Abstract
Background
Blood-based immune-inflammation indexes have been widely used to predict survival in a variety of cancers. In this research, we seeked to evaluate a novel immune-inflammation marker, named the pan-immune-inflammation value (PIV), in patients with nasopharyngeal carcinoma (NPC) undergoing definitive radiotherapy.
Methods
A group of 377 patients with NPC was retrospectived analyzed. Clinical data and laboratory data were collected. Receiver operating characteristic (ROC) curve analysis was performed in order to determine the optimal PIV cut-off value. Survival curves were estimated by Kaplan-Meier method, and prognostic variables were identified using a Cox regression model. Additionally, we developed a nomogram and assessed its acuracy using the concordance index (C-index) and a calibration curve.
Results
The optimal PIV cut-off value was 146.24 according to ROC analysis. High PIV was related to poorer Eastern Cooperative Oncology Group Performance Status (ECOG PS) score (p = 0.017), more advanced T (p<0.001) and clinical stages (p = 0.024). In univariate analysis, older Age, poorer ECOG PS, higher Epstein–Barr virus DNA (EBV-DNA), advanced T, N and clinical stage, and higher PIV levels were related to patients' poorer overall survival (OS). Poorer ECOG PS, higher EBV-DNA, later T stage, later clinical stage, and higher PIV were associated with patients' poorer progression free survival (PFS). Male sex and later T stage were associated with patients' poorer locoregional recurrence free survival (LRRFS). Poorer ECOG PS, higher EBV-DNA, later T stage, later clinical stage, and higher PIV were associated with patients’ poorer distant metastasis free survival (DMFS). Multivariate analysis demonstrated that PIV was an independent prognostic index for OS (HR 2.231, 95 % CI 1.241–4.011, P = 0.007), PFS (HR 1.664, 95 % CI 1.003–2.760, P = 0.049), and DMFS(HR 2.081, 95 % CI 1.071–4.044, P = 0.031). Nomogram C-indexes for the nomogram of OS were 0.684, and PFS were 0.62, respectively. Survival predictions and actual survival were consistent according to the calibration curve.
Conclusions
Pre-treatment PIV is a promising biomarker for predicting survival in patients with NPC.
Keywords: Pan-immune-inflammation value, Nasopharyngeal carcinoma, Prognosis, Nomogram
1. Introduction
Nasopharyngeal carcinoma (NPC), a type of squamous cell cancer, exhibits a distinct geographical prevalence, with over three-quarters of cases occurring predominantly in Southern and Southeast Asia [1]. Most patients with NPC (75.4 %) are diagnosed with locally advanced disease [2], and the standard treatment regimen at this stage is concurrent chemoradiotherapy (CCRT) [3]. Although 15%–30 % of patients with locally advanced NPC will relapse or develop metastases [4], there is no standardized prognostic predictor for these patients. Prognostic biomarkers are urgently needed to help optimize treatment.
Research is increasingly highlighting the association between systemic inflammation and carcinogenesis and cancer prognosis [5]. Complete blood cell counts are research hotspots since they can reveal the underlying inflammation. Studies indicate that ratios such as neutrophil-to-lymphocyte ratio (NLR) platelet-to-lymphocyte ratio (PLR), have been identified as significant prognostic indicators in a vartiety of cancer types [[6], [7], [8]]. The pan-immune-inflammatory value (PIV) is a novel biomarker of systemic inflammation, formulated using complete blood counts. Research indicates that PIV serves as an indicator of the balance between the immune response and inflammatory state, and has potential in prognosticating outcomes in cancer patients. PIV is reported to be a valuable prognostic predictor for lung cancer [9], melanoma [10], and breast cancer [11], etc. However, research on the prognstic significance of PIV in cases of locally advanced NPC remains sparse.
Inflammation is an important component of tumor microenvironment (TME), and the changes in inflammatory cells may affect tumor development. Given that the PIV mirrors the immune and inflamatory conditions within a tumor, it holds potential as a prognositic indicator in NPC. To explore this potential, we carried out a retrospective investigation into PIV's prognostic relevance in NPC patients. Additionally, we developed a nomogram to aid in predicting outcomes for these patients based on PIV values.
2. Methods and materials
2.1. Patients
A cohort of 377 patients with NPC was retrospectively analyzed. All patients were diagnosed at the Second Xiangya Hospital, Central South University, between July 2014 and December 2019. The patients in our study were identified as having NPC, their disease staging was determined based on the criteria set forth in the 8th edition of the American Joint Committee on Cancer (AJCC) Staging System, specially tailored for nasopharyngeal carcinoma. Patients who have historys of autoimmune diseases, chronic inflammatory diseases, or acute infectious diseases, were excluded. This study adhered to the ethical standards of the 1975 Declaration of Helsinki, as revised in 2008, and received approval from the Second Xiangya Hospital at Central South University. This compliance underscores the study's commitment to ethical research practices.
