Abstract
Olanzapine (OLZ) is a widely prescribed antipsychotic drug with a relatively ideal effect in the treatment of schizophrenia (SCZ). However, its severe metabolic side effects often deteriorate clinical therapeutic compliance and mental rehabilitation. The peripheral mechanism of OLZ-induced metabolic disorders remains abstruse for its muti-target activities. Endoplasmic reticulum (ER) stress is implicated in cellular energy metabolism and the progression of psychiatric disorders. In this study, we investigated the role of ER stress in the development of OLZ-induced dyslipidemia. A cohort of 146 SCZ patients receiving OLZ monotherapy was recruited, and blood samples and clinical data were collected at baseline, and in the 4th week, 12th week, and 24th week of the treatment. This case-control study revealed that OLZ treatment significantly elevated serum levels of endoplasmic reticulum (ER) stress markers GRP78, ATF4, and CHOP in SCZ patients with dyslipidemia. In HepG2 cells, treatment with OLZ (25, 50 μM) dose-dependently enhanced hepatic de novo lipogenesis accompanied by SREBPs activation, and simultaneously triggered ER stress. Inhibition of ER stress by tauroursodeoxycholate (TUDCA) and 4-phenyl butyric acid (4-PBA) attenuated OLZ-induced lipid dysregulation in vitro and in vivo. Moreover, we demonstrated that activation of PERK-CHOP signaling during ER stress was a major contributor to OLZ-triggered abnormal lipid metabolism in the liver, suggesting that PERK could be a potential target for ameliorating the development of OLZ-mediated lipid dysfunction. Taken together, ER stress inhibitors could be a potentially effective intervention against OLZ-induced dyslipidemia in SCZ.
Keywords: antipsychotic drug, olanzapine, lipid metabolism disorder, endoplasmic reticulum stress, SREBPs, PERK-CHOP signaling pathway
Introduction
Schizophrenia is a severe mental illness characterized by psychotic symptoms and social deficits, which has become a public burden worldwide [1, 2]. Antipsychotic drugs (APDs) are the mainstay of treatment and rehabilitation of schizophrenia [3]. Accumulating evidence indicated that APDs frequently induce weight gain, obesity, and metabolic disorders, such as dyslipidemia, insulin resistance, and hyperglycemia [4, 5]. APD-induced metabolic adverse effects increase the risk of developing metabolic syndrome and/or cardiovascular accidents in individuals with schizophrenia, leading to treatment interruption and symptom relapse [6]. These adverse metabolic events can be partially attributed to psychopathologies [7] but are largely due to the negative effects of APDs [8, 9]. Therefore, the side-effect mechanism of APDs remains elusive, and new predictive biomarkers or therapeutic strategies are urgently needed to face the clinical challenge.
Olanzapine (OLZ) is one of the most commonly prescribed atypical antipsychotics which has shown high efficacy in clinical practice [10]. However, they display a greater risk of metabolic side effects. The most severe metabolic side effects that occurred in OLZ-treated schizophrenia patients include obesity or dyslipidemia, increased levels of triglyceride (TG), total cholesterol (TCHO), low-density lipoprotein cholesterol (LDL), and/or declined high-density lipoprotein cholesterol (HDL), constantly occurred in OLZ-treated schizophrenia patients [11–13]. Previous studies have shown that excessive food intake and reduced energy expenditure are two major contributors to OLZ-induced metabolic dysregulation [14, 15]. However, a considerable proportion of patients with dyslipidemia have not experience obvious weight gain after OLZ treatment. Clinical studies have also indicated that dyslipidemia can be independent of weight gain [16] in schizophrenia. Moreover, acute administration of OLZ has been found to cause early changes in the parameters of glucose and lipid metabolism, as well as endocrine indices in humans [17, 18]. These intriguing findings suggest the existence of complex peripheral mechanisms that are independent of OLZ-induced obesity.
Mechanically, as a weak base amphiphile, OLZ can easily penetrate membranes and accumulate in the cytoplasm, affecting lipid metabolism [19]. To date, the mechanisms underlying OLZ-induced intracellular lipid dysfunction remain unclear. Cellular lipid metabolic homeostasis in hepatic cells is primarily regulated by sterol regulatory-element binding proteins (SREBPs), which are key helix-loop-helix leucine zipper transcription factors that function as dimers to regulate lipids homeostasis by binding to target genes [20]. Previous in vitro and in vivo studies have documented the effects of OLZ on the expression of SREBP target genes [21–24]. In vitro, OLZ has been found to induce SREBP1c and SREBP2 activation and increase the expression of genes involved in cholesterol and fatty acid biosynthesis, including the rate-limiting enzyme in cholesterol biosyntheses, such as HMGCR, LDLRs, the fatty acid synthase (FASN), and acetyl-CoA carboxylase (ACC) in various cell lines [25, 26]. In addition, in vivo studies [21, 27] have revealed that OLZ upregulates lipogenic SREBP target genes in both the liver and adipose tissue, which further increases circulating triglycerides. Activation of SREBP1c determines the expression of fatty acid biosynthesis genes and elevates the levels of free fatty acids and TGs in APD-treated cells [8]. Furthermore, pharmacogenomic studies have indicated that genetic variants of SREPF1 and SREBF2 are significant factors influencing APD-induced metabolic dysfunction in patients [28–30]. Taken together, these findings suggest that the SREBPs pathway may be a therapeutic target for OLZ-induced obesity and obesity-related metabolic disorders. However, the upstream regulatory mechanisms of OLZ-induced SREBPs activation remain unknown.
SREBPs activities are controlled at both transcriptional and post-transcriptional levels [31]. SREBPs are initially synthesized as inactive membrane-bound precursors in the endoplasmic reticulum (ER) and then cleaved in the Golgi to produce an active fragment that translocates to the nucleus, where it activates the expression of genes involved in cholesterol and fatty acid biosynthesis [20, 32]. ER homeostasis plays a crucial role in maintaining the stability and function of SREBPs in hepatic lipid synthesis. ER stress, a well-known unfolded protein reaction, can be induced by various intra- and extra-cellular insults and is a significant contributor to SREBPs activation [33, 34]. Previous studies have well documented that inhibition of ER stress protects against hepatic lipotoxicity by blocking the de novo lipogenesis induced by SREBPs [35–37]. Studies also investigated the association between ER stress and SCZ and ER stress inhibitor 4-phenylbutyric acid (4-PBA) has been suggested as an important therapy to treat SCZ-related manifestations [38, 39]. Moreover, the most widely used but obesogenic antipsychotics such as OLZ induce activation of hypothalamic ER stress in rodents. Inhibition of ER stress suppresses OLZ-induced hyperphagia and weight gain [40]. In summary, these findings suggest the importance of ER stress in APD-induced metabolic disorders and provide insights into the search for targets that could alleviate OLZ-induced dyslipidemia.
In this study, we aim to investigate the role of ER stress biomarkers in patients with and without dyslipidemia following OLZ treatment. Additionally, to prove the mechanism of PERK-CHOP signaling during ER stress in the regulation of SREBPs activation to the development of OLZ-induced lipid disorder.
Materials and methods
Patients study
Inclusion criteria: 1. According to the DSM-V criteria, all patients recruited for this study were diagnosed with schizophrenia spectrum disorder by at least two specialized psychiatrists who were blinded to clinical data. 2. Patients with first-episode or recurrence were required to undergo a drug washout period of at least 4 weeks. Exclusion criteria: Patients with severe organic diseases or lipid metabolism disorders were excluded. All enrolled patients had received OLZ monotherapy in flexible doses based on their clinical symptoms, with the allowance of small doses of sedative and hypnotic drugs. Clinical data and blood samples were obtained from schizophrenia patients at baseline, 4 weeks, 12 weeks, and 24 weeks during OLZ treatment. Dyslipidemia was defined according to the WHO criteria for metabolic syndrome. The OLZ tablets used in this study were manufactured by QILU Pharmaceutical and HANSOH Pharmaceutical (20183501 and H20052688, China). The study was approved by the Institutional Ethics Committee of Nanchong Psychosomatic Hospital (Approval No: 2021009). Detailed characteristics of schizophrenia are listed in Supplementary Table S1, S2. All researches were performed in accordance with government policies and the Helsinki Declaration. Informed written consent was obtained from each patient before participating in the study.
