Abstract
Background:
Consuming ultra-processed foods may increase exposure to phthalates, a group of endocrine disruptors prevalent in food contact materials.
Objectives:
Investigate associations between ultra-processed food intake and urinary phthalates during pregnancy, and evaluate whether ultra-processed foods mediate socioeconomic disparities in phthalate exposures.
Methods:
In a socioeconomically diverse sample of 1031 pregnant women from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) Study in the urban South, the Block Food Frequency Questionnaire was administered and urinary phthalate metabolites were measured in the second trimester. Linear regressions modeled associations between phthalates and overall ultra-processed food consumption, individual ultra-processed foods, and exploratory factor analysis dietary patterns. Causal mediation analyses examined whether ultra-processed food intake mediates relationships between socioeconomic disparities and phthalate exposures.
Results:
Ultra-processed foods constituted 9.8–59.0 % (mean = 38.6 %) of participants’ diets. 10 % higher dietary proportion of ultra-processed foods was associated with 13.1 % (95 %CI: 3.4 %–22.9 %) higher molar sum concentrations of di(2-ethylhexyl) phthalate metabolites (ΣDEHP). 10 % higher consumption of minimally-processed foods was associated with lower ΣDEHP (10.8 %: 3.4 %–22.9 %). Ultra- and minimally-processed food consumption were not associated with non-DEHP metabolites. Standard deviation higher consumptions of hamburger/cheeseburger, French fries, soda, and cake were associated with 10.5 % (4.2 %–17.1 %), 9.2 % (2.6 %–16.2 %), 7.4 % (1.4 %–13.6 %), and 6.0 % (0.0 %–12.4 %), respectively, higher ΣDEHP. Exploratory factor analysis corroborated positive associations of processed food with ΣDEHP, and uncovered a healthy dietary pattern associated with lower urinary ΣDEHP, mono(2-ethyl-5-hydroxyhexyl) (MEHHP), mono(2-ethyl-5-carboxypentyl) (MECPP), mono(2-carboxymethylhexyl) (MCMHP), and mono-isononyl (MINP) phthalates. Significant indirect effects indicated that lower income and education levels were associated with 1.9 % (0.2 %–4.2 %) and 1.4 % (0.1 %–3.3 %) higher ΣDEHP, respectively, mediated via increased ultra-processed food consumption.
Conclusions:
Consumption of ultra-processed foods may increase exposure to phthalates. Policies to reduce dietary phthalate exposures from food packaging and processing are needed, as socioeconomic barriers can preclude dietary recommendations as a sole means to reduce phthalate exposures.
Keywords: Phthalates, Processed food, NOVA, Fast food, Maternal diet, Endocrine disruptors, Socioeconomic status
1. Background
Humans have developed various techniques to process food throughout history, evolving from basic methods of preservation including salting, smoking, and pickling to more sophisticated industrial physical, chemical, or biological processes, including hydrogenation, hydrolysis, and bleaching (Srour et al., 2022). Recent increases in global intake of highly processed foods have coincided with an increase in chronic diseases like metabolic syndrome and inflammatory bowel disease, prompting scientists to explore potential connections between levels of food processing and the risk of chronic illnesses (Srour et al., 2022). Processed diets may impact health through mechanisms other than nutritional quality, and the mechanisms underlying those impacts can vary greatly across processed foods (Hess et al., 2023). Accumulating evidence suggests that chemicals deliberately added to processed foods (e.g., to extend shelf life or to impart certain properties including color and texture), or which inadvertently contaminate food through contact during processing and packaging, including phthalates (Bang et al., 2012), may contribute to human disease (Trasande et al., 2018). In particular, owing to the common use of phthalates in food processing and packaging, processed food diets may be a major source of human phthalate exposures (Bang et al., 2012; Fromme et al., 2007; Engel et al., 2021).
Phthalates are a group of chemical compounds widely used in the production of plastics, personal care items, and other commercial products (Katsikantami et al., 2016; Serrano et al., 2014). These chemicals are not covalently bound to consumer goods, and thereby leach into the environment, resulting in ubiquitous human exposures across the globe (Katsikantami et al., 2016; Serrano et al., 2014). Phthalate exposures may increase risks for low birth weight (Golestanzadeh et al., 2019; Marie et al., 2015), preterm birth (Welch et al., 2022), adverse reproductive development (Swan et al., 2015), and other child health outcomes (Swan, 2008). Thus, advancing our understanding of common sources of phthalate exposures, especially during pregnancy when the developing fetus is particularly sensitive to these exposures, is a critical public health priority.
Diets high in processed and packaged foods could result in higher phthalate exposures compared with diets high in fresh and unprocessed foods. Analyses using data from the National Health and Nutrition Examination Survey (NHANES) have shown that consumption of fast foods (Zota et al., 2016) ultra-processed foods (Buckley et al., 2019; Martínez Steele et al., 2020), and eating outside of the home (Varshavsky et al., 2018) are associated with increased exposure to certain phthalate metabolites in United States (US) children and adults. However, more studies are needed to confirm these potential sources of dietary phthalate exposures. Because prenatal phthalates are associated with adverse birth and other health outcomes (Golestanzadeh M, Riahi R, Kelishadi RJES, Research P, 2019; Marie et al., 2015; Welch et al., 2022; Swan et al., 2015; Swan, 2008) an examination of the degree to which ultra-processed foods increase phthalate exposures during pregnancy is needed.
Prior cohort studies examining the impacts of ultra-processed food consumption have utilized the NOVA (not an acronym) food processing classification system (Buckley et al., 2019; Martínez Steele et al., 2020; Khandpur et al., 2021). Instead of focusing on nutrients, NOVA is a system that classifies foods based on the degree and intention of their processing (Monteiro, 2009; Monteiro et al., 2016). NOVA includes four groups of foods: 1) unprocessed or minimally processed foods; 2) processed culinary ingredients; 3) processed foods; and 4) ultra-processed foods. There is the potential for bias in the manual categorization of foods by researchers into pre-defined food groups, and data-driven approaches can potentially mitigate this bias. However, data-driven methods can result in less interpretable groups compared with manual classification. Thus, both manual and data-driven classification systems should be utilized as complementary approaches to advance our understanding of potential dietary sources of phthalate exposures.
Communities of low socioeconomic status may be disproportionately impacted by food-associated chemical exposures, as highest level of educational attainment, individual poverty, and living in a low resource neighborhood are factors well known to limit access to affordable and healthy foods (Walker et al., 2010). The increased cost of healthy food is a widely acknowledged barrier to following a healthy diet, especially for those with lower incomes (Rao et al., 2013; Darmon and Drewnowski, 2015; Glanz et al., 1998). Similarly, several studies have shown that diets with a higher proportion of energy from minimally processed foods are more expensive than diets high in ultra-processed foods (Vandevijvere et al., 2020; Vellinga et al., 2022). In addition to the higher monetary costs of minimally processed foods, other factors may limit access. Lower income individuals may be more likely to live in food deserts, residential areas with poor availability of affordable and nutritious food (Zenk et al., 2005; Moore and Diez Roux, 2006). The costs of car ownership and public transportation can prevent access to grocery stores (Rose and Richards, 2004; Guy et al., 2004; Cotterill and Franklin, 1995). Additionally, single parents and individuals with long and/or inflexible work schedules may lack the time required to visit the supermarket or prepare meals (Rose and Richards, 2004. Easy access to unhealthy foods in food swamps (Rose et al., 2009) may also predict adverse health outcomes (Cooksey-Stowers et al., 2017; Bevel et al., 2023) Moreover, food swamps are more likely to occur in lower socioeconomic status areas, which can have higher fast food and convenience store densities (Block et al., 2004; Richardson et al., 2014). Owing to these socioeconomic barriers to food access, ultra-processed food consumption is lower among individuals with higher income and education levels in the US general population (Baraldi et al., 2018), yet no studies have examined whether ultra-processed food consumption mediates links between socioeconomic status and phthalate exposures.
To advance the literature on sources of phthalate exposures, our primary objective was to conduct the first analysis modeling associations between ultra-processed food consumption and phthalate exposures in pregnant women rather than the general population. To account for the potential for bias in manual food categorization and the potential for limited interpretability of data-driven food categorization, we employed complementary NOVA and exploratory factor analysis food grouping approaches. Additionally, to explore potential mechanisms of exposure, we conducted the first analysis evaluating whether ultra-processed food consumption mediates socioeconomic disparities in phthalate exposures.
2. Methods
2.1. Study population
Analyses are based on data from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) cohort, a prospective pregnancy cohort of mother–child dyads in Shelby County, Tennessee (Memphis area) (Sontag-Padilla et al., 2015; Palmer et al., 2013). Between 2006 and 2011, 1503 women aged 16–40 with healthy singleton pregnancies were enrolled in their second trimester. Exclusion criteria included an existing chronic disease requiring medication (e.g., hypertension, diabetes, and sickle cell disease), known pregnancy complications (e.g., complete placenta previa and oligohydramnios), or plans to deliver at a nonparticipating hospital. Among the enrolled participants, 1318 had complete maternal dietary data. Among these participants, the study sample consisted of 1031 CANDLE mothers who also had urinary phthalate metabolite data available. The Institutional Review Boards of the University of Tennessee Health Science Center and the University of Washington granted approval for the study. Participants aged 18 years or older provided informed consent, while assent and consent from legally authorized representatives were obtained for those under 18 years prior to enrollment.
2.2. Maternal diet during pregnancy
During the second trimester of pregnancy (between 15.3 and 28.7 weeks’ gestation (mean [SD] = 23.0 [3.0]), trained interviewers administered the Block Food Frequency Questionnaire (FFQ) to evaluate participants’ habitual consumption 114 food and beverage items over the preceding three months (Block et al., 1986; Subar et al., 2001). To ensure accuracy, interviewers received training from registered dietitians and underwent periodic re-certification through recorded interviews every six months. The FFQ data were processed by NutritionQuest (Berkeley, CA, USA) to assess total daily energy intake. Instead of focusing on nutrients, NOVA is a system that classifies foods based on the degree and intention of their processing (Monteiro, 2009; Monteiro et al., 2016). Block FFQ food items were categorized according to a list of NOVA food groups generated previously by Khandpur and colleagues (2021) (Khandpur et al., 2021). Briefly, Khandpur and colleagues (2021) created a food list from the FFQs employed in five different cohort studies and grouped them into four NOVA groups based on their grade of processing. These groups included 1) unprocessed or minimally processed foods; 2) processed culinary ingredients; 3) processed foods; and 4) ultra-processed foods. Initially, three researchers working independently assigned foods to one of the NOVA groups. Foods for which there was disagreement in categorization were reviewed and assigned to a NOVA group by three senior nutrition epidemiologists in consultation with a team of research dietitians (Khandpur et al., 2021). In the present study, one author coded all of the items according to the groupings generated by Khandpur and colleagues (2021), and a second author with nutritional epidemiology expertise reviewed the groupings to flag individual items for sensitivity analyses. Flagged items included fried chicken, fried fish, breakfast sandwiches, and macaroni and cheese, which could belong to different NOVA groups depending on the ingredients and how they are prepared (e.g., fried fish sticks or chicken nuggets vs. pan fried alternatives). One sensitivity analysis categorized all flagged foods as ultra-processed, and another sensitivity analysis categorized them as minimally processed.