2.2. Data collection
We meticulously gathered clinical, pathological, and laboratory data from hospital records. This included each patients’ age, Eastern Cooperative Oncology Group performance status (ECOG PS) score, sex, T and N stages, overall clinical stage, height, weight, treatment approach, and pre-treatment counts of neutrophils, lymphocytes, platelets, and monocytes, and EBV-DNA levels. For calculating the PIV, we used the formula: PIV= (neutrophil count × platelet count × monocyte count)/lymphocyte count [12].
2.3. RT procedure
All participants in the study underwent intensity-modulated radio-therapy (IMRT). The definition of gross and clinical tumor volume adhered to specific guidelines [13]. Radiation doses were tailored as follows: 70Gy for the primary tumor, 60Gy for planning target volume (PTV) 1, and 54Gy for PTV2. Patients received a daily radiation dose ranging from 1.82 to 2.12Gy daily, five days a week. Depending on the tumor stage and patient tolerance, targeted therapy (such as nimotuzumab) and concurrent chemotherapy regimen (using cisplatin or nedaplatin) were performed.
2.4. Follow-up
All patients have been follow-up until April 30, 2023. Overall survival (OS) was defined from the date of diagnosis to their last follow-up date or death from any cause. Progression free survival (PFS) was determined based on the date of diagnosis up to the occurrence of disease progression or death.
2.5. Statistical analysis
SPSS (version 22.0) and R software (version 4.1.3) were used for data analysis. The optimal PIV cut-off value was determined via ROC curves. Chi-square tests were utilized to examine the correlation between PIV and clinicopathological characteristics. Kaplan–Meier method was used to estimate survival curves. A Cox regression model was used for multivariate analysis. P-value less than 0.05 was considered statistical significance. Additionally, a nomogram was developed to illustrate the predictive power of the index for OS and PFS.
3. Results
3.1. Patient characteristics
All the 377 patients’ characteristics are presented in Table 1. The median age was 49 years (range from 16 to 83 years). Among these patients, a majority of 266 (70.6 %) were male, ECOG PS scores of 229 (60.7 %) patients were 0. 6.4 % of patients were underweight. 165 patients (43.8 %) were presented with EBV-DNA more than 400 copies/ml. 47 patients (12.4 %) had stage I-II disease, 236 (62.6 %) had stage III, and 94 (24.9 %) had stage IVa. A total of 121 patients (32.1 %) received CCRT, 87 (23.1 %) received radiotherapy concurrent with nimotuzumab, and the remainder received radiotherapy alone. Over a median follow-up time of 55.5 months (range from 8.8 to 112.9 months), 32 (8.5 %) patients had a recurrence, 61 (16.2 %) developed metastasis, and 78 (20.7 %) died.
Table 1.
The baseline clinico-pathological characteristics of NPC patients (n = 377).
| Characteristics | Number (%) |
|---|---|
| Age | |
| Median | 49 |
| Range | 16–83 |
| Gender | |
| Male | 266 (70.6 %) |
| Female | 111 (29.4 %) |
| ECOG PS | |
| 0 | 229 (60.7 %) |
| 1 | 142 (37.7 %) |
| 2 | 6 (1.6 %) |
| T stage | |
| 1 | 47 (12.5 %) |
| 2 | 149 (39.5 %) |
| 3 | 124 (32.9 %) |
| 4 | 57 (15.1 %) |
| N stage | |
| 0 | 25 (6.6 %) |
| 1 | 60 (15.9 %) |
| 2 | 251 (66.6 %) |
| 3 | 41 (10.9 %) |
| Clinical stage | |
| I | 2 (0.5 %) |
| II | 45 (11.9 %) |
| III | 236 (62.6 %) |
| IVa | 94 (24.9 %) |
| BMI(kg/m2) | |
| <18.5 | 24 (6.4 %) |
| 18.5–24.0 | 191 (50.6 %) |
| >24.0 | 162 (43.0 %) |
| Mean ± SD | 23.43 ± 3.30 |
| EBV-DNA (copy/ml) | |
| <400 | 212 (56.2 %) |
| ≥400 | 165 (43.8 %) |
| Treatment | |
| CCRT | 121 (32.1 %) |
| RT + nimotuzumab | 87 (23.1 %) |
| RT | 169 (44.8 %) |
| Relapse | |
| Yes | 32 (8.5 %) |
| No | 345 (91.5 %) |
| Metastasis | |
| Yes | 61 (16.2 %) |
| No | 316 (83.8 %) |
| Death | |
| Yes | 78 (20.7 %) |
| No | 299 (79.3 %) |
ECOG PS Eastern Cooperative Oncology Group Performance Status, BMI Body Mass Index, SD Standard Deviation, CCRT concurrent chemo-radiotherapy, RT radiotherapy.
