediated JAK2-STAT3 signaling in macrophages.
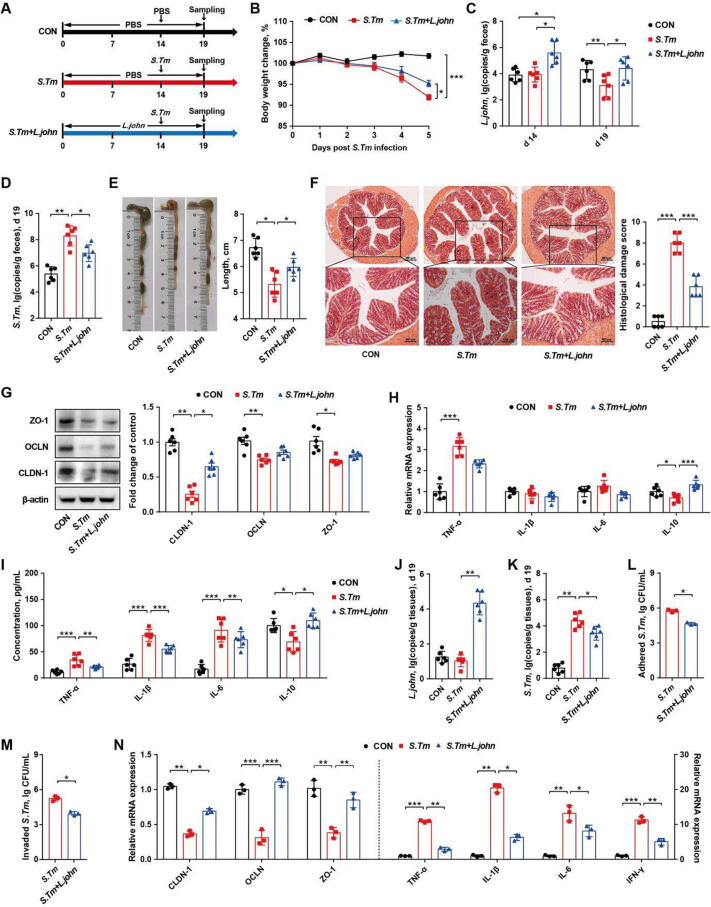
L. johnsonii, but not L. reuteri, alleviates Salmonella infection by inhibiting pathogen adhesion and invasion
Although L. johnsonii and L. reuteri failed to prevent Salmonella infection, we next examined whether both Lactobacillus species could alleviate Salmonella infection by providing them during Salmonella challenge (Fig. 7 A, Fig. S9 A). Interestingly, L. johnsonii, but not L. reuteri, was able to attenuate weight loss of Salmonella-infected mice (Fig. 7 B, Fig. S9 B). L. johnsonii was increased in the feces of L. johnsonii-treated mice (Fig. 7 C) and the fecal burden of Salmonella was also reduced (Fig. 7 D). In addition, L. johnsonii, but not L. reuteri, attenuated Salmonella-induced intestinal barrier dysfunction by reducing colonic pathology, upregulating tight junction protein gene expression (Fig. 7 E-G), and decreasing the colonic and plasma levels of pro-inflammatory cytokines in Salmonella-infected mice (Fig. 7 H, I).
Fig. 7.
L. johnsonii alleviates Salmonella infection by inhibiting pathogenic adhesion and invasion of intestinal epithelial cells. (A) Experimental scheme. (B) Body weight changes. (C) Fecal titers of Salmonella on d 14. (D) Fecal titers of Salmonella on d 19. The colon length (E), histological score (F), tight junction protein expression levels (G), and cytokine mRNA expression levels (H) in the colon as well as plasma immune cytokine levels (I) were indicated. Copies of L. johnsonii (J) and Salmonella (K) on the colonic mucosa on d 19. Adhesive (L) and invasive (M) abilities of Salmonella to the epithelial cells. (N) Relative mRNA expression of tight junction proteins and immune cytokines in HT-29 cells. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; L. john, mice received both L. johnsonii and Salmonella. OCLN, occludin; CLDN-1, claudin-1; ZO-1, zonula occluden-1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10. * P<0.05, ** P<0.01, *** P<0.001.