Cell culture and treatment
Human hepatic carcinoma HepG2 cells (ATCC, STR identification, mycoplasma-free) were purchased from Procell (CL-0103, Wuhan, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, 04-001-1ACS, BI, Israel) and 1% penicillin-streptomycin, and maintained in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were stimulated with a mixture of oleic acid (OA, 0.4 mM, MACKLIN, China) and palmitic acid (PA, 0.2 mM, MACKLIN, China) in a ratio of 2:1 for 36 h and then treated with tunicamycin for 12 h (5 μg/mL in DMSO, HY-A0098, MCE, NJ, USA) to induce ER stress as a positive control. Olanzapine (OLZ, 132539-06-1, Sigma-Aldrich, Germany) was dissolved in DMSO (276855, Sigma-Aldrich, German) and diluted to working concentrations of 25 μM and 50 μM (with DMSO ≤ 0.01% as a placebo vehicle). HepG2 cells were co-incubated with OLZ and various compounds for 48 h including tauroursodeoxycholate (TUDCA, 2 mM in PBS, HY-19696, MCE, NJ, USA), 4-phenyl butyric acid (4-PBA, 2 mM in PBS, HY-A0281, MCE, NJ, USA) [41, 42], CCT020312 (3 μM in DMSO, HY-119240, MCE, NJ, USA), and GSK2606414 (2 μM in DMSO, HY-18072, MCE, NJ, USA) [43] respectively.
Cell viability assays
The cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HepG2 cells were seeded in 96-well culture plates at a concentration of 1.0 × 104 cells/well and allowed to attach overnight. After treating the cells with different concentrations of OLZ for 48 h, they were incubated with 0.5 mg/mL MTT at 37 °C for 1 h. The formazan granules generated by alive cells were dissolved in DMSO and the absorbance of the converted dye was measured at 490 nm using a microplate reader (Multiskan FC, Thermo Scientific, MA, USA). The results were presented as a percentage (%) of the vehicle-treated cell results.
Animal experiments
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Southwest University and followed National Institutes of Health guidelines (Approval No: IACUC-20181020-02). Female C57BL/6 J mice weighing 17–19 g, were purchased from SiPeiFu Biotechnology Co., LTD (SCXK (Jing) 2019-0010, Beijing, China). Mice were housed in sterile laboratory cages at a controlled temperature of 22 ± 2 °C and humidity of 45% ± 5%, with a 12 h artificial light/dark cycle (9:00–21:00) and ad libitum access to water and diet. After one week of adaption, 40 mice were randomly divided into four groups (Con group, OLZ group, OLZ + 4-PBA group, and 4-PBA group). OLZ, used for all animal treatments, was freshly prepared and dissolved in a vehicle comprising 10% DMSO, 40% PEG300, 5% Tween-80, and 45% saline. Mice were gavaged with OLZ at a dose of 3 mg/kg body weight twice a day (b.i.d) to maintain a steady-state concentration of the drug and minimize its sedative effects, in accordance with animal’s circadian rhythms [44]. 4-PBA was dissolved in the same vehicle as OLZ and administered at a dose of 200 mg/kg by gavage once a day (q.d) for 8 weeks. Control mice received the same vector treatment. Throughout the entire research process, the weight of the animals was measured weekly. At the end of the experiment, the mice were fasted for 6 h, anesthetized with isoflurane, and euthanized. Blood samples were collected to detect plasma lipid levels, including TG, TCHO, HDL, and LDL. In addition, liver tissue, heart, abdominal fat, and gonadal fat were weighed and collected. Liver samples were dissected immediately and frozen in liquid nitrogen, then stored in a −80 °C freezer for further analysis.
Lipid biochemical detections
The levels of serum TG, TCHO, HDL, and LDL in all patients were measured using an automated chemical analyzer (AU680, Beckman, CA, USA). In the mouse study, the lipids in blood and liver tissues were also measured using commercial kits for TG (YX-W-8408, Sinobestbio, China), TC (YX-W-B409, Sinobestbio, China), HDL (ml092766, MLBIO, China), and LDL (ml063218, MLBIO, China). To extract lipids from fresh liver tissues, a lipid extraction solution containing normal heptane and isopropanol (1:1, volume/volume) was used at a mass ratio of 1:4. The liver tissues were homogenized in this solution, and cells were sonicated (5 × 106 cells/mL solution) for 2 min at 4 °C. The supernatants were obtained by centrifugation for 10 min at 4 °C, and further procedures were carried out according to the manufacturer’s instructions.
Histological analysis
To perform Oil Red O staining, HepG2 cells and frozen liver sections (10 μm) were rinsed with 60% isopropanol and then incubated with freshly prepared Oil Red O solution (G1260, Solarbio, China) for 15 min. The sections were then rinsed again with 60% isopropanol. Nuclei were subsequently counterstained with hematoxylin, following the manufacturer’s instructions, and observed under a microscope. To perform H&E staining, fresh liver tissues were fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and sectioned into slices (4 μm). Samples were analyzed using an inverted microscope (DMI3000, Leica, Germany).
RNA extraction and real-time quantitative PCR (qPCR)
Total mRNA was isolated from cells or liver tissues using TRIzol reagent (Invitrogen, CA, USA) and reverse transcribed with a high-capacity cDNA reverse transcription kit (RR047A, TAKARA, Japan) following the manufacturer’s instructions. Quantitative PCR was then performed using TB Green (RR820A, TAKARA, Japan). The mRNA levels were quantified relative to GAPDH using the 2-△△Ct method. The primer pairs used are listed in Supplementary Table S3. The experiments themselves were repeated 3 times independently. Each experiment was independently repeated three times, and each sample was tested in triplicate in each experiment.
Western blot assays
Whole-cell lysates were obtained as previously described [44]. Cytoplasm and nuclear proteins were extracted using Nuclear and Cytoplasmic Extraction Kits (R0050, Solarbio, China) according to the manufacturer’s instructions. Western blot analyses were performed using the following antibodies: anti-GRP78 (M1506-2, HUABIO, China), anti-PERK (PA5-79193, Invitrogen, CA, USA), anti-p-PERK (Thr982, PA5-40294, Invitrogen, CA, USA), anti-EIF2A (#5324, CST, MA, USA), anti-p-EIF2A (#3098, CST, MA, USA), anti-ATF4 (#11815, CST, MA, USA), anti-CHOP (YM3668, Immunoway, CA, USA), anti-SREBP1 (ab28481, Abcam, MA, USA), anti-SREBP2 (ab30682, Abcam, MA, USA), anti-FAS (ab22759, Abcam, MA, USA), anti-SCD1 (#3098, CST, MA, USA), anti-HMGCR (ET1702-41, HUABIO, China), anti-β-Tubulin (SR2504, HUABIO, China), anti-rabbit IgG (HA1031, HUABIO, China), anti-mouse IgG (HA1006, HUABIO, China), anti-ATF6-α (SC-166659, Santa, Japan), anti-XBP-1 (SC-8015, Santa, Japan), anti-CD36 (ET1701-24, HUABIO, China), anti-FABP4 (ET1703-98, HUABIO, China), anti-ATGL (RT1058, HUABIO, China), anti-CPT1A (YN3388, Immunoway, CA, USA). Immunoprecipitation assays were performed using mouse anti-rabbit IgG (Conformation Specific) (#3678, CST, MA, USA) and rabbit IgG (Abcam, ab172730, MA, USA). Data were analyzed using Image Lab software and normalized to the corresponding β-tubulin levels. Phosphorylated proteins were normalized to their total proteins.
Immunoprecipitation and mass spectrometric analysis
To perform immunoprecipitation, SREBP1 antibodies were mixed and incubated on a rotating shaker for 1 h at room temperature. The antibody-coupled magnetic beads (1614023, Bio-Rad, CA, USA) were washed with elution buffer to remove any impurities. Proteins from HepG2 cells were extracted using a nondenaturing kit (R0030, Solarbio, China), and the resulting lysate was diluted and added to a clean tube containing the antibody-coupled magnetic beads. The mixture was then incubated according to the manufacturer’s recommendations. After the separation of proteins by SDS-PAGE and silver impregnation staining, bands that interacted with SREBP1 were excised and subjected to mass spectrometric peptide sequence analysis at OMICS SPACE Co. Ltd (Shanghai, China). The resulting spectra were evaluated using the National Center for Biotechnology Information protein database with Mascot and Sequest. To confirm the interactions, cell lysates and immuno-complexes were analyzed by Western blot using the indicated primary antibodies.
Immunofluorescence and Enzyme-linked immune absorbance assay (ELISA)
Cells cultured on glass coverslips were fixed with 4% paraformaldehyde, incubated with the aforementioned primary antibodies, and Alexa Fluor 488-conjugated IgG (1:500, HA1121, HUABIO, China). DAPI (C1005, Beyotime, China) was used to counterstain the nuclei, and images were captured using an IX71 fluorescence microscope equipped with an inverted Microscope camera (Olympus, Japan). Quantification analysis was performed by ImageJ software. Human serum levels of GRP78, ATF4, and CHOP were determined using ELISA kits (YX-071816H, YX-070408H, YX-030815H, Sinobestbio, China) according to the manufacturer’s instructions.