3.2. PIV cut-off and association with clinicopathological characteristics
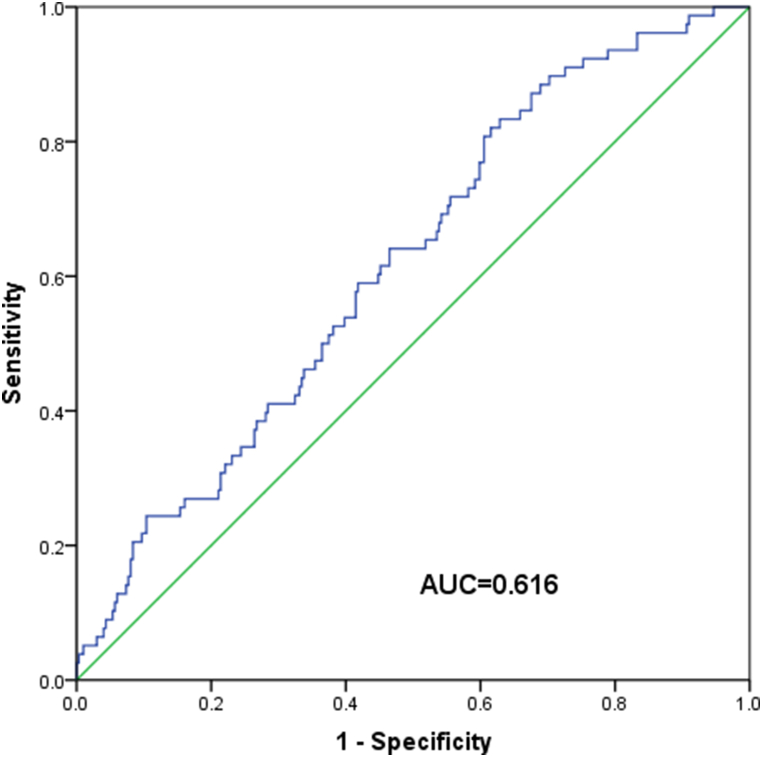
In the study, ROC analysis with OS as the endpoint indicated that the optimal PIV cut-off value was 146.24 (Fig. S1). Based on this, patients were categorized into low- and high-PIV groups. The association between PIV levels and patients’ characteristics was detailed in Table 2. Notably, a higher PIV was linked to poorer ECOG PS score (p = 0.017) and more advanced T (p<0.001) and clinical stages (p = 0.024). However, PIV did not showed a significant association with factors like age, sex, BMI, EBV-DNA levels, N stage, or the type of treatment received (all p>0.05).
Table 2.
The clinico-pathological characteristics of 377 patients with NPC.
| Variables | Low PIV(%) | High PIV (%) | P Value |
|---|---|---|---|
| Age | |||
| ≤60 | 112 (86.8 %) | 215 (86.7 %) | 0.972 |
| >60 | 17 (13.2 %) | 33 (13.3 %) | |
| Gender | |||
| Male | 83 (64.3 %) | 183 (73.8 %) | 0.056 |
| Female | 46 (35.7 %) | 65 (26.2 %) | |
| ECOG PS | |||
| 0 | 91 (70.5 %) | 138 (55.6 %) | 0.017 |
| 1 | 37 (28.7 %) | 105 (42.4 %) | |
| 2 | 1 (0.8 %) | 5 (2.0 %) | |
| BMI | |||
| <18.5 | 10 (7.8 %) | 14 (5.6 %) | 0.545 |
| 18.5–24 | 61 (47.2 %) | 130 (52.4 %) | |
| ≥24 | 58 (45.0 %) | 104 (41.9 %) | |
| EBV-DNA | |||
| <400 | 78 (60.5 %) | 134 (54.0 %) | 0.274 |
| ≥400 | 51 (39.5 %) | 114 (46.0 %) | |
| T stage | |||
| 1-2 | 86 (66.7 %) | 110 (44.4 %) | <0.001 |
| 3-4 | 43 (33.3 %) | 138 (55.6 %) | |
| N stage | |||
| 0-1 | 31 (24.0 %) | 54 (21.8 %) | 0.697 |
| 2-3 | 98 (76.0 %) | 194 (78.2 %) | |
| Clinical stage | |||
| I-III | 106 (82.2 %) | 177 (71.4 %) | 0.024 |
| IVa | 23 (17.8 %) | 71 (28.6 %) | |
| Treatment | |||
| CCRT | 43 (33.3 %) | 78 (31.5 %) | 0.728 |
| Non-CCRT | 86 (66.7 %) | 170 (68.5 %) | |
ECOG PS Eastern Cooperative Oncology Group Performance Status, BMI Body Mass Index, CCRT concurrent chemo-radiotherapy.