In addition to being taken up by macrophages, Salmonella also adheres and invades epithelial cells, thereby triggering inflammation and barrier dysfunction [26]. We found that mucosa-associated L. johnsonii was increased, while the Salmonella burden was decreased in L. johnsonii-treated mice (Fig. 7 J, K), implying that L. johnsonii and Salmonella might compete with each other to colonize in the intestinal mucosa. Consistently, L. johnsonii-treated epithelial cells markedly suppressed Salmonella adhesion and invasion (Fig. 7 L, M). In addition, Salmonella- induced pro-inflammatory cytokine gene expression and impairment of epithelial barrier function were alleviated by L. johnsonii (Fig. 7 N). These results suggested that L. johnsonii protects mice from Salmonella infections through competitive exclusion by inhibiting Salmonella adhesion and invasion of epithelial cells.
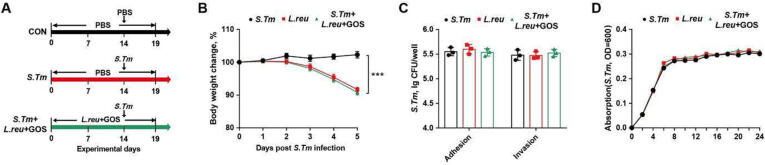
L. reuteri fails to alleviate Salmonella infection in mice
Probiotics and prebiotics have been reported to synergistically amply their individual beneficial effect against intestinal infection [27]. L. reuteri was enriched by GOS, but failed to either prevent or treat Salmonella infection (Fig. S5 C, D; Fig. S9 A, B). To evaluate a possible synergy between L. reuteri and GOS, mice was supplemented with both L. reuteri (BNCC 186135) and GOS, followed by Salmonella infection (Fig. 8 A). However, body weight loss could not be reversed by the combination (Fig. 8 B). Furthermore, cell culture experiments also confirmed a combination of L. reuteri (BNCC 186135) and GOS failed to suppress the adhesion and invasion of Salmonella to epithelial cells (Fig. 8 C) or Salmonella growth (Fig. 8 D). Taken together, these results showed that, although it is induced by GOS, L. reuteri provides little benefits to mitigating Salmonella infection.
Fig. 8.
L. reuteri failes to alleviate Salmonella infection in mice. (A) Experimental scheme. (B) Body weight changes. (C) Adhesive and invasive abilities of Salmonella to epithelial cells. (D) Proliferation of Salmonella co-cultured with L. reuteri. OD600 was read after 12-h incubation. CON, mice treated with PBS; S. Tm, Salmonella-infected mice; L. reu + GOS, mice received L. reuteri and GOS with Salmonella infection. GOS, galactooligosaccharides. *** P<0.001.
Discussion
As a prebiotic, GOS selectively stimulates the growth of resident Lactobacillus and Bifidobacterium to elicit health benefits or shape the microbial community through cross feeding [28], [29]. Therefore, the intestinal health-promoting properties of prebiotic GOS has been receiving growing attention [30], [31], however, the underlying mechanism of GOS remains largely unknown. In this study, we have found that GOS improves the intestinal health of piglets and mice through specific enrichment of L. delbrueckii, L. johnsonii, and L. reuteri, consistent with a multitude of benefits that Lactobacillus provides to the host [32], [33], [34]. However, different species and even different strains vary greatly in their mode of action [17], [32]. Consistently, we revealed a differential involvement of GOS-enriched lactobacilli in Salmonella infection. L. delbrueckii strain promotes intestinal propionate synthesis, which in turn contributes to alleviate Salmonella-induced intestinal disorders by suppressing JAK2-STAT3 signaling and M1 macrophage polarization. On the other hand, L. johnsonii strain alleviates Salmonella infection through competitive exclusion by inhibiting the adhesion and invasion of Salmonella to epithelial cells. However, L. reuteri strain is incapable of enhancing either propionate production or competitive exclusion, providing little protection against Salmonella infection.
In this study, we observed that GOS promotes the growth and health of piglets by enriching specific lactobacilli species and associated microbial SCFAs (acetate and propionate). In agreement with previous studies that GOS can improve gastrointestinal microbiota and barrier function [29], [30], [31], we observed GOS promotes tight junction protein expression in piglets and Salmonella-infected mice. Additionally, GOS-mediated downregulation of pro-inflammatory cytokine genes and upregulation of anti-inflammatory cytokine genes is also a good indication of improved immunological defense and barrier function in piglets and mice.