Our primary analyses modeled food intake using the frequency data from the Block FFQ. Prior work has expressed intake of ultra-processed foods either in terms of frequency of intake (Dehghan et al., 2023) or proportion of energy (caloric) intake (Buckley et al., 2019). Analyzing food frequency rather than proportion of energy was preferable here for several reasons: 1) converting the FFQ frequency and quantity data into proportions of total energy requires unverifiable assumptions about the calories per serving for each food item; (Naska et al., 2017) 2) the frequency data can account for the intake of ultra-processed foods without energy (artificially sweetened drinks, sauces, salad dressings, etc.); (Dehghan et al., 2023) and 3) frequency of intake may be a more reasonable measure of non-nutritional factors (Dehghan et al., 2023; Juul et al., 2021), especially those pertaining to additives, processing, and packaging. Nevertheless, we performed sensitivity analyses (described below) modeling food intake for several FFQ items in terms of dietary energy proportion.
For each participant, we summed the frequency of intake of all food items, NOVA ultra-processed foods, and NOVA minimally processed foods. Variables reflecting the percent dietary intake of ultra-processed and minimally processed foods were calculated by dividing the frequencies of intake for these NOVA groups by the intake of all food items and multiplying by 100.
2.3. Maternal urinary phthalate metabolites
Phthalate metabolites were measured in single spot urine samples collected using phthalate-free polypropylene containers from women during a clinical visit between 15.3 and 28.7 weeks’ gestation (mean [SD] = 23.0 [3.0]). Samples were processed and stored at − 80 °C in the study repository of the University of Tennessee Health Science Center Department of Pathology. Solid-phase extraction and high-performance liquid chromatography-tandem mass spectrometry were employed to analyze the samples for phthalic acid and 21 phthalate metabolites; quality control was ensured by incorporating process and instrument blanks (Rocha et al., 2017). Specific gravity was determined using a handheld refractometer, and phthalate measurements were adjusted according to a formula previously described (Adgent et al., 2020). Individual phthalate metabolites were included in this study only if they exceeded the LOD in over 70 % of samples. Phthalic acid and 16 phthalate metabolites met this cut-off, including monomethyl phthalate (MMP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), monoisobutyl phthalate (MIBP), monobenzyl phthalate (MBZP), monohexyl phthalate (MHXP), mono(4-hydroxypentyl) phthalate (MHPP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-carboxymethylhexyl) phthalate (MCMHP), mono(3-carboxypropyl) phthalate (MCPP), monoisononyl-phthalate (MINP), monocarboxyisooctyl phthalate (MCIOP), and monocarboxyisononyl phthalate (MCINP). Of those metabolites included, individual values below the limit of detection (LOD) were imputed by the LOD divided by the square root of 2 (Hornung and Reed, 1990). Di(2-ethylhexyl) phthalate molar sum (ΣDEHP) was calculated as the molar sum of five metabolites: MEHP, MEOHP, MEHHP, MECPP, and MCMHP.
2.4. Covariates
Covariate data on maternal characteristics were collected via questionnaires and medical record abstraction. All models adjusted for maternal age, education, race, ethnicity, household income, number of individuals in the household, neighborhood deprivation index (which includes indicators related to poverty, unemployment, educational attainment and home ownership derived from the US Census) (Messer et al., 2006), pre-pregnancy body mass index (BMI), tobacco and alcohol use, and total daily caloric intake (calculated by NutritionQuest). Maternal alcohol use was based on self-report. The positive tobacco exposure group included individuals with maternal urine cotinine above 200 ng/mL (Schick et al., 2017), as well as individuals who were below this cut-off but self-reported tobacco use during pregnancy.
2.5. Statistical analysis
Our primary analyses included 827 complete cases with full covariate data and excluding 98 pregnant women who reported questionably low (<1000) or high (>5000) calories/day of total energy intake (Willet, 1990; Völgyi et al., 2013). We performed sensitivity analyses that 1) included all 925 complete cases, regardless of calories/day intake, and 2) included all 1031 individuals in the study by applying Multivariate Imputation by Chained Equations (MICE) to handle missing data (Van Buuren and Groothuis-Oudshoorn, 2011).
Descriptive statistic summaries were calculated for the 827 complete cases in our primary analyses according to quartiles of percent of dietary intake from ultra-processed foods. We examined baseline characteristics by quartiles of ultra-processed food intake using analysis of variance and chi-square goodness of fit tests for continuous and categorical variables, respectively. Descriptive statistic summaries were additionally calculated for the entire 1503-individual CANDLE cohort, the 1031 total individuals with FFQ and phthalate data available, and the 472 individuals who were missing this data and excluded from this study.
We modeled associations of 10-percent increases in ultra-processed or minimally processed food intakes with urinary phthalate metabolite levels in separate linear models for each natural log-adjusted, specific gravity corrected phthalate metabolite. From the regression models, we calculated percent difference in urinary phthalate metabolite levels by ultra- or minimally processed food intake using the formula: (eβ − 1) × 100 % (Zota et al., 2016; Buckley et al., 2019). Additionally, we modeled associations of individual ultra-processed food items with natural log-adjusted ΣDEHP. In these models, individual food items were scaled and centered, and the formula above was used to convert coefficients into percent ΣDEHP differences. Thus, the reported estimates reflect ΣDEHP percent changes per standard deviation higher food item intake.
In a sensitivity analysis, the top four food items associated with increased ΣDEHP (hamburgers/cheeseburgers, French fries, sodas, and cake) were instead modeled in terms of proportion of total dietary energy (USDA Food and Nutrient Database for Dietary Studies, 2022). For each of these food items, we converted participant reported quantity of intake (i.e., serving size) into calories using the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (Table S1). These calorie values were multiplied by daily frequencies of intake to obtain daily caloric intakes, then divided by total daily calories (calculated by NutritionQuest) to determine the percentages of daily calories attributable to each food item. For individuals reporting consumption of diet sodas, percentage of total energy from sodas was set to 0 %. Using linear regressions and the formula above, we modeled ΣDEHP percent changes per one-percent increase in daily calories attributable to each food item. Rather than modeling 10 % differences as described for proportion dietary intake of ultra-processed foods, we modeled one-percent differences for individual food items because the mean intake for individual foods was much lower (following approach by Buckley et. al., 2019) (Buckley et al., 2019). The mean percent of total energy intake from these four food items ranged from 1.6 to 2.7 %, while mean ultra-processed food intake was 38.6 %.
Causal mediation analysis was used to examine the role of percent dietary intake of ultra-processed food as a mediator on the pathway between socioeconomic status variables and ΣDEHP exposure during pregnancy. Separate mediation models were employed for each measure of socioeconomic status, including household income, maternal education, and neighborhood deprivation index. These analyses were conducted using the regression-based approach in the ‘CMAverse’ R package (Shi et al., 2021) with closed-form parameter function estimation and bootstrap inference with 1,000 iterations. The active and control values for continuous variables (income and neighborhood deprivation index) were set to the 75th and 25th percentiles, respectively. Education categories were collapsed into two groups and the active and control values were set to higher education versus no higher education. The higher education group included individuals self-reporting “some graduate work or graduate/professional degree” and “graduated college or technical school” (N = 385 in complete case analysis), while the no higher education group included individuals self-reporting “High School completion” and “<High School” (N = 442 in complete case analysis). Coefficients were expressed as percent changes in ΣDEHP using the formula above.
Data driven maternal dietary pattern groups were created using exploratory factor analysis. This unsupervised method was used to reduce the dimensionality of the Block FFQ independently of any covariates or the outcome of interest. In this case, factor analysis assumes that food frequencies arise from a specific number of latent factors. These latent factors represent common sources of variation and account for correlation structure in the FFQ data. The analysis was performed by inputting all frequency data from the Block FFQ into the ‘fa’ function from the ‘psych’ R package (Revelle, 2015) using the maximum likelihood factoring method, varimax rotation, and regression scores estimator. The number of factors to retain in the final solution was determined to maximize interpretability of the factors in relation to dietary patterns. A steep drop-off in eigenvalues beyond 7 factors was observed, and factor analysis solutions ranging from 2 to 7 factors were tested. Loadings for the factors in each solution were plotted, and a 5-factor solution was chosen as the best balance between variance explained and interpretability of the dietary patterns. We plotted the top 12 loadings for each factor, and modeled associations of the 5 factor variables with urinary phthalate levels in separate linear models for each phthalate outcome. Owing to the subjective nature of relying on interpretability for selecting the number of factors to retain, sensitivity analyses were conducted by repeating exploratory factor analysis with 4 and 6 factors. Analyses were conducted using R version 4.1.2 (R Core Team, 2021).
3. Results
On average, pregnant women were 26.8 years of age (range = 16–40), and most participants self-identified as Black/African American (60.6 %) or White (33.6 %). The majority of women reported no tobacco (91.4 %) or alcohol (91.1 %) use during pregnancy and consumed an average of 2,410 calories per day during early pregnancy (Table 1). Urinary measures of Phthalic acid and 16 phthalate metabolites exceeded the LOD in over 70 % of samples (Table S2). Compared to the included study sample with available phthalates and FFQ data, CANDLE cohort mothers excluded from this study were on average younger during pregnancy, and lower socioeconomic status according to educational attainment, neighborhood deprivation index, and familial income (Table S3). Distributions of the 16 phthalate metabolites, phthalic acid, and ΣDEHP are shown in Fig. 1.
Table 1.
