Abstract
Hand-arm vibration injury is a well-known occupational disorder that affects many workers globally. The diagnosis is based mainly on quantitative psychophysical tests and medical history. Typical manifestations of hand-arm vibration injury entail episodes of finger blanching, Raynaud’s phenomenon (RP) and sensorineural symptoms from affected nerve fibres and mechanoreceptors in the skin. Differences in serum levels of 17 different biomarkers between 92 patients with hand-arm vibration injury and 51 controls were analysed. Patients with hand-arm vibration injury entailing RP and sensorineural manifestations showed elevated levels of biomarkers associated with endothelial injury or dysfunction, inflammation, vaso- or neuroprotective compensatory, or apoptotic mechanisms: intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1); thrombomodulin (TM), heat shock protein 27 (HSP27); von Willebrand factor, calcitonin gene-related peptide (CGRP) and caspase-3. This study adds important knowledge on pathophysiological mechanisms that can contribute to the implementation of a more objective method for diagnosis of hand-arm vibration injury.
Subject terms: Diseases, Health occupations, Medical research, Neurology, Rheumatology, Risk factors
Background
The clinical assessment of the vascular and sensorineural components of hand-arm vibration injury, diagnosis and grading are based mainly on the subjectively reported medical history and the psychophysical assessments of quantitative sensory tests1. An objective method, e.g., measuring serum levels of biomarkers, is therefore desirable. Furthermore, biomarkers could help us understand the pathophysiology and prognosis better. Several pathophysiological mechanisms have been proposed, e.g., localized injury to blood vessels and nerves, as well as systemic inflammatory processes2–5.
The vascular component of hand-arm vibration injury entails episodic attacks of vasoconstriction, described as Raynaud’s phenomenon (RP). For correct diagnosis of vibration-induced RP, other conditions causing RP, e.g., connective tissue diseases, have to be ruled out6. The pathophysiology of RP has been suggested to include both structural changes and dysfunction of the blood vessel wall with loss of the control of the vascular tone6. Various biomarkers related to endothelial cell function and vascular integrity, as well as inflammation, have been suggested as early markers of disease. Elevated levels of von Willebrand factor (vWf) have been shown in individuals with RP who subsequently developed a connective tissue disease7. Elevated levels of biomarkers associated with angiogenesis, tissue remodelling, fibrosis and wound repair, i.e., basic-fibroblast growth factor (b-FGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP-1 and MMP-12), have been detected in cases of RP caused by a connective tissue disease8. It is not clear whether elevated levels of such biomarkers can also be detected in individuals with vibration-induced RP, and if the serum levels resemble those in individuals with primary or with secondary RP due to a connective tissue disease. However, in a study on individuals with vibration injuries, levels of thrombomodulin (TM) were found to be within the range in individuals with RP due to connective tissue disease9–11. Studies of blood levels of vWf in vibration-injured individuals have shown varying results12,13.
The blood vessel walls are constantly exposed to shear stress and cyclic stretch by hemodynamic forces, physiologically generated by the vascular tone, blood viscosity and cardiac output14. Reduced luminal radius size, as an acute effect of vibration exposure, has been shown in a rat tail model15, after continuous vibration4, and in an experimental setting in fingers of exposed individuals, as well as in the non-exposed hand16,17. Endothelial cells have shown to respond to increased mechanical strain associated with mechanical ventilation by promoting inflammation, adhesion, and contractility leading to vascular dysfunction14. Intercellular adhesion molecule 1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1) are biomarkers that have shown to be upregulated under conditions of stress or injury and enhance the inflammatory response by attracting and facilitating the migration of inflammatory cells from the blood stream into the injured tissue18. Changes in cyclic stretch and shear stress to endothelial cells has shown changes in levels of biomarkers associated with angiogenesis, vascular remodelling, adhesion and inflammation (i.e., MMP-1, HGF, VEGF, ICAM-1)14. Elevated plasma levels of ICAM-1 have also been shown in vibration injured patients compared to controls19. Serum levels of ICAM-1 have been correlated to disease activity in patients with RP due to scleroderma and in fact decreasing levels were observed upon drug-induced vasodilation with a prostaglandin analogue [29]. MCP-1 has to the best of our knowledge not been studied in relation to vibration injury.
The vascular tone is balanced by vasoconstricting and vasodilating factors. Overproduction of endothelin-1 (Et-1), a potent vasoconstrictor, has been found in patients with primary RP8. Elevated levels of Et-1 were associated with structural changes in arteries in rat tail exposed to vibration: i.e., vacuole formation in endothelial and smooth muscle cells, disruption of the endothelial cells with discontinuity of the internal elastic membrane, and swelling and cavitation of mitochondria20 in individuals with vibration-induced RP varying blood levels of Et-1 have been reported12,21.
Calcitonin gene-related peptide (CGRP), a well-known vasodilator, has been studied in patients with RP22. Interestingly, neurogenic vasodilation via CGRP has been linked to transient receptor potential ankyrin 1 (TRPA-1)23. TRPA-1 acts as a sensor to cold and is associated with cold intolerance and inflammatory pain. In an animal model cold hypersensitivity could be induced in mice by subcutaneous administration of a TRPA-1 agonist, while TRPA-1 antagonist reduced cold hypersensitivity24. TPRA-1 expression in serum has not been studied in vibration-injured patients, but, since cold intolerance, a prominent symptom among these patients has been association with the sensorineural component of injury25, it is of considerable interest.