SCFAs are the key microbial health-promoting bacterial metabolites. Acetate, propionate, and butyrate in particular contribute to regulating intestinal barrier function, immunity, and metabolism [35]. To our surprise, intestinal propionate, was increased in L. delbrueckii-supplemented mice. Thus, we hypothesized that propionate is a major metabolite responsible for amelioration of intestinal inflammation and dysbiosis. Indeed, supplementation of exogenous propionate attenuates intestinal disorders by upregulating major genes involved in tight junction such as claudin-1 and ZO-1 and suppressing pro-inflammatory cytokine expression.
Intestinal macrophages are a key component of the innate immune system responsible for shaping the inflammatory microenvironment and protection against infections [36]. Macrophages can be polarized into either classically activated macrophages (M1) or alternatively activated macrophages (M2), with the former being mainly pro-inflammatory and the latter being anti-inflammatory [25]. Consistent with a previous study [37], we observed that Salmonella-triggered intestinal inflammation is attenuated by exogenous propionate through reduced M1 macrophage polarization and pro-inflammatory cytokine expression. We further confirmed that macrophages mediate much of the beneficial effect of propionate. NF-κB and JAK/STAT pathways are among the major signaling pathways involved in inflammation [38]. NF-κB signaling is also critical for the induction of STAT3 and IL-6 [39]. In the present study, we found that propionate blocks the activation of both NF-κB and STAT3 signaling, thus decreasing pro-inflammatory cytokine expression (IL-1β, IL-6, and iNOS) in macrophages. Because of a central role of macrophages in intestinal infection and inflammation, it is tempting to speculate the potential benefit of modulating macrophage functions in intestinal disorders such as inflammatory bowel disease [40].
Upon infection, Salmonella first adheres to the intestinal mucosal surface, followed by invasion into epithelial cells using the pathogenicity island-1 type III secretion system [41]. Hence, inhibition of adhesion and invasion of epithelial cells is the first step to prevent Salmonella infection [42]. We found that L. johnsonii strain attenuates Salmonella infection mainly through competitive exclusion by blocking the adhesion and invasion of Salmonella to intestinal epithelial cells. However, although it is drastically induced by GOS, L. reuteri strain is incapable of protecting mice from Salmonella infection because it has no capacity to promote propionate synthesis or preventing epithelial adhesion or invasion of Salmonella.
A major finding of this study is the revelation of differential roles of lactobacilli in protection of animals against Salmonella infection. L. delbrueckii strain, but not L. johnsonii strain or L. reuteri strain, elevates luminal propionate through cross-feeding with SCFA-producing bacteria. Consistently, L. delbrueckii has been found to be enriched with the genes for nutrient metabolism [43], resulting in the synthesis of necessary substrates to promote the production of propionate by SCFA producers. Among three GOS-enriched Lactobacillus species, only L. johnsonii strain is capable of inhibiting Salmonella adhesion and invasion of epithelial cells. It is plausible that the presence of unique extracellular molecules on L. johnsonii such as exopolysaccharides [44], S-layer protein [45], and elongation factor thermal unstable protein (EF-Tu) [46] might be involved in the competitive exclusion of Salmonella.
However, it is noteworthy that our conclusion was only based on our observations with one specific Lactobacillus strain in each species. Whether other strains of the same species act in the same remains to be investigated. For example, L. reuteri (BNCC 186135) fails to alleviate intestinal inflammation in our study, multiple other strains of L. reuteri have demonstrated anti-inflammatory and antimicrobial activities [47], [48]. Therefore, it is possible both species- and strain-specific host-modulatory effect of different lactobacilli exist. It is also noted that a mouse model of Salmonella infection was used in our study. Whether L. delbrueckii and L. johnsonii utilize the same mechanism against Salmonella to mitigate other intestinal pathogens and disorders warrants further investigation.