Descriptive statistics of study population according to quartiles of percent of dietary intake from ultra-processed foods (N = 827).
| Quartile 1: (N = 207) | Quartile 2: (N = 207) | Quartile 3: (N = 206) | Quartile 4: (N = 207) | Total (N = 827)1 | P value2 | |
|---|---|---|---|---|---|---|
| Gestational age at study visit | 0.470 | |||||
| Mean (SD) | 22.8 (2.88) | 22.8 (2.97) | 23.2 (3.13) | 23.0 (3.00) | 23.0 (3.00) | |
| Range | 16.7–28.0 | 16.7–28.0 | 15.3–28.7 | 16.7–27.9 | 15.3–28.7 | |
| Percent of dietary intake from ultra-processed foods | < 0.001 | |||||
| Mean (SD) | 30.8 (3.55) | 36.7 (1.14) | 40.5 (1.07) | 46.4 (3.28) | 38.6 (6.23) | |
| Range | 9.77–34.7 | 34.7–38.6 | 38.7–42.7 | 42.7 – 59.0 | 9.77 – 59.0 | |
| Maternal age (years) | < 0.001 | |||||
| Mean (SD) | 28.7 (5.40) | 27.3 (5.24) | 26.1 (5.55) | 25.0 (5.20) | 26.8 (5.51) | |
| Range | 16–40 | 16–39 | 16–38 | 16–39 | 16–40 | |
| Family income (USD) | < 0.001 | |||||
| Mean (SD) | 51,200 (27,000) | 47,700 (28,100) | 36,600 (27,600) | 30,500 (24,200) | 41,500 (29,000) | |
| Range | 2,490–83,400 | 2,550–83,400 | 2,550–83,400 | 2,550–82,600 | 2,490 – 83,400 | |
| Maternal education | < 0.001 | |||||
| <High School | 8 (3.9 %) | 16 (7.7 %) | 20 (9.7 %) | 26 (12.6 %) | 70 (8.5 %) | |
| High School completion | 68 (32.9 %) | 82 (39.6 %) | 105 (51.0 %) | 117 (56.5 %) | 372 (45.0 %) | |
| Graduated college or technical school | 92 (44.4 %) | 76 (36.7 %) | 60 (29.1 %) | 53 (25.6 %) | 281 (34.0 %) | |
| Some graduate work or graduate/professional degree | 39 (18.8 %) | 33 (15.9 %) | 21 (10.2 %) | 11 (5.3 %) | 104 (12.6 %) | |
| Neighborhood deprivation index | < 0.001 | |||||
| Mean (SD) | 0.056 (0.783) | 0.182 (0.826) | 0.308 (0.805) | 0.456 (0.771) | 0.251 (0.809) | |
| Range | −0.883–2.80 | −1.22–2.80 | −1.04–2.45 | −0.963–3.08 | −1.22–3.08 | |
| Maternal race | < 0.001 | |||||
| Black/African American | 89 (43.0 %) | 111 (53.6 %) | 141 (68.4 %) | 160 (77.3 %) | 501 (60.6 %) | |
| White | 98 (47.3 %) | 84 (40.6 %) | 56 (27.2 %) | 40 (19.3 %) | 278 (33.6 %) | |
| Asian | 5 (2.4 %) | <5 | <5 | <5 | 8 (1.0 %) | |
| Multiple Race | 15 (7.2 %) | 9 (4.3 %) | 6 (2.9 %) | 7 (3.4 %) | 37 (4.5 %) | |
| Other | <5 | <5 | <5 | <5 | <5 | |
| Maternal ethnicity | 0.687 | |||||
| Not Hispanic/Latino | 202 (97.6 %) | 204 (98.6 %) | 203 (98.5 %) | 205 (99.0 %) | 814 (98.4 %) | |
| Hispanic/Latino | 5 (2.4 %) | <5 | <5 | <5 | 13 (1.6 %) | |
| Household size (# of persons) | 0.163 | |||||
| Mean (SD) | 4.35 (1.23) | 4.23 (1.18) | 4.51 (1.53) | 4.45 (1.43) | 4.39 (1.35) | |
| Range | 2–9 | 2–8 | 2–10 | 2 – 10 | 2–10 | |
| Prepregnancy BMI (kg/m 2 ) | 0.227 | |||||
| Mean (SD) | 27.7 (8.13) | 28.6 (7.63) | 28.7 (8.32) | 27.4 (6.97) | 28.1 (7.79) | |
| Range | 17–72 | 18–62 | 14–57 | 16–55 | 14–72 | |
| Maternal tobacco use during pregnancy | 0.646 | |||||
| No | 193 (93.2 %) | 190 (91.8 %) | 187 (90.8 %) | 186 (89.9 %) | 756 (91.4 %) | |
| Yes | 14 (6.8 %) | 17 (8.2 %) | 19 (9.2 %) | 21 (10.1 %) | 71 (8.6 %) | |
| Maternal alcohol use during pregnancy | 0.154 | |||||
| No | 187 (90.3 %) | 182 (87.9 %) | 189 (91.7 %) | 195 (94.2 %) | 753 (91.1 %) | |
| Yes | 20 (9.7 %) | 25 (12.1 %) | 17 (8.3 %) | 12 (5.8 %) | 74 (8.9 %) | |
| Total daily calories | < 0.001 | |||||
| Mean (SD) | 2,110 (831) | 2,330 (896) | 2,490 (915) | 2,700 (960) | 2,410 (926) | |
| Range | 1,010 – 4,850 | 1,040 – 4,820 | 1,040 – 4,920 | 1,030 – 4,800 | 1,010 – 4,920 |
Total sample size with full covariate data included in complete case analyses.
P values calculated from analysis of variance and chi-square goodness of fit tests for continuous and categorical variables, respectively.
Fig. 1.
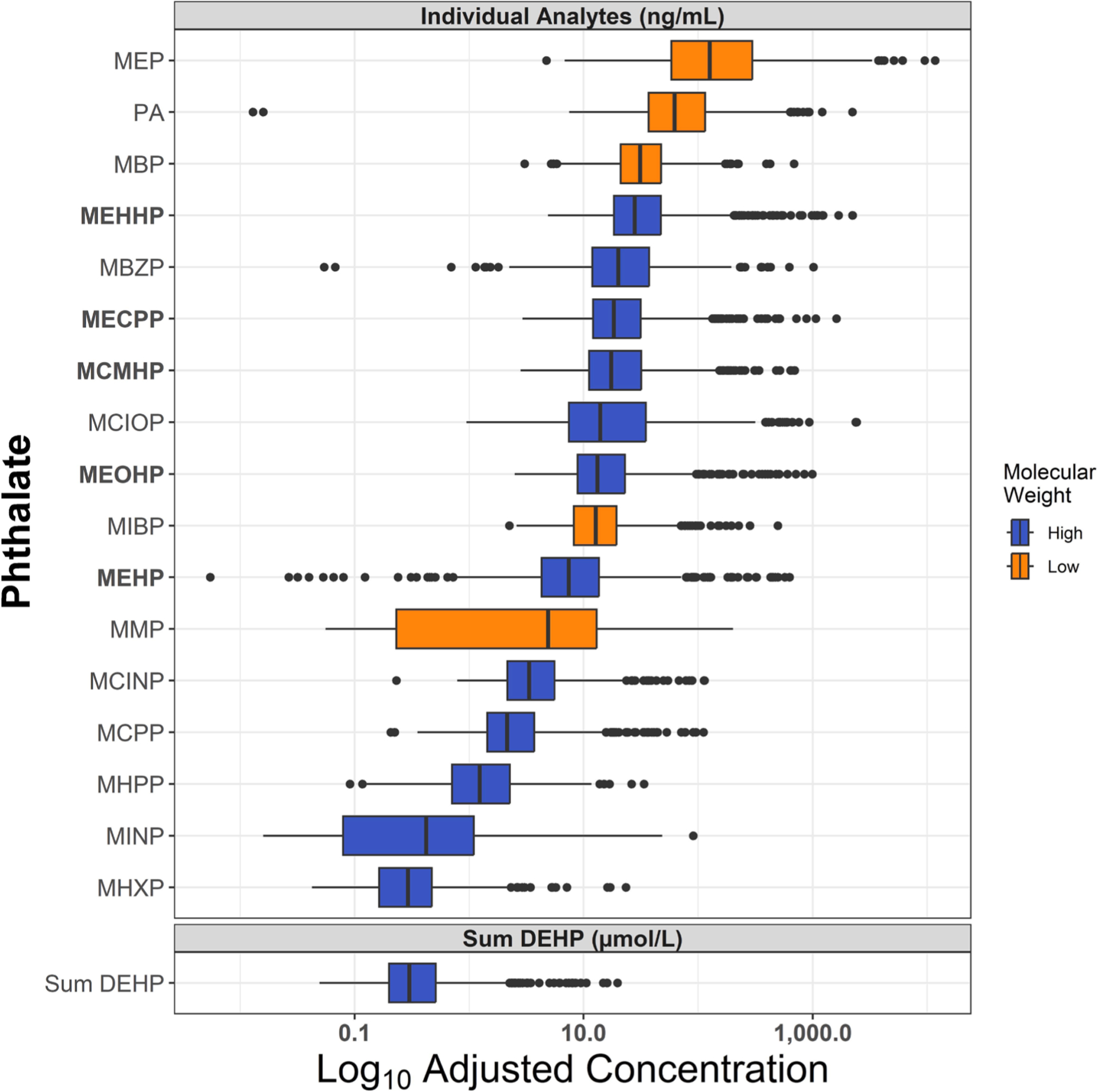
Distributions of 2nd trimester urinary phthalate metabolite levels among CANDLE participants (N = 827). Boxplots with 1.5 interquartile range (IQR) whiskers show distribution of log10-adjusted, specific gravity corrected urinary phthalate levels, and log10-adjusted DEHP molar sum. DEHP metabolites indicated in bold. DEHP molar sum calculated as (MEHP * (1/278.34)) + (MEHHP * (1/294.34)) + (MEOHP * (1/292.33)) + (MECPP * (1/308.33)) + (MCMHP * (1/308.33)).
Fig. 2 details our categorization of the Block FFQ line items into the four NOVA food groups. Most mothers reported more frequent consumption of minimally processed foods compared with ultra-processed foods (Fig. 3A). Ultra-processed foods constituted 9.8 to 59.0 % (mean = 38.6 %) of the total items in participants’ diets, while minimally processed foods constituted 30.8 to 80.5 % (mean = 49.6 %). Mothers in the highest ultra-processed food quartile had 42.7 to 59.0 % of total food intake from ultra-processed foods compared with 9.8 to 34.7 % for mothers in the lowest quartile (Table 1). Mothers in the highest quartile of ultra-processed food consumption were more likely to be younger, have lower family income, lower educational attainment, higher neighborhood deprivation levels, and higher daily caloric intakes (Table 1).
Fig. 2.