Electron microscopy
HepG2 cells were fixed in 2.5% glutaraldehyde and stored overnight at 4 °C. Samples were then rinsed with the same buffer and post-fixed for 1 h in 1% osmium tetroxide and 1% potassium ferrocyanide in 0.1 M cacodylate buffer to enhance the staining of membranes. Cells were then rinsed in distilled water, dehydrated in low-temperature acetone, and finally embedded in epoxy resin. Contrasted ultrathin sections (70 nm) were analyzed under a transmission electron microscope (HT7700, HITACHI, Japan). TIA-TEM imaging was used to analyze major cellular structures.
Cell transfection and hepatic-specific PERK knockdown
To knock down PERK expression in HepG2 cells, lentiviral plasmid vectors (hU6-MCS-CBh-gcGFP-IRES-Puromycin) encoding short hairpin RNAs (shRNAs) targeting PERK or negative control shRNA were generated at Shanghai Genechem Co. Ltd. Human PERK shRNA and Control Target sequences used in this study were listed in Supplementary Table S4. In the animal experiment, liver-specific adeno-associated virus serotype 9 (AAV9) capsid was used to specifically knock down PERK in the livers of mice. Female C57BL/6 J mice (5 – 6 weeks old) were randomly divided into four groups (Con-AAV + Vehicle, Perk-AAV + Vehicle, Con-AAV + OLZ, and Perk-AAV + OLZ, n = 8 for each group). Mice were injected with either an AAV vector carrying PERK shRNA (pAAV-ApoE/hAATp-PERK-shRNA) or a negative control vector (pAAV-ApoE/hAATp-null, Genechem, China) through tail vein at a dose of 1×1011 vg/200 μL per mouse. The AAVs were injected twice a week for 3 weeks starting from 3 weeks before OLZ administration. To ensure the efficiency of PERK knockdown in mice livers, both mRNA and protein levels of PERK were detected in 2 randomly selected mice during the third week of this experiment. After 3 weeks of AAV injection, mice with liver-specific PERK knockdown and control groups were sequentially administered OLZ or Vehicle along with AAV injection once a week for 5 weeks. All reagents and protocols used in this animal experiment followed the principles as previously described.
RNA-sequencing
Total RNA was extracted from the HepG2 cells using the TRIzol reagent. Then RNA quality was determined by a 5300 bioanalyzer (Agilent) and quantified using the Nanodrop 2000 (OD260/280 = 1.8–2.2, OD260/230 ≥ 2.0, RIN ≥ 6.5, 28 S: 18 S ≥ 1.0, Total RNA > 1 μg). RNA purification, reverse transcription, library construction, and sequencing were performed at Majorbio Bio-pharm Biotechnology Co., Ltd (Shanghai, China) according to the manufacturer’s instructions. The RNA-seq transcriptome library was prepared following Illumina stranded mRNA Prep. Ligation from Illumina using 1 μg of total RNA. Quality control and read mapping were strictly conducted by HISAT2 software. To identify DEGs (differential expression genes) between OLZ and control samples, the expression level of each transcript was calculated by the transcript per million reads (TPM) method. RESM was used to quantify gene abundances. Essentially, differential expression analysis was performed using the DESeq2 or DEGseq. DEGs with ∣log2FC∣ ≥ 1 and FDR ≤ 0.05 (DESeq2) or FDR ≤ 0.001 (DEGseq) were considered to be significantly different expressed genes. In addition, functional enrichment analysis including GO and KEGG was performed to identify which DEGs were significantly enriched in GO terms and metabolic pathways at Bonferroni-corrected P-value ≤ 0.05 compared with the whole-transcriptome background. GO functional analysis, KEGG pathway, and GSEA analysis were carried out by the cluster Profiler R package.
Statistical analysis
All statistical analyses were performed with either SPSS software (version 17.0) or GraphPad Prism (version 8.0). Clinical data analyses were executed using the R project (version 4.3.1). Results were presented as the mean ± standard deviation (SD). Statistical significance was assessed using a two-tailed Student’s t-test for comparison between the two groups. For comparisons among multiple groups, one-way ANOVA followed by Bonferroni’s post hoc test was used. P < 0.05 was considered statistically significant. P > 0.05 was considered not significant (NS). The number of animals used for each experiment was indicated in the figure captions. Data were representative of at least three independent experiments.
Results
Schizophrenia patients with dyslipidemia have higher serum levels of ER stress markers after OLZ treatment
To observe the severity of lipid metabolism dysfunction induced by OLZ, we first analyzed the demographic data and blood lipid profiles of 146 schizophrenia patients who received OLZ monotherapy at baseline, 4 weeks, 12 weeks, and 24 weeks, respectively (Fig. 1a). Patients with dyslipidemia showed significantly higher BMI compared to those without dyslipidemia after 4 weeks of OLZ treatment (Fig. 1b, P < 0.001). Additionally, central obesity was observed in dyslipidemia patients 12 weeks after OLZ treatment, as indicated by an increase in waist-to-hip ratio (Fig. 1c, P < 0.001). Dyslipidemia of varying degrees may occur in approximately one-third of patients receiving OLZ treatment for up to 24 weeks. Gender differences should also be taken into account, as female patients were twice as likely to develop dyslipidemia compared to male patients. While antipsychotics-derived obesity (defined as weight gain of more than 7% of basal body weight) is a well-recognized high-risk factor for lipid metabolism disorders in schizophrenia, other factors may also contribute to the development of dyslipidemia. However, 36% of non-obese patients remain developed dyslipidemia after OLZ treatment within 24 weeks (Fig. 1d), suggesting the existence of obscure peripheral mechanisms that are independent of body weight gain in OLZ-induced lipid dysregulation. To further investigate the involvement of ER stress in OLZ-induced dyslipidemia, we measured the levels of ER stress-related markers (GRP78, ATF4, and CHOP) in blood samples using ELISA kits from 97 non-dyslipidemia patients and 49 dyslipidemia patients who received OLZ monotherapy at various time points over 24 weeks. Multiple logistic regression analysis showed that female was a risk factor for OLZ-induced dyslipidemia. Meanwhile, the ER stress biomarker GRP78 was identified as another risk factor in patients with dyslipidemia after OLZ treatment (Fig. 1e, P < 0.05). The serum levels of GRP78, ATF4, and CHOP were significantly higher in patients with dyslipidemia compared to those without dyslipidemia after 4 weeks of OLZ medication (Fig. 1f–h, P < 0.001). Taken together, these findings indicated that ER stress biomarkers (GRP78, ATF4, and CHOP) were upregulated in the serum of schizophrenia patients with dyslipidemia who were treated with OLZ. These results are interesting because they imply that ER stress may play a pivotal role in the lipid dysfunction caused by OLZ.
Fig. 1. The serum level of endoplasmic reticulum (ER) stress biomarkers is elevated in schizophrenia patients with dyslipidemia after olanzapine (OLZ) treatment.
a Schematic showing the clinical design protocol for the cohort study. b, c The tendencies of BMI and WHR change in schizophrenia patients with dyslipidemia or non-dyslipidemia at different time points after OLZ medication. d The split pie chart shows the proportions of obese or non-obese patients with dyslipidemia after the OLZ remedy. e Multiple logistic regression analysis showing the odds ratios (ORs) with 95% confidence intervals (CIs) and P-values in 146 OLZ-treated schizophrenia patients (female versus male). f–h The changes in serum level of ER stress biomarkers (GRP78, ATF4, CHOP) were measured by ELISA in schizophrenia patients with Dyslipidemia or Non-dyslipidemia at different time points after OLZ therapy (Dyslipidemia group, n = 49; Non-dyslipidemia group, n = 97). P-values < 0.05 are presented numerically. P1 indicates the significance at 4 weeks, P2 indicates the significance at 12 weeks, and P3 indicates the significance at 24 weeks. Data are expressed as mean ± SD. For (e–h), significance was determined by Student’s two-tailed t-test.