Conclusion
GOS improves intestinal health in piglets and Salmonella-infected mice by specific enrichment of L. delbrueckii, L. johnsonii, and L. reuteri. Three lactobacilli strains protect animals against Salmonella infection through different mechanisms. L. delbrueckii strain (ATCC®BAA 365™), but not L. johnsonii or L. reuteri, ameliorates Salmonella-induced intestinal inflammation by enhancing propionate synthesis to suppress the JAK2-STAT3 signaling and M1 macrophage polarization. L. johnsonii strain (BNCC 186110), on the other hand, inhibited Salmonella adhesion and invasion of epithelial cells through competitive exclusion. However, L. reuteri strain (BNCC 186135) failed to promote propionate synthesis or exert competitive exclusion, thus incapable of protecting mice against Salmonella infection. Our results provide novel insights into the mechanism of action of GOS and individual lactobacilli species in the control and prevention of intestinal inflammatory disorders.
Funding
This research was supported by the National Natural Science Foundation of China (32125036, 31902170, and 31972596), the National Key Research and Development Program (2021YFD1300201), China Postdoctoral Science Foundation (2022M723423), the earmarked fund for the China Agriculture Research System (CARS-35), and the Higher Education Discipline Innovation Project (B16044).
Compliance with Ethics
All experiments involving animals were conducted according to the ethical policies and procedures approved by the Institutional Animal Care and Use Committee of China Agricultural University, China (Approval No. AW07040202-1).
CRediT authorship contribution statement
Yujun Wu: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Xiangyu Zhang: Data curation, Formal analysis, Investigation. Xiaoyi Liu: Formal analysis, Investigation, Methodology. Yi Li: Formal analysis, Investigation, Methodology. Dandan Han: Investigation, Methodology, Validation, Visualization, Writing – review & editing. Yu Pi: Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. Melanie A. Whitmore: Writing – review & editing. Xingmiao Lu: Formal analysis, Investigation, Methodology. Guolong Zhang: Writing – review & editing. Jinkai Zheng: Formal analysis, Investigation, Methodology. Junjun Wang: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We appreciated Prof. Jiangchao Zhao from University of Arkansas for the revision of the manuscript.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.03.001.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Gibson G., Hutkins R., Sanders M., Prescott S., Reimer R., Salminen S., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastro Hepat. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 2.Hoeflinger J., Kashtanov D., Cox S., Dowd S., Jouni Z., Donovan S., et al. Characterization of the intestinal Lactobacilli community following galactooligosaccharides and polydextrose supplementation in the neonatal piglet. PLoS One. 2015;10:e0135494. doi: 10.1371/journal.pone.0135494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuki T., Tajima S., Hara T., Yahagi K., Ogawa E., Kodama H. Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants. Benef Microbes. 2016;7:453–461. doi: 10.3920/BM2015.0168. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Zhang X., Han D., Ye H., Tao S., Pi Y., et al. Short administration of combined prebiotics improved microbial colonization, gut barrier, and growth performance of neonatal piglets. ACS Omega. 2020;5:20506–20516. doi: 10.1021/acsomega.0c02667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopalakrishnan A., Clinthorne J., Rondini E., McCaskey S., Gurzell E., Langohr I., et al. Supplementation with galacto-oligosaccharides increases the percentage of NK cells and reduces colitis severity in Smad3-deficient mice. J Nutr. 2012;142:1336–1342. doi: 10.3945/jn.111.154732. [DOI] [PubMed] [Google Scholar]
- 6.Searle L.E.J., Cooley W.A., Jones G., Nunez A., Crudgington B., Weyer U., et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium adhesion and invasion in vitro and in vivo. J Med Microbiol. 2010;59:1428–1439. doi: 10.1099/jmm.0.022780-0. [DOI] [PubMed] [Google Scholar]