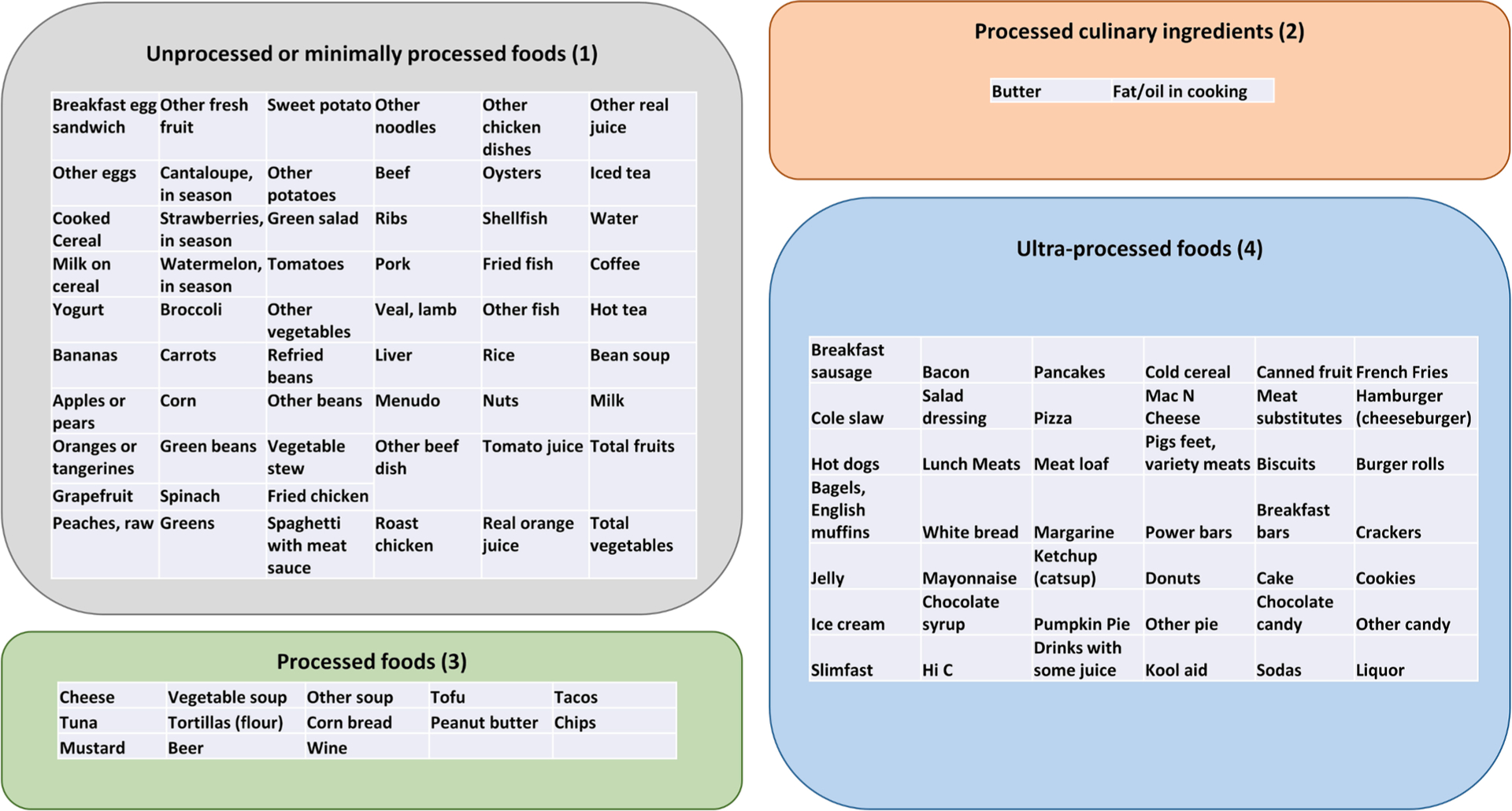
NOVA food groups. Manual categorization of Block food frequency questionnaire frequency data into NOVA food groups.
Fig. 3.
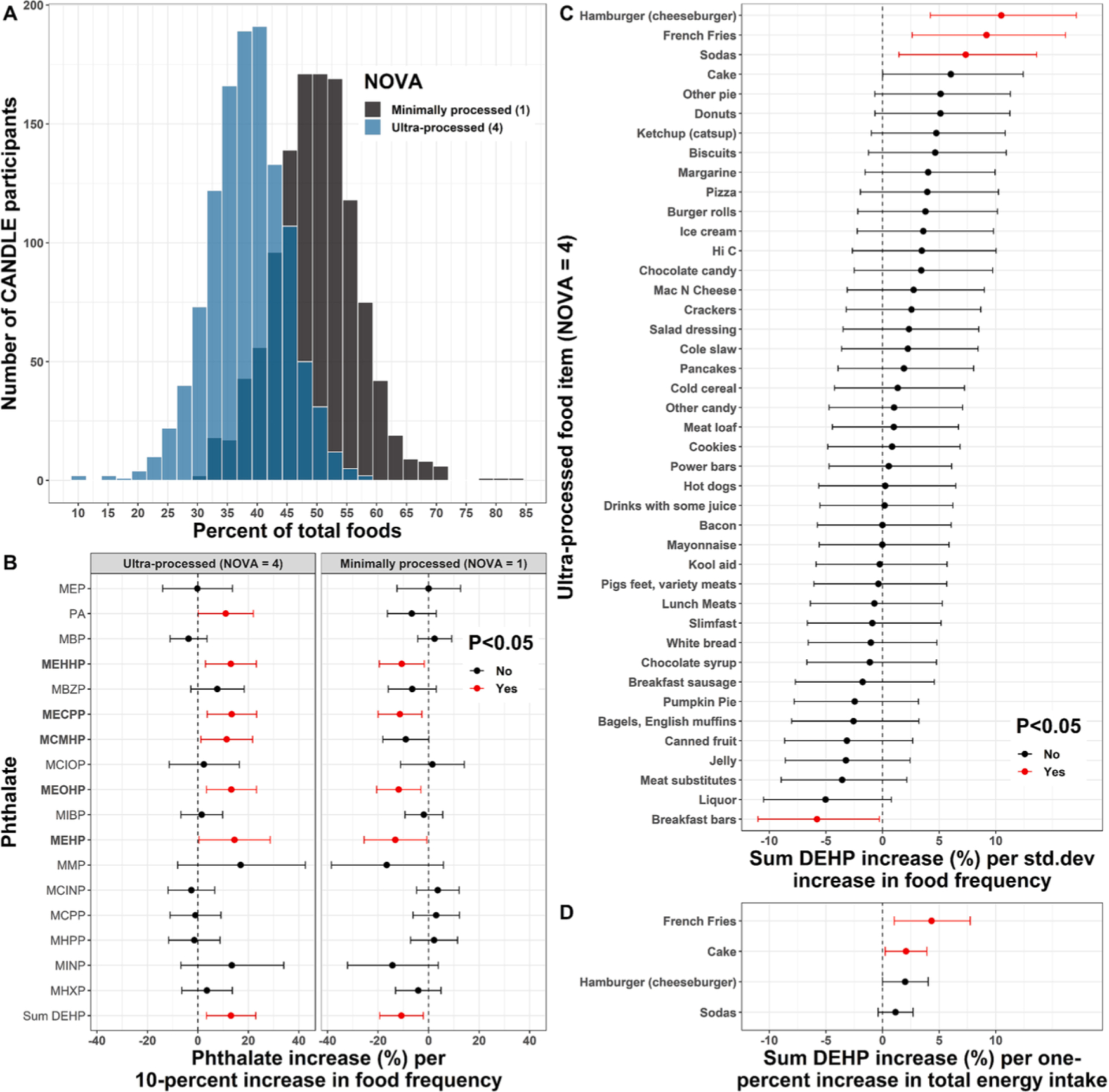
Associations of NOVA groups and ultra-processed foods with phthalate levels. Histogram (A) shows the distribution of CANDLE participants with respect to food frequency for ultra-processed and minimally processed food groups as a percentage of total food frequency. Linear model coefficients and 95% confidence intervals depict: (B) Associations of ultra-processed and minimally processed NOVA food group dietary proportions with phthalates from separate models for each NOVA group by phthalate outcome combination; (C) Associations of individual ultra-processed food items with DEHP molar sum from separate models per food item; and (D) A sensitivity analysis for the top four ultra-processed foods associated with sum DEHP where food items were modeled as percent of total daily energy intake rather than frequency of intake. All models in B-D were adjusted for maternal age, education, race, ethnicity, household income, number of individuals in the household, neighborhood deprivation index, pre-pregnancy BMI, tobacco and alcohol use, and daily caloric intake.
3.1. Associations of NOVA food groups and ultra-processed foods with urinary phthalate concentrations
Each 10 % higher dietary proportion of ultra-processed foods was associated with 13.1 % (95 % CI: 3.4 %, 22.9 %) higher urinary ΣDEHP (Fig. 3B, Table S4). Ultra-processed food intake was also significantly associated with 11.0 % higher urinary phthalic acid concentrations (95 % CI: 0.2 %, 22.0 %) and higher levels of all five DEHP metabolites (Fig. 3B). Conversely, each 10 % higher dietary proportion of minimally processed food intake was associated with 10.8 % (95 % CI: 3.4 %, 22.9 %) lower urinary ΣDEHP (Fig. 3B, Table S4). Higher minimally processed food intake was also significantly associated with lower levels of all five DEHP metabolites (Fig. 3B, Table S4), but there was no association with phthalic acid (−6.7 %; 95 % CI: −16.3 %, 3.1 %). There were no significant associations of consumption of ultra- or minimally-processed foods with non-DEHP phthalate metabolites (Fig. 3B).
Because the overall intake of ultra-processed foods was associated with higher urinary ΣDEHP and DEHP metabolite concentrations, we further explored associations of individual ultra-processed foods with ΣDEHP (Fig. 3C). Standard deviation increases in the frequency of intake of hamburgers/cheeseburgers, French fries, sodas, and cakes were each associated with 10.5 % (95 % CI: 4.2 %, 17.1 %), 9.2 % (95 % CI: 2.6 %, 16.2 %), 7.4 % (95 % CI: 1.4 %, 13.6 %), and 6.0 % (95 % CI: 0.0 %, 12.4 %) higher urinary ΣDEHP, respectively (Fig. 3C, Table S5). Each standard deviation higher frequency of breakfast bar intake was associated with 5.8 % (95 % CI: 0.3 %, 11.0 %) lower ΣDEHP. No other ultra-processed food items were significantly associated with ΣDEHP (Fig. 3C). Similar ΣDEHP increments were seen in a sensitivity analysis modeling hamburgers/cheeseburgers, French fries, sodas, and cakes in terms of percentage of total energy intake, although associations were attenuated and not statistically significant for hamburgers/cheese-burgers and sodas (Fig. 3D, Table S6).