3.3. Prognostic value of PIV
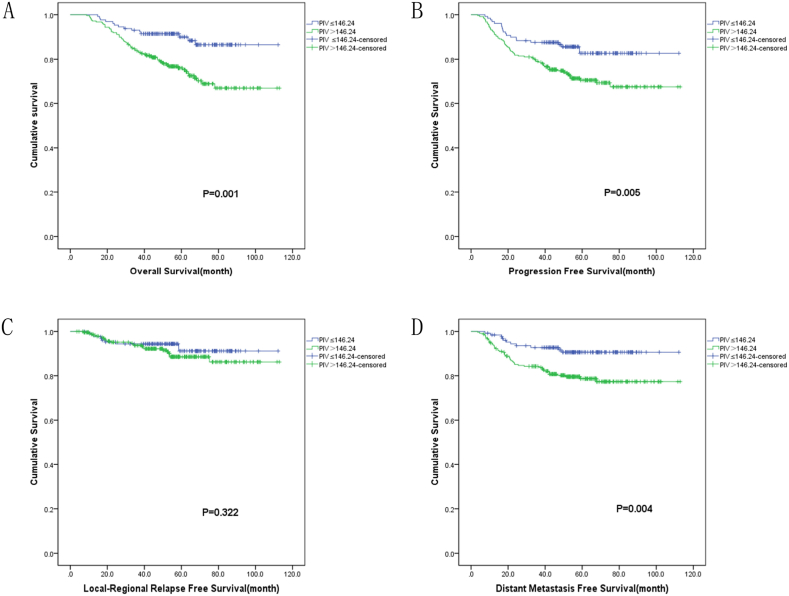
The Kaplan-Meier analysis in the study revealed several factors significantly associated with patient outcomes. Specially, variables like age (p = 0.035), EBV-DNA (P = 0.011), ECOG PS (p<0.001), T stage (p = 0.003), N stage (p = 0.038), clinical stage (p = 0.040), and PIV(p = 0.001) were linked to OS. (Table 3, Fig. 1A). Additionally, EBV-DNA (p = 0.001), ECOG PS(p<0.001), T stage (p = 0.001), clinical stage (p = 0.030), and PIV(P = 0.005) were correlated with patients’ PFS(Table 3, Fig. 1B). The analysis also identified connections between sex (p = 0.023), T stage (p = 0.008) and LRRFS. (Table 3, Fig. 1C), as well as between EBV-DNA (p<0.001), ECOG PS(p<0.001), T stage (p = 0.009), clinical stage (p = 0.009), PIV(P = 0.004) and DMFS(Table 3, Fig. 1D).
Table 3.
Univariate analysis of potential factors associated with OS, PFS, LRRFS, and DMFS.
| Variables |
OS |
PFS |
LRRFS |
DMFS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | 5y-(%) | P | Case | 5y-(%) | P | Case | 5y-(%) | P | Case | 5y-(%) | P | |
| Age | ||||||||||||
| ≤ 60 | 327 | 81.8 | 0.035 | 327 | 76.2 | 0.054 | 327 | 89.4 | 0.923 | 327 | 84.3 | 0.073 |
| >60 | 50 | 73.6 | 50 | 64.3 | 50 | 90.7 | 50 | 72.5 | ||||
| Gender | ||||||||||||
| Male | 266 | 78.4 | 0.065 | 266 | 71.9 | 0.112 | 266 | 86.6 | 0.023 | 266 | 82.6 | 0.854 |
| Female | 111 | 85.8 | 111 | 80.7 | 111 | 96.1 | 111 | 83.2 | ||||
| ECOG PS | ||||||||||||
| 0 | 229 | 85.5 | <0.001 | 229 | 80.5 | <0.001 | 229 | 90.9 | 0.095 | 229 | 88.5 | <0.001 |
| 1-2 | 148 | 73.3 | 148 | 65.4 | 148 | 87.2 | 148 | 73.9 | ||||
| BMI | ||||||||||||
| <18.5 | 24 | 79.2 | 0.664 | 24 | 79.2 | 0.392 | 24 | 95.2 | 0.509 | 24 | 83.1 | 0.328 |
| 18.5–24.0 | 191 | 79.1 | 191 | 72.0 | 191 | 88.0 | 191 | 80.3 | ||||
| >24.0 | 162 | 82.9 | 162 | 77.3 | 162 | 90.8 | 162 | 85.7 | ||||
| EBV-DNA | ||||||||||||
| <400 | 212 | 86.1 | 0.011 | 212 | 79.9 | 0.001 | 212 | 91.1 | 0.180 | 212 | 88.2 | <0.001 |
| ≥400 | 165 | 73.9 | 165 | 67.8 | 165 | 84.0 | 165 | 75.8 | ||||
| T stage | ||||||||||||
| 1-2 | 196 | 85.4 | 0.003 | 196 | 81.6 | 0.001 | 196 | 93.5 | 0.008 | 196 | 87.9 | 0.009 |
| 3-4 | 181 | 75.6 | 181 | 67.1 | 181 | 85.1 | 181 | 77.2 | ||||
| N stage | ||||||||||||
| 0-1 | 85 | 91.4 | 0.038 | 85 | 82.8 | 0.054 | 85 | 95.1 | 0.121 | 85 | 87.0 | 0.170 |
| 2-3 | 292 | 77.6 | 292 | 72.2 | 292 | 87.8 | 292 | 81.5 | ||||
| Clinical stage | ||||||||||||
| I-III | 283 | 83.2 | 0.040 | 283 | 76.9 | 0.030 | 283 | 90.2 | 0.280 | 283 | 85.6 | 0.009 |
| IVa | 94 | 73.1 | 94 | 67.8 | 94 | 87.6 | 94 | 74.5 | ||||
| Treatment | ||||||||||||
| CCRT | 121 | 83.6 | 0.571 | 121 | 79.0 | 0.370 | 121 | 93.0 | 0.548 | 121 | 84.9 | 0.402 |