The neurosensory component of hand-arm vibration injury entails clinical manifestations from small (Aδ and unmyelinated C) and large (Aβ) sensory nerve fibres, as well as sensory receptors in the skin. Structural changes, such as demyelination and axonal degeneration, have been observed in nerve biopsies from vibration-exposed individuals26 and in experimental models27, suggesting that certain nerve biomarkers may be elevated. Structural changes may be the mechanism(s) underlying increased susceptibility to nerve entrapment in vibration-exposed individuals, according to light microscopic findings in individuals with diabetes, where a higher prevalence of nerve entrapment has been observed28,29. Finger biopsies from patients with vibration injuries have shown Schwann cell activation, an increase in fibroblast cell number, fibrosis, loss of the myelin sheath, and reductions in the elastic membrane and axonal size2,30–32. These findings have been confirmed in experimental studies4,33. Nerve degeneration induces a variety of biological processes in the affected nerve, including inflammatory response with macrophage recruitment, Schwann cell proliferation and apoptosis with compensatory neuroprotective mechanisms, as seen in various traumatic injuries and in neuropathies. The apoptotic response in Schwann cells, for example, can be measured as increased levels of caspase-3, which balance the proliferative response after a nerve injury. Some vibration-injured patients suffer badly from pain in their hands, interestingly, plasma levels of galanin, an important substance in a variety of functions, including nociception, is increased after traumatic nerve injuries and in individuals after vibration exposure34. Furthermore, glial fibrillary acidic protein (GFAP), a proposed marker for axonal damage35, has been detected in nerve biopsies in patients with type 2 diabetes and controls36, and elevated serum levels of GFAP were found to correlate to reduced nerve action potentials and disease severity in chronic neuropathies37. Furthermore, a tendency of decreased levels of myelin basic protein (MBP), a lipid-interacting protein of myelin, and higher levels of GFAP have been observed in nerve biopsies from subjects with type 2 diabetes compared to healthy subjects36. GFAP and MBP have, to the best of our knowledge, not been studied in relation to hand-arm vibration injury. Neuronal survival, axonal outgrowth, synaptic plasticity and neurotransmission have been reported to be regulated by neurotrophins, including β-nerve growth factor (β-NGF), brain-derived neurotrophic factor, neurotrophin-3 (NT-3), and neurotrophin-4/5, which bind to tyrosine kinase receptors TrkA, TrkB, and TrkC, and the common neurotrophin receptor, p75NTR38. Neurotrophins may act as axonal guidance molecules during nerve regeneration, as well as having neuroprotective properties after injury, and may even stimulate nerve regeneration38. Neuroprotection includes activation of heat shock proteins (HSPs) such as HSP27, detected in human nerve biopsies, which act as chaperones to protect nerve structures under stress36,39 to preserve nerve function. Hypothetically, HSPs may be upregulated in individuals with hand-arm vibration injuries to protect the nerves and to prevent apoptosis40. Hence, biomarkers reflecting endothelial injury or dysfunction, inflammation, nerve injury and tissue remodelling of extracellular matrix were selected for assessment of injury.
The aim of this study was to assess serum levels of several of these biomarkers in individuals with hand-arm vibration injuries to improve our knowledge on pathophysiological mechanisms, to identify objective markers as candidates for accurate and timely diagnosis. We hypothesized that the levels of selected biomarkers indicating endothelial injury or dysfunction, inflammation, cell apoptosis, nerve injury with neuroprotection would be different in patients and in controls. Since the profile of biomarkers may differ according to the clinical expression of vascular or neural manifestations, we also aimed to do sub-group analyses.
Results
Descriptive characteristics of the patients and controls are presented in Table 1. Serum levels of ICAM-1, MCP-1, TM and HSP27 were found to be elevated in patients with a vibration injury compared to controls (Table 2). Difference in serum levels of TM remained almost statistically significant when including only men (p = 0.07), and when including only individuals without previous frostbite (p = 0.07, not in table). All other differences remained after sensitivity analyses.
Table 1.
Descriptive characteristics of the 92 patients with hand-arm vibration injury, with and without Raynaud’s phenomenon (RP) and 51 controls.
| Patients | Controls | |||
|---|---|---|---|---|
| All (n = 92) | With RP (n = 45) | Without RP (n = 47) | n = 51 | |
| Age (years) | 45 (21–64) | 45 (24–64) | 45 (21–64) | 42 (26–62) |
| Females | 6 (7) | 1 (2) | 5 (11) | 9 (18) |
| Ongoing cigarette smoking | 14 (15) | 8 (18) | 6 (13) | 2 (4) |
| Other medical conditions | ||||
| Previous frostbite | 107 (11) | 64 (13) | 43 (9) | 31 (6) |
| Cardiovascular disease | 18 (20) | 10 (22) | 8 (17) | 7(14) |
| Diabetes | 7(8) | 5 (11) | 2 (4) | 2 (4) |
| Thyroid diseases | 5 (5) | 4 (9) | 1 (2) | 1 (2) |
| Rheumatic disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Polyneuropathy | 4 (4) | 4 (9) | 0(0) | –51 |
| ADHD or migraine medication | 4 (4) | 2 (4) | 2(4) | 1 (2) |
| Symptomsa | ||||
| White fingers when exposed to cold or damp (RP) | 45 (49) | 100 (100) | 0 (0) | 5 (10) |
| Numbness/tingling | 90 (98) | 45 (100) | 45 (96) | 7 (14) |
| Duration of RP (years) | 5 (1–25)57 | 5 (1–25)10 | 4.5 (4–25)47 | –51 |
| Duration of numbness (years) | 3 (0.2–25)7 | 4 (1–25)2 | 2 (0.2–25)5 | –51 |
| Pain/discomfort in fingers/hands when exposed to cold | 80 (87) | 44 (98) | 36 (77) | 6 (12) |
| Poor fine motor skills | 65 (71) | 36 (80) | 29 (62) | 4 (8) |
| Poor grip strength | 72 (78) | 36 (80) | 36 (77) | 4 (8) |
| Clinical findinga | ||||
| Impaired perception of touchb | 45 (49) | 29 (64) | 16 (34) | 6 (12) |
Data presented as median (range) or n (%).
1,2,3,4,5,7,10,47,51,57denote the number of participants with missing data. The controls were not asked about polyneuropathy or duration of numbness or RP.
aLeft and/or right hand.
bUnable to detect Semmes–Weinstein Monofilament No, 3.61, corresponding to a force of 0.4 g.
Table 2.
Serum levels of biomarkersa in 92 patients with vibration injury and 51 controls.