Fried chicken, fried fish, breakfast sandwiches, and macaroni and cheese were assigned to different NOVA groups in sensitivity analyses. Compared with the main analysis, associations of ultra-processed food consumption with phthalates were the same when all flagged foods were categorized as minimally processed (Fig. S1A). When all flagged foods were categorized as ultra-processed, results were the same except that ultra-processed food consumption was no longer associated with phthalic acid (Fig. S1B). None of the flagged foods were individually associated with ΣDEHP (Fig. S1C). We found similar results in a sensitivity analysis including all 925 complete cases with full covariate data, without filtering based on daily caloric intake. Consistent with the main analysis, ultra-processed foods were positively associated with four DEHP metabolites and ΣDEHP. Unlike the main analysis, however, ultra-processed foods were not associated with MEHP or phthalic acid (Fig. S2A). The following ultra-processed food items were positively associated with urinary ΣDEHP: hamburgers/cheeseburgers, French fries, donuts, sodas, and cake (Fig. S1B). In another sensitivity analysis, we included all 1031 individuals by imputing missing covariate data with MICE. Consistent with the main analysis, ultra-processed foods were associated with phthalic acid, four DEHP metabolites, and ΣDEHP. Unlike the main analysis, however ultra-processed foods were not associated with MEHP (Fig. S3A). The following ultra-processed food items were positively associated with urinary ΣDEHP: hamburgers/cheeseburgers, French fries, donuts, chocolate candy, and sodas (Fig. S3B).
Causal mediation analyses (N = 827) examined associations of three socioeconomic variables with urinary ΣDEHP, mediated through ultra-processed food intake. Consistent with the main analysis, the outcome regressions in all three causal mediation models indicated that each 10 % increase in the proportion of dietary intake from ultra-processed foods was significantly associated with approximately 13 % higher urinary ΣDEHP (Fig. 4). Being in the 75th ($71,440.5) versus 25th ($17,909.0) percentile of income was associated with 1.4 % reduced ultra-processed food intake (95 % CI: 0.3 %, 2.6 %), and the indirect effect estimate indicated that being in the 75th versus 25th percentile of income was associated with 1.9 % (95 % CI: 0.2 %, 4.2 %) lower ΣDEHP mediated through reduced ultra-processed food intake (Fig. 4A; proportion mediated = 0.66). Compared with self-report of educational attainment beyond high school, no higher education was associated with 1.0 % increased ultra-processed food intake (95 % CI: 0.3 %, 2.3 %), and the indirect effect estimate indicated that no higher education was associated with 1.4 % (95 % CI: 0.1 %, 3.3 %) higher ΣDEHP mediated through increased ultra-processed food intake (Fig. 4B; proportion mediated = −0.20). Neighborhood deprivation index was neither associated with ultra-processed food consumption nor indirectly associated with ΣDEHP through the ultra-processed food consumption pathway (Fig. 4C), and the direct and total effects were non-significant in all mediation models (Fig. 4).
Fig. 4.
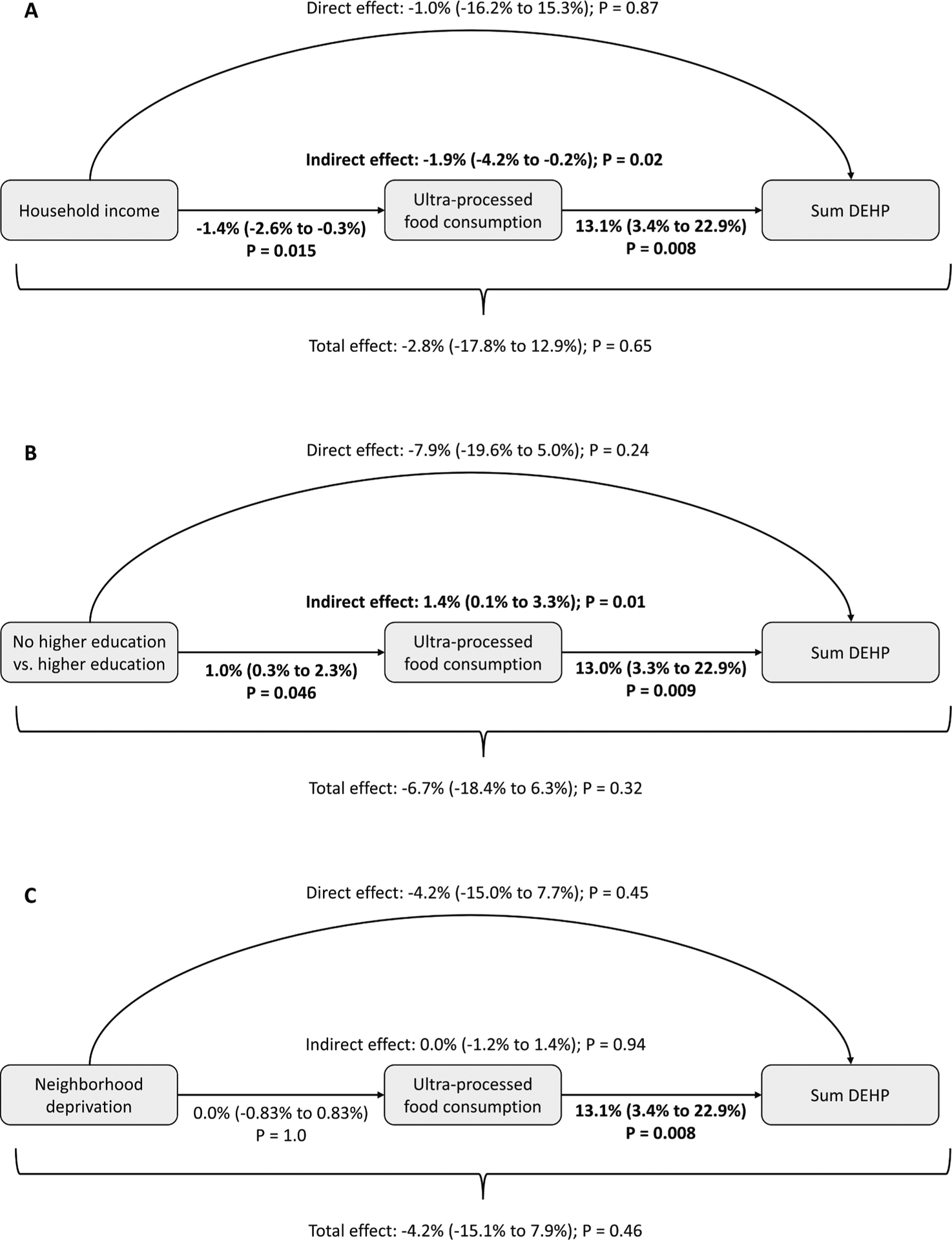
Mediation analyses of sociodemographic characteristics, ultra-processed foods, and sum DEHP. Causal mediation analysis examining the role of ultra-processed food intake as a mediator on the pathway between socioeconomic factors and DEHP exposure during pregnancy. Regression-based mediation approach was implemented in the ‘CMAverse’ R package with closed-form parameter function estimation and bootstrap inference with 1,000 iterations. Models estimated the percent change in DEHP (95 % confidence interval) associated with: (A) being in the 75th ($71,440.5) versus 25th ($17,909.0) percentiles of income, (B) higher education versus no higher education, and (C) being in the 75th versus 25th percentiles of neighborhood deprivation index. The no higher education group includes individuals self-reporting “High School completion” and “<High School” while the higher education group includes individuals self-reporting “Some graduate work or graduate/professional degree” and “Graduated college or technical school.” P values < 0.05 indicated in bold.
3.2. Associations of latent dietary pattern variables with urinary phthalate concentrations
Loadings for 5 factors from exploratory factor analysis are presented in Fig. 5A. Based on the loadings, we interpreted these factors to represent latent variables for the following 5 dietary patterns (% variance explained): ‘Cereal’ (7.1 %), characterized by higher consumption of cold cereal and milk on cereal; ‘Healthy’ (6.1 %), characterized by higher total fruit and total vegetable consumption; ‘US Southern’ (3.2 %), characterized by higher consumption of foods commonly associated with the US south including greens, fried fish and chicken, and corn bread; ‘Salad’ (2.3 %), characterized by higher consumption of green salad, salad dressing, and tomatoes; and ‘Processed’ (2.1 %), characterized by higher consumption of pre-packaged foods, refined grains, high-sugar drinks, candy and sweets. The data-driven, ‘Processed’ dietary pattern was associated with increases in the same urinary phthalates as the manually categorized NOVA ultra-processed food group: phthalic acid, ΣDEHP, and all five DEHP metabolites (Fig. 5, Table S7). The strongest association was a 14.1 % (95 % CI: 3.5 %, 25.9 %) increase in the DEHP metabolite, MEHP, per standard deviation increase in the ‘Processed’ pattern. Green salad and salad dressing were the top loadings for the ‘Salad’ dietary pattern, which was significantly associated with increased MCIOP, and there were suggestive positive associations with MCMHP (P = 0.064), and MCINP (P = 0.067). The ‘US Southern’ pattern was associated with increased phthalic acid, MCPP, and MHXP. The ‘Healthy’ dietary pattern was associated with decreased urinary levels of MEHHP, MECPP, MCMHP, MINP, and ΣDEHP. There were no phthalate associations with the Cereal pattern.
Fig. 5.