OLZ promotes hepatic de novo lipogenesis and activates ER stress in vitro
To demonstrate the role of ER stress in OLZ-induced hepatic lipid dysregulation, we conducted experiments using tunicamycin (TUN), an ER stress inducer, as a positive control in palmitate-pretreated HepG2 cells. First, we assessed the effect of OLZ on HepG2 cell viability using MTT assays. The results showed that OLZ had little effect on cell viability within the concentration range of 0.1–100 μM after 48 h of treatment (Data not shown). To ensure the effects of OLZ in different metabolic pathways, we performed the transcriptome analysis by RNA-sequencing between OLZ-treated HepG2 cells and Control subjects. GO and KEGG enrichment analysis showed that OLZ treatment significantly upregulated cholesterol biosynthesis pathway-related genes and Gene Set Enrichment Analysis (GSEA) also indicated that OLZ remarkedly upregulated fatty acid synthesis in these elevated differential expression genes (Supplementary Fig. S1a–c, P < 0.05). Our results demonstrated that the fatty acid and cholesterol biosynthesis pathways were dominantly disrupted by OLZ treatment. To further validate the effects of OLZ in the de novo lipogenic pathway, We then conducted Oil Red O staining to visualize intracellular lipid droplet accumulation in HepG2 cells treated with OLZ or TUN. Results revealed that both 25 μM and 50 μM OLZ, as well as TUN, remarkably induced intracellular lipid droplet accumulation in HepG2 cells after 48 h of treatment (Fig. 2a). Quantification analysis indicated a significant increase in the contents of TG in HepG2 cells treated with 25 μM and 50 μM OLZ, as compared to the control group (Fig. 2d, P < 0.05, P < 0.001). Moreover, transmission electron microscopy (TEM) uncovered that treatment with 50 μM OLZ and TUN resulted in the enlargement of the ER lumen (Fig. 2a). To further validate the potential role of ER stress in OLZ-induced hepatic lipogenesis, we measured the de novo lipogenic and ER stress-related proteins using Western bolts. The results demonstrated a significant increase in the expression levels of de novo lipogenic markers (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) in both 50 μM OLZ group and TUN group, as compared to the control group (Fig. 2b, i, P < 0.05, P < 0.01, P < 0.001). Additionally, the expression levels of ER stress-related markers (GRP78, p-PERK, p-EIF2A, ATF4, and CHOP) were markedly upregulated in both 50 μM OLZ and TUN groups as compared to the control group (Fig. 2c, j). In comparison to the control group, the 25 μM OLZ only upregulated the expression levels of p-PERK, p-EIF2A, and ATF4, but slightly increased the protein levels of SREBP1c-N. In contrast, neither CHOP nor lipogenic markers (SREBP2, SCD1, and HMGCR) was increased by 25 μM OLZ treatment. To determine the effect of OLZ on the expression of the key transcription factor in hepatic de novo lipogenesis, we measured the expression levels of SREBP1c-N and SREBP2-N using immunofluorescence (IF) assays. IF staining revealed that both the 50 μM OLZ and the TUN prominently enhanced the expression levels of SREBP1c-N and SREBP2-N in HepG2 cells compared to the control group (Fig. 2e, f). Quantitative analysis also revealed a significant upregulation of SERBP1c-N and SREBP2-N protein levels in these two groups (Fig. 2g, h, P < 0.05, P < 0.001). However, only the protein levels of SREBP1c-N were upregulated in the 25 μM OLZ group. Therefore, subsequent experiments were performed using 50 μM OLZ based on these results. In summary, our results demonstrated that 50 μM OLZ could significantly promote abnormal hepatic lipid accumulation by activating endogenous SREBP1c-N and SREBP2-N de novo lipogenic pathways in vitro. Furthermore, we observed concomitant activation of ER stress in the OLZ-induced hepatic lipid dysregulation phenotype.
Fig. 2. OLZ promotes hepatic de novo lipogenesis and ER stress in vitro.
a Representative Oil Red O staining and TEM images in HepG2 cells after adding different concentrations of olanzapine for 48 h. Tunicamycin (TUN) as an ER stress inducer. (N indicates nuclei; Red arrows indicate the swollen lumen of the endoplasmic reticulum). Scale bar: 50 μm in Oil Red O staining and 2 μm, 0.5 μm in TEM images. b The protein levels of SREBP1c, SREBP2, FASN, SCD1, and HMGCR were detected by Western blot in HepG2 cells after 48 h of treatment. c The protein levels of GRP78, PERK, p-PERK, EIF2A, p-EIF2A, ATF4, and CHOP were measured by Western blot in HepG2 cells after 48 h of administration. d The quantification of Hepatic TG is represented in a bar graph (n = 3/group, *P < 0.05, ***P < 0.001 as indicated). e, f Representative immunofluorescence staining showing the expression of SREBP1c (left panel green) and SREBP2 (right panel green) in HepG2 cells. Scale bar: 50 μm. g, h The quantifications of the relative fluorescence intensity of SREBP1c and SREBP2 are represented as a bar graph. n = 3/group, *P < 0.05, ***P < 0.001 as indicated. i, j Statistical analysis of Western blotting assays in (b, c). (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated). All data are presented as mean ± SD, and significance was determined by one-way ANOVA.
Targeting ER stress prevents OLZ-induced hepatic lipid disorder in vitro
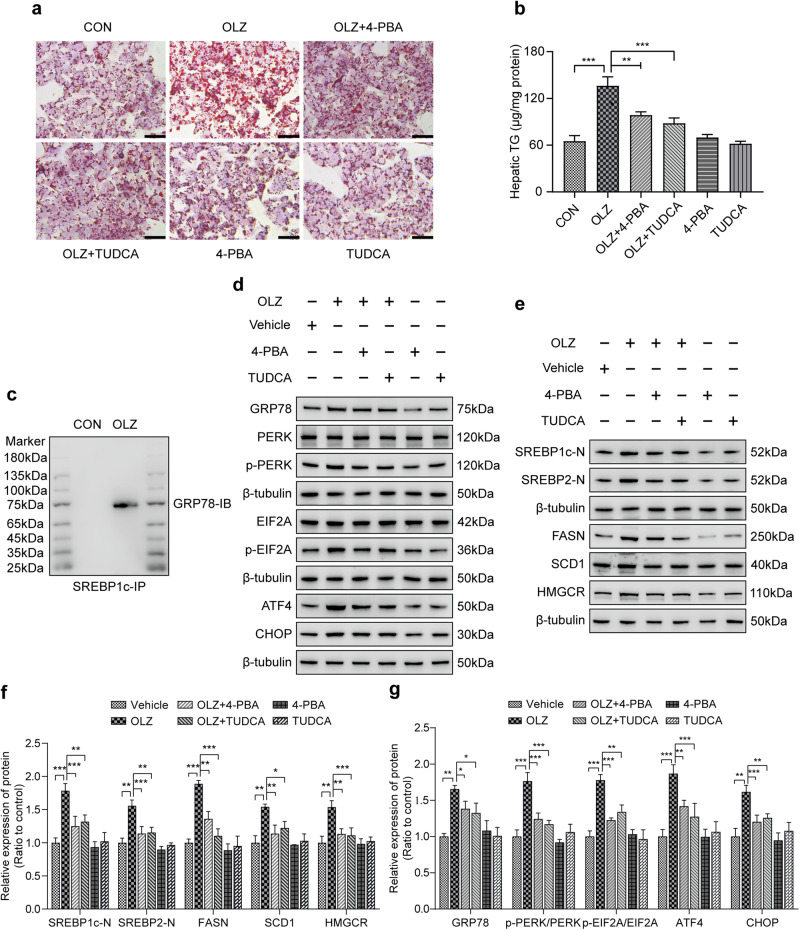
To investigate whether inhibition of ER stress could prevent OLZ-induced lipid dysfunction, we co-incubated HepG2 cells with OLZ and two recognized ER stress alleviators, tauroursodeoxycholate (TUDCA) and 4-phenyl butyric acid (4-PBA). Oil Red O staining revealed that the OLZ group resulted in more aggregation of larger lipid droplets compared to the control group. However, the increase mediated by OLZ could be reversed by the administration of both TUDCA and 4-PBA. Quantification analysis showed a significant increase in the cellular content of TG in OLZ-treated cells compared to control cells, and this effect was reversed by treatment with both TUDCA and 4-PBA treatment (Fig. 3a, b, P < 0.01, P < 0.001). To explore the molecular mechanisms underlying the function of SREPB1c in OLZ-induced lipid metabolism imbalance, we performed co-immunoprecipitation (Co-IP) experiments and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to qualitatively analyze SREBP1c binding protein. LC-MS/MS analysis identified GRP78 as a protein that interacts with SREBP1c. The Co-IP of SREBP1c was qualitatively validated by Western blot (Fig. 3c). More importantly, our data from Western blot assays showed that the expression levels of ER stress-related proteins (GRP78, p-PERK, p-elF2α, ATF4, and CHOP) and hepatic de novo lipogenic markers (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) were significantly increased in OLZ group compared to the control group. However, these increases were ameliorated by both TUDCA and 4-PBA treatment (Fig. 3d–f, P < 0.05, P < 0.01, P < 0.001). Overall, these findings suggest that OLZ promotes hepatic lipid accumulation by inducing ER stress in vitro.