| Biomarker | Patients (n = 92) | Controls (n = 51) | P-valueb |
|---|---|---|---|
| ICAM-1 (ng/ml) | 170 (130–290) | 140 (80–180) | < 0.001 |
| MCP-1 (pg/ml) | 38 (10–100) | 34 (13–73) | 0.02 |
| TM (ng/ml) | 5.5 (2.3–39) | 4.4 (0.31–34) | 0.02 |
| vWF (µg/ml) | 16 (6.2–34) | 17 (6.6–31) | 0.81 |
| VEGF (pg/ml) | < LOD (< LOD–1500) | < LOD (< LOD– < LOD) | 0.19 |
| b-FGF (pg/ml) | < LOD (< LOD–18) | < LOD (< LOD–4.8) | 0.42 |
| HGF (pg/ml) | 180 (10–390) | 180 (59–470) | 0.64 |
| MMP-1 (ng/ml) | 3.3 (0.60–29) | 3.0 (0.46–9.8) | 0.77 |
| Et-1 (pg/ml) | 17 (< LOD–1800) | < LOD (< LOD–660) | 0.37 |
| CGRP (pg/ml) | < LOD (< LOD–3700) | 50 (< LOD–3600) | 0.93 |
| TRPA-1 (ng/ml) | < LOD (< LOD–2.9) | < LOD (< LOD–2.4) | 0.68 |
| HSP27 (ng/ml) | 3.2 (0.70–45) | 2.0 (0.10–290) | < 0.001 |
| GFAP (pg/ml) | < LOD (< LOD–3100) | < LOD (< LOD–2800) | 0.51 |
| MBP (ng/ml) | < LOD (< LOD–410) | < LOD (< LOD–100) | 0.79 |
| Caspase-3 (ng/ml) | 1.8 (0.63–85) | 2.1 (0.66–330) | 0.17 |
| Caspase-8 (ng/ml) | < LOD (< LOD–1.7) | < LOD (< LOD–7.2) | 0.95 |
| Galanin (ng/ml) | 7.1 (0.7–36) | 6.8 (0.6–25) | 0.90 |
aIntercellular adhesion molecule 1 (ICAM-1) limit of detection (LOD), 0.002 ng/ml; monocyte chemoattractant protein 1 (MCP-1), LOD 0.6 pg/ml; thrombomodulin (TM), LOD 0.63 ng/ml; von Willebrand factor (vWf) LOD, 0.025 µg/ml; vascular endothelial growth factor (VEGF) LOD, 23 pg/ml; basic fibroblast growth factor basic (b-FGF) LOD, 2 pg/ml; hepatocyte growth factor (HGF), LOD 20 pg/ml; matrix metalloproteinase 1 (MMP-1), LOD 45 ng/ml; endothelin 1 (Et-1), LOD 10 pg/ml; calcitonin gene related peptide (CGRP), LOD 15 pg/ml; transient receptor potential ankyrin 1 (TRPA-1), LOD 0.625 ng/ml; heat shock protein 27 (HSP-27), LOD 0.21 ng/ml; glial fibrillary acidic protein (GFAP), LOD 31 pg/ml; myelin basic protein (MBP), LOD 0.16 ng/ml; caspase-3, LOD 0.041 ng/ml; caspase-8 LOD, 0.08 ng/ml; galanin LOD, 16 ng/ml.
Levels < LOD were assigned a value of half the LOD in the statistical analyses.
bMann-Whitney U test used for comparison of distributions between groups. P-values in boldface denote statistically significant differences.
When comparing patients with RP to patients without RP; TM, vWF, CGRP, HSP27 and caspase-3 were elevated in the former (Table 3). The difference in TM remained almost statistically significant when including only individuals without previous frostbite (p = 0.06, not in table). However, when excluding participants with concurrent diseases, the difference for HSP27 was no longer statically significant (p = 0.57, not in table).
Table 3.
Serum levels of biomarkersa in patients with and without Raynaud’s Phenomenon (RP).
| Patients with RP (n = 45) | Patients without RP (n = 47) | P-valuesb | |
|---|---|---|---|
| ICAM-1 (ng/ml) | 170 (130–240) | 170 (130–290) | 0.58 |
| MCP-1(pg/ml) | 37 (12–95) | 39 (10–104) | 0.69 |
| TM (ng/ml) | 6.1 (2.7–30) | 5.2 (2.3–39) | 0.01 |
| vWF (µg/ml) | 18 (6.2–33) | 14 (6.9–34) | 0.01 |
| VEGF (pg/ml) | < LOD (< LOD–1500) | < LOD (< LOD–510) | 0.53 |
| b-FGF (pg/ml) | < LOD (LOD–13) | < LOD (< LOD–18) | 0.81 |
| HGF (pg/ml) | 180 (10–390) | 180 (67–290) | 0.11 |
| MMP-1 (ng/ml) | 3.7 (0.63–29) | 2.9 (0.60–11) | 0.09 |
| ET-1 (pg/ml) | 37 (< LOD–1800) | < LOD (< LOD–1700) | 0.23 |
| CGRP (pg/ml) | 94 (< LOD–3700) | < LOD (< LOD–1400) | < 0.001 |
| TRPA-1(ng/ml) | < LOD (< LOD– < LOD) | < LOD (< LOD–2.9) | 0.33 |
| HSP-27 (ng/ml) | 3.4 (1.3–16) | 2.8 (0.70–45) | 0.02 |
| GFAP (pg/ml) | < LOD (< LOD–3100) | < LOD (< LOD–2500) | 0.28 |
| MBP (ng/ml) | < LOD (< LOD–410) | < LOD (< LOD–180) | 0.09 |
| Caspase-3 (ng/ml) | 2.0 (1.0–61) | 1.6 (0.63–85) | 0.01 |
| Caspase-8 (ng/ml) | < LOD (< LOD–1.7) | < LOD (< LOD– < LOD) | 0.15 |
| Galanin (ng/ml) | 7.4 (0.73–36) | 6.5 (0.67–19) | 0.23 |
aIntercellular adhesion molecule 1 (ICAM-1) limit of detection (LOD), 0.002 ng/ml; monocyte chemoattractant protein 1 (MCP-1), LOD 0.6 pg/ml; thrombomodulin (TM), LOD 0.63 ng/ml; von Willebrand factor (vWf) LOD, 0.025 µg/ml; vascular endothelial growth factor (VEGF) LOD, 23 pg/ml; basic fibroblast growth factor basic (b-FGF) LOD, 2 pg/ml; hepatocyte growth factor (HGF), LOD 20 pg/ml; matrix metalloproteinase 1 (MMP-1), LOD 45 ng/ml; endothelin 1 (Et-1), LOD 10 pg/ml; calcitonin gene related peptide (CGRP), LOD 15 pg/ml; transient receptor potential ankyrin 1 (TRPA-1), LOD 0.625 ng/ml; heat shock protein 27 (HSP-27), LOD 0.21 ng/ml; glial fibrillary acidic protein (GFAP), LOD 31 pg/ml; myelin basic protein (MBP), LOD 0.16 ng/ml; caspase-3, LOD 0.041 ng/ml; caspase-8 LOD, 0.08 ng/ml; galanin LOD, 16 ng/ml.
Levels < LOD were assigned a value of half the LOD in the statistical analyses.
bMann-Whitney U test used for comparison of distributions between groups. P-values in boldface denote statistically significant differences.
Patients without RP, compared to 45 controls without RP, showed elevated serum levels of ICAM-1, MCP-1 and HSP27 (Table 4). When including only individuals without previous frostbites the p-value for MCP-1 was 0.06, i.e., almost statistically significant. Caspase-3 was lower in patients than in controls, but this difference disappeared when including only participants without concurrent disease (p = 0.25, not in table). All other results remained statistically significant according to the sensitivity analyses.
Table 4.