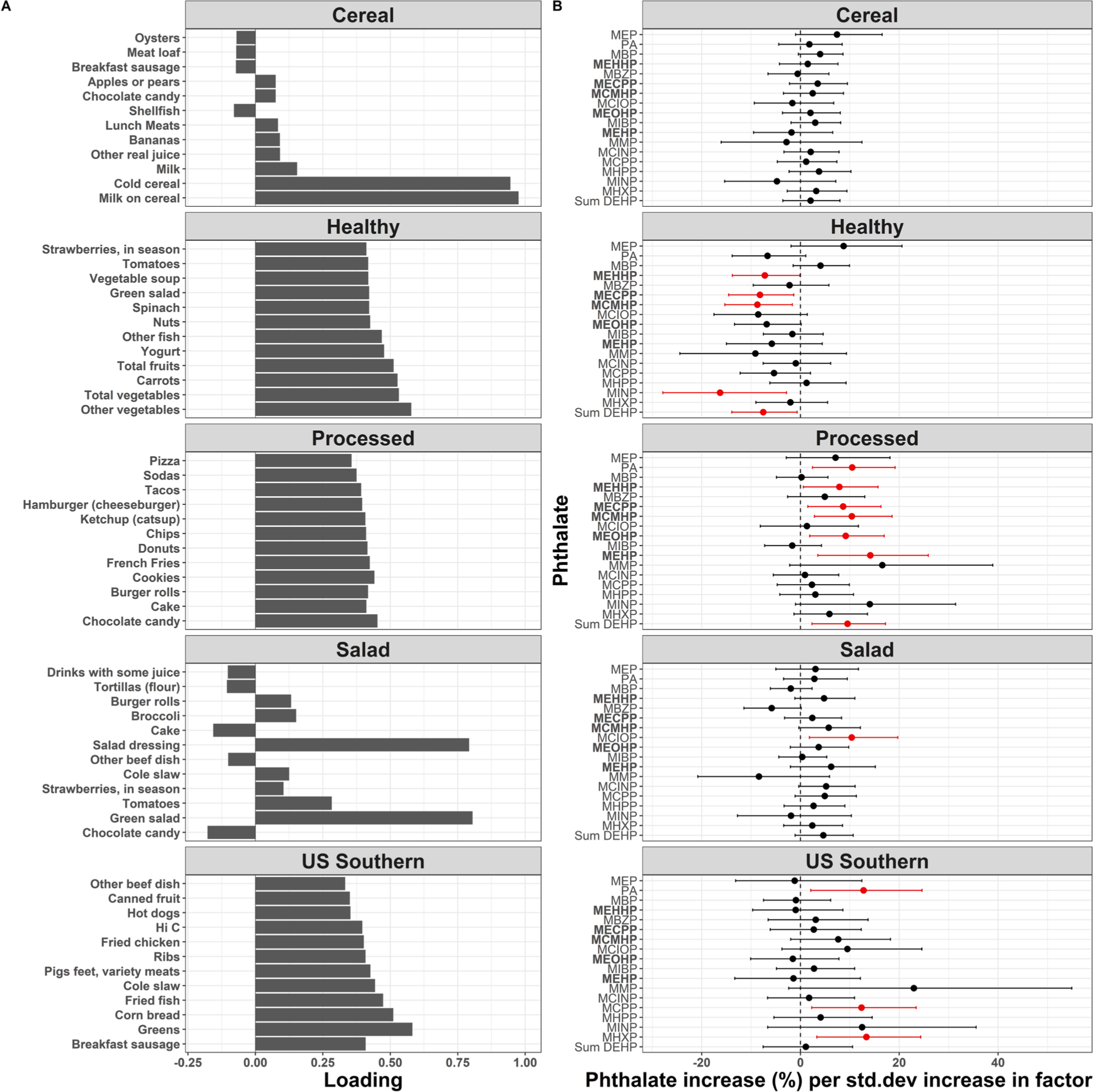
Associations of FFQ factors with phthalate levels. Exploratory factor analysis with 5 factors for 114 food item frequencies from the Block FFQ (A). Top 12 loadings for each factor shown. Loadings are approximately equal to Pearson correlations between the food items and factors (i.e., milk on cereal is nearly 100% correlated with the “Cereal” factor). Coefficients and 95% confidence intervals depict one-percent increase in phthalate exposures per one-standard deviation (std. dev) increase in FFQ factors (B). Linear models include all 5 factors and covariates as predictors of phthalates, with one model per metabolite. Covariates included age, education, race, ethnicity, household income, number of individuals in the household, neighborhood deprivation index, pre-pregnancy BMI, tobacco and alcohol use, and daily caloric intake.
A sensitivity analysis with 4 rather than 5 factors uncovered similar latent variables for the ‘Cereal’, ‘Healthy’, ‘US Southern’, and ‘Processed’ dietary patterns, and their associations with urinary phthalates were similar compared to the main analysis (Figure S4). For instance, the ‘Processed’ pattern was similarly associated with increases in phthalic acid, ΣDEHP, and all five DEHP metabolites. A sensitivity analysis with 6 factors uncovered similar latent variables for the ‘Cereal’, ‘Healthy’, ‘US Southern’, ‘Salad’, and ‘Processed’ dietary patterns, along with an additional ‘Unknown’ pattern related to increased consumption of coffee, vegetables, fish, meat substitutes, and tofu, potentially reflecting a pescatarian diet (Fig. S5A). Associations were generally similar between phthalates and the 5 factors uncovered in the primary analysis, and the ‘Unknown’ pattern was associated with decreased urinary phthalic acid and MCIOP (Fig. S5B).
4. Discussion
In this socioeconomically diverse, US Southern cohort study of 1031 pregnant women, more frequent ultra-processed food intake during pregnancy was associated with higher urinary concentrations of phthalic acid, ΣDEHP, and five DEHP metabolites, while more frequent intake of minimally processed foods was associated with lower levels of ΣDEHP and five DEHP metabolites. In our analysis of individual ultra-processed food items, hamburgers/cheeseburgers, French fries, sodas, and cake were each individually associated with higher urinary ΣDEHP. Unsupervised, exploratory factor analysis identified a pattern consistent with more frequent processed food intake, and this data-driven processed food pattern was positively associated with urinary concentrations of phthalic acid, ΣDEHP, and five DEHP metabolites. Lower household income and lower maternal education levels were associated with higher urinary ΣDEHP mediated through higher ultra-processed food intake, implicating socioeconomic barriers to accessing affordable, minimally processed foods. To our knowledge, this study is the first to evaluate the proportion of dietary intake from NOVA ultra-processed foods during pregnancy as a potential source of prenatal phthalate exposures, and the first to show that ultra-processed food consumption partially mediates socioeconomic disparities in phthalate exposures. Moreover, these findings in a racially and socioeconomically diverse, US Southern population enhances inclusion of groups traditionally under-represented in research.
Prior to this study, the only analyses of links between ultra-processed food consumption and phthalates were conducted in the US (NHANES) (Buckley et al., 2019; Martínez Steele et al., 2020) and Taiwanese (Huang et al., 2021) general populations. Higher consumption of minimally processed food was associated with lower concentrations of MEP and MBP in the Asian Taiwanese adult population (Huang et al., 2021). In an analysis of NHANES 2013–2014, greater ultra-processed food intake was associated with higher urinary MCPP, MCNP, and MCOP (Buckley et al., 2019). Including data from 2009 to 2016, a second NHANES study found that greater ultra-processed food intake was associated with higher MCPP, MCNP, MBZP, and DINP molar sum (Martínez Steele et al., 2020). In contrast to those studies, where no associations with DEHP were observed, we found strong associations of ultra-processed foods with urinary DEHP metabolites. Our study sample was recruited earlier (2006–2011) than the participants in these NHANES analyses, and temporal trends in phthalate exposures likely explains the discrepant findings. Declines in DEHP over time have been observed as it is increasingly replaced with other high molecular weight phthalate substitutes such as DINP and DIDP (Zota et al., 2014; Koch et al., 2017). Exemplifying this trend, we detected MEHP in 99.1 % of our study sample, while MEHP was only detected in 57.8 % in the NHANES 2013–2014 study (Buckley et al., 2019). Additionally, for three phthalate metabolites significantly associated with ultra-processed foods in NHANES (MCPP, MCNP, and MCOP), concentrations were significantly lower in this cohort compared with national estimates (Adgent et al., 2020).
We observed that consumption of food items commonly found at fast food restaurants was associated with increased urinary ΣDEHP. These foods included hamburgers/cheeseburgers, French fries, and sodas. This result is consistent with prior studies showing higher phthalate exposures in association with fast foods (Zota et al., 2016). Although positive associations between ultra-processed foods and DEHP observed here appear to be primarily driven by fast foods, our results suggest additional phthalate exposure sources such as cakes and pies. Similarly, prior studies have also shown that consumption of foods prepared away from home may increase phthalate exposures (Varshavsky et al., 2018). Interestingly, the association we found between the frequency of soda consumption and urinary ΣDEHP was substantially attenuated and no longer statistically significant when sodas were modeled in terms of percent of total energy intake. We explicitly accounted for diet and non-diet soda consumption. Thus, modeling energy intakes could have resulted in classifying diet soda drinkers as having zero soda exposure. Modeling consumption frequencies rather than calories may have advantages in such cases where phthalate contributions from low calorie foods, such as diet sodas, could be missed.
DEHP remains ubiquitous, particularly in fast foods, despite the general trend toward lower levels of exposure and replacement by alternative plasticizers (Schecter et al., 2013). More than 20 years ago, disposable gloves used in the preparation of foods were identified as a major source of DEHP in packaged foods (Tsumura et al., 2001). Tsumura and colleagues found that these gloves were upwards of 41 % by weight of DEHP, detected DEHP in 100 % of packed lunches purchased from 10 convenience stores and 10 restaurants, and observed that DEHP could readily migrate into handled foods in a lab experiment (Tsumura et al., 2001). In a recent study, 70 % of food samples collected in 2017–2018 from six of the most popular fast food restaurants contained detectable levels of DEHP, yet DEHP was not found in gloves collected from the same restaurants (Edwards et al., 2022), which could reflect shifts from vinyl to safer alternative glove materials such as poly-ethylene or nitrile (Olson et al., 2019), and suggests other prominent sources of exposure. Recent analyses of plasticizers in US market products detected DEHP in food contact materials including tubing, the gaskets found in metal cap closures used on glass jars and bottles, and paper-based fast food packaging (Carlos et al., 2018; Carlos et al., 2021). Food contact materials may be sources of exposure to additional environmental chemicals, including phthalate replacements like di(2-ethylhexyl) terephthalate (DEHT) (Edwards et al., 2022), per- and polyfluoroalkyl substances (PFASs) (Susmann et al., 2019), and bisphenols (Martínez Steele et al., 2020; Hartle et al., 2016).
We found that urinary phthalates during pregnancy were not only associated with ultra-processed foods and individual fast food-related items, but also with data-driven dietary patterns. Our dietary patterns based on exploratory factor analysis have several advantages over NOVA. For instance, NOVA requires manual classification of food items. Individuals, including those involved in scientific research, possess unique relationships with food, and these individualized perspectives could be a source of bias during manual food categorization. Additionally, each FFQ item can only be assigned to a single NOVA group, and exposure misclassification could occur for foods that may belong to different groups depending on how they are prepared. A possible disadvantage of exploratory factor analysis is the potential to produce uninterpretable factor variables. However, the FFQ data factors generated here were relatively distinct and interpretable. Furthermore, one of the factors was characterized by increased processed food consumption, and as with increased NOVA ultra-processed food intake, this processed food factor was associated with higher urinary phthalic acid, ΣDEHP, and DEHP metabolites.