| nonCCRT | 256 | 79.3 | 256 | 72.5 | 256 | 87.8 | 256 | 81.9 | ||||
| PIV | ||||||||||||
| Low | 129 | 90.0 | 0.001 | 129 | 82.7 | 0.005 | 129 | 91.2 | 0.322 | 129 | 90.7 | 0.004 |
| High | 248 | 75.9 | 248 | 70.5 | 248 | 88.7 | 248 | 78.7 | ||||
OS overall survival, PFS progression free survival, LRRFS local regional recurrence free survival, DMFS distant metastasis free survival, ECOG PS Eastern Cooperative Oncology Group Performance Status, BMI Body Mass Index, CCRT concurrent chemo-radiotherapy, PIV pan-immune-inflammation value.
Fig. 1.
Prognostic value of PIV in NPC patients. (A) Survival curves for OS, (B) Survival curves for PFS, (C) Survival curves for LRRFS, (D) Survival curves for DMFS.
According to univariate analysis, variables significantly correlated with patients’ prognosis were included in multivariate analysis. Cox regression analysis showed that ECOG PS (p = 0.026), N stage (p = 0.039), and PIV(p = 0.007) were significant independent predictors for OS. ECOG PS (p = 0.019), EBV-DNA (p = 0.007), and PIV (p = 0.049) were significant independent predictors for PFS. Only T stage (p = 0.011) was an significant independent predictor for LRRFS. ECOG PS (p = 0.013), EBV-DNA (p = 0.003), and PIV (p = 0.031) were significant independent predictors for DMFS(Table 4).
Table 4.
Multi-variable Cox regression analyses for OS, PFS, LRRFS, and DMFS.
| Variables | OS |
PFS |
LRRFS |
DMFS |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | HR (95 % CI) | P | HR (95 % CI) | P | |
| Age | ||||||||
| ≤60 | ref | 0.088 | ||||||
| >60 | 1.643 (0.930–2.905) | |||||||
| ECOG PS | ||||||||
| 0 | ref | 0.026 | ref | 0.019 | ref | 0.013 | ||
| 1-2 | 1.756 (1.070–2.881) | 1.718 (1.092–2.701) | 2.042 (1.159–3.596) | |||||
| EBV-DNA | ||||||||
| <400 | ref | 0.075 | ref | 0.007 | ref | 0.003 | ||
| ≥400 | 1.519 (0.959–2.408) | 1.803 (1.175–2.768) | 2.229 (1.302–3.814) | |||||
| T stage | ||||||||
| 1-2 | ref | 0.390 | ref | 0.174 | ref | 0.011 | ref | 0.645 |
| 3-4 | 1.257 (0.746–2.117) | 1.394 (0.863–2.250) | 2.637 (1.248–5.569) | 1.149 (0.636–2.077) | ||||
| N stage | ||||||||
| 0-1 | ref | 0.039 | ||||||
| 2-3 | 1.974 (1.036–3.762) | |||||||
| Clinical stage | ref | 0.438 | ||||||
| I-III | ref | 0.596 | ref | 0.799 | 1.247 (0.714–2.179) | |||
| IVa | 1.147 (0.691–1.904) | 1.063 (0.665–1.699) | ||||||
| PIV | ||||||||
| Low | ref | 0.007 | ref | 0.049 | ref | 0.031 | ||
| High | 2.231 (1.241–4.011) | 1.664 (1.003–2.760) | 2.081 (1.071–4.044) | |||||
OS overall survival, PFS progression free survival, LRRFS local regional recurrence free survival, DMFS distant metastasis free survival, ECOG PS Eastern Cooperative Oncology Group Performance Status, PIV pan-immune-inflammation value.