Fig. 3. Targeting ER stress prevents OLZ-induced hepatic de novo lipogenesis in vitro.
a Representative Oil Red O staining indicated that TUDCA and 4-PBA prevented OLZ-induced lipid droplet accumulation in HepG2 cells after 48 h of co-incubation. Scale bar: 50 μm. Both TUDCA and 4-PBA are ER stress alleviators. b The quantification of hepatic lipid is presented in a bar graph (n = 3/group, **P < 0.01, ***P < 0.001 as indicated). c Co-IP assays showed that GRP78 interacted with the critical de novo lipogenic transcription factor SREBP1c in OLZ-treated HepG2 cells. d The expression of ER stress markers in HepG2 cells treated with ER stress alleviators (TUDCA and 4-PBA) under OLZ addition or not. e Representative images showing the expression levels of de novo lipogenic markers in HepG2 cells treated with ER stress antagonists during OLZ remedy (n = 3 independent experiments for the in vitro study). f, g Statistical analysis of Western blotting assays in (d, e). (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated). All data are presented as mean ± SD, and significance was determined by one-way ANOVA.
Inhibiting ER stress ameliorates OLZ-induced lipid dysregulation in mice
Similarly, we investigated whether an ER stress alleviator could reduce blood lipid levels in OLZ-treated mice. Female C57BL/6 J mice were administered either the vehicle, OLZ, OLZ + 4-PBA, or 4-PBA. The body weight of mice was monitored once a week for 8 weeks, and the data indicated that the body weight of mice was significantly increased in the OLZ group compared to the control group. However, this effect was significantly reduced by 4-PBA in a time-dependent manner (Fig. 4a, P < 0.001). After the experiment, the liver weight and visceral fat weight were also examined. Data showed that the liver weight and visceral fat weight were significantly higher in the OLZ group compared to the control group. However, the increase in weight caused by OLZ could be rescued by administering 4-PBA to mice (Supplementary Table S3, P < 0.001). Oil Red O and H&E staining revealed that the degree of lipid droplet accumulation and steatosis were more severe in the livers of OLZ-treated mice than that of control mice. However, 4-PBA administration ameliorated these effects (Fig. 4b, P < 0.001). Quantitative analysis demonstrated that the levels of lipids in liver tissues were notably increased in OLZ-treated mice than in control mice. These elevations induced by OLZ were suppressed by 4-PBA treatment (Fig. 4c, P < 0.01, P < 0.001). In addition, biochemical assay results manifested that serum levels of TG, TC, and LDL-C were significantly elevated in the OLZ group compared to the control group, while HDL-C levels were significantly reduced. However, 4-PBA administration remarkably weakened these changes (Fig. 4d–g, P < 0.05, P < 0.01, P < 0.001). Our Western blot data showed that the levels of ER stress-related proteins (GRP78, p-PERK, p-elF2α, ATF4, and CHOP) and de novo lipogenic proteins (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) were drastically increased in mice livers of the OLZ group compared to the control group while administering 4-PBA effectively reversed these upregulations caused by OLZ (Fig. 4h–k, P < 0.05, P < 0.01, P < 0.001). Furthermore, we also detected the expression levels of ER stress ATF6-α, XBP-1 pathways, fatty acid uptake (CD36 and FABP4), lipolysis (ATGL), and β-oxidation (CPT1A) marker proteins by Western blot in mice livers. Although the protein levels of ATF6-α were mildly elevated in OLZ-treated mice livers, there were still no significant differences after quantified to housekeeping proteins. Considering the real de novo lipogenesis affected by OLZ in mice, OLZ simultaneously enhanced the expression levels of fatty acid uptake proteins (CD36 and FABP4), reduced the expression levels of β-oxidation (CPT1A) and lipolysis (ATGL) (Supplementary Fig. S1d, e, P < 0.05, P < 0.01, P < 0.001). Collectively, our findings confirm that OLZ has a significant impact on increasing body weight, visceral fat weight, blood lipid levels, and liver steatosis in mice. More importantly, we also demonstrated that administering 4-PBA to mice effectively improved OLZ-induced lipid dysfunction by suppressing ER stress-driven SREBPs activation, suggesting that the ER stress signaling pathway plays a crucial role in the development of dyslipidemia.
Fig. 4. Inhibiting ER stress ameliorates OLZ-induced lipid metabolism dysfunction in mice.
a OLZ-induced body weight increase in mice was ameliorated by ER stress reliever 4-PBA (n = 8/groups, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated, OLZ + 4-PBA versus OLZ). b Representative Oil Red O staining showed that 4-PBA improved OLZ-induced lipid droplet aggregation in mice liver. Paraffin-embedded liver sections were stained with H&E. OLZ-elicited positive steatosis area of the liver was decreased by 4-PBA medication. Scale bar: 50 μm. c The quantification of liver TG is shown in a bar graph (n = 6/groups, **P < 0.01, ***P < 0.001 as indicated, OLZ versus CON, OLZ + 4-PBA versus OLZ). d–g The graph exhibited the serum TG, TCHO, HDL, and LDL-C of mice in each group (n = 8/groups, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated, OLZ versus CON, OLZ + 4-PBA versus OLZ). h, i The expression levels of ER stress and de novo lipogenic marker proteins in mice liver were determined by Western blot after 4-PBA intervention (n = 3 independent experiments for the in vivo study). j, k Statistical analysis of Western blotting assays in (h, i). (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated). All data are presented as mean ± SD, and significance was determined by one-way ANOVA.
Loss of PERK attenuates OLZ-induced lipid dysfunction in vitro
Previous studies revealed that ER stress, specifically PERK-CHOP pathway activation, contributes to ER stress-mediated hepatic lipid imbalance. To further confirm whether the PERK-CHOP pathway is the primary signaling mechanism responsible for OLZ-induced lipid metabolism disorder, we used both a PERK-specific agonist (CCT020312) and antagonist (GSK2606414) to rescue the lipid side-effect of OLZ. Consistent with our hypothesis, Oil Red O staining exhibited that OLZ or CCT020312 dramatically increased lipid droplet accumulation in HepG2 cells compared to control cells, while these effects were further enhanced by CCT020312 or attenuated by GSK2606414 (Fig. 5a). Quantitive analysis of cellular TG levels revealed that both OLZ and CCT020312 treatment resulted in a significant increase in TG content compared to control cells, whereas the OLZ-induced elevation could be exacerbated by CCT020213 administration and ameliorated by GSK2606414 (Fig. 5b, P < 0.05, P < 0.01, P < 0.001). Furthermore, Western blot data showed that OLZ or CCT020213 resulted in a significant increase in the protein levels of ER stress signaling (p-PERK, p-elF2α, ATF4, and CHOP) and de novo lipogenic markers (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) in HepG2 cells, compared to control cells. Notably, the upregulations induced by OLZ were further exacerbated by CCT020213, but rescued by GSK2606414 (Fig. 5d, i, j, P < 0.05, P < 0.01, P < 0.001). To further investigate the molecular switch effect of PERK in OLZ-induced hepatic lipid dysfunction, lentivirus-mediated sh-RNA interferences with different target sequences were employed to knockdown Perk in HepG2 cells, which were then screened with puromycin after 72 h infection (Fig. 5c). Similarly, the mRNA and protein levels of PERK were markedly downregulated in HepG2 cells transfected with Perk-shRNA2 and Perk-shRNA3 compared to control sh-RNA treated cells (Fig. 5e, P < 0.001). Interestingly, Oil Red O staining and cellular TG quantitive analysis results showed that Perk knockdown remarkably attenuated the accumulation of lipid droplets and decreased the intracellular TG contents in OLZ-treated HepG2 cells (Fig. 5f, h, P < 0.001, Perk-shRNA2 and Perk-shRNA3 versus con-shRNA). Additionally, Western blot assays also demonstrated that Perk silencing notably decreased the levels of ER stress markers (p-PERK, p-elF2α, ATF4, and CHOP) and downregulated the de novo lipogenic proteins (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) in OLZ-treated HepG2 cells (Fig. 5g, k, l, P < 0.05, P < 0.01, P < 0.001, Perk-shRNA2 and Perk-shRNA3 versus con-shRNA). In summary, our results demonstrated that the PERK-CHOP signaling pathway of ER stress is the main mechanism responsible for OLZ-induced hepatic de novo lipogenesis. As a sensor, PERK may serve as a potential molecular target for controlling the switch in OLZ-induced lipid dysregulation.