Serum levels of biomarkersa in patients without Raynaud’s phenomenon (RP) compared to controls without RP.
| Patients without RP (n = 47) | Controls without RPc (n = 46) | P-valuesb | |
|---|---|---|---|
| ICAM-1 (ng/ml) | 170 (130–290) | 140 (80–180) | < 0.001 |
| MCP-1 (pg/ml) | 39 (10–100) | 34 (13–68) | 0.02 |
| TM (ng/ml) | 5.2 (2.3–39) | 4.4 (0.31–28) | 0.39 |
| vWF (µg/ml) | 14 (6.9–34) | 16 (6.6–31) | 0.11 |
| VEGF (pg/ml) | < LOD (< LOD–510) | < LOD (< LOD– < LOD) | 0.32 |
| b-FGF (pg/ml) | < LOD (< LOD–18) | < LOD (< LOD–3.7) | 0.28 |
| HGF (pg/ml) | 180 (67–290) | 190 (59–470) | 0.24 |
| MMP-1 (ng/ml) | 2.9 (0.6–11) | 3.0 (1.0–9.8) | 0.43 |
| Et-1 (pg/ml) | < LOD (< LOD–1700) | < LOD (< LOD–580) | 0.80 |
| CGRP (pg/ml) | < LOD (< LOD–1400) | 29 (< LOD–3600) | 0.14 |
| TRPA-1 (ng/ml) | < LOD (< LOD–2.9) | < LOD (< LOD–2.4) | 1.0 |
| HSP27 (ng/ml) | 2.8 (0.70–45) | 1.8 (< LOD–290) | < 0.001 |
| GFAP (pg/ml) | < LOD (< LOD–2500) | < LOD (< LOD–2800) | 0.66 |
| MBP (ng/ml) | < LOD (< LOD–180) | < LOD (< LOD–0.95) | 0.46 |
| Caspase-3 (ng/ml) | 1.6 (0.63–85) | 2.0 (0.66–330) | 0.02 |
| Caspase-8 (ng/ml) | < LOD (< LOD– < LOD) | < LOD (< LOD–7.2) | 0.31 |
| Galanin (ng/ml) | 6.5 (0.67–19) | 5.9 (0.57–25) | 0.96 |
aIntercellular adhesion molecule 1 (ICAM-1) limit of detection (LOD), 0.002 ng/ml; monocyte chemoattractant protein 1 (MCP-1), LOD 0.6 pg/ml; thrombomodulin (TM), LOD 0.63 ng/ml; von Willebrand factor (vWf) LOD, 0.025 µg/ml; vascular endothelial growth factor (VEGF) LOD, 23 pg/ml; basic fibroblast growth factor basic (b-FGF) LOD, 2 pg/ml; hepatocyte growth factor (HGF), LOD 20 pg/ml; matrix metalloproteinase 1 (MMP-1), LOD 45 ng/ml; endothelin 1 (Et-1), LOD 10 pg/ml; calcitonin gene related peptide (CGRP), LOD 15 pg/ml; transient receptor potential ankyrin 1 (TRPA-1), LOD 0.625 ng/ml; heat shock protein 27 (HSP-27), LOD 0.21 ng/ml; glial fibrillary acidic protein (GFAP), LOD 31 pg/ml; myelin basic protein (MBP), LOD 0.16 ng/ml; caspase-3, LOD 0.041 ng/ml; caspase-8 LOD, 0.08 ng/ml; galanin LOD, 16 ng/ml.
Levels < LOD were assigned a value of half the LOD in the statistical analyses.
bMann-Whitney U test used for comparison of distributions between groups. P-values in boldface denote statistically significant differences.
cFive individuals in the control group had RP and were excluded.
Serum levels of VEGF, b-FGF, HGF, MMP-1 and caspase-8, GFAP, MBP and galanin showed no differences among the studied groups. Serum levels of MMP-12, β-NGF and NT-3 were below the detection limits in all samples.
Discussion
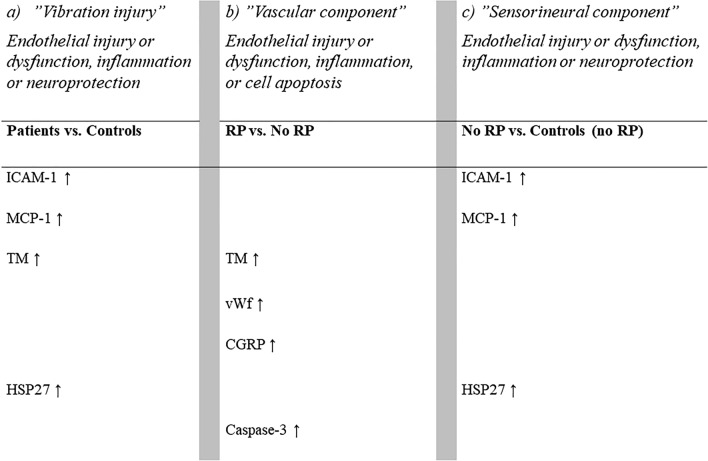
Patients with hand-arm vibration injury had elevated serum levels of biomarkers related to endothelial injury or dysfunction, inflammation (TM, ICAM-1 and MCP-1), and to neuroprotection in relation to nerve fibre injury (HSP27) compared to controls (Fig. 1a). Comparing patients with and without RP revealed a different pattern, with elevated levels of TM, vWf, CGRP and caspase-3, indicating a higher degree of endothelial injury, endothelial dysfunction, inflammation, and cell apoptosis among patients with the vascular component of vibration injury than among those without RP (Fig. 1b). When comparing patients with only the sensorineural component of injury (without RP) to controls (without RP), elevated levels of ICAM-1, MCP-1 and HSP27 were shown (Fig. 1c).
Figure 1.
Schematic illustration of elevated serum levels of biomarkers (after sensitivity analyses) for: (a) patients vs. controls; (b) patients with Raynaud’s phenomenon (RP) vs. patients without RP; (c) patients without RP vs. controls without RP.
The elevated levels of ICAM-1 in patients compared with controls are in line with a previous study19, however, the elevated levels of MCP-1 in patients with vibration injuries is a novel finding18,41. Upregulation of ICAM-1 has been shown in response to both low and high amplitude of cyclic strain applied14,42. The elevated levels of ICAM-1 and MCP-1 suggests an inflammatory response in vibration injuries. No difference in serum levels between patients with and without RP suggests that these biomarkers are not primarily linked to the clinical expression of RP, but rather the “sensorineural component” of injury (Fig. 1).
The elevated levels of TM found in patients with vibration injuries compared with controls is in accordance with previous findings9,19,43. Since TM was elevated in patients with RP, but not in the patient group with only the sensorineural component of injury, it appears that TM is involved in the clinical presentation of RP. Under normal conditions, TM is present in the blood at low concentrations, but it is elevated in several pathological conditions associated with endothelial dysfunction, such as cardiovascular, inflammatory, infectious and metabolic diseases44. Although patients with RP reported higher prevalence of e.g., cardiovascular diseases and current smoking, the results remained after sensitivity analyses.