3.4. Prognostic nomogram construction
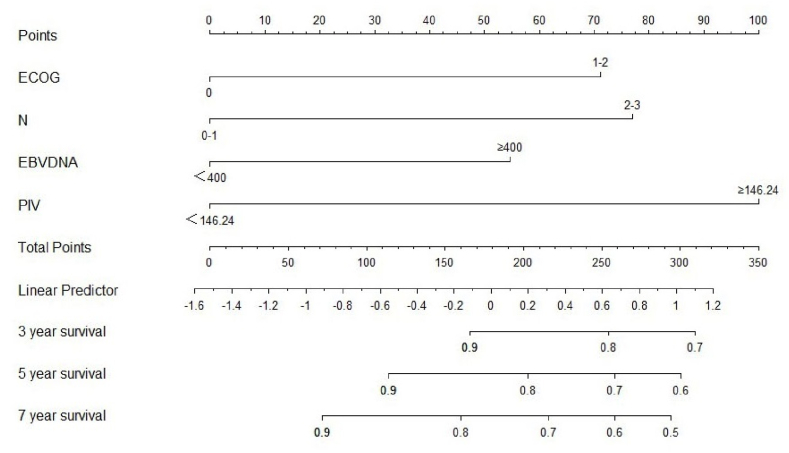
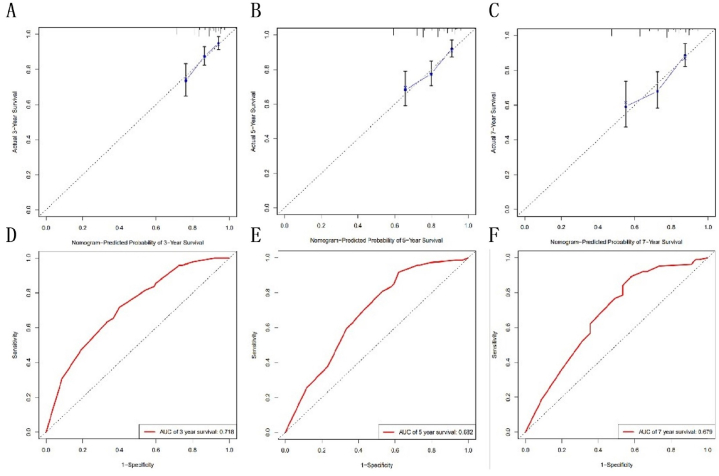
The independent prognostic factors identified from multivariate analysis were utilized to develop a nomogram for predicting 3, 5, and 7 OS(Fig. 2). The nomogram's accuracy, measured by the C-index, was 0.684 for OS. Additionally, the AUCs values for 3, 5, and 7 years OS preditions were retrospectively 0.718, 0.682, and 0.679 (Fig. 3). Calibration curves demonstrated good consistence between the nomogram's predicted OS and the actual observed values for these time frames (Fig. 4).
Fig. 2.
Nomogram for OS predicting at 3,5, and 7 years.
Fig. 3.
The nomogram-predicted probablity of 3 years OS (A), 5 years OS (B), 7 years OS (C). The AUCs for 3 years OS(D), 5 years OS (E), 7 years OS (F).
Fig. 4.
The calibration curves for 3 years OS (A), 5 years OS (B), 7 years OS (C).
4. Discussion
In this study, the prognostic value of pretreatment PIV in NPC patients undergoing definitive radiotherapy was investigated for the first time. Findings revealed that PIV serves as an independent prognostic index of OS, PFS and DMFS. A nomogram incorporating PIV and other clinical parameters was created, exhibiting strong predictive performance and accuracy, as evidenced by its C-index value and calibration curve. This suggests that PIV could be a valuable blood-based immune-inflammation biomarker for forecasting NPC patient prognosis.
Systemic inflammation has long been accepted as a hallmark of cancer, and is considered to be closely associated with oncogenesis, cancer progression, and prognosis of cancer patients [14]. Uncontrolled systemic inflammation modulates the TME by secreting pro-inflammatory cytokines and angiogenesis factors, leading to immune exhaustion and evasion [[15], [16], [17]]. Cell components of peripheral blood, including lymphocytes, neutrophils and platelets, are important mediators of host anti-tumor immunity, and can reflect the systemic and TME immune-inflammation status. Circulating and infiltrating lymphocytes are major mediators of the anti-tumor adaptive immune response [18]. Neutrophils could promote tumor progression via multiple mechanisms, including the enhancement of cell proliferation, angiogenesis, and epithelial-mesenchymal transition [19]. Moreover, tumor-associated neutrophils (TANs) can impair CD8+ T cell anti-tumor activity [20]. Monocytes, the source of tumor associated macrophages, drive immunosuppression in the TME, which impairs immunity and promotes tumor progression [21]. Platelets may coat the circulating tumor cells, allowing them to evade immune attack, and activated platelets release cytokines that support tumor development [21]. Since all pro-inflammatory cells in the blood count are included in PIV calculation, there is a strong biological rationale for PIV as a biomarker.