Fig. 5. Loss of PERK reverses OLZ-induced lipid disorder in vitro.
a Representative Oil Red O staining displayed that the OLZ-induced cellular lipid droplet accumulation was aggravated by a PERK agonist (CCT020312) and reversed by a PERK antagonist (GSK2606414) in HepG2 cells after 48 h of incubation. Scale bar: 50 μm. b The quantification of hepatic cellular TG content is represented in a bar graph (n = 3 independent experiments for the in vivo study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated, OLZ versus CON, OLZ + CCT020312 versus OLZ, OLZ + GSK2606414 versus OLZ). c Representative fluorescence images exhibited the lentiviral plasmid transfection with different shRNA sequences in HepG2 cells after 72 h of processing. Scale bar: 50 μm. d Western blot images indicated that the expression levels of ER stress and de novo lipogenic marker proteins were enhanced by the PERK agonist (CCT020312) and decreased by the PERK antagonist (GSK2606414) in OLZ-treated HepG2 cells. e The expression levels of PERK in HepG2 cells were measured by qPCR and Western blot (n = 3 independent experiments for the in vivo study, ***P < 0.001 as indicated, Perk-shRNA versus Con-shRNA). f Oil Red O staining showed the changes of OLZ-elicited lipid droplet accumulation in HepG2 cells after 48 h of interference by Perk-shRNA lentivirus plasmid with different target sequences. Scale bar: 50 μm. g The expression levels of ER stress and cellular de novo lipogenic marker proteins were determined by Western blot under PERK silencing condition in HepG2 cells (n = 3 independent experiments for the in vivo study). h The quantification of hepatic cellular TG content is represented in a bar graph (n = 3 independent experiments for the in vivo study, ***P < 0.001 as indicated, Perk-shRNA versus Con-shRNA). i–l Statistical analysis of Western blotting assays in (d, g). (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated). All data are presented as mean ± SD, and significance was determined by one-way ANOVA.
Liver-specific PERK silencing improves OLZ-induced lipid metabolism imbalance in mice
To investigate the potential for liver Perk knockdown to improve blood lipid perturbations in OLZ-treated mice, female C57BL/6 J mice were administered liver-specific serotype AAVs by tail vein injection during OLZ treatment (Fig. 6a). To confirm the efficacy of knockdown in livers, we validated the expression levels of Perk on the 21st day. AAV interference significantly downregulated Perk at both mRNA and protein levels (Fig. 6c, P < 0.01). Our results also revealed that the Perk-AAV + OLZ group exhibited a time-dependent reduction in body weight increase compared to the Con-AAV + OLZ group, following OLZ treatment (Fig. 6b, P < 0.01, P < 0.001). The liver weight and visceral fat weight were also measured at the end of the experiment. Results showed that the Perk-AAV + OLZ group had a significant reduction in both liver weight and visceral fat weight compared to the Con-AAV + OLZ group (Supplementary Table S3, P < 0.05). Oil Red O and H&E staining uncovered that the aggregation of lipid droplets and steatosis induced by OLZ were significantly attenuated in the livers of Perk-AAV-treated mice compared to those of the Con-AAV + OLZ group. These findings were further supported by liver TG quantification analysis (Fig. 6d, e, P < 0.001). Lipid biochemical analysis results demonstrated a significant decrease in serum levels of TG, TC, and LDL-C, and a remarkable increase in HDL-C in the Perk-AAV + OLZ group compared to the Con-AAV + OLZ group (Fig. 6f, P < 0.05, P < 0.01, P < 0.001). Furthermore, Western blot assays showed that the levels of ER stress markers (p-PERK, p-elF2α, ATF4, and CHOP) and de novo lipogenic proteins (SREBP1c-N, SREBP2-N, FASN, SCD1, and HMGCR) were noteworthily downregulated in Perk-AAV + OLZ group compared to the Con-AAV + OLZ group (Fig. 6g, h, P < 0.05, P < 0.01, P < 0.001). Taken together, these data provided further evidence for the essential role of PERK in promoting OLZ-induced lipid dysfunction and showed that liver-specific knockdown of PERK protected against the development of dyslipidemia in OLZ-induced metabolic syndrome.
Fig. 6. Liver-specific PERK silencing improves OLZ-induced lipid dysregulation in mice.
a The graphic description shows the protocol for Con-AAV, Perk-AAV, and OLZ or Vehicle administration in mice. b Body weight gain was represented as a line chart (n = 6/groups, **P < 0.01, ***P < 0.001 as indicated, Con-AAV + OLZ versus Con-AAV + Vehicle, Perk-AAV + OLZ versus Con-AAV + OLZ). c The expression levels of PERK in mice livers were measured by qPCR and Western blot (n = 3 independent experiments for the in vivo study, ***P < 0.001 as indicated, Perk-AAV versus Con-AAV). d Paraffin-embedded liver sections were stained with Oil Red O and H&E. Scale bar: 50 μm. e The quantification of TG content in mice livers was shown in the bar graph (n = 6 independent experiments for the in vitro study, **P < 0.05, ***P < 0.001 as indicated). f The Graph presented the serum TG, TCHO, HDL-C, and LDL-C of mice in each group (n = 6/groups, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated, Con-AAV + OLZ versus Con-AAV + Vehicle, Perk-AAV + OLZ versus Con-AAV + OLZ). g The expression levels of ER stress and de novo lipogenic marker proteins were determined by Western blot in mice livers of each group (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated, Con-AAV + OLZ versus Con-AAV + Vehicle, Perk-AAV + OLZ versus Con-AAV + OLZ). h Statistical analysis of Western blotting assays in (g). (n = 3 independent experiments for the in vitro study, *P < 0.05, **P < 0.01, ***P < 0.001 as indicated). All data are presented as mean ± SD, and significance was determined by one-way ANOVA.
Discussion
This study demonstrates the critical involvement of the PERK-CHOP branch of ER stress in the development of lipid disorders induced by OLZ treatment. Clinically, the ER stress sensor (GRP78) was a potential predictive biomarker for OLZ-induced dyslipidemia in schizophrenia patients. Functionally, targeting ER stress was found to be protective against SREBP-mediated lipogenesis triggered by OLZ. Mechanistically, the knockdown of PERK resulted in a significant improvement in lipid droplet accumulation, hepatic steatosis, and blood lipids in cell and mouse models treated with OLZ (Fig. 7).
Fig. 7. The graphical mechanism of OLZ causes lipid disorder.
OLZ disrupted ER homeostasis and activated PERK-CHOP signaling leading to the activation of SREBPs in the liver. Moreover, the consequent activation of FASN, SCD1, and HMGCR driven by SREBPs enhanced cellular de novo lipogenesis and eventually resulted in dyslipidemia.
Intriguingly, our case-control study revealed that gender, particularly female, may represent a risk factor for developing dyslipidemia during OLZ treatment. This could be due to slower drug absorption, metabolism, and excretion in women, leading to higher plasma levels of APDs than in men [45]. Moreover, the increased dopamine sensitivities caused by estrogens result in higher dopamine receptor occupancy at the same dose in women compared to men, leading to a relative overdose of APDs in women [46]. These two theories offer better insight into why female subjects are more vulnerable to side effects. APD-induced obesity and obesity-related metabolic syndrome have been well-documented in previous studies [4, 9]. However, dyslipidemia caused by OLZ can not be fully explained by weight gain, as our clinical data has confirmed. To explore novel potential peripheral mechanisms for OLZ-induced lipid imbalance, we hypothesized that ER stress may be involved in the regulation of lipid disorders. Intriguingly, our case-control study revealed that ER stress biomarkers were remarkably elevated in schizophrenia patients with dyslipidemia after OLZ treatment. Multiple logistic regression analysis implied that serum GRP78 levels may represent a potential predictive risk factor in the development of OLZ-induced dyslipidemia. Although there were statistical differences in the levels of GRP78 and ATF4 between dyslipidemia and non-dyslipidemia subjects at baseline, the levels of ER stress biomarkers were notably higher in dyslipidemia subjects receiving OLZ compared to non-dyslipidemia subjects. This may be due to the differences in specific psychiatric symptoms between the two groups. In other words, schizophrenia patients with higher serum levels of GRP78 seem may be more susceptible to dyslipidemia when receiving OLZ treatment. Realistically, the limitations of our study include the sample size and group representativeness. GRP78 is a sensor of ER stress and participates in diverse pathophysiological processes. Recent studies have also revealed that serum GRP78 levels could be a representative marker in different human pathologies, including COVID-19, ovarian cancer, and even metabolic disease [47–49]. Therefore, these elevations in biomarkers suggest that ER stress plays an important role in OLZ-induced dyslipidemia to a certain extent.