- 7.Sangwan V., Tomar S., Singh R., Singh A., Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- 8.Ost K., Round J. Communication between the microbiota and mammalian immunity. Annu Rev Microbiol. 2018;72:399–422. doi: 10.1146/annurev-micro-090817-062307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Q., Yuan L., Deng J., Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Microbiol. 2015;5:26. doi: 10.3389/fcimb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T., Sun H., Chen J., Luo L., Gu Y., Wang X., et al. Anti-adhesion effects of Lactobacillus strains on caco-2 cells against Escherichia coli and their application in ameliorating the symptoms of dextran sulfate sodium-induced colitis in mice, Probiotics Antimicrob. Proteins. 2021;13:1632–1643. doi: 10.1007/s12602-021-09774-8. [DOI] [PubMed] [Google Scholar]
- 11.Hai D., Lu Z., Huang X., Lv F., Bie X. In vitro screening of chicken-derived Lactobacillus strains that effectively inhibit Salmonella colonization and adhesion. Foods. 2021;10:569. doi: 10.3390/foods10030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervantes-Barragan L., Chai J., Tianero M., Luccia B., Ahern P., Merriman J., et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang S., Hyam S., Han M., Kim S., Lee B., Kim D. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J Appl Microbiol. 2013;115:888–896. doi: 10.1111/jam.12273. [DOI] [PubMed] [Google Scholar]
- 14.Aditya A., Peng M., Young A., Biswas D. Antagonistic mechanism of metabolites produced by Lactobacillus casei on lysis of enterohemorrhagic Escherichia coli. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.574422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X., Lee A., Huang S., Gao J., Spence J., Owyang C. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes. 2019;10:59–76. doi: 10.1080/19490976.2018.1479625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., Ma L., Zhao Z., Luo S., Gong B., Li J., et al. IL-25-induced shifts in macrophage polarization promote development of beige fat and improve metabolic homeostasis in mice. PLoS Biol. 2021;19:e3001348. doi: 10.1371/journal.pbio.3001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Hu D., Chen Y., Huang H., Zhang H., Zhao J., et al. Strain-specific properties of Lactobacillus plantarum for prevention of Salmonella infection. Food Funct. 2018;9:3673–3682. doi: 10.1039/c8fo00365c. [DOI] [PubMed] [Google Scholar]
- 18.de Keersmaecker S., Verhoeven T., Desair J., Marchal K., Vanderleyden J., Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett. 2006;259:89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 19.Li N., Zuo B., Huang S., Zeng B., Han D., Li T., et al. Spatial heterogeneity of bacterial colonization across different gut segments following inter-species microbiota transplantation. Microbiome. 2020;8:161. doi: 10.1186/s40168-020-00917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N., Huang S., Jiang L., Dai Z., Li T., Han D., et al. Characterization of the early life microbiota development and predominant Lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front Microbiol. 2019;10:797. doi: 10.3389/fmicb.2019.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Zhang X., Pi Y., Han D., Feng C., Zhao J., et al. Maternal galactooligosaccharides supplementation programmed immune defense, microbial colonization and intestinal development in piglets. Food Funct. 2021;12:7260–7270. doi: 10.1039/d1fo00084e. [DOI] [PubMed] [Google Scholar]
- 22.Schultz B., Salazar G., Paduro C., Pardo-Roa C., Pizarro D., Salazar-Echegarai F., et al. Persistent Salmonella enterica serovar Typhimurium infection increases the susceptibility of mice to develop intestinal inflammation. Front Immunol. 2018;9:1166. doi: 10.3389/fimmu.2018.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y., Zhang X., Tao S., Pi Y., Han D., Ye H., et al. Maternal supplementation with combined galactooligosaccharides and casein glycomacropeptides modulated microbial colonization and intestinal development of neonatal piglets. J Funct Foods. 2020;74 doi: 10.1016/j.jff.2020.104170. [DOI] [Google Scholar]
- 24.Xie Z., Zhang Y., Huang X. Evidence and speculation: the response of Salmonella confronted by autophagy in macrophages. Future Microbiol. 2020;15:1277–1286. doi: 10.2217/fmb-2020-0125. [DOI] [PubMed] [Google Scholar]
- 25.Murray P. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 26.LaRock D., Chaudhary A., Miller S. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner S., Chassard C., Zihler B., Lacroix C. Synergistic effects of Bifidobacterium thermophilum RBL67 and selected prebiotics on inhibition of Salmonella colonization in the swine proximal colon PolyFermS model. Gut Pathog. 2014;6:44. doi: 10.1186/s13099-014-0044-y3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez J., Moreno F., Olano A., Clemente A., Villar C., Lombo F. A galacto-oligosaccharides preparation derived from lactulose protects against colorectal cancer development in an animal model. Front Microbiol. 2018;9:2004. doi: 10.3389/fmicb.2018.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang F., Wei J., Lu Y., Sun Y., Wang Q., Zhang R. Galacto-oligosaccharides modulate gut microbiota dysbiosis and intestinal permeability in rats with alcohol withdrawal syndrome. J Funct Foods. 2019;60 doi: 10.1016/j.jff.2019.103423. [DOI] [Google Scholar]