Exploratory factor analysis revealed additional dietary patterns associated with phthalate exposures. For instance, pregnant women with diets high in vegetables, fruits, yogurt, fish, and nuts had reduced ΣDEHP and MINP concentrations. This finding corroborates inverse associations between phthalate levels and consumption of fruit in NHANES (Trasande et al., 2013; Colacino et al., 2010), and results from a study of pregnant women in the Tongji Birth Cohort, where a fruit-nut-vegetable pattern constructed by principal component analysis correlated with lower MEOHP exposure (Luo et al., 2022). However, positive associations between fruits and DEHP have also been reported (Husøy et al., 2019), and a previous NHANES analysis found no associations between healthy diet patterns and phthalate exposures, suggesting that factors beyond food selection (such as production, storage, and preparation) may be stronger predictors of contamination (Melough et al., 2022). Interestingly, we found another factor characterized by increased consumption of salad and salad dressing which was associated with increased urinary MCIOP. MCIOP is the primary metabolite of DINP, which has been detected in cap gaskets (Carlos et al., 2018; Fankhauser-Noti and Grob, 2006) and vegetable oils (Nanni et al., 2011), implicating potential contamination in salad and salad dressings.
Our finding that diets high in minimally processed foods, vegetables, fruits, yogurt, fish, and nuts correlate with lower urinary phthalate concentrations suggests opportunities for intervention. However, the results of prior dietary interventions aimed at reducing phthalate exposures are mixed (Rudel et al., 2011; Sathyanarayana et al., 2013; Barrett et al., 2015). One study found that a 3-day fresh, unprocessed food intervention significantly decreased levels of urinary DEHP metabolites during the intervention compared with the pre- and post-intervention periods (Rudel et al., 2011). By contrast, another intervention study randomized participants to a 5-day complete dietary replacement designed to reduce phthalate and bisphenol exposures, and found an unanticipated rise in DEHP during the intervention compared with the pre- and post-intervention periods (Sathyanarayana et al., 2013). Follow-up testing of the food samples used in the dietary replacement revealed substantial levels of DEHP in the individual spices, especially ground coriander. Complicating this, a previous cohort study found negative associations between consumption of spices and phthalate metabolite levels (Serrano et al., 2014). Thus, although the consensus of prior research suggests that consumption of ultra-processed and fast foods may drive phthalate exposures, switching to a minimally processed diet may not guarantee decreased exposure owing to unpredictable sources of phthalate contamination. Dietary recommendations are a short-term solution that places burdens on consumers, and regulatory policies promoting safer alternatives (rather than regrettable substitution with equally hazardous phthalates, as has already been done with DINP for DEHP) (Engel et al., 2021) may be a more effective strategy to reduce harmful prenatal phthalate exposures.
This is the first study to show that ultra-processed food consumption partially mediates links between lower socioeconomic status and greater phthalate exposures. Individual (Rao et al., 2013; Darmon and Drewnowski, 2015; Glanz et al., 1998) and neighborhood level (Zenk et al., 2005; Moore and Diez Roux, 2006; Block et al., 2004; Richardson et al., 2014) socioeconomic factors are known to affect food access. In this study, associations of lower household income and maternal education levels with higher phthalate exposures were partially mediated through higher levels of ultra-processed food intake. However, this mediation pathway was not found for neighborhood deprivation index. These disparate results for individual versus neighborhood level socioeconomic indicators can possibly be explained by differing constraints faced by low and high-income consumers. Individual financial constraints are among the top factors influencing health-related food-shopping behaviors (Cannuscio et al., 2014). For instance, low-income individuals in food deserts may be unable to afford transportation costs to facilitate access to supermarkets, while higher income individuals living in the same residential areas may not face this constraint (Rose and Richards, 2004; Guy et al., 2004; Cotterill and Franklin, 1995). Beyond income, some individuals choose to shop in stores where other shoppers align with their self-perceived socioeconomic status (Cannuscio et al., 2014). It is possible that these individual-level socioeconomic factors may, in some cases, override neighborhood characteristics in guiding food choices. These hypotheses align with several studies reporting that many individuals do not shop at the supermarket closest to them (Cannuscio et al., 2013; Drewnowski et al., 2012; LeDoux and Vojnovic, 2013). Thus, regulatory action aimed at enhancing economic access, rather than just physical access, to minimally processed foods could be an effective strategy to curb ultra-processed food-associated phthalate exposures.
These results should be considered in the context of several limitations. First, our study focused on ultra-processed foods and did not incorporate information on food contact materials. We grouped foods based on their level of processing following NOVA, but future work could apply a similar strategy by designing a manual food categorization system specifically related to the degree of food contact with plastics during processing. Food contact with plastics may also result from consumer practices, and prior studies have examined associations of phthalate exposures with self-reported use of plastics in food contact materials including microwave containers, tableware, and food packaging (Serrano et al., 2014; Yan et al., 2009; Valvi et al., 2015; Cantonwine et al., 2014). Second, self-reported dietary intake is subject to intentional and unintentional misreporting and thus the potential for misclassification bias. To address these errors, dietary biomarkers have been used in nutritional epidemiology (Naska et al., 2017), although it is unclear how such measures could be employed in our study of ultra-processed foods and phthalates. Third, most phthalate metabolites are subject to short half-lives and high variability with respect to the time and date of sample collection (Preau et al., 2010; Sugeng et al., 2020). A 24-hour recall reflecting diet in the hours preceding urine collection would likely better capture which foods contributed to phthalate exposure. Alternatively, multiple spot urine collections throughout pregnancy and at different times of day could also increase the precision of the diet-phthalate associations reported here. Fourth, because we conducted many statistical tests, false discoveries are possible. Finally, although we controlled for many potential confounding variables, residual confounding cannot be ruled out in any observational study.
In summary, our study demonstrates associations between maternal dietary patterns, particularly the consumption of ultra-processed foods and fast-food items, and higher urinary phthalate concentrations during pregnancy. These findings expand upon previous research conducted in the general US and Taiwanese populations and highlight dietary sources of phthalates in pregnant women rather than the general population. Moreover, our exploratory factor analysis approach provides a data-driven internal validation of associations of processed food intake with increased phthalate exposures. We additionally corroborate findings from prior studies by showing associations of healthy diets high in vegetables, fruits, yogurt, fish, and nuts with lower levels of some phthalates. Although these findings have important implications for modifiable lifestyle factors regarding dietary choices during pregnancy, unpredictable contamination and socioeconomic barriers to dietary modification preclude the use of dietary recommendations as a sole means to reduce phthalate exposures. Policy reforms to reduce dietary phthalate exposures, especially from food packaging, are critically needed.
Supplementary Material
Acknowledgements
This research was conducted using specimens and data collected from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study which was funded by the Urban Child Institute. This study was conducted with additional support from ECHO PATHWAYS, funded by the National Institutes of Health (NIH; UG3/UH3OD023271). BHB was supported in part by the UW NIEHS sponsored Biostatistics, Epidemiologic and Bioinformatic Training in Environmental Health (BEBTEH) Training Grant: NIEHS T32ES015459. AGP was supported by NICHD K99/R00HD096112 and 1R01ES033785. ESB was supported by NIH P30ES005022. The authors would like to thank the study staff, data teams, and co-investigators involved in the CANDLE cohort as well as the ECHO-PATHWAYS consortium for their invaluable contributions. We are also grateful to the study participants who generously volunteered their time for this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications.
Footnotes
CRediT authorship contribution statement
Brennan H. Baker: Conceptualization, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Melissa M. Melough: Conceptualization, Formal analysis, Methodology, Writing – review & editing. Alison G. Paquette: Methodology, Writing – review & editing. Emily S. Barrett: Methodology, Writing – review & editing. Drew B. Day: Methodology, Writing – review & editing. Kurunthachalam Kannan: Methodology, Writing – review & editing. Ruby HN Nguyen: Methodology, Writing - Review & Editing. Nicole R. Bush: Funding acquisition, Methodology, Writing – review & editing. Kaja Z. LeWinn: Funding acquisition, Methodology, Writing – review & editing. Kecia N. Carroll: Methodology, Funding acquisition, Writing - Review & Editing. Shanna H. Swan: Methodology, Writing – review & editing. Qi Zhao: Methodology, Writing – review & editing. Sheela Sathyanarayana: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2024.108427.
Data availability
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement.