To confirm the mechanism behind OLZ-induced activations of SREBPs, HepG2 cells, and mice were used to demonstrate that ER stress contributed to the pathogenesis of lipogenesis. There are two major isoforms of SREBPs involved in OLZ-induced lipid disruption: SREBP1c, which mainly regulates fatty acid biosynthesis and energy storage, and SREBP2, which is responsible for cholesterol metabolism and has some overlapping functions with SREBP1c in different physiological processes. As expected, our results confirmed that OLZ dramatically upregulated the expression levels of SREBP1c and its downstream genes (FASN and SCD1), as well as SREBP2 and its target gene (HMGCR) in hepatocytes and mouse livers, and promoted lipid droplet accumulation in hepatocytes. These findings are consistent with previous studies [21, 23, 25, 50, 51]. SREBPs are transcription factors containing a helix-loop-helix leucine zipper domain that binds as dimers to the SRES, inducing downstream genes that contain SRE elements in their promoters, ultimately regulating cellular lipid homeostasis. The activity of SREBPs is controlled at the transcriptional and post-transcriptional levels within the ER and Golgi apparatus [31]. Given the chemical constitution of lipid droplets and higher TG levels observed in the clinical settings, we regarded SREBP1c as a key regulator. To illustrate the interaction of ER stress and SREBP1c, we used Co-IP and LC-MS/MS analysis, which demonstrated that GRP78 interacts with SREBP1c. However, the underlying mechanism is still unknown. Studies have well documented that SREBP1c is anchored by SREBP cleavage-activating protein (SCAP) and insulin-induced genes (INSIGs) in an inactive form within the ER. Different pathogenic factors are involved in the activations of SREBP1c, including inflammations, ER stress, autophagy, and apoptosis, ultimately leading to metabolic disease [20, 33, 35, 36]. Among those factors, ER stress plays a significant role in SREBP1c-mediated lipogenesis. Our data confirmed that the degree of lipid droplet accumulation induced by OLZ could be significantly reduced by ER stress inhibitors such as 4-PBA and TUDCA. Similarly, the administration of 4-PBA to OLZ-treated mice significantly attenuated OLZ-induced weight gain, dyslipidemia, and liver steatosis. Overall, our results confirmed that targeting ER stress could ameliorate OLZ-mediated lipid dysfunction in vivo and in vitro.
The ER serves as a central universal platform for nutrient sensing, metabolic adaptation, and stress response. ER stress, also known as disrupted ER homeostasis or unfolded protein reaction (UPR), is a key feature of metabolic disorders. When ER homeostasis is interrupted by different insults, including drugs, the UPR serves as an adaptive response for cell survival. Different types of ER stress branches are intricately linked and closely related to proteotoxicity, lipotoxicity, and glucotoxicity [52]. Among them, the PERK-ATF4-CHOP signaling axis of ER stress exerts a pivotal role in the lipid metabolism of the liver [53]. To further affirm the mechanism of PERK-CHOP signaling in OLZ-induced SREBP1c activation, we validated that the effects of lipid droplet accumulation mediated by OLZ were enhanced by PERK agonist (CCT020312) and rescued by PERK antagonist (GSK2606414), as well as the expression levels of de novo lipogenic markers. Similarly, the effect of OLZ-induced ER stress and subsequent activation of SREBP1c could be remarkably diminished by PERK knockdown via lentivirus-carried shRNA in HepG2 cells. Likewise, liver-specific knockdown of PERK by AAV injection also improved body mass, liver steatosis, and blood lipids in OLZ-treated mice. Antipsychotic drug-induced ER stress is a double-edged sword and is involved in complex tissue specificity [40, 54–56]. Although Perk arm inhibition of ER stress was also validated to ameliorate metabolic dysfunction in some preclinical models [57–59], more profound mechanistic studies remain needed in the regulation of ER stress and dyslipidemia. It is important to note that we did not investigate the underlying mechanisms for OLZ-induced ER stress, which is a limitation of our research. The latest clinical trials demonstrated that OLZ- samidorphan (a κ-opioid receptor antagonist) combination tablets could better improve OLZ-induced weight gain and blood lipid parameters in patients [60, 61]. The κ-opioid receptor inhibition in the lateral hypothalamic area ameliorated melanin-concentrating hormone-induced liver steatosis by reducing ER stress in a parasympathetic nervous-controlling manner [62]. This interesting finding provides a novel direction between the crosstalk of ER stress and OLZ-induced metabolic dysfunction. Collectively, our results demonstrated that PERK-CHOP signaling activation is the main upstream contributor to OLZ-induced SREBPs upregulation in livers. We propose that targeting ER stress would be a potential strategy for the prevention and management of metabolic complications associated with OLZ treatment.
Supplementary information
Acknowledgements
This study was supported by the Chongqing Basic Research and Frontier Exploration Project (cstc2022ycjh-bgzxm0119).
Author contributions
CHH and LL conducted the literature searches and designed the experiments; JML, LT, SYC, CYY, and LL collected patient blood samples and clinical data; LL conducted the experiments and drafted the article in this study; CHH, XML, and LL analyzed and interpreted data, and revised the article; All authors approved the final version for submission.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01180-w.
References
- 1.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jauhar S, Johnstone M, Mckenna PJ. Schizophrenia. Lancet. 2022;399:473–86. doi: 10.1016/S0140-6736(21)01730-X. [DOI] [PubMed] [Google Scholar]
- 3.Ceraso A, Lin JJ, Schneider-Thoma J, Siafis S, Tardy M, Komossa K, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;2020:Cd008016. doi: 10.1002/14651858.CD008016.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cernea S, Dima L, Correll CU, Manu P. Pharmacological management of glucose dysregulation in patients treated with second-generation antipsychotics. Drugs. 2020;80:1763–81. doi: 10.1007/s40265-020-01393-x. [DOI] [PubMed] [Google Scholar]
- 5.Wada M, Noda Y, Iwata Y, Tsugawa S, Yoshida K, Tani H, et al. Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment. Mol Psychiatry. 2022;27:2950–67. doi: 10.1038/s41380-022-01572-0. [DOI] [PubMed] [Google Scholar]
- 6.Lähteenvuo M, Tiihonen J. Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs. 2021;81:1273–84. doi: 10.1007/s40265-021-01556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–64. doi: 10.1016/S2215-0366(15)00115-7. [DOI] [PubMed] [Google Scholar]
- 8.Carli M, Kolachalam S, Longoni B, Pintaudi A, Baldini M, Aringhieri S, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14:238. doi: 10.3390/ph14030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazereel V, Detraux J, Vancampfort D, Van Winkel R, De Hert M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front Endocrinol. 2020;11:573479. doi: 10.3389/fendo.2020.573479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vantaggiato C, Panzeri E, Citterio A, Orso G, Pozzi M. Antipsychotics promote metabolic disorders disrupting cellular lipid metabolism and trafficking. Trends Endocrinol Metab. 2019;30:189–210. doi: 10.1016/j.tem.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Rojo LE, Gaspar PA, Silva H, Risco L, Arena P, Cubillos-Robles K, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: A global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85. doi: 10.1016/j.phrs.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Ono S, Sugai T, Suzuki Y, Yamazaki M, Shimoda K, Mori T, et al. High-density lipoprotein-cholesterol and antipsychotic medication in overweight inpatients with schizophrenia: post-hoc analysis of a Japanese nationwide survey. BMC Psychiatry. 2018;18:1–7. doi: 10.1186/s12888-018-1764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. 2019;76:1052–62. doi: 10.1001/jamapsychiatry.2019.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira V, Folgueira C, Guillén M, Zubiaur P, Navares M, Sarsenbayeva A, et al. Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight. Metabolism. 2022;137:155335. doi: 10.1016/j.metabol.2022.155335. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Hei GR, Yang Y, Liu CC, Xiao JM, Long YJ, et al. Increased appetite plays a key role in olanzapine-induced weight gain in first-episode schizophrenia patients. Front Pharmacol. 2020;11:739. doi: 10.3389/fphar.2020.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handen BL, Anagnostou E, Aman MG, Sanders KB, Chan J, Hollway JA, et al. A randomized, placebo-controlled trial of metformin for the treatment of overweight induced by antipsychotic medication in young people with autism spectrum disorder: open-label extension. J Am Acad Child Adolesc Psychiatry. 2017;56:849–56. doi: 10.1016/j.jaac.2017.07.790. [DOI] [PubMed] [Google Scholar]
- 17.Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS One. 2011;6:e22662. doi: 10.1371/journal.pone.0022662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn MK, Wolever TM, Arenovich T, Teo C, Giacca A, Powell V, et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol. 2013;33:740–6. doi: 10.1097/JCP.0b013e31829e8333. [DOI] [PubMed] [Google Scholar]