Patients with RP showed elevated levels of vWf and CGRP, both of which are associated with primary and secondary forms of RP22,31,45–47. Increased levels of vWf have previously been associated with inflammation and intimal hyperplasia48; thus, such mechanisms may be at play in these patients. In conditions with high shear stress, it has been shown that globular-shaped vWF unfolds into long-chain structure, making the adherence of platelets to the injured endothelial surface easy and enhancing further leukocyte recruitment48. At low shear stress, on the other hand, with intact endothelium, smooth muscle cell proliferation occurs without platelet activation and the degree of intimal hyperplasia is proportional to the expression of vWF48. Structural injury with increased smooth muscle thickness has been shown as result of vibration injury in animal models2,20,49.
CGRP is a potent vasodilator released from perivascular sensory nerve endings of unmyelinated C-fibres and myelinated thin Aδ-fibres50. On administration of CGRP antagonists for migraine treatment, subjects have reported new onset of RP51. Interestingly, in this study patients with RP showed elevated, instead of decreased levels, which is in line with a previous study on patients with scleroderma52. However, a reduction of CGRP-staining nerves in histopathological examination of biopsies from patients with primary RP, secondary RP due to scleroderma, and in patients with vibration-induced RP has been shown22,31,46. Also, lower serum levels of CGRP were shown in patients with long standing scleroderma, possibly due to chronic inflammation-induced depletion of CGRP50. A plausible explanation for the present elevated levels of CGRP, could be an increased CGRP-release as a vaso- or neuroprotective mechanism. CGRP has been shown to inhibit intima hyperplasia and expression of the inflammatory marker MCP-1 in chronic induced inflammation50. A normalised function of sensory nerves was seen on upregulation of CGRP in the dorsal root ganglion neuron on capsaicin administration in diabetic induced neuropathy in rats53.
Furthermore, experimentally induced activation of TRPA-1, channels, associated with cold intolerance and inflammatory pain24,54, showed a CGRP-induced neurogenic vasodilation23. A possible link with the prevalent symptom cold intolerance in patients with hand-arm vibration injury is therefore highly interesting and warrants further investigation.
Patients with RP showed higher serum levels of caspase-3 than patients without RP. This is to the best of our knowledge a novel finding. Disrupted vascular endothelial cells with vacuole formation and altered levels in myosin light chain kinase as a result of vibration induced trauma have been observed in an animal model20. Inhibiting myosin light chain kinase was shown to induce apoptosis in vitro and in vivo55. Although caspase-3 seems to be related to the clinical presentation of RP among vibration-injured patients, its exact origin from vascular or cells related to nerve fibres, e.g., Schwann cells, cannot completely be revealed.
Finally, in contrast with a previous study, where no elevated levels of HSP27 were shown in hand-arm vibration exposed workers34, HSP27 was elevated both in patients compared to controls, and in patients with only the sensorineural component of injury compared to controls. This indicates that compensatory neuroprotective mechanisms, such as upregulation of HSPs to preserve function, as seen in subjects with diabetes neuropathy36,56, are present in patients with hand-arm vibration injury57,58 despite no increased levels of GFAP as a sign of nerve injury. In fact, similar structural changes in nerve biopsies from the posterior interosseous nerve, i.e., nerve fibre degeneration, demyelination and fibrosis, as seen in subjects with diabetes neuropathy have been shown in patients with hand-arm vibration injury26.
In this study we have looked at biomarkers associated the vascular and neurosensory components of injury including markers indicating remodelling of the extracellular matrix (growth factors, MMP-1 and MMP-12). There are yet other interesting biomarkers that could reflect the site of injury, i.e., bone and cartilage metabolism. Today there is a suspicion, but not sufficient scientific evidence concerning an association between vibration exposure and osteoarthritis in the hands59.A strength of this study is the large number of patients in whom hand-arm vibration injury had been ascertained by a specialist in OEM. Also, a vast number of biomarkers with a theoretically hypothesis driven approach of plausible pathophysiological mechanisms were assessed. The control group was smaller and due to practical reasons, had a lower proportion of smokers. To assess confounding, we performed sensitivity analyses on subgroups without smokers, as well as other factors that may have influenced the results. A limitation of this study is the way used to assess some known risk factors for RP, e.g., previous frostbite60. In this study, information on frostbite was based on answering yes or no to a single question, with no further assessment, and many failed to answer this question, which could have biased the results. Differences in blood biomarker concentrations due to diurnal variations were avoided by collecting all blood samples before noon. A limitation of the study is that blood samples were not collected from patients and controls during the same time-period. The recruitment of the controls was delayed due to the Covid-19 pandemic, and the samples from the controls were collected two years later than those in the patient group. Samples were stored at − 80 °C, but the concentrations of biomarkers may potentially be biased towards higher biomarker concentrations in samples from controls, depending on the stability of the biomarkers, due to a shorter storage time However, for the differences presented in this study, the serum levels of biomarkers were more elevated in patients than controls. Despite this, the difference in storage time before analysis could add to the uncertainty of the results.
Conclusions
Patients with hand-arm vibration injuries showed elevated serum levels of biomarkers related to endothelial injury or dysfunction, inflammation (ICAM-1, MCP-1, TM), and neuroprotection in relation till nerve injury (HSP27) compared to controls. In addition, patients with RP showed elevated levels of biomarkers associated with intima hyperplasia (vWf) and possibly vaso- or neuroprotective compensatory (CGRP and caspase-3), or apoptotic mechanisms (caspase-3). This study adds important knowledge on pathophysiological mechanisms that can contribute to implementation of a more objective method for diagnosis of hand-arm vibration injury.
Methods
Study design
This study has an observational case–control design. Cases were patients with hand-arm vibration injury. Blood samples were collected from patients diagnosed with hand-arm vibration injury by a specialist in occupational and environmental medicine (OEM), in conjunction with their visit to an OEM outpatient clinic in southern Sweden. The patients filled in a questionnaire before their visit, and then underwent a clinical examination. They were instructed not to use vibrating tools during the twelve hours before attending the clinic, and not to use nicotine in any form one hour before their visit, and to keep their hands warm. Controls without a known diagnosis of hand-arm vibration injury, and without ongoing exposure to vibration at work were enrolled and examined at their workplaces. They filled in a short version of the questionnaire and were examined regarding their perception of touch.
Study group
Ninety-two patients (86 men and 6 women) were enrolled from August 2018 until February 2020. Eight of them were no longer exposed to hand-arm vibration at the time of the study. Their characteristics have been described in a previous study61. One participant claimed not to have diabetes, but were on diabetes medication and had elevated HbA1c, and was thus classified as having diabetes in the present study.