- 30.Wang J., Tian S., Yu H., Wang J., Zhu W. Response of colonic mucosa-associated microbiota composition, mucosal immune homeostasis, and barrier function to early life galacto-oligosaccharides intervention in suckling piglets. J Agric Food Chem. 2019;67:578–588. doi: 10.1021/acs.jafc.8b05679. [DOI] [PubMed] [Google Scholar]
- 31.Tian S., Wang J., Yu H., Wang J., Zhu W. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J Anim Sci Biotechnol. 2018;9:75. doi: 10.1186/s40104-018-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos C., Thorsen L., Schwan R., Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013;36:22–29. doi: 10.1016/j.fm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Liu Z., Li Y., Yang L., Yin J., He J., et al. Effects of dietary supplementation of Lactobacillus delbrueckii on gut microbiome and intestinal morphology in weaned piglets. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.692389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh A., de Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 36.Wynn T., Chawla A., Pollard J. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J., Kim H., Kim M., Jeong S., Yun C., Han S. Short-chain fatty acids inhibit staphylococcal lipoprotein-induced nitric oxide production in murine macrophages. Immune Netw. 2019;19:e9. doi: 10.4110/in.2019.19.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayele T., Muche Z., Teklemariam A., Kassie A., Abebe E. Role of JAK2/STAT3 signaling pathway in the tumorigenesis, chemotherapy resistance, and treatment of solid tumors: A systemic review. J Inflamm Res. 2022;15:1349–1364. doi: 10.2147/JIR.S353489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y., Dai Z., Sun S., Ma X., Yang Y., Tso P., et al. Hydroxyproline attenuates dextran sulfate sodium-induced colitis in mice: Involvment of the NF-κB signaling and oxidative stress. Mol Nutr Food Res. 2018;62:e1800494. doi: 10.1002/mnfr.201800494. [DOI] [PubMed] [Google Scholar]
- 40.Duan B., Shao L., Liu R., Msuthwana P., Hu J., Wang C. Lactobacillus rhamnosus GG defense against Salmonella enterica serovar Typhimurium infection through modulation of M1 macrophage polarization. Microb Pathog. 2021;156 doi: 10.1016/j.micpath.2021.104939. [DOI] [PubMed] [Google Scholar]
- 41.Velge P., Wiedemann A., Rosselin M., Abed N., Boumart Z., Chausse A., et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiologyopen. 2012;1:243–258. doi: 10.1002/mbo3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birhanu B., Park N., Lee S., Hossain M., Park S. Inhibition of Salmonella Typhimurium adhesion, invasion, and intracellular survival via treatment with methyl gallate alone and in combination with marbofloxacin. Vet Res. 2018;49:101. doi: 10.1186/s13567-018-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makarova K., Slesarev A., Wolf Y., Sorokin A., Mirkin B., Koonin E., et al. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A. 2006;103:15611–15616. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dertli E., Mayer M.J., Narbad A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol. 2015;15:8. doi: 10.1186/s12866-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Zhang L., Ma W., Yi H., Yang X., Du M., et al. Screening of probiotic lactobacilli for inhibition of Shigella sonnei and the macromolecules involved in inhibition. Anaerobe. 2012;18:498–503. doi: 10.1016/j.anaerobe.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Granato D., Bergonzelli G.E., Pridmore R.D., Marvin L., Rouvet M., Corthésy-Theulaz I.E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect Immun. 2004;72:2160–2169. doi: 10.1128/IAI.72.4.2160-2169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D., Li R., Li J. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res Vet Sci. 2012;93:366–373. doi: 10.1016/j.rvsc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Mu Q., Tavella V.J., Luo X. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. 2018;9:757. doi: 10.3389/fmicb.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.