- 19.Vucicevic L, Misirkic-Marjanovic M, Paunovic V, Kravic-Stevovic T, Martinovic T, Ciric D, et al. Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy. 2014;10:2362–78. doi: 10.4161/15548627.2014.984270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–30. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Lian J, Hu CH, Deng C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol Res. 2015;100:36–46. doi: 10.1016/j.phrs.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Lian J, Huang XF, Pai N, Deng C. Ameliorating antipsychotic-induced weight gain by betahistine: mechanisms and clinical implications. Pharmacol Res. 2016;106:51–63. doi: 10.1016/j.phrs.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Deng C, Cao S, Gong J, Wang BC, Hu CH. Acute effects of oral olanzapine treatment on the expression of fatty acid and cholesterol metabolism-related gene in rats. Life Sci. 2015;128:72–8. doi: 10.1016/j.lfs.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Raeder MB, Fernø J, Vik-Mo AO, Steen VM. SREBP activation by antipsychotic-and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects. Mol Cell Biochem. 2006;289:167–73. doi: 10.1007/s11010-006-9160-4. [DOI] [PubMed] [Google Scholar]
- 25.Yang LH, Chen TM, Yu ST, Chen YH. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol Res. 2007;56:202–8. doi: 10.1016/j.phrs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, et al. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–42. doi: 10.1016/j.schres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Skrede S, Fernø J, Vázquez MJ, Fjær S, Pavlin T, Lunder N, et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol. 2012;15:163–79. doi: 10.1017/S1461145711001271. [DOI] [PubMed] [Google Scholar]
- 28.Le Hellard S, Mühleisen T, Djurovic S, Fernø J, Ouriaghi Z, Mattheisen M, et al. Polymorphisms in SREBF1 and SREBF2, two antipsychotic-activated transcription factors controlling cellular lipogenesis, are associated with schizophrenia in German and Scandinavian samples. Mol Psychiatry. 2010;15:463–72. doi: 10.1038/mp.2008.110. [DOI] [PubMed] [Google Scholar]
- 29.Vassas TJ, Burghardt KJ, Ellingrod VL. Pharmacogenomics of sterol synthesis and statin use in schizophrenia subjects treated with antipsychotics. Pharmacogenomics. 2014;15:61–7. doi: 10.2217/pgs.13.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Chen J, Liu D, Yu S, Cong E, Li Y, et al. Association between SREBF2 gene polymorphisms and metabolic syndrome in clozapine-treated patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:136–41. doi: 10.1016/j.pnpbp.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Debose-Boyd RA, Ye J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem Sci. 2018;43:358–68. doi: 10.1016/j.tibs.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–5. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Garcia-Carbonell R, Yamachika S, Zhao P, Dhar D, Loomba R, et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell. 2018;175:133–45. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. 2021;33:319–33. doi: 10.1016/j.cmet.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura S, Matsushita Y, Kurosaki S, Tange M, Fujiwara N, Hayata Y, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis. J Clin Invest. 2022;132:e151895. doi: 10.1172/JCI151895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, Wang LQ, Sladky VC, Oh TG, Liu J, Trinh K, et al. PIDDosome-SCAP crosstalk controls high-fructose-diet-dependent transition from simple steatosis to steatohepatitis. Cell Metab. 2022;34:1548–60. doi: 10.1016/j.cmet.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng ZG, Zhu ST, Cheng HM, Zhang X, Cheng G, Thu PM, et al. Discovery of a potent SCAP degrader that ameliorates HFD-induced obesity, hyperlipidemia and insulin resistance via an autophagy-independent lysosomal pathway. Autophagy. 2021;17:1592–613. doi: 10.1080/15548627.2020.1757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S, Sharma D, Kalia K, Tiwari V. Crosstalk between endoplasmic reticulum stress and oxidative stress in schizophrenia: The dawn of new therapeutic approaches. Neurosci Biobehav Rev. 2017;83:589–603. doi: 10.1016/j.neubiorev.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Zhou R, He M, Fan J, Li R, Zuo Y, Li B, et al. The role of hypothalamic endoplasmic reticulum stress in schizophrenia and antipsychotic-induced weight gain: a narrative review. Front Neurosci. 2022;16:947295. doi: 10.3389/fnins.2022.947295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He M, Huang XF, Gao G, Zhou T, Li W, Hu J, et al. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology. 2019;104:286–99. doi: 10.1016/j.psyneuen.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Wu H, Tang X, Chen L. 4-Phenylbutyrate protects against rifampin-induced liver injury via regulating MRP2 ubiquitination through inhibiting endoplasmic reticulum stress. Bioengineered. 2022;13:2866–77. doi: 10.1080/21655979.2021.2024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YR, Lee EJ, Shin KO, Kim MH, Pewzner-Jung Y, Lee YM, et al. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp Mol Med. 2019;51:1–16. doi: 10.1038/s12276-019-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Zhang M, Yu H, Lu J, Cheng KK, Zhou J, et al. Activation of G0/G1 switch gene 2 by endoplasmic reticulum stress enhances hepatic steatosis. Metabolism. 2019;99:32–44. doi: 10.1016/j.metabol.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Liu XM, Zhao XM, Deng C, Zeng YP, Hu CH. Simvastatin improves olanzapine-induced dyslipidemia in rats through inhibiting hepatic mTOR signaling pathway. Acta Pharmacol Sin. 2019;40:1049–57. doi: 10.1038/s41401-019-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brand BA, De Boer JN, Dazzan P, Sommer IE. Towards better care for women with schizophrenia-spectrum disorders. Lancet Psychiatry. 2022;9:330–6. doi: 10.1016/S2215-0366(21)00383-7. [DOI] [PubMed] [Google Scholar]
- 46.Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Girona J, Rodríguez-Borjabad C, Ibarretxe D, Vallvé JC, Ferré R, Heras M, et al. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med. 2019;8:1793. doi: 10.3390/jcm8111793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paris EA, Bahr JM, Abramowicz JS, Basu S, Barua A. Glucose-regulated protein 78 is a potential serum and imaging marker for early detection of ovarian cancer. Cancers. 2023;15:1140. doi: 10.3390/cancers15041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabirli R, Koseler A, Goren T, Turkcuer I, Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265:118781. doi: 10.1016/j.lfs.2020.118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristiana I, Sharpe L, Catts V, Lutze-Mann L, Brown A. Antipsychotic drugs upregulate lipogenic gene expression by disrupting intracellular trafficking of lipoprotein-derived cholesterol. Pharmacogenomics J. 2010;10:396–407. doi: 10.1038/tpj.2009.62. [DOI] [PubMed] [Google Scholar]
- 51.Su Y, Liu X, Lian J, Deng C. Epigenetic histone modulations of PPARγ and related pathways contribute to olanzapine-induced metabolic disorders. Pharmacol Res. 2020;155:104703. doi: 10.1016/j.phrs.2020.104703. [DOI] [PubMed] [Google Scholar]
- 52.Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Q, Chen Y, Wang J, Hao L, Huang C, Griffiths A, et al. ER stress-induced upregulation of NNMT contributes to alcohol-related fatty liver development. J Hepatol. 2020;73:783–93. doi: 10.1016/j.jhep.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matteoni S, Matarrese P, Ascione B, Ricci-Vitiani L, Pallini R, Villani V, et al. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J Exp Clin Cancer Res. 2021;40:347. doi: 10.1186/s13046-021-02144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi L, Chen H, Chen K, Zhong C, Song C, Huang Y, et al. The DRD2 antagonist haloperidol mediates autophagy-induced ferroptosis to increase temozolomide sensitivity by promoting endoplasmic reticulum stress in glioblastoma. Clin Cancer Res. 2023;29:3172–88. doi: 10.1158/1078-0432.CCR-22-3971. [DOI] [PubMed] [Google Scholar]
- 56.Weston-Green K, Babic I, De Santis M, Pan B, Montgomery MK, Mitchell T, et al. Disrupted sphingolipid metabolism following acute clozapine and olanzapine administration. J Biomed Sci. 2018;25:1–11. doi: 10.1186/s12929-018-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chattopadhyay A, Guan P, Majumder S, Kaw K, Zhou Z, Zhang C, et al. Preventing cholesterol-induced perk (protein kinase RNA-like endoplasmic reticulum kinase) signaling in smooth muscle cells blocks atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol. 2022;42:1005–22. doi: 10.1161/ATVBAHA.121.317451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30:1141–51. doi: 10.1016/j.cmet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Wang B, Zhang M, Urabe G, Huang Y, Chen G, Wheeler D, et al. PERK inhibition mitigates restenosis and thrombosis: a potential low-thrombogenic antirestenotic paradigm. JACC Basic Transl Sci. 2020;5:245–63. doi: 10.1016/j.jacbts.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Correll CU, Newcomer JW, Silverman B, Dipetrillo L, Graham C, Jiang Y, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177:1168–78. doi: 10.1176/appi.ajp.2020.19121279. [DOI] [PubMed] [Google Scholar]
- 61.Correll CU, Stein E, Graham C, Dipetrillo L, Akerman S, Stanford AD, et al. Reduction in multiple cardiometabolic risk factors with combined olanzapine/samidorphan compared with olanzapine: post hoc analyses from a 24-week phase 3 study. Schizophr Bull. 2023;49:454–63. doi: 10.1093/schbul/sbac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imbernon M, Sanchez-Rebordelo E, Romero-Picó A, Kalló I, Chee MJ, Porteiro B, et al. Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology. 2016;64:1086–104. doi: 10.1002/hep.28716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.