Controls were enrolled from five different workplaces from March 2022 to October 2022. All the employees at these workplaces were invited to participate, and the exclusion criteria were having a diagnosis of vibration injury or ongoing exposure to vibration at work. The controls worked with logistics, as warehouse workers, janitors, chefs, and office workers. Fifty-three individuals were recruited as controls, but two men were excluded since they had been exposed to vibration previously, reported tingling/numbness, or showed impaired perception of touch, i.e., a hand-arm vibration injury could not be ruled out. The control group thus consisted of 44 men and 9 women. As the blood samples collected from the controls were also used in another study on exposure to chromium at work, they were required not to be heavy smokers.
Symptoms
The part of the questionnaire completed by both the patients and the controls included six questions on symptoms, where the responses (Yes) or (No) were indicated separately for the right and left hand: Do you experience: (a) numbness or tingling?, (b) numbness or tingling during the night?, (c) pain/discomfort in fingers/hands during cold exposure?, (d) white fingers when exposed to cold or dampness?, (e) poor grip strength?, (f) poor fine motor skills or clumsiness?. The patients were also asked about the duration of symptoms of RP and numbness and tingling.
Perception of touch
The patients were examined with Semmes–Weinstein monofilaments according to a previously described protocol61. The controls were only tested with filament No. 3.61 (corresponding to 0.4 g force). Those who could not detect filament stimulation with filament No. 3.61, were considered to have impaired perception of touch.
Biomarkers
Blood samples were collected after clinical examination, before noon, in 7 ml serum separation tubes with gel. After 30 min, serum was removed by centrifugation at 2000 × g for 10 min, and the samples were stored at − 80 °C until analysed. Serum concentrations of TM, vWf, ET-1, GFAP, HSP27, CGRP, MBP and TRPA-1 were determined using commercially available ELISA kits, following the instructions provided by the manufacturers (TM from BioVendor, Brno, Czech Republic; vWf from Abnova, Taipei City, Taiwan; ET-1 and caspase-3 from RayBiotech, Norcross, GA, USA; GFAP from Proteintech, Manchester, UK; HSP27 from Millipore, Saint Louis, MO, USA; CGRP, MBP, caspase-8 and galanin from FineTest, Hubei, China and TRPA-1 from Cloud-Clone Corp., Katy, TX, USA). All samples were diluted 1:2 except HSP27 and caspase-3 (1:3 dilution), galanin (1:10 dilution) and vWf (1:100 dilution), and samples with levels exceeding the highest calibration point were also further diluted.
Serum concentrations of ICAM-1 (1:100 dilution), MCP-1 and VEGF (1:4 dilution) were determined using multiplexed immunoassays from Bio-Rad (Hercules, CA, USA), while serum concentrations of MMP-1, MMP-12, b-FGF, β-nerve growth factor (β-NGF), HGF and neurotrophin (NT)-3 (1:2 dilution) were analysed using multiplexed Luminex discovery assays from Bio-Techne (Minneapolis, MN, USA). The multiplexed assays were prepared and analysed on a Luminex platform (Bio-Plex 200, Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. All samples were analysed in duplicate. Samples with values below the limit of detection (LOD) were assigned a value of half the LOD in the statistical analyses.
Data handling and statistical analyses
SPSS IBM Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analysis. Data on serum levels of biomarkers were evaluated and found not to be normally distributed. The results are therefore presented as medians with ranges. Mann–Whitney U tests were used to compare serum levels in patients and controls, in patients with and without RP, and in patients who exhibited only the sensorineural component of injury (i.e., no RP) and controls without RP (five controls with RP were excluded from this comparison). Male sex, smoking, previous frostbite, and concurrent disease (cardiovascular disease, diabetes, thyroid disease, polyneuropathy or pharmacologically treated ADHD or migraine) were more common among the patients than among the controls, and in patients with, than without, RP. Therefore, sensitivity analyses were performed. The Mann–Whitney U-test was repeated in subgroups including only men, only non-smokers, only individuals without previous frostbite, and finally only individuals without concurrent disease. A p-value below 0.05 was considered to indicate that there was a statistically significant difference between two groups.
Informed consent
Participants gave their informed consent to participate in the study before taking part.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Ethics Board in Lund, Sweden (No. 2018/15).
Acknowledgements
The authors would like to thank Ms Ulla Andersson, Ms Else Åkerberg Krook, Ms Eva Assarsson and Ms Anna Larsson for collection of the blood samples and handling of the data. We would also like to thank all participants in the patient and control groups.
Abbreviations
- RP
Raynaud’s Phenomenon
- TM
Thrombomodulin
- vWF
von Willebrand factor
- ICAM-1
Intercellular adhesion molecule 1
- MCP-1
Monocyte chemoattractant protein 1
- CGRP
Calcitonin gene-related peptide
- HSP27
Heat shock protein 27
- Et-1
Endothelin 1
- MMP-1
Matrix metalloproteinase 1
- MMP-12
Metalloproteinase 12
- HGF
Hepatocyte growth factor
- b-FGF
Basic fibroblast growth factor
- VEGF
Vascular endothelial growth factor
- MBP
Myelin basic protein
- GFAP
Glial fibrillary acidic protein
- TRPA-1
Transient receptor potential ankyrin 1
- GAL
Galanin
- β-NGF
β-Nerve growth factor
- NT-3
Neurotrophin
- LOD
Limit of detection
Author contributions
Conceptualization: E.T., C.N., M.K.; methodology: MK., A.A.; chemical analyses: E.H., M.K.; formal analysis: E.T., C.N., M.K.; data curation: E.T., C.N.; writing–original draft preparation: E.T., C.N., M.K; L.B.D.; T.N.; writing, review and editing: E.T., C.N., M.K., L.B.D., A.A., J.R., T.N.; supervision: T.N., L.B.D.; funding acquisition: C.N., LBD. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Lund University. The study was funded by AFA Insurance (Grant number 170124), the Swedish Research Council [2022-01942], The Swedish Diabetes Foundation [DIA2020-492], the Regional Agreement on Medical Training and Clinical Research (ALF) between Region Skåne and Lund University, Region Skåne, and funds from Skåne University Hospital.