The utility of blood-cell-based biomarkers for prognostic prediction has been intensely investigated over the past decades. Previous studies have demonstrated that lymphocyte [22], neutrophil [23], monocyte [24], and platelet counts [25], each predict survival in various cancers. However, due to the complexity of the immune system, other parameters combining different blood cell populations have been explored to more accurately predict outcomes for patients with cancer. PLR, NLR, systemic immune inflammation index (SII) and systemic inflammation response index (SIRI) are the most comprehensively studied biomarkers, and mounting evidence has demonstrated their prognostic significance in cancer patients [[26], [27], [28], [29]]. However, since PLR, NLR, SII and SIRI are each composed of two or three peripheral blood cell components, they cannot fully represent immune status. PIV is a novel index that reflects immune-inflammation status more comprehensively because it includes four cell components. Giovanni Fucà et al. first reported in 2020 that PIV can act as predictor for OS and PFS in colorectal cancer patients with metastatic disease, patients with high PIV presented with worse OS and PFS [12]. The prognostic role of PIV was subsequently explored in breast cancer [11,30], head and neck squamous cell carcinoma [31], small cell lung cancer [9], pancreatic adenocarcinoma [32], and prostate cancer [33], with high PIV consistently predicting a poorer prognosis. In the present study, we demonstrated that PIV is an independent prognostic index of OS, PFS and DMFS for patients with NPC, which is in agreement with these studies. A nomogram was constructed that confirmed the accuracy of the PIV prediction.
PIV includes all the inflammatory peripheral blood cells and therefore reflects the complex interactions between cancer and host immune-inflammatory responses. It is a strong predictor of cancer survival outcomes, and our research has confirmed PIV as a promising prognostic biomarker in patients with NPC. However, our research has several shortcomings. First, it is a single center retrospective research, which may have introduced bias. Second, the sample size is too small, internal and external validation were not considered. Finally, patients who receive CCRT may have experienced inflammatory adverse reactions such as radiation-induced oral mucositis and mandibular osteoradionecrosis [34], which may have influenced the results. Therefore, a further prospective multicenter study may be needed to further confirm our findings.
5. Conclusions
In summary, this retrospective study concluded that high pre-treatment PIV is an indicator of poorer OS, PFS and DMFS in patients with NPC. The study suggests PIV as a convenient and practical biomarker that could assist in better patient stratification and treatment planning. It also propose that targeting systemic inflammation might be a viable approach to enhance NPC prognosis, a hypothesis that merits further research.
Ethics statement
This research was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (certification for clinical research 2023 [No. 092]). The Ethics Committee waived the requirement of written informed consent since this was a retrospective study.
Funding declaration
This research was sponsored by the Clinical Medical Technology Innovation Guidance Project of Hunan Province (2021SK53515), the Natural Science Foundation of Hunan Province (2021JJ40882, 2022JJ40682), and the National Natural Science Foundation of China (81802476).
Data availability statement
The raw data supporting the conclusions of this article will be made available on request from the authors.
CRediT authorship contribution statement
Na Zhang: Writing – original draft, Methodology, Conceptualization. Tao Hou: Methodology, Data curation. Sujuan Zhang: Methodology, Data curation. Jie Ling: Methodology, Data curation. Shun Jiang: Software, Data curation. Yangchun Xie: Writing – review & editing, Data curation. Xianling Liu: Supervision, Investigation. Chunhong Hu: Supervision, Investigation. Yuhua Feng: Writing – review & editing, Software, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24804.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
References
- 1.Chow J.C., et al. Immunotherapeutic approaches in nasopharyngeal carcinoma. Expet Opin. Biol. Ther. 2019;19(11):1165–1172. doi: 10.1080/14712598.2019.1650910. [DOI] [PubMed] [Google Scholar]
- 2.Jiromaru R., Nakagawa T., Yasumatsu R. Advanced nasopharyngeal carcinoma: current and emerging treatment options. Cancer Manag. Res. 2022;14:2681–2689. doi: 10.2147/CMAR.S341472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossi P., et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(dagger) Ann. Oncol. 2021;32(4):452–465. doi: 10.1016/j.annonc.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.W., et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J. Clin. Oncol. 2015;33(29):3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 5.Saputra H.M., et al. Prognostic value of neutrophil-to-lymphocyte ratio (NLR) in penile cancer: a systematic review and meta-analysis. Ann Med Surg (Lond) 2022;81 doi: 10.1016/j.amsu.2022.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo A., et al. Neutrophil-to-Lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (nsclc): a large retrospective multicenter study. Adv. Ther. 2020;37(3):1145–1155. doi: 10.1007/s12325-020-01229-w. [DOI] [PubMed] [Google Scholar]