References
- Adgent MA, Carroll KN, Hazlehurst MF, et al. , 2020. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ. Int 143, 105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang DY, Kyung M, Kim MJ, et al. , 2012. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Compr. Rev. Food Sci. Food Saf 11 (5), 453–470. [Google Scholar]
- Baraldi LG, Steele EM, Canella DS, Monteiro CA, 2018. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open 8 (3), e020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Velez M, Qiu X, Chen S-R, 2015. Reducing prenatal phthalate exposure through maternal dietary changes: results from a pilot study. Matern. Child Health J 19, 1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevel MS, Tsai M-H, Parham A, Andrzejak SE, Jones S, Moore JX, 2023. Association of food deserts and food swamps with obesity-related cancer mortality in the US. JAMA Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L, 1986. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol 124 (3), 453–469. [DOI] [PubMed] [Google Scholar]
- Block JP, Scribner RA, DeSalvo KB, 2004. Fast food, race/ethnicity, and income: a geographic analysis. Am. J. Prev. Med 27 (3), 211–217. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E, Rebholz CM, 2019. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int 131, 105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannuscio CC, Tappe K, Hillier A, Buttenheim A, Karpyn A, Glanz K, 2013. Urban food environments and residents’ shopping behaviors. Am. J. Prev. Med 45 (5), 606–614. [DOI] [PubMed] [Google Scholar]
- Cannuscio CC, Hillier A, Karpyn A, Glanz K, 2014. The social dynamics of healthy food shopping and store choice in an urban environment. Soc Sci Med 122, 13–20. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-González LO, et al. , 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ. Int 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos KS, de Jager LS, Begley TH, 2018. Investigation of the primary plasticisers present in polyvinyl chloride (PVC) products currently authorised as food contact materials. Food Addit. Contaminants: Part A 35 (6), 1214–1222. [DOI] [PubMed] [Google Scholar]
- Carlos KS, de Jager LS, Begley TH, 2021. Determination of phthalate concentrations in paper-based fast food packaging available on the US market. Food Additives & Contaminants: Part A 38 (3), 501–512. [DOI] [PubMed] [Google Scholar]
- Colacino JA, Harris TR, Schecter A, 2010. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect 118 (7), 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey-Stowers K, Schwartz MB, Brownell KD, 2017. Food swamps predict obesity rates better than food deserts in the United States. Int. J. Environ. Res. Public Health 14 (11), 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterill RW, Franklin AW (1995). The urban grocery store gap (Food Marketing Policy Issue Paper No. 8). Storrs, CT: Food Marketing Policy Center, University of Connecticut. 1995;199. [Google Scholar]
- Darmon N, Drewnowski A, 2015. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr. Rev 73 (10), 643–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Rangarajan S, et al. , 2023. Ultra-processed foods and mortality: Analysis from the Prospective Urban and Rural Epidemiology study. Am. J. Clin. Nutr 117 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Aggarwal A, Hurvitz PM, Monsivais P, Moudon AV, 2012. Obesity and supermarket access: proximity or price? Am. J. Public Health 102 (8), e74–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L, McCray NL, VanNoy BN, et al. , 2022. Phthalate and novel plasticizer concentrations in food items from US fast food chains: a preliminary analysis. J. Eposure Sci. Environ. Epidemiol 32 (3), 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Patisaul HB, Brody C, et al. , 2021. Neurotoxicity of ortho-phthalates: recommendations for critical policy reforms to protect brain development in children. Am. J. Public Health 111 (4), 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser-Noti A, Grob K, 2006. Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: Amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends Food Sci. Technol 17 (3), 105–112. [Google Scholar]
- Fromme H, Gruber L, Schlummer M, et al. , 2007. Intake of phthalates and di (2-ethylhexyl) adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ. Int 33 (8), 1012–1020. [DOI] [PubMed] [Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, Snyder D, 1998. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J. Am. Diet. Assoc 98 (10), 1118–1126. [DOI] [PubMed] [Google Scholar]
- Golestanzadeh M, Riahi R, Kelishadi R, 2019. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environmental Science and Pollution Research 26, 35670–35686. [DOI] [PubMed] [Google Scholar]
- Guy C, Clarke G, Eyre H, 2004. Food retail change and the growth of food deserts: a case study of Cardiff. Int. J. Retail Distrib. Manage 32 (2), 72–88. [Google Scholar]
- Hartle JC, Navas-Acien A, Lawrence RS, 2016. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res 150, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JM, Comeau ME, Casperson S, et al. , 2023. Dietary guidelines meet NOVA: developing a menu for a healthy dietary pattern using ultra-processed foods. J. Nutr [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5 (1), 46–51. [Google Scholar]
- Huang Y-C, Huang P-R, Lo Y-TC, et al. , 2021. Food processing and phthalate exposure: The nutrition and health survey in Taiwan (1993–1996 and 2005–2008). Front. Nutrit 2021;8:766992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husøy T, Andreassen M, Hjertholm H, et al. , 2019. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ. Int 132, 105103. [DOI] [PubMed] [Google Scholar]
- Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N, 2021. Ultra-processed foods and incident cardiovascular disease in the Framingham Offspring Study. J. Am. Coll. Cardiol 77 (12), 1520–1531. [DOI] [PubMed] [Google Scholar]
- Katsikantami I, Sifakis S, Tzatzarakis MN, et al. , 2016. A global assessment of phthalates burden and related links to health effects. Environ. Int 97, 212–236. [DOI] [PubMed] [Google Scholar]
- Khandpur N, Rossato S, Drouin-Chartier J-P, et al. , 2021. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Rüther M, Schütze A, et al. , 2017. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 220 (2), 130–141. [DOI] [PubMed] [Google Scholar]
- LeDoux TF, Vojnovic I, 2013. Going outside the neighborhood: The shopping patterns and adaptations of disadvantaged consumers living in the lower eastside neighborhoods of Detroit, Michigan. Health & Place 19, 1–14. [DOI] [PubMed] [Google Scholar]
- Luo C, Deng J, Chen L, et al. , 2022. Phthalate acid esters and polycyclic aromatic hydrocarbons concentrations with their determining factors among Chinese pregnant women: a focus on dietary patterns. Sci. Total Environ 852, 158344. [DOI] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat M-P, 2015. Obstetrical outcomes and biomarkers to assess exposure to phthalates: a review. Environ. Int 83, 116–136. [DOI] [PubMed] [Google Scholar]
- Martínez Steele E, Khandpur N, da Costa Louzada ML, Monteiro CA, 2020. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS One 15 (7), e0236738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melough MM, Maffini MV, Otten JJ, Sathyanarayana S, 2022. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res 211, 113049. [DOI] [PubMed] [Google Scholar]
- Messer LC, Laraia BA, Kaufman JS, et al. , 2006. The development of a standardized neighborhood deprivation index. J. Urban Health 83, 1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CA, 2009. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 12 (5), 729–731. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Cannon G, Levy R, et al. , 2016. NOVA. The star shines bright. World Nutrition 7 (1–3), 28–38. [Google Scholar]
- Moore LV, Diez Roux AV, 2006. Associations of neighborhood characteristics with the location and type of food stores. Am. J. Public Health 96 (2), 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni N, Fiselier K, Grob K, et al. , 2011. Contamination of vegetable oils marketed in Italy by phthalic acid esters. Food Control 22 (2), 209–214. [Google Scholar]
- Naska A, Lagiou A, Lagiou P, 2017. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000 Research 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L, Miller GZ, Belliveau M, 2019. Taking off the toxic gloves: An investigation of phthalates and other chemicals of concern in food-handling gloves. Ecology Center. [Google Scholar]
- Palmer FB, Anand KJ, Graff JC, et al. Early adversity, socioemotional development, and stress in urban 1-year-old children. The Journal of pediatrics. 2013;163(6):1733–1739. e1731. [DOI] [PubMed] [Google Scholar]
- Preau JL Jr, Wong L-Y, Silva MJ, Needham LL, Calafat AM, 2010. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di (2-ethylhexyl) phthalate among eight adults: an observational study. Environ. Health Perspect 118 (12), 1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2021. Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rao M, Afshin A, Singh G, Mozaffarian D, 2013. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open 3 (12), e004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W, 2015. Package ‘psych’. The Comprehensive R Archive Network. 337, 338. [Google Scholar]
- Richardson AS, Meyer KA, Howard AG, et al. , 2014. Neighborhood socioeconomic status and food environment: a 20-year longitudinal latent class analysis among CARDIA participants. Health Place 30, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Barbosa F Jr, Kannan K, 2017. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ 586, 152–162. [DOI] [PubMed] [Google Scholar]
- Rose D, Bodor JN, Swalm CM, Rice JC, Farley TA, Hutchinson PL, 2009. Deserts in New Orleans? Illustrations of urban food access and implications for policy. University of Michigan National Poverty Center/USDA Economic Research Service Research, Ann Arbor, MI. [Google Scholar]
- Rose D, Richards R, 2004. Food store access and household fruit and vegetable use among participants in the US Food Stamp Program. Public Health Nutr. 7 (8), 1081–1088. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, et al. , 2011. Food packaging and bisphenol A and bis (2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect 119 (7), 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Alcedo G, Saelens BE, et al. , 2013. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J. Eposure Sci. Environ. Epidemiol 23 (4), 378–384. [DOI] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, et al. , 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect 121 (4), 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob P 3rd, et al. 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol.-Lung Cell. Mol. Physiol 2017; 313(3):L425–L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S, 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ. Health 13, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Karr CJ, Seixas NS, et al. , 2014. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int. J. Environ. Res. Public Health 11 (6), 6193–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Choirat C, Coull BA, VanderWeele TJ, Valeri L, 2021. CMAverse: a suite of functions for reproducible causal mediation analyses. Epidemiology. 2021;32(5):e20–e22. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, et al. , 2015. The urban child institute CANDLE study. RAND Corporation, Santa Monica, CA. [Google Scholar]
- Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B, 2022. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol [DOI] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, et al. , 2001. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am. J. Epidemiol 154 (12), 1089–1099. [DOI] [PubMed] [Google Scholar]
- Sugeng EJ, Symeonides C, O’Hely M, et al. , 2020. Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: The Barwon infant study. Environ. Int 139, 105700. [DOI] [PubMed] [Google Scholar]
- Susmann HP, Schaider LA, Rodgers KM, Rudel RA, 2019. Dietary habits related to food packaging and population exposure to PFASs. Environ. Health Perspect 127 (10), 107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, 2008. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res 108 (2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S, Sathyanarayana S, Barrett E, et al. , 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod 30 (4), 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Messito MJ, Gross RS, Attina TM, Mendelsohn AL, 2013. Phthalates and the diets of US children and adolescents. Environ. Res 126, 84–90. [DOI] [PubMed] [Google Scholar]
- Trasande L, Shaffer RM, Sathyanarayana S, et al. , 2018. Food additives and child health. Pediatrics 142, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura Y, Ishimitsu S, Kaihara A, Yoshii K, Nakamura Y, Tonogai Y, 2001. Di (2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit. Contam 18 (6), 569–579. [DOI] [PubMed] [Google Scholar]
- USDA Food and Nutrient Database for Dietary Studies 2019–2020. U.S. Department of Agriculture, Agricultural Research Service. Food Surveys Research Group Home Page, http://www.ars.usda.gov/nea/bhnrc/fsrg. Published 2022. Accessed. [Google Scholar]
- Valvi D, Monfort N, Ventura R, et al. , 2015. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int. J. Hyg. Environ. Health 218 (2), 220–231. [DOI] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate imputation by chained equations in R. J. Stat. Softw 45, 1–67. [Google Scholar]
- Vandevijvere S, Pedroni C, De Ridder K, Castetbon K, 2020. The cost of diets according to their caloric share of ultra-processed and minimally processed foods in Belgium. Nutrients 12 (9), 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR, 2018. Dietary sources of cumulative phthalates exposure among the US general population in NHANES 2005–2014. Environ. Int 115, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga RE, van Bakel M, Biesbroek S, et al. , 2022. Evaluation of foods, drinks and diets in the Netherlands according to the degree of processing for nutritional quality, environmental impact and food costs. BMC Public Health 22 (1), 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi E, Carroll KN, Hare ME, et al. , 2013. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 5 (5), 1511–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RE, Keane CR, Burke JG, 2010. Disparities and access to healthy food in the United States: A review of food deserts literature. Health Place 16 (5), 876–884. [DOI] [PubMed] [Google Scholar]
- Welch BM, Keil AP, Buckley JP, et al. , 2022. Associations between prenatal urinary biomarkers of phthalate exposure and preterm birth: a pooled study of 16 US cohorts. JAMA Pediatr. 176 (9), 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLET W Nutritional Epidemiology Oxford, Ed. In: University Press; 1990. [Google Scholar]
- Yan X, Calafat A, Lashley S, et al. , 2009. Phthalates biomarker identification and exposure estimates in a population of pregnant women. Hum. Ecol. Risk Assess 15 (3), 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML, 2005. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in metropolitan Detroit. Am. J. Public Health 95 (4), 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 122 (3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Phillips CA, Mitro SD, 2016. Recent fast food consumption and bisphenol A and phthalates exposures among the US population in NHANES, 2003–2010. Environ. Health Perspect 124 (10), 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement.