Data availability
The datasets presented in this article are not readily available as public access to data is restricted to Swedish Authorities (Public Access to Information and Secrecy Act), but data can be made available for researchers after a special review that includes approval of the research project by both an Ethics Committee and the Authorities’ Data Safety Committees. Contact the corresponding author for request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nilsson T. Neurological diagnosis: Aspects of bedside and electrodiagnostic examinations in relation to hand-arm vibration syndrome. Int. Arch. Occup. Environ. Health. 2002;75:55. doi: 10.1007/s004200100278. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi T, Futatsuka M, Imanishi H, Yamada S. Pathological changes observed in the finger biopsy of patients with vibration-induced white finger. Scand. J. Work Environ. Health. 1986;12:280–283. doi: 10.5271/sjweh.2140. [DOI] [PubMed] [Google Scholar]
- 3.Stoyneva Z, Lyapina M, Tzvetkov D, Vodenicharov E. Current pathophysiological views on vibration-induced Raynaud's phenomenon. Cardiovasc. Res. 2003;57:615–624. doi: 10.1016/S0008-6363(02)00728-9. [DOI] [PubMed] [Google Scholar]
- 4.Govindaraju SR, Curry BD, Bain JL, Riley DA. Comparison of continuous and intermittent vibration effects on rat-tail artery and nerve. Muscle Nerve. 2006;34:197–204. doi: 10.1002/mus.20578. [DOI] [PubMed] [Google Scholar]
- 5.Krajnak K, Waugh S. Systemic effects of segmental vibration in an animal model of hand-arm vibration syndrome. J. Occup. Environ. Med. 2018;60:886–895. doi: 10.1097/JOM.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wigley FM, Flavahan NA. Raynaud’s phenomenon. N. Engl. J. Med. 2016;375:556–565. doi: 10.1056/NEJMra1507638. [DOI] [PubMed] [Google Scholar]
- 7.Gualtierotti R, et al. Detection of early endothelial damage in patients with Raynaud's phenomenon. Microvasc. Res. 2017;113:22–28. doi: 10.1016/j.mvr.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Silva I, Teixeira A, Oliveira J, Almeida R, Vasconcelos C. Peripheral vasculopathy in Raynaud phenomenon: Vascular disease biomarkers. Angiologia e Cirurgia Vascular. 2016;12:77–84. doi: 10.1016/j.ancv.2016.02.004. [DOI] [Google Scholar]
- 9.Kanazuka M, Shigekiyo T, Toibana N, Saito S. Increase in plasma thrombomodulin level in patients with vibration syndrome. Thromb. Res. 1996;82:51–56. doi: 10.1016/0049-3848(96)00050-3. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto N, Iwasaki T, Kitano M, Ogata A, Hamano T. Levels of vascular endothelial growth factor and hepatocyte growth factor in sera of patients with rheumatic diseases. Mod. Rheumatol. 2003;13:129–134. doi: 10.3109/s10165-002-0211-8. [DOI] [PubMed] [Google Scholar]
- 11.Manetti M, et al. Increased serum levels and tissue expression of MMP-12 in patients with systemic sclerosis: Correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann. Rheum. Dis. 2012;71:A48. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson K, Burström L, Nilsson T. Blood biomarkers for vibration-induced white fingers. A case-comparison study. Am. J. Ind. Med. 2020;63:779–786. doi: 10.1002/ajim.23148. [DOI] [PubMed] [Google Scholar]
- 13.Blann AD, et al. Vibration and induction of endothelial injury. Lancet. 1992;340:616–617. doi: 10.1016/0140-6736(92)92156-A. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Wu D, Birukov KG. Mechanosensing and mechanoregulation of endothelial cell functions. Compr. Physiol. 2019;9:873–904. doi: 10.1002/cphy.c180020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry BD, et al. Evidence for frequency-dependent arterial damage in vibrated rat tails. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005;284A:511–521. doi: 10.1002/ar.a.20186. [DOI] [PubMed] [Google Scholar]
- 16.Ye Y, Griffin MJ. Reductions in finger blood flow induced by 125-Hz vibration: Effect of location of contact with vibration. Int. Arch. Occup. Environ. Health. 2016;89:425–433. doi: 10.1007/s00420-015-1081-7. [DOI] [PubMed] [Google Scholar]
- 17.Noe̋l C, Settembre N. Assessing mechanical vibration-altered wall shear stress in digital arteries. J. Biomech. 2022;131:110893. doi: 10.1016/j.jbiomech.2021.110893. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021;101:107598. doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao DS, et al. Serological tests for diagnosis and staging of hand-arm vibration syndrome (HAVS) Hand. 2008;3:129–134. doi: 10.1007/s11552-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei N, et al. Local vibration induced vascular pathological structural changes and abnormal levels of vascular damage indicators. Microvasc. Res. 2021;136:104163. doi: 10.1016/j.mvr.2021.104163. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, et al. Blood endothelin-1 and cold-induced vasodilation in patients with primary Raynauld's phenomenon and workers with vibration-induced white finger. Int. Angiol. J. Int. Union Angiol. 2003;22:243–249. doi: 10.1007/s00547-004-0999-5. [DOI] [PubMed] [Google Scholar]
- 22.Bunker CB, et al. Calcitonin gene-related peptide, endothelin-1, the cutaneous microvasculature and Raynaud's phenomenon. Br. J. Dermatol. 1996;134:399–406. doi: 10.1046/j.1365-2133.1996.22757.x. [DOI] [PubMed] [Google Scholar]
- 23.Aubdool AA, et al. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016;173:2419–2433. doi: 10.1111/bph.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Camino D, et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke RA, Lawson IJ. Cold intolerance and hand-arm vibration syndrome. Occup. Med.-Oxford. 2022;72:152–153. doi: 10.1093/occmed/kqab071. [DOI] [PubMed] [Google Scholar]
- 26.Strömberg T, Dahlin LB, Brun A, Lundborg G. Structural nerve changes at wrist level in workers exposed to vibration. Occup. Environ. Med. 1997;54:307–311. doi: 10.1136/oem.54.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raju SG, Rogness O, Persson M, Bain J, Riley D. Vibration from a riveting hammer causes severe nerve damage in the rat tail model. Muscle Nerve. 2011;44:795–804. doi: 10.1002/mus.22206. [DOI] [PubMed] [Google Scholar]
- 28.Rydberg M, et al. Diabetes mellitus as a risk factor for compression neuropathy: A longitudinal cohort study from southern Sweden. BMJ Open Diabetes Res. Care. 2020;8:e001298. doi: 10.1136/bmjdrc-2020-001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlin LB, et al. Low myelinated nerve-fibre density may lead to symptoms associated with nerve entrapment in vibration-induced neuropathy. J. Occup. Med. Toxicol. 2014;9:7. doi: 10.1186/1745-6673-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi T, Takeya M, Imanishi H. Ultrastructural changes in peripheral nerves of the fingers of three vibration-exposed persons with Raynaud's phenomenon. Scand. J. Work, Environ. Health. 1988;14:31–35. doi: 10.5271/sjweh.1953. [DOI] [PubMed] [Google Scholar]