- 7.Qi W.X., et al. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol. Immunother. 2021;70(11):3199–3206. doi: 10.1007/s00262-021-02926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng R., et al. PIV and PILE score at baseline predict clinical outcome of anti-PD-1/PD-L1 inhibitor combined with chemotherapy in extensive-stage small cell lung cancer patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.724443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambichler T., et al. Prognostic potential of the baseline pan-immune-inflammation value and neutrophil/lymphocyte ratio in stage I to III melanoma patients. Cancers. 2022;14(18) doi: 10.3390/cancers14184410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ligorio F., et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-Positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers. 2021;13(8) doi: 10.3390/cancers13081964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fucà G., et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer. 2020;123(3):403–409. doi: 10.1038/s41416-020-0894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W.F., et al. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin. J. Cancer. 2012;31(12):579–587. doi: 10.5732/cjc.012.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar-Cazares D., et al. Contribution of angiogenesis to inflammation and cancer. Front. Oncol. 2019;9:1399. doi: 10.3389/fonc.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basheer A.S., et al. Role of inflammatory mediators, macrophages, and neutrophils in glioma maintenance and progression: mechanistic understanding and potential therapeutic applications. Cancers. 2021;13(16) doi: 10.3390/cancers13164226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S.C., et al. Evaluation of cytotoxic T lymphocyte-mediated anticancer response against tumor interstitium-simulating physical barriers. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L., et al. Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11(4) doi: 10.3390/cancers11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M., et al. Tumour-associated neutrophils orchestrate intratumoural IL-8-driven immune evasion through Jagged2 activation in ovarian cancer. Br. J. Cancer. 2020;123(9):1404–1416. doi: 10.1038/s41416-020-1026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibutani M., et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer. 2017;17(1):404. doi: 10.1186/s12885-017-3395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S., et al. Severe lymphopenia as a prognostic factor in rectal cancer patients receiving adjuvant chemoradiotherapy: a retrospective study. Sci. Rep. 2023;13(1):7566. doi: 10.1038/s41598-023-34145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R., et al. Absolute neutrophil count in the peripheral blood predicts prognosis in lung cancer patients treated with anlotinib. Cancer Manag. Res. 2021;13:3619–3627. doi: 10.2147/CMAR.S307368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigeta K., et al. High absolute monocyte count predicts poor clinical outcome in patients with castration-resistant prostate cancer treated with docetaxel chemotherapy. Ann. Surg Oncol. 2016;23(12):4115–4122. doi: 10.1245/s10434-016-5354-5. [DOI] [PubMed] [Google Scholar]
- 25.Taucher S., et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemostasis. 2003;89(6):1098–1106. [PubMed] [Google Scholar]
- 26.Imai H., et al. Using the neutrophil-to-lymphocyte ratio to predict the outcome of individuals with nonsquamous non-small cell lung cancer receiving pembrolizumab plus platinum and pemetrexed. Thorac Cancer. 2023;14(25):2567–2578. doi: 10.1111/1759-7714.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H., et al. Prognostic value of EBV DNA and platelet-to-lymphocyte ratio in patients with non-metastatic nasopharyngeal carcinoma: a retrospective study. BMC Cancer. 2023;23(1):673. doi: 10.1186/s12885-023-11117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey-Whyte M., et al. Systemic inflammation indices and association with prostate cancer survival in a diverse patient cohort. Cancers. 2023;15(6):1869. doi: 10.3390/cancers15061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schietroma M., et al. Systemic inflammation response index (SIRI) as predictor of anastomotic leakage after total gastrectomy for gastric cancer. Surg Oncol. 2022;43 doi: 10.1016/j.suronc.2022.101791. [DOI] [PubMed] [Google Scholar]
- 30.Sahin A.B., et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-94184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guven D.C., et al. The association between pan-immune-inflammation value and survival in head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2023;280(5):2471–2478. doi: 10.1007/s00405-022-07804-x. [DOI] [PubMed] [Google Scholar]
- 32.Topkan E., et al. Low pre-ChemoradiotherapyPan-immune-inflammation value (PIV) measures predict better survival outcomes in locally advanced pancreatic adenocarcinomas. J. Inflamm. Res. 2022;15:5413–5423. doi: 10.2147/JIR.S385328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazgan S.C., et al. Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors. Prostate. 2022;82(15):1456–1461. doi: 10.1002/pros.24419. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz B., et al. Utility of pre-chemoradiotherapy Pan-Immune-Inflammation-Value for predicting the osteoradionecrosis rates in locally advanced nasopharyngeal cancers. Strahlenther. Onkol. 2023;199(10):910–921. doi: 10.1007/s00066-023-02119-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on request from the authors.