- 31.Goldsmith PC, et al. Cutaneous nerve fibre depletion in vibration white finger. J. R. Soc. Med. 1994;87:377–381. doi: 10.1177/014107689408700703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundborg G, Dahlin LB, Hansson HA, Kanje M, Necking LE. Vibration exposure and peripheral nerve fiber damage. J. Hand Surg. 1990;15:346–351. doi: 10.1016/0363-5023(90)90121-7. [DOI] [PubMed] [Google Scholar]
- 33.Dahlin LB, Necking LE, Lundstrom R, Lundborg G. Vibration exposure and conditioning lesion effect in nerves: An experimental study in rats. J. Hand Surg. 1992;17:858–861. doi: 10.1016/0363-5023(92)90456-Y. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman M, Nilsson P, Dahlin LB. Exposure to hand-held vibrating tools and biomarkers of nerve injury in plasma: A population-based, observational study. BMJ Open. 2023;13:e070450. doi: 10.1136/bmjopen-2022-070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossor AM, Reilly MM. Blood biomarkers of peripheral neuropathy. Acta Neurolo. Scand. 2022;146:325–331. doi: 10.1111/ane.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ising E, et al. Quantitative proteomic analysis of human peripheral nerves from subjects with type 2 diabetes. Diabet. Med. 2021;38:e14658. doi: 10.1111/dme.14658. [DOI] [PubMed] [Google Scholar]
- 37.Notturno F, Capasso M, DeLauretis A, Carpo M, Uncini A. Glial fibrillary acidic protein as a marker of axonal damage in chronic neuropathies. Muscle Nerve. 2009;40:50–54. doi: 10.1002/mus.21323. [DOI] [PubMed] [Google Scholar]
- 38.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovascular Res. 2007;4:143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- 39.de Los Reyes T, Casas-Tintó S. Neural functions of small heat shock proteins. Neural Regen. Res. 2022;17:512–515. doi: 10.4103/1673-5374.320975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidyasagar A, Wilson NA, Djamali A. Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair. 2012;5:7. doi: 10.1186/1755-1536-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 42.Suo J, et al. Hemodynamic shear stresses in mouse aortas. Arterioscler. Thromb. Vasc. Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 43.Toibana N, Kanazuka M, Shigekiyo T. High level of plasma thrombomodulin (TM) concentration and correlation with endothelin (ET)-1 in vibration-exposed patients. Cent. Eur. J. Public Health. 1995;3:40–42. [PubMed] [Google Scholar]
- 44.Boron M, Hauzer-Martin T, Keil J, Sun XL. Circulating thrombomodulin: Release mechanisms, measurements, and levels in diseases and medical procedures. TH Open. 2022;6:e194–e212. doi: 10.1055/a-1801-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro SV, Jimenez SA. Biomarkers in systemic sclerosis. Biomark. Med. 2010;4:133–147. doi: 10.2217/bmm.09.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunker CB, Terenghi G, Springall DR, Polak JM, Dowd PM. Deficiency of calcitonin gene-related peptide in Raynaud's phenomenon. Lancet. 1990;336:1530–1533. doi: 10.1016/0140-6736(90)93307-B. [DOI] [PubMed] [Google Scholar]
- 47.Scheja A, et al. Von Willebrand factor propeptide as a marker of disease activity in systemic sclerosis (scleroderma) Arthritis Res. 2001;3:178–182. doi: 10.1186/ar295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gragnano F, et al. The role of von Willebrand factor in vascular inflammation: From pathogenesis to targeted therapy. Mediat. Inflamm. 2017 doi: 10.1155/2017/5620314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goenka S, Peelukhana SV, Kim J, Stringer KF, Banerjee RK. Dependence of vascular damage on higher frequency components in the rat-tail model. Ind. Health. 2013;51:373–385. doi: 10.2486/indhealth.2012-0060. [DOI] [PubMed] [Google Scholar]
- 50.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bedrin K, Ailani J, Dougherty C. Raynaud's phenomenon associated with calcitonin gene-related peptide receptor antagonists case report. Headache J. Head Face Pain. 2022;62:1419–1423. doi: 10.1111/head.14417. [DOI] [PubMed] [Google Scholar]
- 52.Bartosik I, Eskilsson J, Ekman R, Akesson A, Scheja A. Correlation between plasma concentrations of calcitonin gene related peptide and pulmonary pressure in patients with systemic sclerosis. Ann. Rheum. Dis. 2002;61:261–263. doi: 10.1136/ard.61.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang XY, Guo Z, Li TP, Sun T. Dietary capsaicin normalizes CGRP peptidergic DRG neurons in experimental diabetic peripheral neuropathy. Sci. Rep. 2021;11:1704. doi: 10.1038/s41598-021-81427-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takayama Y, Derouiche S, Maruyama K, Tominaga M. Emerging perspectives on pain management by modulation of TRP channels and ANO1. Int. J. Mol. Sci. 2019;20(14):3411. doi: 10.3390/ijms20143411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazal F, et al. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol. Cell. Biol. 2005;25:6259–6266. doi: 10.1128/MCB.25.14.6259-6266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen NOB, Mojaddidi M, Malik RA, Dahlin LB. Reduced myelinated nerve fibre and endoneurial capillary densities in the forearm of diabetic and non-diabetic patients with carpal tunnel syndrome. Acta Neuropathol. 2009;118:785–791. doi: 10.1007/s00401-009-0578-0. [DOI] [PubMed] [Google Scholar]
- 57.Pourhamidi K, Dahlin LB, Boman K, Rolandsson O. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia. 2011;54:3143–3149. doi: 10.1007/s00125-011-2303-5. [DOI] [PubMed] [Google Scholar]
- 58.Ising E, et al. Quantification of heat shock proteins in the posterior interosseous nerve among subjects with type 1 and type 2 diabetes compared to healthy controls. Front. Neurosci. 2023;17:1227557. doi: 10.3389/fnins.2023.1227557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson, T., Wahlström, J., Reierth, E. & Burström, L. Radiographic hand osteoarthritis in relation to exposure to hand-transmitted vibration: A systematic review and meta-analysis. in Proceedings, Vol. 86 (2023).
- 60.Stjernbrandt A, et al. Incidence, remission, and persistence of Raynaud's phenomenon in the general population of northern Sweden: A prospective study. BMC Rheumatol. 2022;6:41. doi: 10.1186/s41927-022-00272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tekavec, E., Nilsson, T., Riddar, J., Axmon, A. & Nordander, C. Concordance between the Stockholm workshop scale and the international consensus criteria for grading the severity of neurosensory manifestations in hand-arm vibration syndrome in a Swedish clinical setting. Occup. Environ. Med. (2023). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available as public access to data is restricted to Swedish Authorities (Public Access to Information and Secrecy Act), but data can be made available for researchers after a special review that includes approval of the research project by both an Ethics Committee and the Authorities’ Data Safety Committees. Contact the corresponding author for request.